Abstract
The major hurdle to be cleared in active immunotherapy of cancer is the poor immunogenicity of cancer cells. In previous attempts to overcome this problem, whole tumor cells have been used as vaccines, either admixed with adjuvant(s) or genetically engineered to express nonself proteins or immunomodulatory factors before application. We have developed a novel approach to generate an immunogeneic, highly effective vaccine: major histocompatibility complex (MHC) class I-positive cancer cells are administered together with MHC class I-matched peptide ligands of foreign, nonself origin, generated by a procedure we term transloading. Murine tumor lines of the H2-Kd or the H2-Db haplotype, melanoma M-3 and B16-F10, respectively, as well as colon carcinoma CT-26 (H2-Kd), were transloaded with MHC-matched influenza virus-derived peptides and applied as irradiated vaccines. Mice bearing a deposit of live M-3 melanoma cells were efficiently cured by this treatment. In the CT-26 colon carcinoma and the B16-F10 melanoma, high efficacies were obtained against tumor challenge, suggesting the universal applicability of this new type of vaccine. With foreign peptide ligands adapted to the requirements of a desired MHC class I haplotype, this concept may be used for the treatment of human cancers.
Full text
PDF
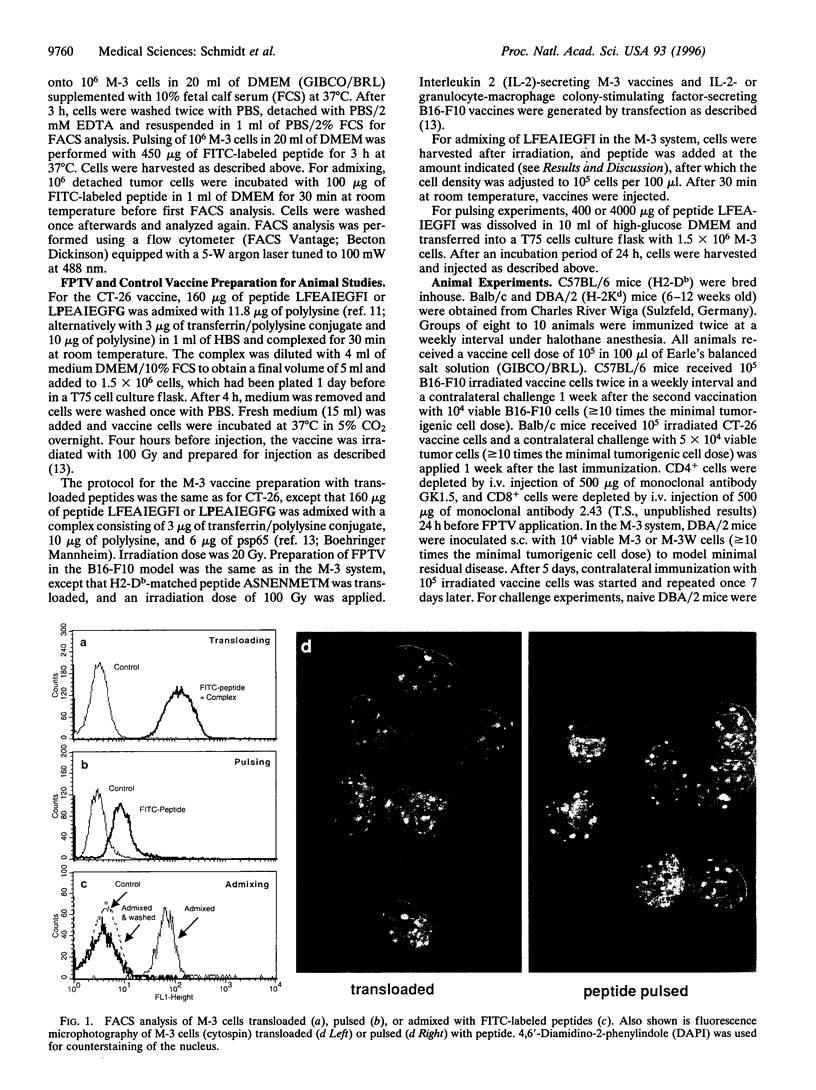
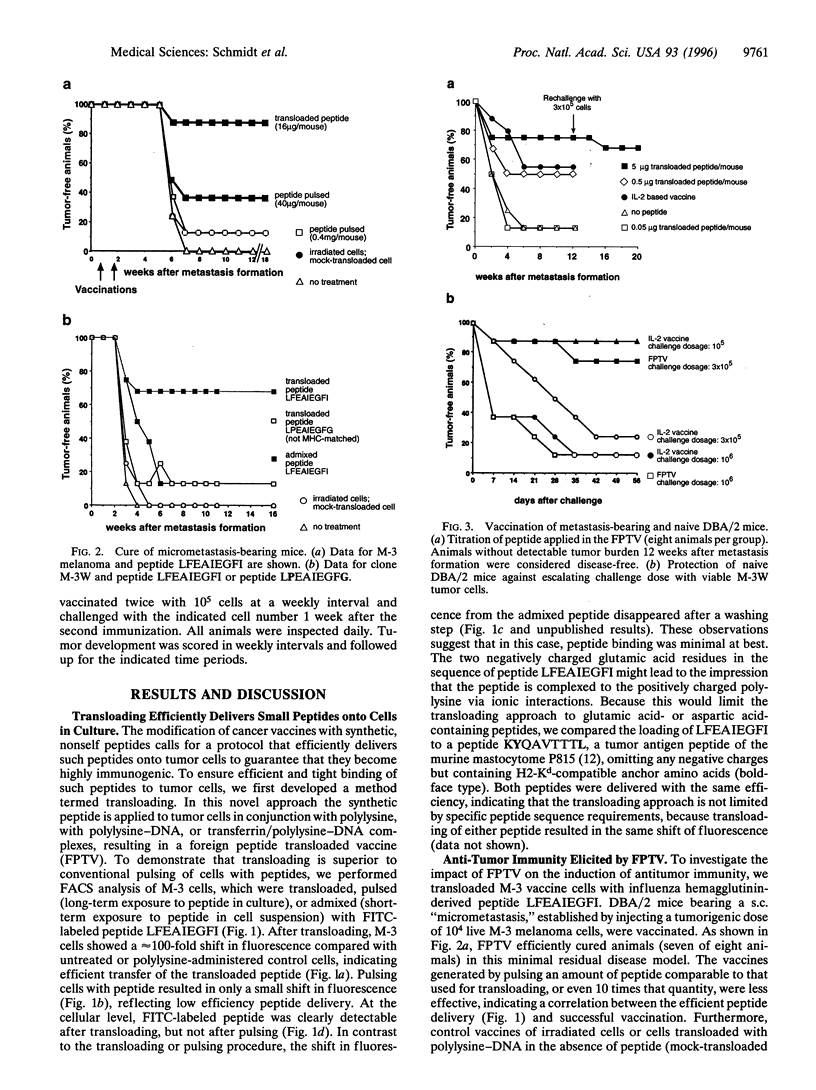
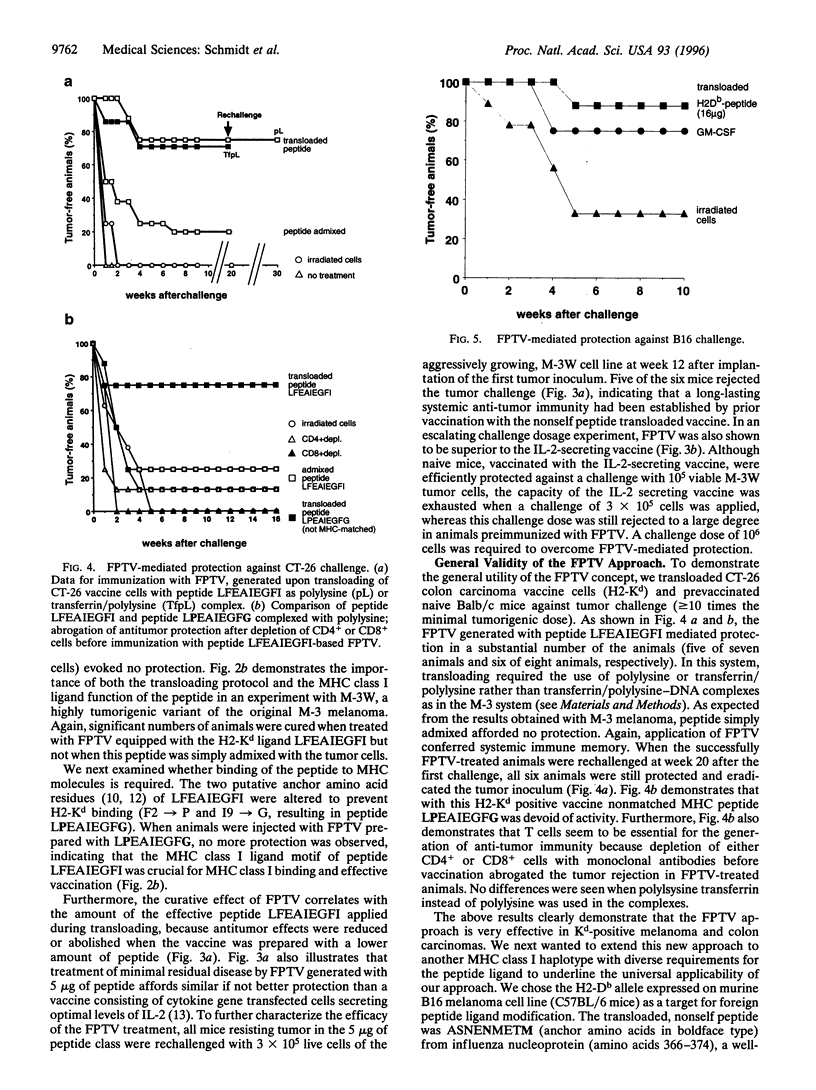

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Itaya T., Hunt B., Vogelstein B., Frost P. Induction in a murine tumor of immunogenic tumor variants by transfection with a foreign gene. Cancer Res. 1988 Jun 1;48(11):2975–2980. [PubMed] [Google Scholar]
- Liebrich W., Schlag P., Manasterski M., Lehner B., Stöhr M., Möller P., Schirrmacher V. In vitro and clinical characterisation of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer. 1991;27(6):703–710. doi: 10.1016/0277-5379(91)90170-i. [DOI] [PubMed] [Google Scholar]
- Mandelboim O., Vadai E., Fridkin M., Katz-Hillel A., Feldman M., Berke G., Eisenbach L. Regression of established murine carcinoma metastases following vaccination with tumour-associated antigen peptides. Nat Med. 1995 Nov;1(11):1179–1183. doi: 10.1038/nm1195-1179. [DOI] [PubMed] [Google Scholar]
- Mayordomo J. I., Zorina T., Storkus W. J., Zitvogel L., Celluzzi C., Falo L. D., Melief C. J., Ildstad S. T., Kast W. M., Deleo A. B. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995 Dec;1(12):1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- Paglia P., Chiodoni C., Rodolfo M., Colombo M. P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996 Jan 1;183(1):317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank C., Oberhauser B., Mechtler K., Koch C., Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994 Apr 29;269(17):12918–12924. [PubMed] [Google Scholar]
- Plautz G. E., Yang Z. Y., Wu B. Y., Gao X., Huang L., Nabel G. J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Friede T., Stevanoviíc S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41(4):178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Wallny H. J., Faath S., Rammensee H. G. Characterization of naturally occurring minor histocompatibility peptides including H-4 and H-Y. Science. 1990 Jul 20;249(4966):283–287. doi: 10.1126/science.1695760. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Schweighoffer T., Herbst E., Maass G., Berger M., Schilcher F., Schaffner G., Birnstiel M. L. Cancer vaccines: the interleukin 2 dosage effect. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4711–4714. doi: 10.1073/pnas.92.10.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Von Hoegen P., Weber E., Schirrmacher V. Modification of tumor cells by a low dose of Newcastle disease virus. Augmentation of the tumor-specific T cell response in the absence of an anti-viral response. Eur J Immunol. 1988 Aug;18(8):1159–1166. doi: 10.1002/eji.1830180803. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal K., Schneeberger A., Berger M., Schmidt W., Koszik F., Kutil R., Cotten M., Wagner E., Buschle M., Maass G. Elicitation of a systemic and protective anti-melanoma immune response by an IL-2-based vaccine. Assessment of critical cellular and molecular parameters. J Immunol. 1995 Apr 1;154(7):3406–3419. [PubMed] [Google Scholar]
- Zitvogel L., Mayordomo J. I., Tjandrawan T., DeLeo A. B., Clarke M. R., Lotze M. T., Storkus W. J. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996 Jan 1;183(1):87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]