Fluxes of NH3, but not NH4+, account for futile cycling across both plasmalemma and tonoplast in roots of barley, resulting in a thermodynamic NH3 equilibrium between cytosol, vacuole, and external solution, and aquaporins are likely mediators of these fluxes.
Abstract
Futile transmembrane NH3/NH4+ cycling in plant root cells, characterized by extremely rapid fluxes and high efflux to influx ratios, has been successfully linked to NH3/NH4+ toxicity. Surprisingly, the fundamental question of which species of the conjugate pair (NH3 or NH4+) participates in such fluxes is unresolved. Using flux analyses with the short-lived radioisotope 13N and electrophysiological, respiratory, and histochemical measurements, we show that futile cycling in roots of barley (Hordeum vulgare) seedlings is predominately of the gaseous NH3 species, rather than the NH4+ ion. Influx of 13NH3/13NH4+, which exceeded 200 µmol g–1 h–1, was not commensurate with membrane depolarization or increases in root respiration, suggesting electroneutral NH3 transport. Influx followed Michaelis-Menten kinetics for NH3 (but not NH4+), as a function of external concentration (Km = 152 µm, Vmax = 205 µmol g–1 h–1). Efflux of 13NH3/13NH4+ responded with a nearly identical Km. Pharmacological characterization of influx and efflux suggests mediation by aquaporins. Our study fundamentally revises the futile-cycling model by demonstrating that NH3 is the major permeating species across both plasmalemma and tonoplast of root cells under toxicity conditions.
Ammonia/ammonium (NH3/NH4+) toxicity in higher plants has resulted in crop reduction and forest decline (Pearson and Stewart, 1993; Vitousek et al., 1997; Britto and Kronzucker, 2002), biodiversity loss (Stevens et al., 2004; Bobbink et al., 2010), and species extirpation (de Graaf et al., 1998; McClean et al., 2011). These major ecological and economic problems have been aggravated by an accelerated global nitrogen (N) cycle caused primarily by the industrialized production and use of N fertilizers (Galloway et al., 2008; Gruber and Galloway, 2008). With increasing global population and demands on agricultural production, there is no sign of this trend easing: anthropogenic N fixation has reached 210 teragrams year–1, an approximately 12% increase from 2005 and an approximately 1,300% rise from 150 years ago (Galloway et al., 2008; Fowler et al., 2013).
Although considerable knowledge of the causes and mechanisms of NH3/NH4+ toxicity has accrued in recent years, our understanding of the key processes remains rudimentary (Gerendas et al., 1997; Britto and Kronzucker, 2002). A major hypothesis is that of futile transmembrane NH4+ cycling, which proposes a pathological inability of root cells to restrict the primary entry of NH4+ at high external concentrations ([NH4+]ext); many downstream toxicological events are contingent upon this entry (Britto et al., 2001b). In this model, a rapid, thermodynamically passive influx of NH4+ is coupled to an active efflux of NH4+ that is nearly as rapid, constraining normal cellular function and energetics and resulting in plant growth decline and mortality. This phenomenon is thought to occur in NH4+-sensitive species such as barley (Hordeum vulgare) and, to a lesser extent, in tolerant species such as rice (Oryza sativa), which can be susceptible at higher thresholds (Balkos et al., 2010; Chen et al., 2013).
Most soils are typically acidic, especially when [NH4+] is high (i.e. in the millimolar range; Van Breemen et al., 1982; Bobbink et al., 1998; Britto and Kronzucker, 2002), and given the pKa of 9.25 for the conjugate pair NH3/NH4+, [NH3] is generally low (Izaurralde et al., 1990; Weise et al., 2013). Consequently, the fluxes of NH3 have largely been considered negligible (Britto et al., 2001a; Britto and Kronzucker, 2002; Loqué and von Wirén, 2004), in contrast to NH4+ fluxes, which are well characterized physiologically (Lee and Ayling, 1993; Wang et al., 1993a, 1993b; Kronzucker et al., 1996) and at the molecular level (Rawat et al., 1999; von Wirén et al., 2000; Ludewig et al., 2007), at least at lower concentrations. However, the transport of NH3 across membranes has received new attention in the light of evidence that some members of the aquaporin (AQP) family of transporters, a diverse and ubiquitous class of major intrinsic proteins (Maurel et al., 2008; Hove and Bhave, 2011), can mediate NH3 fluxes in single-cell systems (Jahn et al., 2004; Holm et al., 2005; Loqué et al., 2005; Saparov et al., 2007). However, a convincing demonstration that AQPs transport NH3 in planta is currently lacking. Given the unusually high capacity of AQP-mediated fluxes relative to those of ion channels and other transporters (Kozono et al., 2002), it is possible that sizable NH3 fluxes can be conducted through AQPs, even at very low external NH3 concentration ([NH3]ext).
Here, we have critically reexamined the hypothesis that futile cycling is composed of cationic NH4+ fluxes across the plasmalemma, of which an active efflux mechanism accounts for energetic demands directly contributing to toxicity (Britto et al., 2001b). We present evidence for the following alternative scenario: 1) futile cycling consists mainly of the passive electroneutral flux of the conjugate base NH3; 2) such fluxes rapidly span both major membrane systems in root cells (i.e. plasmalemma and tonoplast); 3) AQPs mediate such fluxes; and 4) a thermodynamic equilibrium of NH3 is established throughout the cell, resulting in hyperaccumulation of NH4+ in the acidic vacuole. This evidence comes primarily from positron emission tracing with the short-lived radioisotope 13N, used to characterize the component fluxes of futile cycling at the cellular level in the model species barley. We have coupled this with 42K+ radiotracing, to provide comparison with a well-understood cationic flux, as well as electrophysiological, respiratory, pharmacological, and histochemical analyses.
RESULTS
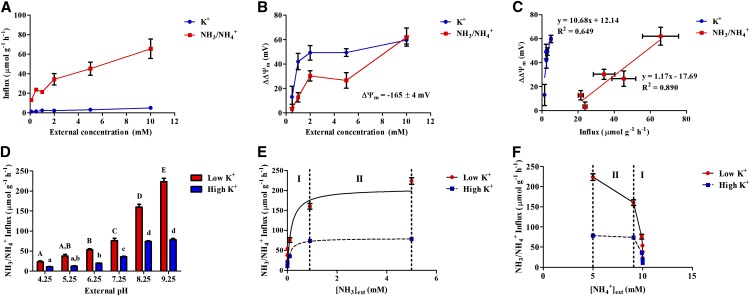
To gauge the relative contributions of NH3 and NH4+ transport, concentration-dependent root NH3/NH4+ influxes and their associated plasma membrane depolarization (change in membrane potential [∆∆Ψm]) were compared with those of the macronutrient ion potassium (K+; Fig. 1, A–C). In these experiments, plants were grown under nontoxic conditions, using a complete nutrient medium with K+ and NH4+ both provided at 0.1 mm (pH 6.25). Direct influx measurements with 42K+ and 13NH3/13NH4+, determined between 0.1 and 10 mm for each (with the other held constant at 0.1 mm), show influx of the two ions to have vastly different rates and isotherm shapes. For instance, steady-state NH3/NH4+ influx (i.e. measured at the growth concentrations of 0.1 mm K+ and NH4+) was 11.5-fold higher than that of K+ (13.12 ± 0.85 versus 1.26 ± 0.06 µmol g–1 h–1, respectively). As each substrate’s concentration independently rose to 10 mm, its influx increased 4- to 5-fold, peaking with a NH3/NH4+ influx 13-fold higher than that of K+ (Fig. 1A). Interestingly, however, the rise in NH3/NH4+ influx was not commensurate with membrane depolarization, indicating that most of the influx observed was not electrogenic and supporting the idea that NH3, not NH4+, is the main transported N species. The significance of this result was underscored when compared with changes in ∆∆Ψm observed with K+: increases in external K+ concentration resulted in up to 4-fold greater depolarization than seen with comparable changes in NH3/NH4+ (Fig. 1B), despite NH3/NH4+ influx being more than 10 times higher than K+ influx. Only at 10 mm were depolarizations of similar magnitude observed (approximately 60 mV). Figure 1C further illustrates this disproportion, by showing ∆∆Ψm as a function of influx for each substrate. The 9-fold steeper slope with K+ relative to NH3/NH4+ illustrates the much greater electrical response elicited by K+ transport.
Figure 1.
NH3 (not NH4+) is the main permeating species in barley roots. A and B, Concentration dependence of influx (A) and ∆∆Ψm (B) of NH3/NH4+ (red) and K+ (blue) in plants grown at 0.05 mm (NH4)2SO4 and K2SO4. C, Data from A and B replotted to show relationship between ∆∆Ψm and influx. D, Root NH3/NH4+ influx as a function of external pH. E and F, Data from D replotted to show dependence of NH3/NH4+ influx on NH3 (E) or NH4+ (F) concentrations, which were predicted from solution pH, according to the Henderson-Hasselbalch equation (pKa of NH3/NH4+ = 9.25). Area I represents pH 4.25 to 8.25; area II represents pH 8.25 to 9.25. Plants were grown at 5 mm (NH4)2SO4 and either low (0.01 mm, red) or high (2.5 mm, blue) K2SO4. For all sections, error bars represent ± se of the mean (n ≥ 3). Letters in D denote significantly different means (P < 0.05) as determined by a one-way ANOVA with Tukey’s posthoc test. [See online article for color version of this figure.]
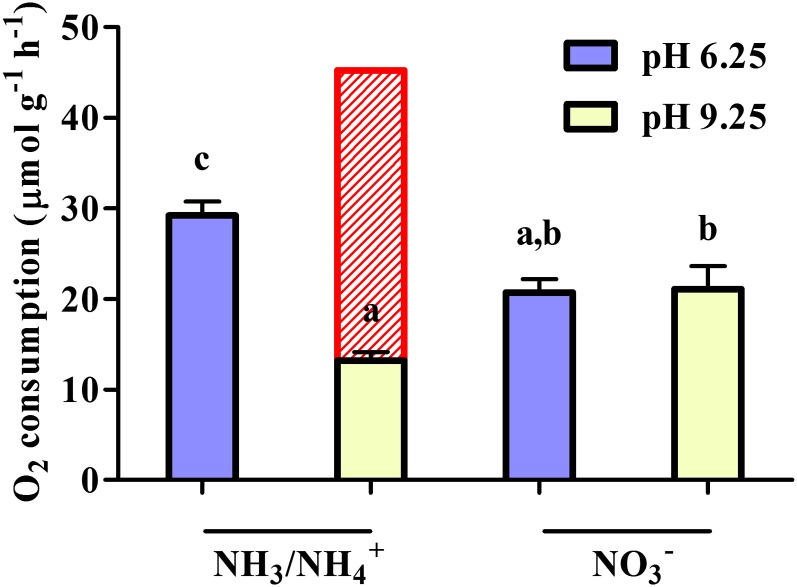
To further test the capacity of NH3 transport in planta, we monitored NH3/NH4+ influx as a function of each conjugate species’ external concentration independently, by adjusting solution pH and thus the [NH3] to [NH4+] ratio (pKa = 9.25; Fig. 1, D–F). Seedlings were grown under high (10 mm) external NH3/NH4+ concentration ([NH3/NH4+]ext) in a full-nutrient medium (pH 6.25), then placed in growth solution with pH ranging between 4.25 and 9.25 for 10 min, prior to influx measurement with 13NH3/13NH4+. Influx showed significant stimulations with rising pH over the entire range (Fig. 1D) and followed clear Michaelis-Menten kinetics with rising [NH3]ext (derived using the Henderson-Hasselbalch equation; Fig. 1E). By contrast, influx as a function of rising [NH4+]ext showed a declining pattern, particularly above 9 mm (Fig. 1F). These effects were observed under both low (0.02 mm) and high (5 mm) external K+ concentrations, which were applied in context of the known regulation of NH3/NH4+ fluxes by K+ (Szczerba et al., 2008; Balkos et al., 2010; ten Hoopen et al., 2010). Under low K+, where NH3/NH4+ toxicity is most severe (Britto and Kronzucker, 2002; Balkos et al., 2010), total influx plateaued at approximately 200 µmol g–1 h–1, the highest transmembrane flux of NH3/NH4+ hitherto reported in any plant system. Under high K+, where relief from toxicity is observed (Britto and Kronzucker, 2002), [NH3]ext-dependent influx was significantly lower, as apparent in the decrease in Vmax (from 204.8 ± 14.5 to 80.0 ± 4.5 µmol g–1 h–1 for low- and high-K+ plants, respectively). By contrast, no significant differences in Km were observed between K+ conditions (0.15 ± 0.05 versus 0.09 ± 0.03 mm for low- and high-K+ plants, respectively). The energetic consequences of increases in NH3/NH4+ influx with pH were also tested using root respiration measurements. We found that, despite the much higher influx observed when pH was changed from 6.25 to 9.25, steady-state root O2 consumption decreased by approximately 55% within 5 min of this change in low-K+, high-NH3/NH4+ plants (Fig. 2). By contrast, no such changes were observed when nitrate (NO3–) was the sole N source.
Figure 2.
Effect of 5-min exposure to elevated pH (pH 9.25) on root respiration in barley plants grown with 0.01 mm K2SO4 and 5 mm of either (NH4)2SO4 or Ca(NO3)2. Red bar represents O2 consumption, predicted if NH3/NH4+ influx at pH 9.25 is comprised entirely of cationic NH4+ fluxes (see Fig. 1F). Letters denote significantly different means (P < 0.05) as determined by one-way ANOVA with Tukey’s posthoc test. [See online article for color version of this figure.]
As with influx, efflux of 13NH3/13NH4+ from prelabeled roots was strongly stimulated by alkaline solution pH (and thus, higher external [NH3] to [NH4+] ratios; Supplemental Fig. S1A). In plants grown on high NH3/NH4+ and low K+, sudden (at 8 min; Supplemental Fig. S1A, see arrow) upward shifts in external pH immediately and significantly stimulated 13NH3/13NH4+ efflux, with greater stimulations observed at higher pH values. When tracer release was plotted as a function of the concomitant NH3/NH4+ influx (measured at the identical pH shift), we observed a strong linear relationship between efflux and influx (Supplemental Fig. S1B). Moreover, tracer release as a function of [NH3]ext (which we suggest may be equivalent to cytosolic [NH3]; see below) resulted in Michaelis-Menten kinetics similar to those seen with influx, having Km values ranging from 0.10 to 0.36 mm [NH3] (Supplemental Fig. S1B, inset).
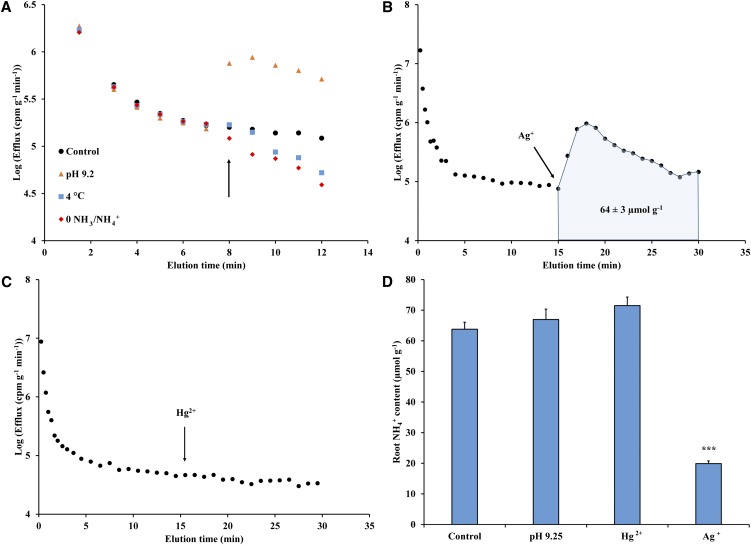
We sought to further characterize 13NH3/13NH4+ efflux in intact roots under toxicity conditions (low K+, high NH3/NH4+) to identify the compartmental origin of tracer release (Britto and Kronzucker, 2003; Coskun et al., 2010). In roots prelabeled with tracer, 13NH3/13NH4+ efflux was immediately suppressed by sudden (at 8 min; Fig. 3A, see arrow) exposure to 4°C or upon withdrawal of external NH3/NH4+, while an external pH shift to 9.25 (from 6.25) resulted in an immediate and sizable efflux stimulation. Importantly, these findings demonstrate that efflux analysis under toxic conditions captures physiological (i.e. transmembrane) events, not artifacts of apoplastic exchange (Coskun et al., 2010, 2013). Thus, compartmental analysis by tracer efflux could be applied (Lee and Clarkson, 1986; Kronzucker et al., 1997; Britto and Kronzucker, 2003), revealing efflux to influx ratios of approximately 80% and extremely rapid rates of both unidirectional fluxes characteristic of futile cycling (Britto et al., 2001b).
Figure 3.
Characterization of NH3/NH4+ efflux from roots of intact barley seedlings. A, Effect of sudden (at 8 min; see arrow) exposure to either pH 9.25, 4°C, or N withdrawal from the external medium. Each point represents the mean of three to seven replicates (se of the mean <15% of the mean). B, Effect of sudden (at 15 min; see arrow) exposure to 500 µm Ag+. Integration range of tracer release due to Ag+ and results of integration are given in shaded area. Each point represents the mean of four replicates (se of the mean <15% of the mean). C, Lack of effect of 500 µm Hg2+ application (at 15.5 min; see arrow) on root 13NH3/13NH4+ efflux. Each point represents the mean of three replicates (se of the mean <15% of the mean). D, Root NH4+ content measured using OPA and its effect due to 15-min exposure to pH 9.25, 500 µm Hg2+, or 500 µm Ag+. Each bar represents mean ± se of the mean (n ≥ 6). Asterisks denote significant difference from control (P ≤ 0.001) as determined by one-way ANOVA with Dunnett’s posthoc test. In all sections, plants were grown at 5 mm (NH4)2SO4 and 0.01 mm K2SO4. [See online article for color version of this figure.]
Further evidence for the intracellular origin of effluxed tracer was seen in a silver (Ag+)-induced stimulation of 13NH3/13NH4+ release (Fig. 3B). We have previously shown that sudden exposure to Ag+ causes extensive damage to both major membrane systems (plasmalemma and tonoplast) in barley roots (Coskun et al., 2012). By contrast, we observed no effect of mercury (Hg2+) application on tracer release (Fig. 3C), suggesting a lack of membrane disintegrity occurring. Importantly, this qualifies the use of Hg2+ as a potential inhibitor of AQPs (see below). With respect to the Ag+-induced stimulation in tracer efflux, this effect allowed for quantification of released substrate (in terms of µmol g–1 root fresh weight) via integration of the 13N loss and estimated intracellular specific activity, as shown previously (Coskun et al., 2012). The chemical quantity of NH3/NH4+ released during Ag+ application (64 ± 3 µmol g–1) was very similar to that of total root tissue NH3/NH4+ content under control conditions, as measured by chemical (orthophthaldehyde [OPA]) analysis (63.8 ± 2.3 µmol g–1; Fig. 3D). We should note that, although efflux still proceeded after termination of the Ag+ treatment (Fig. 3B), the apparent premature curtailment of the treatment resulted in an underestimate of no more than approximately 1 µmol g–1, which was considered negligible. Tissue analysis (determined by OPA assay) revealed that approximately 70% of root NH3/NH4+ was lost during Ag+ exposure, demonstrating that the majority of cellular (i.e. both cytoplasmic and vacuolar) NH3/NH4+ was released (Fig. 3D). By contrast, pH 9.25 and Hg2+ resulted in no change in tissue NH3/NH4+ content (Fig. 3D), despite the significant effects on both influx and efflux of the former (see above).
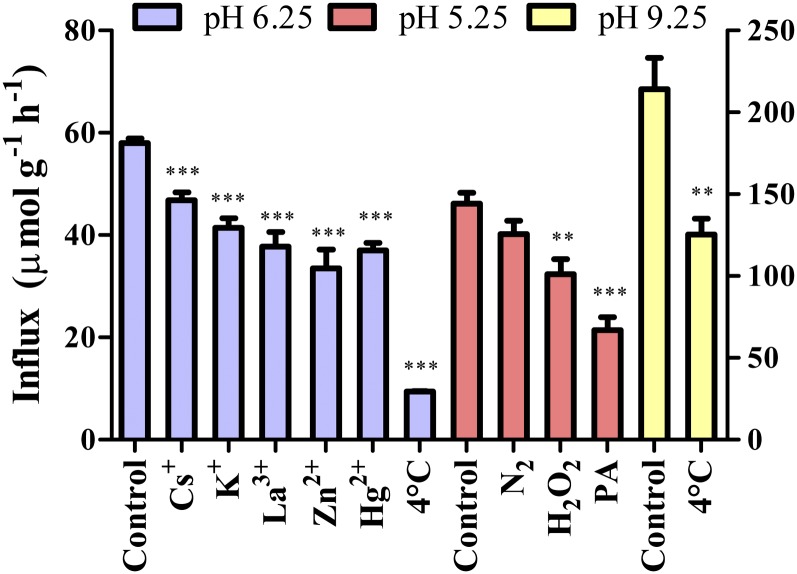
Lastly, to gain mechanistic insight into NH3/NH4+ influx, the possible involvement of different types of membrane transporters in NH3/NH4+ influx was tested by means of pharmacological profiling, in low-K+, high-NH3/NH4+ plants (Fig. 4). Hg2+, a well-known blocker of AQP activity, was applied with significant effect (36% inhibition at pH 6.25), while further support for AQP involvement was observed with treatments known to induce intracellular acidosis, which can cause closure of AQPs via protonation of conserved His residues on the cytoplasmic side (Tournaire-Roux et al., 2003; Törnroth-Horsefield et al., 2006; Ehlert et al., 2009). Hydrogen peroxide (H2O2) and propionic acid (PA) were two such effective treatments (30% and 54% inhibition relative to control, respectively). N2 treatment, however, was not as effective, despite its efficacy in other systems (Tournaire-Roux et al., 2003). Note that these acidifying treatments were only effective at lower external pH (pH 5.25). Also, Hg2+ could not be tested at high pH (pH 9.25) due to hydroxide precipitation (Schuster, 1991). Other significant inhibitors of NH3/NH4+ influx at pH 6.25 included Cs+ < K+ < La3+ < Zn2+ << 4°C. The highest influx, seen at pH 9.25, was also suppressible at 4°C by approximately 44%.
Figure 4.
Pharmacological profile of NH3/NH4+ influx into roots of barley seedlings at varying external pH. Ionic inhibitors were applied as chloride salts, except for K+ (applied as K2SO4). Influx at pH 9.25 corresponds to y axis on the right. Each bar represents mean ± se of the mean (n ≥ 5). Asterisks denote significantly different means (**, P ≤ 0.01; ***, P ≤ 0.001) from respective control, as determined by one-way ANOVA with Dunnett’s posthoc test (at pH 6.25 and 5.25) or Student’s t test (at pH 9.25). Plants were grown as in Figure 3. [See online article for color version of this figure.]
DISCUSSION
This study critically reexamines the nature of futile transmembrane NH3/NH4+ cycling in barley roots, a phenomenon with ties to NH4+ toxicity in a wide range of higher plants (Feng et al., 1994; Britto et al., 2001b, 2002; Chen et al., 2013). We have addressed the fundamental question of which species of the conjugate pair (NH3 or NH4+) is transported in the futile cycle to thus enable insight into mechanisms of transport, compartmentation, and toxicity of NH3/NH4+.
The lack of agreement between ∆∆Ψm and changes in NH3/NH4+ influx, in contrast to K+, suggests that, above a small baseline cationic NH4+ flux no higher than that of K+ (<5 µmol g–1 h–1; Fig. 1A), electroneutral NH3 transport accounts for the observed rapid rates of 13N transport in intact barley roots (Fig. 1, A–C). While previous tracer studies have also demonstrated that NH3/NH4+ fluxes exceed those of K+ at equimolar concentrations (Scherer et al., 1984; Vale et al., 1988; Wang et al., 1996), none have provided parallel membrane potential measurements. A comparison between fluxes and ∆∆Ψm, however, is of great utility in gauging the relative apportionment of NH3 and NH4+ fluxes, as we show here.
Because such rapid NH3 fluxes in planta are simply without precedent, additional investigation was called for. Further evidence in support of NH3 uptake was seen in the Michaelis-Menten character of the [NH3]ext-dependent influx isotherms (Fig. 1E). By contrast, NH4+ influx was seen to decline with rising [NH4+]ext (Fig. 1F), ruling out a sizeable contribution from that N species. We should note, however, that because these isotherms were obtained using changes in external pH, there may be pH-specific and/or NH4+-specific effects on transport. Such effects require examination, although they are inherently difficult to ascertain because pH and [NH3] to [NH4+] ratios are inextricably linked. It should also be noted that in a study on rice, Wang et al. (1993b) observed a decline in 13NH3/13NH4+ influx with rising pH at 10 mm [NH3/NH4+]ext. However, the fluxes in their study were much lower than in this study and also were determined in an NH4+-tolerant species. Further investigation is necessary to determine whether this is a part of the strategy by which a plant may achieve tolerance to this N source.
We also provide evidence for NH3 (but not NH4+) efflux under toxic (low-K+, high-NH3/NH4+) conditions. Firstly, the trans-inhibition and -stimulation of efflux in response to changes in NH3 provision (by substrate withdrawal and pH 9.2, respectively; Fig. 3A) suggests that NH3 efflux is highly dependent on NH3 influx (see also the linear dependence of the fluxes; Supplemental Fig. S1B), which is consistent with observations that efflux to influx ratios increase with rising influx (Wang et al., 1993a; Britto et al., 2002; Britto and Kronzucker, 2006). Such trans-inhibition and -stimulation of efflux have previously been shown in barley (Britto and Kronzucker, 2003) and in the mammalian literature, specifically for amino acids (White and Christensen, 1982; Sweiry et al., 1991). In the latter case, trans-stimulation of efflux has been attributed to a large counterflow through a single transporter mediating bidirectional fluxes and, as such, could in large part explain the futile NH3 cycling in this paper. Further evidence that the 13N efflux trace represents 13NH3 and not 13NH4+ is found in its saturating response to [NH3]ext (Supplemental Fig. S1B, inset), which resembles that of influx (Fig. 1E). Km values for efflux, which were comparable to those for influx (ranging between 0.10–0.36 mm NH3), suggests a similar, if not identical, mechanism of NH3 transport for the two fluxes. It is not clear why the efflux step should respond so readily to changes in external NH3, when substrate binding to an efflux transporter must take place intracellularly. Intriguingly, it may be that NH3 transport responds to [NH3]ext in a manner that leads to a rapid equalization between NH3 pools on either side of the plasma membrane, and thus NH3 efflux kinetics are in fact directly responding to cytosolic [NH3] and only indirectly to [NH3]ext. NH3 may shuttle rapidly among multiple cellular compartments, establishing similar equilibrium concentrations in each, where membrane permeabilities permit (see below).
The Michaelis-Menten analyses under low-K+, high-NH3/NH4+ conditions (see above; Fig. 1E) revealed a Vmax of about 200 µmol g–1 h–1 for NH3 influx, the highest bona fide transmembrane flux hitherto reported in any plant system. Such rapid fluxes are orders of magnitude higher than typical fluxes of mineral (ionic) nutrients (Britto and Kronzucker, 2006). Although fluxes of sodium (Na+) under toxic (saline) conditions have been reported to reach or exceed such values (Lazof and Cheeseman, 1986; Essah et al., 2003; Malagoli et al., 2008), the validity of these fluxes have recently come into question, particularly with respect to their unrealistic energetic requirements (Britto and Kronzucker, 2009; Kronzucker and Britto, 2011); moreover, such fluxes are generally reported at much higher external substrate concentrations (typically, 100 mm or higher). On the other hand, such energetic limitations do not apply to the passive electroneutral fluxes of NH3. In fact, root O2 consumption was found to decline under such conditions (i.e. pH 9.25; Fig. 2), a result that further discounts NH4+-specific futile cycling, which is predicted to involve a thermodynamically active efflux (Britto et al., 2001b). Figure 2 highlights, in red, the theoretical increase in O2 consumption necessary to power an active efflux mechanism of NH4+ when fluxes are as high as 200 µmol g–1 h–1 based on current models of ion transport and O2 consumption (Poorter et al., 1991; Kurimoto et al., 2004; Britto and Kronzucker, 2009). This large energy deficit is consistent with the idea that futile cycling is primarily of the conjugate base, NH3. However, unlike with the previously proposed NH4+ cycling, the term futile here does not refer to an energy-dissipating process (e.g. Amthor, 2000), but more generally to the lack of apparent functional utility in the NH3 cycle. The fact that the pH shift did not affect root O2 consumption in NO3−-grown plants shows an N source specificity of this effect that will require further investigation to explain.
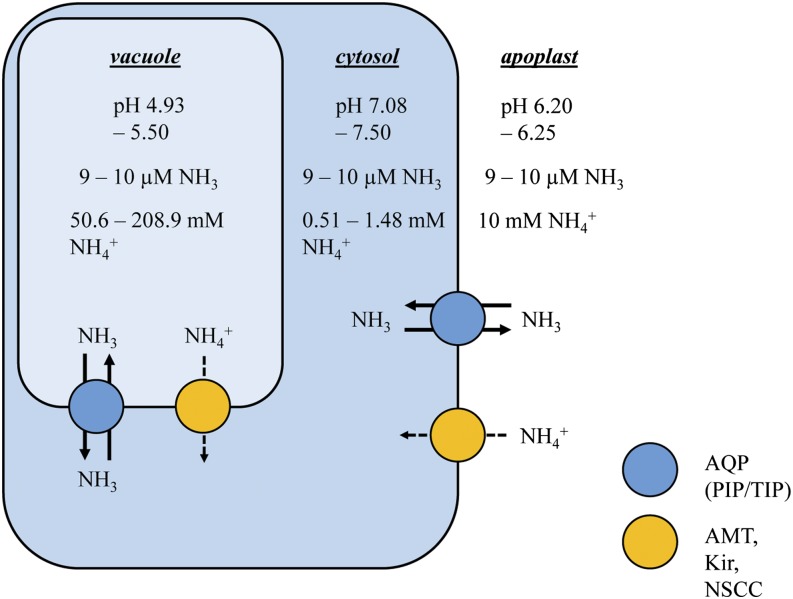
These results have important consequences for the compartmentation and toxicity of NH3/NH4+. Compartmental analyses with 13NH3/13NH4+ in roots and shoots of plants grown under toxic (high-NH3/NH4+) conditions generally yield extremely high “pool sizes” of many hundred millimolar (Britto et al., 2001b, 2002), leading to the speculation that the entire cell, not simply the cytosol, acts as a single compartment of tracer origin (Britto and Kronzucker, 2003; Balkos et al., 2010). This study provides the first evidence in support of this “whole-cell” hypothesis. Because a high-capacity, thermodynamically passive NH3 transport can account for futile cycling, it is feasible that NH3 rapidly equilibrates across intracellular membranes and among cellular compartments, particularly the vacuole and cytosol (Fig. 5). How such rapid unidirectional fluxes can persist given the apparent lack of an NH3 concentration gradient across cellular compartments is an interesting question and can be most simply explained by passive diffusion through high-capacity membrane channels such as AQPs (see below). The NH3 equilibration across cellular compartments can explain why tracer accumulation (measured as counts retained in tissue or released with Ag+ application; Fig. 3B) closely agreed with that of chemical (OPA) analyses measuring tissue NH3/NH4+ content (Fig. 3D). Thus, the NH4+ content within each compartment may be ultimately determined by NH3 permeation and compartmental pH, as illustrated in Figure 5. In this revised model of futile cellular N cycling, the 0.5 to 1.5 mm range of cytosolic [NH4+] agrees well with measured values from studies using methods such as ion-selective microelectrodes and NMR (Lee and Ratcliffe, 1991; Wells and Miller, 2000). Importantly, the model reveals that a hyperaccumulation of NH4+ in the vacuole would ultimately exist (Fig. 5), due to vacuolar acid trapping, and could explain the frequently observed suppressions in cationic nutrients, notably K+ (but also Ca2+ and Mg2+; Barker et al., 1967; Van Beusichem et al., 1988; Lang and Kaiser, 1994; Kronzucker et al., 2003), which may ultimately be the major cause of NH3/NH4+ toxicity in higher plants.
Figure 5.
Revised model of futile transmembrane NH3/NH4+ cycling in root cells of higher plants. Uncharged NH3 rapidly equilibrates across both major membrane systems (plasmalemma and tonoplast) and is likely mediated by AQPs specific to each system. A relatively minor channel/carrier-mediated flux of NH4+ may also occur across both membrane systems. NH4+ concentrations are a function of NH3 equilibration and compartment pH (Roberts et al., 1982; Walker et al., 1996; Kosegarten et al., 1997; Kosegarten et al., 1999). PIP, Plasmalemma-intrinsic protein; TIP, tonoplast-intrinsic protein; AMT, ammonium transporter; Kir, K+ inward rectifier; NSCC, nonselective cation channel. [See online article for color version of this figure.]
It is likely that the rapid NH3 cycling reported here is mediated by AQPs (Fig. 4), which have high transport capacity and are known to conduct NH3 fluxes (Jahn et al., 2004; Holm et al., 2005; Loqué et al., 2005; Saparov et al., 2007; Hove and Bhave, 2011). Kozono et al. (2002) estimated the rate of water transport through AQP1 to be 3 × 109 molecules per subunit per second, roughly 30-fold higher than K+ transport via the potassium crystallographically-sited activation (KcsA) channel, which is among the fastest ion channels (Morais-Cabral et al., 2001). AQP involvement is suggested by the pharmacological profiling of NH3 influx in our study, particularly in the effect of Hg2+, a classic AQP inhibitor (Fig. 4). Importantly, unlike with Ag+ (Fig. 3B), Hg2+ showed no sign of causing membrane damage in our system (as manifest in lack of efflux stimulation or tissue content losses; Fig. 3, C and D; compare with Coskun et al., 2012). The strong suppressions of influx by H2O2 and PA also support AQP involvement (Fig. 4; Tournaire-Roux et al., 2003; Törnroth-Horsefield et al., 2006; Ehlert et al., 2009). It is worth highlighting here, however, that pharmacological profiling, like any method, is not without its caveats. The lack of specificity of several blockers/chemical treatments (Coskun et al., 2013), as well as the need to employ relatively high concentrations at times, can potentially have secondary effects. This by no means invalidates the use of pharmacology, but highlights the importance of a diversity of experimental approaches. Future experiments with AQP antisense/knockout lines (Martre et al., 2002; Javot et al., 2003), particularly for AQPs already shown to mediate NH3 fluxes (Jahn et al., 2004; Holm et al., 2005; Loqué et al., 2005), could help further elucidate their involvement in futile NH3 cycling. In addition, such mutant analyses could provide a critical test of the “whole-cell distribution” hypothesis for NH3 presented above (Fig. 5).
We end by highlighting the suppression of Vmax for NH3 influx by high-plant K+ status, while Km remains unaffected (Fig. 1E). It appears that K+ status has no effect on the substrate affinity of NH3 transporters but regulates NH3 influx by other means. One such mechanism might involve the modulation of AQP activity, which may well be expected, because, in plants, K+ acquisition is the chief means of establishing osmotic balance and cell turgor (Britto and Kronzucker, 2008; Grzebisz et al., 2013). The effects of K+ in the short-term suppression of NH3/NH4+ influx and efflux (Szczerba et al., 2008; Balkos et al., 2010; see also Fig. 4) also suggest a posttranslational regulation of NH3 transporters by K+. Such a mechanism may explain the agriculturally important alleviation of NH3/NH4+ toxicity in higher plants by K+ (Barker et al., 1967; Szczerba et al., 2008; Balkos et al., 2010) and thus pave the way for future studies.
MATERIALS AND METHODS
Plant Culture
Barley (Hordeum vulgare ‘Metcalfe’) seedlings were grown hydroponically for 4 d (after 3-d germination in sand) in a climate-controlled growth chamber (Coskun et al., 2013). Hydroponic tanks contained aerated, N- and K+-free, modified Johnson’s solution (pH = 6.25) and were frequently exchanged to maintain a nutritional steady state. Depending on the experiment, N was supplied as either NH4+ (0.1 or 10 mm, as (NH4)2SO4) or NO3– (10 mm, as Ca(NO3)2) and K+ was supplied at either 0.02, 0.1, or 5 mm as K2SO4 (see “Results”).
Radiotracer Experiments
13N (half-life = 9.98 min) was used to trace the unidirectional fluxes and cellular compartmentalization of NH3/NH4+ in roots of intact seedlings (Kronzucker et al., 1997; Britto et al., 2001b). For steady-state influx experiments, roots were incubated for 5 min in aerated growth solution spiked with 13NH3/13NH4+, then desorbed in two sequential steps (for 5 s and 5 min) in nonradioactive growth solution to release tracer from extracellular spaces (Balkos et al., 2010). Treatment conditions were conducted as above, with some modifications. These included concentration-dependent and pH-dependent isotherms, whereby growth solution [NH3/NH4+]ext and pH were adjusted (with NaOH) as specified in all solutions, including a 10-min-preloading solution. Other treatments included 10 mm CsCl, 5 mm K2SO4, 10 mm LaCl3, 10 mm ZnCl2, and 500 µm HgCl2 and chilling (4°C) in preloading (10 min) and loading solutions. A subset of experiments involved a 2-h pretreatment at pH 5.25 ± N2 bubbling (anoxia treatment), 2 mm H2O2, or 20 mm PA. For all treatments, after the final desorption, roots were separated from shoots, spun in a low-speed centrifuge for 30 s to remove surface water, weighed, and counted for γ-ray emissions. A small subset of experiments were conducted, as above for the concentration-dependent isotherm, but with 42K (half-life = 12.36 h; Coskun et al., 2013).
For compartmental analysis by tracer efflux, roots were exposed for 1 h in loading solution to maximize intracellular-specific activity of the tracer (Kronzucker et al., 1997), then placed in efflux funnels and eluted of radioactivity with successive 20-mL aliquots of fresh, nonlabeled growth solution for various washout periods. The desorption series was timed as follows, from first to final eluate: 1.5 min (twice), 1 min (nine times), for a total of 12 min. A subset of experiments involved either chemical (pH 7.25, 8.25, and 9.25 or complete NH3/NH4+ withdrawal) or cold (4°C) treatment for the final 5 min of elution (see “Results”). Another subset involved a longer elution protocol (30 min; Coskun et al., 2012) and the sudden (at 15 min) application of 500 µm AgNO3 to disrupt membranes and release tracer accumulated in the cell (Coskun et al., 2012). Following elution, roots were handled as above, and radioactivity in roots, shoots, and efflux eluates were counted.
To quantify the chemical amount of released NH3/NH4+ during Ag+ application (above), an integration technique was employed as described in detail elsewhere (Coskun et al., 2012). In brief, the summation of radioactivity released (in counts per min) during Ag+ treatment was divided by the internal specific activity at the time of Ag+ application (taking into account the exponential rise in specific activity during loading and its decline during elution up to the time of Ag+ application) and corrected for root fresh weight.
Electrophysiological Measurements
Membrane potential differences in epidermal and cortical root cells from intact barley seedlings were measured as described in detail elsewhere (Schulze et al., 2012). In brief, roots were immersed in growth solution in a plexiglass cuvette mounted onto a light microscope. Root cells were impaled with a glass microelectrode, and potential differences were recorded with the use of an electrometer. Once stable readings were achieved, growth solution was exchanged by use of peristaltic pumps at approximately 7.5 mL min–1. Treatments included growth solution supplemented with rising concentrations of either NH3/NH4+ or K+ (see “Results”), and ∆∆Ψm was recorded.
Respiration Measurements
Root respiration was measured in intact barley seedlings as described in detail elsewhere (Malagoli et al., 2008). In brief, roots of 7-d-old seedlings were immersed in growth solution in a 3-mL Hansatech cuvette/O2 electrode system, and the decline in dissolved O2 was recorded over 10 min, after which roots were dried and weighed, as described above.
Tissue Content Measurements
Tissue NH4+ content was determined by the OPA method as described in detail elsewhere (Coskun et al., 2012). Briefly, roots of 7-d-old intact seedlings were immersed for 5 min in aerated 10 mm CaSO4 to desorb extracellular NH4+, and plant organs were harvested as described above. For treatments, roots were first immersed for 15 min in aerated growth solution supplemented with either 500 µm AgNO3, 500 µm HgCl2, or pH 9.25 (titrated with NaOH) prior to CaSO4 desorption. From there, root and shoot tissue was pulverized under liquid N2, and NH4+ was extracted with 10 mm formic acid (Husted et al., 2000). Purified supernatant was added to OPA reagent (Goyal et al., 1988; Coskun et al., 2012), and the color was left to develop in the dark at room temperature for 30 min. Sample absorbance was measured at 410 nm using a spectrophotometer.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. pH dependence of root 13NH3/13NH4+ efflux and its relationship to influx.
Supplementary Material
Acknowledgments
We thank the team at the Canadian Association for Mental Health for providing 13N, the McMaster Nuclear Reactor team for providing 42K, and Jessie R. Wong for assistance with experiments.
Glossary
- [NH4+]ext
external NH4+ concentration
- [NH3]ext
external NH3 concentration
- AQP
aquaporin
- ∆∆Ψm
change in membrane potential
- [NH3/NH4+]ext
external NH3/NH4+ concentration
- OPA
orthophthaldehyde
- H2O2
hydrogen peroxide
- PA
propionic acid
References
- Amthor JS. (2000) The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot 86: 1–20 [Google Scholar]
- Balkos KD, Britto DT, Kronzucker HJ. (2010) Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ 33: 23–34 [DOI] [PubMed] [Google Scholar]
- Barker AV, Maynard DN, Lachman WH. (1967) Induction of tomato stem and leaf lesions, and potassium deficiency, by excessive ammonium nutrition. Soil Sci 103: 319–327 [Google Scholar]
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, et al. (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20: 30–59 [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hornung M, Roelofs JG. (1998) The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J Ecol 86: 717–738 [Google Scholar]
- Britto DT, Glass AD, Kronzucker HJ, Siddiqi MY. (2001a) Cytosolic concentrations and transmembrane fluxes of NH4+/NH3. An evaluation of recent proposals. Plant Physiol 125: 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159: 567–584 [Google Scholar]
- Britto DT, Kronzucker HJ. (2003) Trans-stimulation of 13NH4+ efflux provides evidence for the cytosolic origin of tracer in the compartmental analysis of barley roots. Funct Plant Biol 30: 1233–1238 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2006) Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends Plant Sci 11: 529–534 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2008) Cellular mechanisms of potassium transport in plants. Physiol Plant 133: 637–650 [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. (2009) Ussing’s conundrum and the search for transport mechanisms in plants. New Phytol 183: 243–246 [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ. (2001b) Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98: 4255–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. (2002) Subcellular NH4+ flux analysis in leaf segments of wheat (Triticum aestivum). New Phytol 155: 373–380 [DOI] [PubMed] [Google Scholar]
- Chen G, Guo S, Kronzucker HJ, Shi W. (2013) Nitrogen use efficiency (NUE) in rice links to NH4+ toxicity and futile NH4+ cycling in roots. Plant Soil 369: 351–363 [Google Scholar]
- Coskun D, Britto DT, Jean YK, Schulze LM, Becker A, Kronzucker HJ. (2012) Silver ions disrupt K⁺ homeostasis and cellular integrity in intact barley (Hordeum vulgare L.) roots. J Exp Bot 63: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Kronzucker HJ. (2010) Regulation and mechanism of potassium release from barley roots: an in planta 42K+ analysis. New Phytol 188: 1028–1038 [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Li MY, Oh S, Kronzucker HJ. (2013) Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol 162: 496–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf MC, Bobbink R, Roelofs JG, Verbeek PJ. (1998) Differential effects of ammonium and nitrate on three heathland species. Plant Ecol 135: 185–196 [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. (2009) Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol 150: 1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M. (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JN, Volk RJ, Jackson WA. (1994) Inward and outward transport of ammonium in roots of maize and sorghum: contrasting effects of methionine sulfoximine. J Exp Bot 45: 429–439 [Google Scholar]
- Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, et al. (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 368: 20130164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320: 889–892 [DOI] [PubMed] [Google Scholar]
- Gerendas J, Zhu ZJ, Bendixen R, Ratcliffe RG, Sattelmacher B. (1997) Physiological and biochemical processes related to ammonium toxicity in higher plants. Z Pflanzen Bodenk 160: 239–251 [Google Scholar]
- Goyal SS, Rains DW, Huffaker RC. (1988) Determination of ammonium ion by fluorometry or spectrophotometry after on-line derivatization with o-phthalaldehyde. Anal Chem 60: 175–179 [DOI] [PubMed] [Google Scholar]
- Gruber N, Galloway JN. (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451: 293–296 [DOI] [PubMed] [Google Scholar]
- Grzebisz W, Gransee A, Szczepaniak W, Diatta J. (2013) The effects of potassium fertilization on water-use efficiency in crop plants. J Plant Nutr Soil Sci 176: 355–374 [Google Scholar]
- Holm LM, Jahn TP, Møller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T. (2005) NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch 450: 415–428 [DOI] [PubMed] [Google Scholar]
- Hove RM, Bhave M. (2011) Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75: 413–430 [DOI] [PubMed] [Google Scholar]
- Husted S, Hebbern CA, Mattsson M, Schjoerring JK. (2000) A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol Plant 109: 167–179 [Google Scholar]
- Izaurralde R, Kissel D, Cabrera M. (1990) Simulation model of banded ammonia in soils. Soil Sci Soc Am J 54: 917–922 [Google Scholar]
- Jahn TP, Møller AL, Zeuthen T, Holm LM, Klaerke DA, Mohsin B, Kühlbrandt W, Schjoerring JK. (2004) Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett 574: 31–36 [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D, et al. (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosegarten H, Grolig F, Esch A, Glusenkamp KH, Mengel K. (1999) Effects of NH4+, NO3– and HCO3– on apoplast pH in the outer cortex of root zones of maize, as measured by the fluorescence ratio of fluorescein boronic acid. Planta 209: 444–452 [DOI] [PubMed] [Google Scholar]
- Kosegarten H, Grolig F, Wieneke J, Wilson G, Hoffmann B. (1997) Differential ammonia-elicited changes of cytosolic pH in root hair cells of rice and maize as monitored by 2′,7′-bis-(2-carboxyethyl)-5 (and -6)-carboxyfluorescein-fluorescence ratio. Plant Physiol 113: 451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozono D, Yasui M, King LS, Agre P. (2002) Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Invest 109: 1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT. (2011) Sodium transport in plants: a critical review. New Phytol 189: 54–81 [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass AD. (1996) Kinetics of NH4+ influx in spruce. Plant Physiol 110: 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass AD. (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385: 59–61 [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT. (2003) Cytosolic potassium homeostasis revisited: 42K-tracer analysis in Hordeum vulgare L. reveals set-point variations in K+. Planta 217: 540–546 [DOI] [PubMed] [Google Scholar]
- Kurimoto K, Day DA, Lambers H, Noguchi K. (2004) Effect of respiratory homeostasis on plant growth in cultivars of wheat and rice. Plant Cell Environ 27: 853–862 [DOI] [PubMed] [Google Scholar]
- Lang B, Kaiser WM. (1994) Solute content and energy status of roots of barley plants cultivated at different pH on nitrate- or ammonium-nitrogen. New Phytol 128: 451–459 [DOI] [PubMed] [Google Scholar]
- Lazof D, Cheeseman JM. (1986) Sodium transport and compartmentation in Spergularia marina: partial characterization of a functional symplasm. Plant Physiol 81: 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Ratcliffe RG. (1991) Observations on the subcellular distribution of the ammonium ion in maize root tissue using in-vivo 14N-nuclear magnetic resonance spectroscopy. Planta 183: 359–367 [DOI] [PubMed] [Google Scholar]
- Lee RB, Ayling SM. (1993) The effect of methionine sulfoximine on the absorption of ammonium by maize and barley roots over short periods. J Exp Bot 44: 53–63 [Google Scholar]
- Lee RB, Clarkson DT. (1986) Nitrogen-13 studies of nitrate fluxes in barley roots. 1. Compartmental analysis from measurements of 13N efflux. J Exp Bot 37: 1753–1767 [Google Scholar]
- Loqué D, Ludewig U, Yuan L, von Wirén N. (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loqué D, von Wirén N. (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55: 1293–1305 [DOI] [PubMed] [Google Scholar]
- Ludewig U, Neuhäuser B, Dynowski M. (2007) Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett 581: 2301–2308 [DOI] [PubMed] [Google Scholar]
- Malagoli P, Britto DT, Schulze LM, Kronzucker HJ. (2008) Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance. J Exp Bot 59: 4109–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130: 2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- McClean CJ, van den Berg LJ, Ashmore MR, Preston CD. (2011) Atmospheric nitrogen deposition explains patterns of plant species loss. Glob Change Biol 17: 2882–2892 [Google Scholar]
- Morais-Cabral JH, Zhou YF, MacKinnon R. (2001) Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414: 37–42 [DOI] [PubMed] [Google Scholar]
- Pearson J, Stewart GR. (1993) The deposition of atmospheric ammonia and its effects on plants. New Phytol 125: 283–305 [DOI] [PubMed] [Google Scholar]
- Poorter H, Vanderwerf A, Atkin OK, Lambers H. (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiol Plant 83: 469–475 [Google Scholar]
- Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass AD. (1999) AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: evidence for regulation by root glutamine levels. Plant J 19: 143–152 [DOI] [PubMed] [Google Scholar]
- Roberts JK, Wemmer D, Ray PM, Jardetzky O. (1982) Regulation of cytoplasmic and vacuolar pH in maize root tips under different experimental conditions. Plant Physiol 69: 1344–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saparov SM, Liu K, Agre P, Pohl P. (2007) Fast and selective ammonia transport by aquaporin-8. J Biol Chem 282: 5296–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HW, Mackown CT, Leggett JE. (1984) Potassium ammonium uptake interactions in tobacco seedlings. J Exp Bot 35: 1060–1070 [Google Scholar]
- Schulze LM, Britto DT, Li M, Kronzucker HJ. (2012) A pharmacological analysis of high-affinity sodium transport in barley (Hordeum vulgare L.): a 24Na+/42K+ study. J Exp Bot 63: 2479–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster E. (1991) The behavior of mercury in the soil with special emphasis on complexation and adsorption processes: a review of the literature. Water Air Soil Pollut 56: 667–680 [Google Scholar]
- Stevens CJ, Dise NB, Mountford JO, Gowing DJ. (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303: 1876–1879 [DOI] [PubMed] [Google Scholar]
- Sweiry JH, Muñoz M, Mann GE. (1991) Cis-inhibition and trans-stimulation of cationic amino acid transport in the perfused rat pancreas. Am J Physiol 261: C506–C514 [DOI] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ. (2008) Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J Exp Bot 59: 303–313 [DOI] [PubMed] [Google Scholar]
- ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61: 2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. (2006) Structural mechanism of plant aquaporin gating. Nature 439: 688–694 [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C. (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Vale FR, Volk RJ, Jackson WA. (1988) Simultaneous influx of ammonium and potassium into maize roots: kinetics and interactions. Planta 173: 424–431 [DOI] [PubMed] [Google Scholar]
- Van Beusichem ML, Kirkby EA, Baas R. (1988) Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in Ricinus communis. Plant Physiol 86: 914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen N, Burrough PA, Velthorst EJ, Van Dobben HF, De Wit T, Ridder TB, Reijnders HF. (1982) Soil acidification from atmospheric ammonium sulfate in forest canopy throughfall. Nature 299: 548–550 [Google Scholar]
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D. (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7: 737–750 [Google Scholar]
- von Wirén N, Gazzarrini S, Gojon A, Frommer WB. (2000) The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261 [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. (1996) Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA 93: 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Glass AD. (1996) Interactions between K+ and NH4+: effects on ion uptake by rice roots. Plant Cell Environ 19: 1037–1046 [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass AD. (1993a) Ammonium uptake by rice roots. 1. Fluxes and subcellular distribution of 13NH4+. Plant Physiol 103: 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Ruth TJ, Glass AD. (1993b) Ammonium uptake by rice roots. 2. Kinetics of 13NH4+ influx across the plasmalemma. Plant Physiol 103: 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise T, Kai M, Piechulla B. (2013) Bacterial ammonia causes significant plant growth inhibition. PLoS ONE 8: e63538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DM, Miller AJ. (2000) Intracellular measurement of ammonium in Chara corallina using ion-selective microelectrodes. Plant Soil 221: 103–106 [Google Scholar]
- White MF, Christensen HN. (1982) The 2-way flux of cationic amino acids across the plasma membrane of mammalian cells is largely explained by a single transport system. J Biol Chem 257: 69–80 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.