Abstract
Background and Purpose
We sought to demonstrate the contribution of axonal remodeling of the corticospinal tract (CST) in the spinal cord to functional outcome after stroke.
Methods
Bilateral pyramidotomy (BPT) or sham-BPT was performed in mice with transgenic yellow fluorescent protein labeling in the CST subjected to middle cerebral artery occlusion (MCAo). Foot-fault and single pellet reaching tests were performed 3 days after MCAo and weekly thereafter. Mice were euthanized at day 14 or 28 after stroke. Immunofluorescent staining for growth-associated protein-43 and Synaptophysin was performed on cervical sections.
Results
Functional improvements were evident during the initial 14 days in both MCAo-sham-BPT and MCAo-BPT mice (P<0.01, versus day 3). Progressive recovery was present during the subsequent 14 days in MCAo-sham-BPT mice (P<0.001, versus day 14) but not in MCAo-BPT mice. In the stroke-affected cervical gray matter of MCAo-sham-BPT mice, growth-associated protein-43-Cy3 staining on CST axons were significantly increased at day 14 after stroke compared with normal mice (P<0.001), and CST axonal density and Synaptophysin-Cy3 staining of CST-yellow fluorescent protein axonal terminals were significantly increased at day 28 compared with day 14 after MCAo (P<0.001).
Conclusions
Our data demonstrate that voluntary motor recovery is associated with CST axonal outgrowth and synaptic formation in the denervated side of the spinal gray matter during the later phase after stroke, suggesting that the CST axonal plasticity in the spinal cord contributes to neurological recovery.
Keywords: functional recovery, middle cerebral artery occlusion, neuronal plasticity, pyramidotomy
Stroke is the leading cause of long-term disability in adults. Although most patients have some spontaneous behavioral improvements during the first several months after stroke, the recovery of motor function is generally incomplete. Of stroke survivors, 50% have some hemiparesis, and ≈15% to 30% are left permanently disabled.1 Critically, there are no efficacious therapies available for the vast majority of patients with stroke, in part because the mechanisms of brain repair and neuronal plasticity, and their relation to behavioral and functional recovery are not completely understood.
Because the motor deficits after stroke are a consequence of interruption of motor signals from the motor cortex to the spinal motoneurons, re-establishment of synaptic connections between cerebral neurons and their peripheral targets provides a physical substrate for functional recovery. The corticospinal tract (CST), the long axons of the cerebral cortical motor neurons distributed within the gray matter of the spinal cord, and directly or indirectly innervating the spinal motoneurons, is the primary transmission tract from the sensorimotor cortex, and thus, forms the neuroanatomical basis for brain controlled voluntary movements of the peripheral muscles.2 Using a clinically relevant model for ischemic stroke in rodents, middle cerebral artery occlusion (MCAo), we and others have demonstrated that behavioral outcome after stroke highly correlates with CST axonal remodeling in the stroke-impaired side of the spinal cord.3–5
However because the behavioral improvement after stroke involves many processes and multiple neural pathways between the motor cortex and the spinal cord, the specific contribution of the CST to neurological recovery after stroke requires investigation. To demonstrate the contribution directly of CST axonal remodeling in the spinal cord to motor behavioral recovery after stroke, we compared the behavioral outcome between mice subjected to MCAo and MCAo followed by bilateral pyramidotomy (BPT) to eliminate the CST axons in the spinal cord. In this study, we used transgenic mice, in which the CST axons are specifically labeled with yellow fluorescent protein (YFP), combined with an additional Cy3 fluorescent immunostaining, to investigate CST axonal outgrowth and synaptic plasticity in the spinal cord after stroke.
Methods
Animals
Adult male CST-YFP mice (n=39; 2 months old; 25–30 g) were generated using an in-house breeding colony of 2 transgenic mouse strains of B6.Cg-Tg(Thy1-EYFP)15Jrs/J and B6.129-Emx1tm1(cre)Krj/J purchased from Jackson Laboratories (Bar Harbor, ME), in which the CST axons are specifically and completely labeled by YFP.6 Animals were randomly divided into 4 groups: (1) euthanized at 14 days after MCAo-sham-BPT (n=10); (2) euthanized at 28 days after MCAo-sham-BPT (n=12); (3) euthanized 28 days after MCAo-BPT (n=12); and (4) naive mice without surgery used as normal controls (n=5). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
MCAo Model
A method of intraluminal suture vascular occlusion,7 modified in our laboratory, was performed by advancing a 6-0 surgical monofilament nylon suture with an expanded tip (heated) from the right external carotid artery into the lumen of the internal carotid artery for 1 hour, to block the origin of the MCA. The blood flow was restored by withdrawal of the suture.
Pyramidotomy
To examine the functional contribution of the CST to behavioral recovery after stroke, mice were subjected to bilateral pyramidal tract transection rostral to the CST decussation at the medulla level with a ventral surgical procedure after MCAo to eliminate the CST axons in the spinal cord.8 Briefly, when the MCAo surgery was performed, the animal was restricted in a Kopf stereotaxic apparatus in a supine position. Using the neck skin incision made for MCAo, the ventral vertebral column and outer surface of the occipital bone were exposed by bluntly splitting the muscle layers under an operating microscope, and the ventrocaudal part of the bone was partially removed with a high-speed drill and a blunt forceps. The right and the left pyramidal tracts were transected with an iridectomy scissor. Mice subjected to craniotomy only were used as sham control for BPT. The esophagus, trachea, and muscles were then repositioned, and then the skin was closed.
Behavioral Tests
To direct assess the voluntary motor deficits and recovery of the stroke-impaired left forepaw, a foot-fault test and a single pellet reaching tests were performed before surgery, 3 days after MCAo, and weekly thereafter. The foot-fault test measures the accuracy of forepaw placement on a nonequidistant grid as the percentage of foot faults of the left forepaw to total steps.9 The single pellet reaching test measures the ability of skilled forepaw use.10 The mice were placed in a Plexiglas box with a vertical slot on the front wall. Animals were trained for 5 days before surgery to use their left forepaw to extract 14-mg food pellets (Bio-Serv Inc, Frenchtown, NJ) through the slot. The number of the left forepaw extensions through the slot and the number of pellets extracted were counted for each animal during a 10-minute testing period. Performance was defined by the success rate=(number of pellets extracted/number of left forepaw attempts)×100.
Tissue Preparation and Immunohistochemistry
At 14 or 28 days after surgery, animals were transcardially perfused with saline, followed by 4% paraformaldehyde. The brain and the cervical spinal cord were removed and immersed in 4% paraformaldehyde overnight. The brains were cut into 7 equally spaced (1 mm each) coronal blocks using a mouse brain matrix. The brain blocks were embedded in paraffin. A series of adjacent 6-μm-thick sections was cut from each block and stained with hematoxylin and eosin for ischemic lesion volume measurements. The cervical spinal cord segments of C4-7 from MCAo mice and the medulla oblongata from MCAo-BPT mice were cut into consecutive 100-μm-thick vibratome sections. Completeness of BPT surgery was confirmed with YFP labeling in the CST on medulla sections upper and below the transection site.
Because the CST axons were almost completely eliminated in the spinal cord in MCAo-BPT mice, immunostaining was performed only in MCAo-sham-BPT mice. After blocking with normal serum, the spinal vibratome sections were incubated with primary antibodies against growth-associated protein-43 (GAP-43, 1:500; Novus, Littleton, CO) or Synaptophysin (1:1000; Chemicon, Temecula, CA) for 3 days at 4°C, and then incubated with a Cy3-conjugated secondary antibody at 4°C overnight.
Data Analysis and Statistics
A laser-scanning confocal imaging system (Bio-Rad MRC 1024, Cambridge, MA) mounted onto a Zeiss microscope was used to examine the Cy3 immunofluorescent staining and the transgenic YFP labeling in the denervated gray matter of the cervical cord. A horizontal line was drawn at the bottom border of the central gray matter and a vertical line was drawn at the middle between the midline and the lateral rim of the gray matter on traverse spinal sections. The area below the horizontal line and to the left of the vertical line was scanned on 10 sections in each animal using a 40× oil immersion objective for GAP-43-Cy3 staining and a 100× oil immersion objective for Synaptophysin-Cy3 staining. Colocalization of the immunofluorescent staining and YFP was analyzed on single-layer confocal images using a plug-in of NIH ImageJ software, Colocalization (Institut Jacques Monod, Service Imagerie, Paris, France), as percentage of the area scanned. CST axonal density was measured on the green channel of same images using ImageJ. Lesion volume was measured by ImageJ and presented as a volume percentage of the lesion area to the contralesional hemisphere.
All data are presented as mean±SD. The behavior outcomes were evaluated using 1-way ANOVA. Two-sample t test was used to analyze the difference in lesion volume, immunostaining, and CST axonal density between animal groups. A value of P<0.05 was considered significant.
Results
Behavioral Outcome
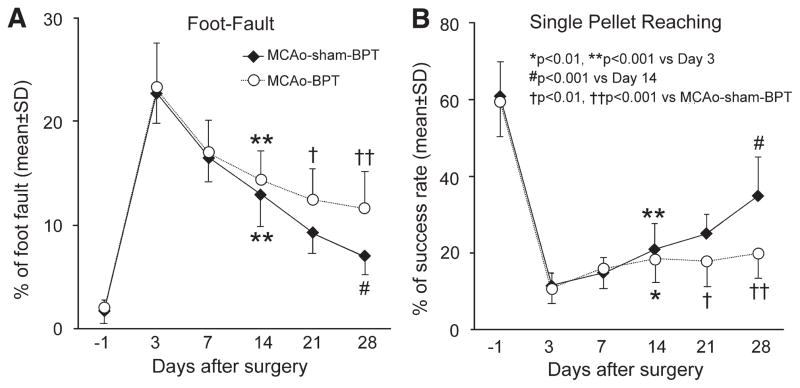
Ischemic infarct was evident in the cortex and striatum in mice subjected to 1-hour MCAo, without significant differences in lesion volume among animal groups (data not shown). In MCAo-BPT mice, the completeness of CST transection was confirmed with >95% loss of YFP labeling caudal to the site of pyramidotomy, and there was no significant CST axonal regeneration in the spinal cord (data not shown). As assessed by the foot-fault test (Figure 1A) for forepaw placing and grasping, and the single pellet reaching test (Figure 1B) for skilled forepaw reaching, grasping, and releasing, severe behavioral deficits of the left forepaw were evident in all animals 3 days after stroke. All animals showed significant improvement 14 days after surgery (P<0.01, versus day 3) with no differences between MCAo-sham-BPT and MCAo-BPT groups. Significant progressive recovery was observed in MCAo-sham-BPT mice between days 14 and 28 (P<0.001, versus day 14). However, in mice subjected to MCAo followed by BPT, there was no significant recovery in both pellet reaching task and grid walking test at day 28 compared with day 14 after MCAo. Thus, the motor performance of the stroke-impaired left forepaw was significantly worse in MCAo-BPT mice than that in MCAo-sham-BPT mice (P<0.01 and P<0.001 at days 21 and 28, respectively).
Figure 1.
Line graphs showing behavioral outcome after MCAo-sham-BPT or MCAo-BPT in mice assessed by foot-fault test (A) and single pellet reaching test (B). Note that significant improvement was observed during the initial 14 days after MCAo in all animals (n=22 for MCAo-sham-BPT; n=12 for MCAo-BPT), whereas progressive recovery for skilled use of the stroke-impaired forepaw was evident during the subsequent 14 days in MCAo-sham-BPT mice (n=12), however, was lost in MCAo-BPT mice (n=12). BPT indicates bilateral pyramidotomy; and MCAo, middle cerebral artery occlusion.
CST Axonal Outgrowth in the Denervated Spinal Cord
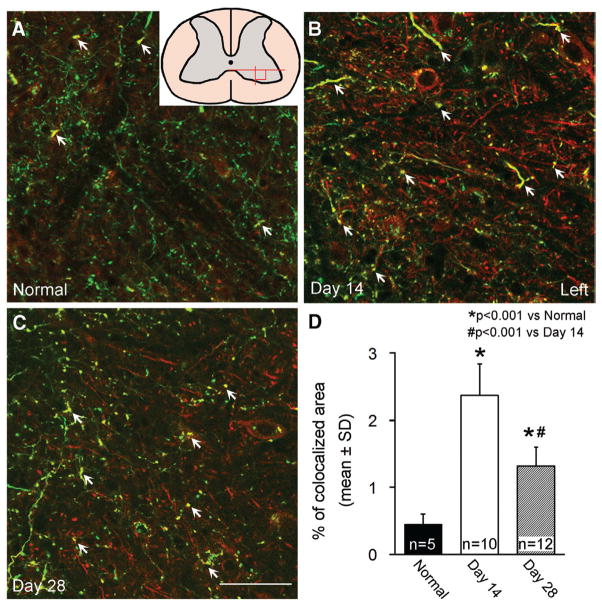
To examine the neuroanatomical basis of motor behavioral recovery found in mice after MCAo, we performed immunostaining for GAP-43 to identify growing CST axons in the stroke-impaired spinal gray matter in the CST-YFP mice, in which the CST axons were specifically and completely labeled with YFP (Figure 2). In the gray matter of the spinal cord in adult naive mice (A), GAP-43-Cy3 staining was very low and only a few CST axons were stained. At day 14 after MCAo, increased GAP-43-Cy3 staining was found in the membrane of the cell body and neurites, including CST axons labeled with YFP and non-CST neurites. The increased GAP-43-Cy3 persisted to 28 days after stroke (C). By measuring the double-labeled area with GAP-43 immunostaining and YFP labeling in single-layer confocal images (D), GAP-43 expression was 5.3- and 2.9-fold increased in the CST axons at 14 and 28 days after MCAo, respectively, compared with normal mice (P<0.001), whereas the upregulation of GAP-43 was significantly decreased from day 14 to 28 (P<0.001).
Figure 2.
Single-layer confocal images showing immunofluorescent staining for GAP-43 in the gray matter of the cervical cord. GAP-43-Cy3 staining (red) was low in the YFP-labeled CST axons (green) in normal adult mice (A), increased in the CST axons and non-CST neurites at day 14 (B), then partially decreased at day 28 (C) after MCAo-sham-BPT in the denervated side of the spinal cord. Quantitative data (D) showed that the double-strained area with Cy3 and YFP were significantly increased at 14 and 28 days after stroke. Arrows indicate GAP-43 immunostaining in the CST axons. A square field in the spinal cord scheme in A indicates the position of the photomicrograph appearing in Figures 2 and 4. Scale bar=50 μm. BPT indicates bilateral pyramidotomy; CST, corticospinal tract; GAP-43, growth-associated protein-43; MCAo, middle cerebral artery occlusion; and YFP, yellow fluorescent protein.
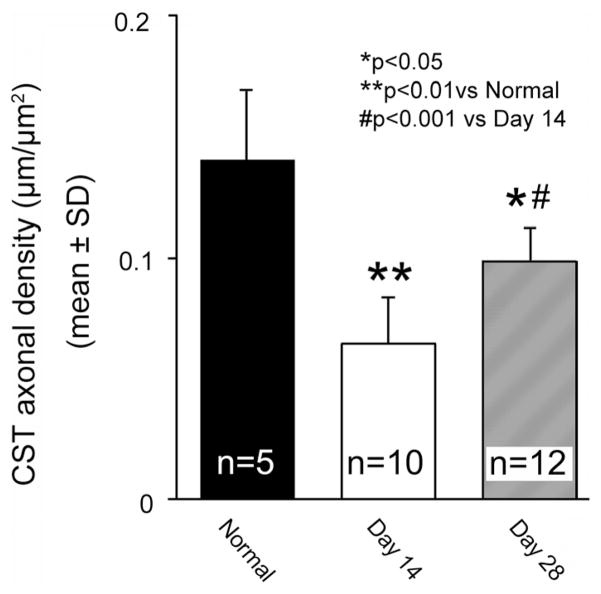
In addition, we measured the CST axonal density in the same images (Figure 3). Approximately 55% of the CST axons were eliminated in the stroke-impaired spinal gray matter 14 days after stroke compared with normal mice (P<0.01). A significant recovery in CST density was observed at 28 days compared with 14 days after MCAo (P<0.001); however, it was still lower than normal level (P<0.05).
Figure 3.
Bar graph showing CST axonal density in the gray matter of the cervical cord. Note that the CST axonal density was decreased in the stroke-impaired gray matter at day 14 compared with normal mice, and significantly recovered at day 28 after MCAo-sham-BPT. BPT indicates bilateral pyramidotomy; CST, corticospinal tract; and MCAo, middle cerebral artery occlusion.
Synaptic Connections of the CST Axons in the Denervated Spinal Cord
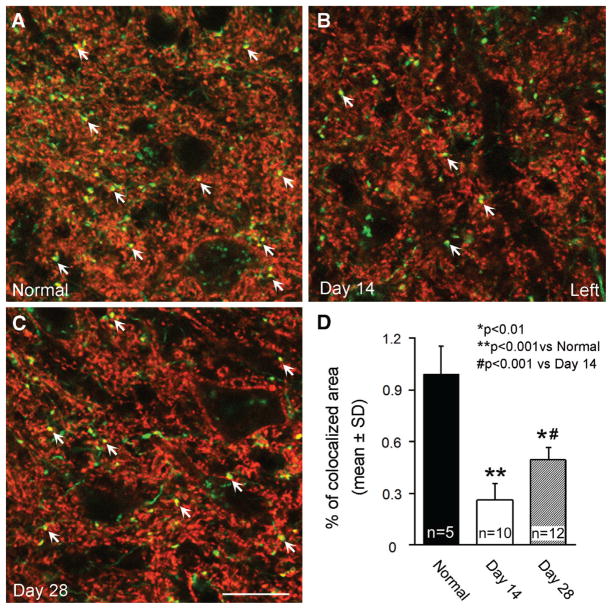
CST axons innervate the spinal motoneurons and interneurons by synaptic connections in the spinal gray matter for peripheral motor control. To test whether the stroke-induced growth of CST axons in the denervated side of the spinal gray matter is involved into the corticospinal innervation, we investigated synaptic remodeling derived from the CST axons with immunostaining for a presynaptic marker, Synaptophysin (Figure 4). Compared with normal mice (A), Synaptophysin-Cy3 staining on CST axons was dramatically reduced in the stroke-impaired gray matter at 14 days (B), and then increased at 28 days after MCAo (C). Quantitative data (D) showed that the Synaptophysin-Cy3 and YFP double-stained positive area was reduced to 26% (P<0.001) at day 14 and returned to 50% at day 28 (P<0.01) compared with the normal level, and there was a significant difference between the early and late time points (P<0.001).
Figure 4.
Single-layer confocal images showing immunofluorescent staining for Synaptophysin in the gray matter of the cervical cord. Compared with normal mice (A), Synaptophysin-Cy3 staining (red) was reduced in the YFP-labeled CST axons (green) at day 14 (B), then recovered at day 28 (C) after MCAo-sham-BPT in the denervated side of the spinal cord. Quantitative data (D) showed that the double-stained area with Cy3 and YFP was significantly decreased at 14 and 28 days after stroke, whereas a significant recovery was evident at day 28 compared with day 14 after MCAo. Arrows indicate Synaptophysin immunostaining in the CST axons. Scale bar=20 μm. BPT indicates bilateral pyramidotomy; CST, corticospinal tract; MCAo, middle cerebral artery occlusion; and YFP, yellow fluorescent protein.
Discussion
A survey performed in chronic spinal cord injury patients demonstrated that regaining arm and hand function is considered the highest priority for improving the quality of life.11 Similarly, for stroke survivors with long-term disability, regaining upper extremity function may lead to greater independence, thereby improving quality of life.12 The present study investigated voluntary motor behavioral outcome of the stroke-impaired forepaw in mice subjected to MCAo followed by sham-BPT or BPT. We found that significant behavioral improvement was evident in all mice during the initial 14 days after stroke. However, elimination of the CST axons in the spinal cord by BPT significantly reduced neurological recovery from 14 to 28 days after stroke compared with MCAo-sham-BPT mice during the same period. In the MCAo-sham-BPT mice, CST axons significantly sprout and form synaptic connections in the denervated gray matter of the spinal cord. Our observations support the hypothesis that in the early stage after stroke, functional improvement is attributable to the resolution of brain edema, absorption of damaged tissue, or reperfusion of the ischemic penumbra, whereas the recovery thereafter is attributable to neuronal plasticity and substantial structural reorganization of the central nervous system.13
Behavioral improvement in patients with stroke with severe impairment may be attributed to motor recovery, that is, the reappearance of elemental motor patterns present before a stroke by restoration of neuronal innervation, and compensatory movement—the appearance of new motor patterns resulting from the adaptation of remaining motor elements or substitution. Thus, functions are taken over, replaced, or substituted by different end effectors or body segments.14 In this study, we used 2 behavioral tests, the foot-fault test and single pellet reaching test, to estimate the neurological outcome of the left forepaw in mice subjected to right MCAo. In both behavioral tests used in this study, the mice need to voluntarily control the paw movement. When mice walk on the nonequidistant grids, each step requires adjustment in stride length and distribution of body weight, to place the limb appropriately on the rung and then grasp it.15 For the skilled reaching task, mice advance the forelimb aimed to the pellet, pronate the paw on it, grasp it, extract it, and release the food into the mouth.10 As the digit grasping and flexing is especially controlled by the pyramidal motor system,16 our observation of no significant behavioral improvement during the late phase in stroke mice with CST axonal elimination by BPT and no axonal regeneration in the spinal cord is in accordance with an earlier study showing that in monkeys subjected to BPT, considerable gross locomotor motor function was recovered, but the fine, independent finger movements were permanently lost.17 Our study is also consistent with a recent electrophysiological study showing that there is no recovery for synaptic responses in spinal motor neurons innervating forearm extensor muscles in the monkey subject to unilateral pyramidotomy.18 In contrast, in mice subjected to MCAo, GAP-43 expressed in the CST axons was significantly upregulated in the denervated side of the spinal gray matter 14 days after stroke. As GAP-43 is an intracellular growth protein playing an important role in axonal sprouting, branching, and path finding,19 these data suggest the presence of CST axonal outgrowth induced by ischemic stroke. Indeed, significantly increased CST axonal density was evident in the stroke-impaired spinal gray matter at 28 days after MCAo. Synapses are the basic units of neuronal connectivity. In immunostaining for the presynaptic marker, Synaptophysin, an integral membrane protein of synaptic vesicles,20 we found that at 28 days after MCAo, synapses formed by CST axonal terminals in the stroke-impaired spinal gray matter significantly recovered from their stroke-induced decrease, suggesting that the new CST axons were functionally integrated into the corticospinal innervation.
In addition, although MCAo-BPT mice lost accuracy of fine voluntary motor control of the left forepaw, especially the digits, motor ability was not completely lost. The mice are still able to walk, and attempt to reach the food pellets, suggesting that such locomotion may be achieved by subcortical motor systems.21,22 For example, the rubrospinal tract originated from the red nucleus participates in the coordination of movements across joints, such as skilled forelimb movements23 and locomotion.24 Because the corticospinal and rubrospinal axons possess similar branching patterns in the spinal cord,25 the corticorubrospinal pathway seems to be a backup to the CST to enhance the behavioral recovery after CST lesion.26 We also found that GAP-43 immunostaining was increased in neurites other than CST axons in the spinal cord after stroke, suggesting a possibility that stroke-induced denervation also enhances axonal plasticity of non-CST pathways and increases the neurite arborization of the spinal neurons. Further studies are warranted to investigate neuronal plasticity in the spinal cord after stroke. However, the lack of significant neurological recovery during the late phase after stroke in MCAo-BPT mice suggests that the CST remodeling in the spinal gray matter is necessary for voluntary motor behavioral recovery and cannot be compensated with other descending neural pathways.
The CST is the principal system for skilled voluntary movement in the human, and motor functional recovery after stroke critically depends on CST integrity.27 The CST is the only descending pathway in which some axons make synaptic contacts directly onto spinal motor neurons. This direct cortical innervation is necessary to allow the powerful processing networks of the cortex to control the activity of the spinal circuits that direct the exquisite movements of the fingers and hands. Although other parallel descending pathways can often recover the function of more coarse movements, for example, vestibulo-, reticulo-, rubro-, and tectospinal tracts, these pathways are not capable of generating fine, skilled movements. The CST anatomy differs between mice and humans in several fundamental ways, for example, in the human, the main CST is located in the lateral white matter, whereas in the rodents it is located in the dorsal funicular. In addition, the termination pattern of CST fibers in the spinal cord, for example, layers of gray matter, the Rexed laminae, also show differences between the 2 species. Moreover, the anatomic distribution of its sites of origin in motor cortices that control the opposite side of the body indicates homology of the CST between rodents and primates. Skilled limb movements, such as reach-to-grasp movement, show very similar motor components in humans and in rodents.28 Therefore, we use the mouse model to investigate CST axonal remodeling and voluntary motor recovery to mimic human stroke.
As the central nervous system in mammals is a highly inhibitory environment for axonal regeneration, it is difficult to expect the CST axons lost in a stroke can be regenerated from the cerebral cortex into the denervated spinal cord. Our previous study has demonstrated that the stroke-induced axonal outgrowth is attributed to local axonal sprouting and outgrowth within the spinal gray matter, rather than long distance regeneration originating from the brain.3 Therefore, as a homeostatic process, enhancing CST axonal remodeling in the spinal gray matter may provide a therapeutic opportunity to improve neurological recovery during the later phase after stroke.
Conclusions
The present study provides substantial evidence that the CST axonal remodeling in the denervated spinal gray matter contributes to re-establishment of functional corticospinal connections and to voluntary motor behavioral recovery after stroke, which may provide a treatment target to develop rational therapeutic approaches to enhance neurological recovery for the mass of chronic stroke patients.
Acknowledgments
Sources of Funding
This work was supported by American Heart Association National Scientist Development Grant 0835397N, National Institutes of Health R01AG037506 and R01NS066041.
Footnotes
Disclosures
None.
References
- 1.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36:e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 2.Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain Behav Evol. 1983;23:165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats following stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zai L, Ferrari C, Dice C, Subbaiah S, Havton LA, Coppola G, et al. Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci. 2011;31:5977–5988. doi: 10.1523/JNEUROSCI.4498-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, et al. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 10.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 12.Zorowitz RD, Chen E, Tong KB, Laouri M. Costs and rehabilitation use of stroke survivors: a retrospective study of Medicare beneficiaries. Top Stroke Rehabil. 2009;16:309–320. doi: 10.1310/tsr1605-309. [DOI] [PubMed] [Google Scholar]
- 13.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 15.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 16.Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci. 2008;28:311–322. doi: 10.1111/j.1460-9568.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 20.Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- 21.Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol. 1993;119:153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- 22.Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behav Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- 23.van Kan PL, McCurdy ML. Role of primate magnocellular red nucleus neurons in controlling hand preshaping during reaching to grasp. J Neurophysiol. 2001;85:1461–1478. doi: 10.1152/jn.2001.85.4.1461. [DOI] [PubMed] [Google Scholar]
- 24.Ruigrok TJ, van der Burg H, Sabel-Goedknegt E. Locomotion coincides with c-Fos expression in related areas of inferior olive and cerebellar nuclei in the rat. Neurosci Lett. 1996;214:119–122. doi: 10.1016/0304-3940(96)12898-6. [DOI] [PubMed] [Google Scholar]
- 25.Shinoda Y, Futami T, Mitoma H, Yokota J. Morphology of single neurones in the cerebello-rubrospinal system. Behav Brain Res. 1988;28:59–64. doi: 10.1016/0166-4328(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 26.Fanardjian VV, Gevorkyan OV, Mallina RK, Melik-Moussian AB, Meliksetyan IB. Enhanced behavioral recovery from sensorimotor cortex lesions after pyramidotomy in adult rats. Neural Plast. 2000;7:261–277. doi: 10.1155/NP.2000.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 28.Sacrey LA, Alaverdashvili M, Whishaw IQ. Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav Brain Res. 2009;204:153–161. doi: 10.1016/j.bbr.2009.05.035. [DOI] [PubMed] [Google Scholar]