Abstract
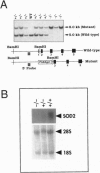
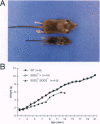
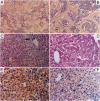
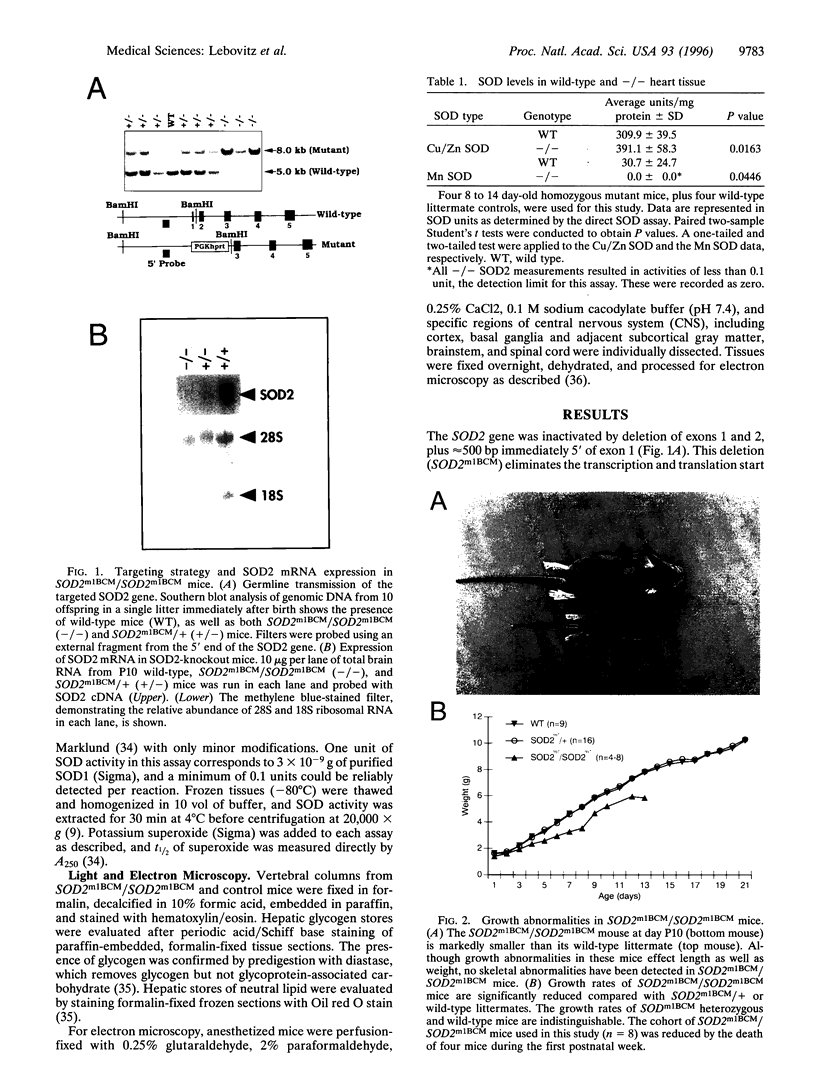
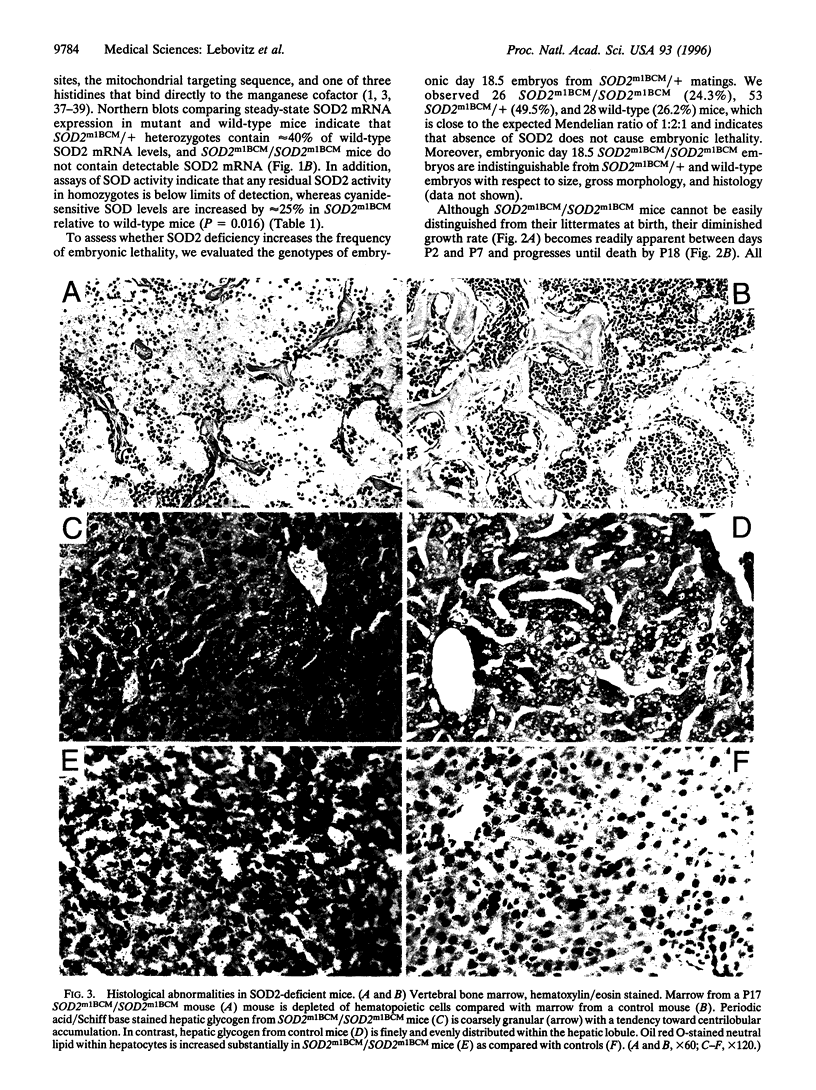
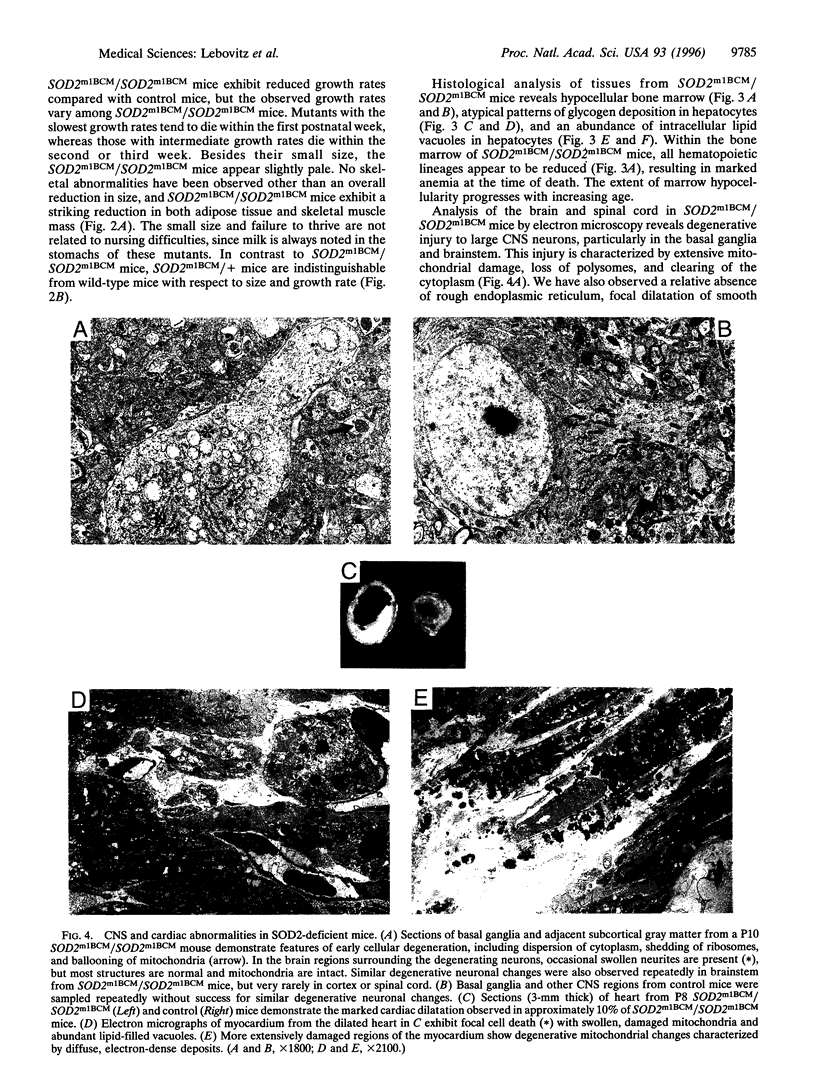
Manganese superoxide dismutase (SOD2) converts superoxide to oxygen plus hydrogen peroxide and serves as the primary defense against mitochondrial superoxide. Impaired SOD2 activity in humans has been associated with several chronic diseases, including ovarian cancer and type I diabetes, and SOD2 overexpression appears to suppress malignancy in cultured cells. We have produced a line of SOD2 knockout mice (SOD2m1BCM/SOD2m1BCM) that survive up to 3 weeks of age and exhibit several novel pathologic phenotypes including severe anemia, degeneration of neurons in the basal ganglia and brainstem, and progressive motor disturbances characterized by weakness, rapid fatigue, and circling behavior. In addition, SOD2m1BCM/SOD2m1BCM mice older than 7 days exhibit extensive mitochondrial injury within degenerating neurons and cardiac myocytes. Approximately 10% of SOD2m1BCM/SOD2m1BCM mice exhibit markedly enlarged and dilated hearts. These observations indicate that SOD2 deficiency causes increased susceptibility to oxidative mitochondrial injury in central nervous system neurons, cardiac myocytes, and other metabolically active tissues after postnatal exposure to ambient oxygen concentrations. Our SOD2-deficient mice differ from a recently described model in which homozygotes die within the first 5 days of life with severe cardiomyopathy and do not exhibit motor disturbances, central nervous system injury, or ultrastructural evidence of mitochondrial injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G. Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med. 1991 Feb;196(2):117–129. doi: 10.3181/00379727-196-43171a. [DOI] [PubMed] [Google Scholar]
- Beyer W., Imlay J., Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr, Price D. L., Sisodia S. S., Cleveland D. W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravard A., Sabatier L., Hoffschir F., Ricoul M., Luccioni C., Dutrillaux B. SOD2: a new type of tumor-suppressor gene? Int J Cancer. 1992 May 28;51(3):476–480. doi: 10.1002/ijc.2910510323. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Walker J. E. Superoxide dismutase from Bacillus stearothermophilus. Complete amino acid sequence of a manganese enzyme. Biochemistry. 1980 Jun 24;19(13):2873–2882. doi: 10.1021/bi00554a009. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L. M., Jonsson J., Edlund T., Marklund S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Meese E. U., Trent J. M. Sublocalization of the gene encoding manganese superoxide dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ hybridization and somatic cell hybrid mapping. Genomics. 1992 Nov;14(3):823–825. doi: 10.1016/s0888-7543(05)80202-2. [DOI] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Ridnour L. A., Oberley L. W., Swanson P. E., Meltzer P. S., Trent J. M. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Frank L. Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med. 1991;11(5):463–494. doi: 10.1016/0891-5849(91)90062-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Gralla E. B., Kosman D. J. Molecular genetics of superoxide dismutases in yeasts and related fungi. Adv Genet. 1992;30:251–319. doi: 10.1016/s0065-2660(08)60322-3. [DOI] [PubMed] [Google Scholar]
- Gualandi F., Morelli C., Pavan J. V., Rimessi P., Sensi A., Bonfatti A., Gruppioni R., Possati L., Stanbridge E. J., Barbanti-Brodano G. Induction of senescence and control of tumorigenicity in BK virus transformed mouse cells by human chromosome 6. Genes Chromosomes Cancer. 1994 Jun;10(2):77–84. doi: 10.1002/gcc.2870100202. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- L'Abbé M. R., Trick K. D. Changes in pancreatic glutathione peroxidase and superoxide dismutase activities in the prediabetic diabetes-prone BB rat. Proc Soc Exp Biol Med. 1994 Nov;207(2):206–212. doi: 10.3181/00379727-207-43808. [DOI] [PubMed] [Google Scholar]
- Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Liu X. F., Elashvili I., Gralla E. B., Valentine J. S., Lapinskas P., Culotta V. C. Yeast lacking superoxide dismutase. Isolation of genetic suppressors. J Biol Chem. 1992 Sep 15;267(26):18298–18302. [PubMed] [Google Scholar]
- Matzuk M. M., Finegold M. J., Mather J. P., Krummen L., Lu H., Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8817–8821. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Bode G., Ditschuneit H., Malfertheiner P. Demonstration of a phospholipid-rich zone in the human gastric epithelium damaged by Helicobacter pylori. Gastroenterology. 1993 Dec;105(6):1698–1704. doi: 10.1016/0016-5085(93)91065-p. [DOI] [PubMed] [Google Scholar]
- Nishida T., Sugiyama T., Kataoka A., Tashiro M., Yakushiji M., Ishikawa M. Serum manganese superoxide dismutase (MnSOD) and histological virulence of ovarian cancer. Asia Oceania J Obstet Gynaecol. 1993 Dec;19(4):427–431. doi: 10.1111/j.1447-0756.1993.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Orrell R., de Belleroche J., Marklund S., Bowe F., Hallewell R. A novel SOD mutant and ALS. Nature. 1995 Apr 6;374(6522):504–505. doi: 10.1038/374504a0. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 A resolution. J Mol Biol. 1988 Feb 20;199(4):649–661. doi: 10.1016/0022-2836(88)90308-7. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociot F., Lorenzen T., Nerup J. A manganese superoxide dismutase (SOD2) gene polymorphism in insulin-dependent diabetes mellitus. Dis Markers. 1993 Dec;11(5-6):267–274. doi: 10.1155/1993/678310. [DOI] [PubMed] [Google Scholar]
- Pociot F., Rønningen K. S., Bergholdt R., Lorenzen T., Johannesen J., Ye K., Dinarello C. A., Nerup J. Genetic susceptibility markers in Danish patients with type 1 (insulin-dependent) diabetes--evidence for polygenicity in man. Danish Study Group of Diabetes in Childhood. Autoimmunity. 1994;19(3):169–178. doi: 10.3109/08916939408995692. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Bradley A. Advances in the use of embryonic stem cell technology. Curr Opin Biotechnol. 1994 Oct;5(5):528–533. doi: 10.1016/0958-1669(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Ripps M. E., Huntley G. W., Hof P. R., Morrison J. H., Gordon J. W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Safford S. E., Oberley T. D., Urano M., St Clair D. K. Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase. Cancer Res. 1994 Aug 15;54(16):4261–4265. [PubMed] [Google Scholar]
- St Clair D. K., Holland J. C. Complementary DNA encoding human colon cancer manganese superoxide dismutase and the expression of its gene in human cells. Cancer Res. 1991 Feb 1;51(3):939–943. [PubMed] [Google Scholar]
- St Clair D. K., Wan X. S., Oberley T. D., Muse K. E., St Clair W. H. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6(4):238–242. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Wakamiya M., Blackburn M. R., Jurecic R., McArthur M. J., Geske R. S., Cartwright J., Jr, Mitani K., Vaishnav S., Belmont J. W., Kellems R. E. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]