Abstract
Objective
To identify the risk of sudden cardiac death (SCD) associated with obstructive sleep apnea (OSA).
Background
Risk stratification for SCD, a major cause of mortality, is difficult. OSA is linked to cardiovascular disease and arrhythmias, and has been shown to increase the risk of nocturnal SCD. It is unknown if OSA independently increases the risk of SCD.
Methods
We included 10,701 consecutive adults undergoing their first diagnostic polysomnogram between 7/1987 and 7/2003. During follow-up up to 15 years, we assessed incident resuscitated or fatal SCD in relationship to the presence of OSA, physiological data including the apnea-hypopnea index (AHI) and nocturnal oxygen saturation (O2sat) parameters, and relevant comorbidities.
Results
During an average follow-up of 5.3 years, 142 patients had resuscitated or fatal SCD (annual rate 0.27%). In multivariate analysis, independent risk factors for SCD were age, hypertension, coronary artery disease, cardiomyopathy or heart failure, ventricular ectopy or nonsustained ventricular tachycardia, and lowest nocturnal O2sat (per -10%, HR 1.14, P=0.029). SCD was best predicted by age >60 years (HR 5.53), AHI >20 (HR 1.60), mean nocturnal O2sat <93% (HR 2.93), and lowest nocturnal O2sat <78% (HR 2.60, all P<0.0001).
Conclusions
In a population of 10,701 adults referred for polysomnography, OSA predicted incident SCD, and the magnitude of risk was predicted by multiple parameters characterizing OSA severity. Nocturnal hypoxemia, an important pathophysiological feature of OSA, strongly predicted SCD independently of well-established risk factors. These findings implicate OSA, a prevalent condition, as a novel risk factor for SCD.
Keywords: Arrhythmia, Heart disease, Risk factor, Sleep apnea, Sudden cardiac death
Introduction
Sudden cardiac death (SCD) accounts for 450,000 deaths annually in the United States (1). The approach to SCD risk stratification is difficult, as modern therapies after myocardial infarction have altered the prognostic power of many risk factors, and also because the vast majority of SCD occurs in people without recognized heart disease (2). Recent population studies suggest that the risk of SCD is largely unrelated to traditional risk factors and may involve yet unrecognized variables that directly affect cardiac function and arrhythmogenesis (3,4).
Obstructive sleep apnea (OSA) may be one such unrecognized risk factor for SCD (5). A growing body of evidence confirms strong associations between OSA and cardiovascular diseases, including coronary artery disease, hypertension, left ventricular dysfunction, and arrhythmias (6). OSA also is associated with increased mortality (7,8). A specific link to SCD was suggested by the finding that SCD is more likely to occur during usual sleep hours in individuals with OSA, which is the time when SCD is least likely in individuals without OSA and in the general population (9). While these data demonstrated that individuals with OSA experience an altered day-night pattern of SCD, whether OSA increases the overall risk of SCD is not known.
We hypothesized that OSA is associated with an increased risk of SCD independently of other risk factors, and that the severity of OSA is directly associated with the magnitude of this risk.
Methods
The Mayo Foundation Institutional Review Board approved the study, and only individuals who authorized their records to be used for research were included. Data were collected from medical records by dedicated cardiovascular research studies unit personnel trained specifically for this study. Standardized, piloted forms were used for data collection, and quality was maintained by direct supervision and feedback by a study physician. A validation series using data from 200 subjects demonstrated agreement (Cohen's kappa 0.91) between data collected separately by a study physician and the research personnel (10).
Subjects
The study population was derived from all individuals referred for sleep studies to the Mayo Clinic Sleep Disorders Center between July 1, 1987 and July 31, 2003. The indication for sleep studies in the vast majority of subjects was suspected sleep disordered breathing, and this remained constant during the study period. We limited the study sample to Minnesota residents who were 18 years or older and undergoing their first-ever overnight sleep study (polysomnogram), which necessarily excluded individuals with a prior diagnosis of OSA. We also excluded individuals with a history of resuscitated SCD.
Data collected at the time of polysomnography included variables that have previously been associated with SCD. These included subjects' age, sex, body mass index, and comorbidities. Coronary artery disease was confirmed if the medical record noted a history of angina, myocardial infarction, abnormal cardiac stress or perfusion study, coronary angiogram revealing one or more stenoses greater than or equal to 70%, percutaneous coronary intervention, or coronary artery bypass graft surgery. Cardiomyopathy or heart failure was confirmed if the medical record documented the presence of cardiomyopathy or clinical heart failure, or if any cardiac imaging study demonstrated a left ventricular ejection fraction less than 50%. Diabetes mellitus was confirmed if the medical record noted the presence of type 1 or type 2 diabetes mellitus or the use of diabetes medications. Hypertension, strokes, transient ischemic attacks, complex ventricular ectopy or nonsustained ventricular tachycardia, prior smoking, and presence of an implantable cardioverter-defibrillator (ICD) were confirmed if the diagnosis was established in the medical record. We did not collect numerical data, such as cholesterol levels, blood pressures, or number of cigarettes smoked. Due to the retrospective methodology, the presence and effects of treatment for these conditions, and the development or resolution of these conditions during follow-up, were not measured.
Polysomnography
All individuals' sleep evaluations were conducted by a board-certified sleep specialist at the Mayo Clinic Sleep Disorder Center. We reviewed each individual's first diagnostic polysomnogram, which included measures of the electroencephalogram, electrooculogram, electromyogram, electrocardiogram, thoracoabdominal excursions, pulse oximetry, and naso-oral airflow. The apnea-hypopnea index (AHI) was calculated as the sum of apneas and hypopneas per hour of sleep. We also collected the awake oxygen saturation, mean nocturnal oxygen saturation, lowest nocturnal oxygen saturation, arousal index, and sleep efficiency. According to American Academy of Sleep Medicine criteria, an AHI ≥ 5 established the diagnosis of OSA (11).
Follow-up
The collection of follow-up data and confirmation of the primary outcome were performed blinded to the collection of baseline and polysomnographic data and classification of OSA. Follow-up data were obtained for each subject from the date of polysomnography to the date of SCD or resuscitated SCD (see definitions below), death from other causes, or last follow-up through December 31, 2003 ascertained with the Mayo Clinic electronic medical record, the Minnesota Department of Health database, and the National Death Index. To ascertain the use of continuous positive airway pressure therapy during follow-up, we considered that continuous positive airway pressure was used by a subject if it was prescribed after the sleep study and if the medical record subsequently confirmed its use. Due to the retrospective methodology, we could not quantify compliance with or effectiveness of continuous positive airway pressure therapy during follow-up.
Classification of SCD
The primary outcome of the study was fatal or resuscitated SCD. Manual review of death certificates and data from the Minnesota Department of Health provided the immediate and underlying causes of death. A state nosologist used the methods of the National Center for Health Statistics to assign causes of death. SCD was established when the cause of death was sudden cardiac death, (fatal) cardiac dysrhythmia, (fatal) cardiac arrhythmia, cardiac arrest, cardiorespiratory arrest; or coronary heart disease or myocardial infarction when the time interval from symptoms to death was specified ≤ 1 hour. SCD was excluded if the medical record or death certificate provided information that explicitly contradicted this definition of SCD: “natural death due to cardiac causes, heralded by abrupt loss of consciousness within one hour of the onset of acute symptoms; preexisting heart disease may have been known to be present, but the time and mode of death are unexpected” and non-traumatic (12). The occurrence of SCD during sleep was an exception to the criteria for “loss of consciousness within one hour of the acute onset of symptoms.”
Resuscitated SCD included out-of-hospital and in-hospital resuscitated cardiac arrest, as well as the first-ever delivery of appropriate ICD therapy for ventricular tachycardia or ventricular fibrillation. By definition, the latter instances occurred in subjects with ICDs implanted for primary prevention of SCD, since individuals with prior SCD were excluded from the study sample.
Statistical Analysis
Analyses were performed using SAS Proprietary Software Release 8.2 (1999-2000 SAS Institute, Cary, NC). A two-sided p-value <0.05 was considered statistically significant for all analyses.
Baseline characteristics of the study population were described by counts (and percentages), means (and standard deviations), or medians (and interquartile ranges). Kaplan-Meier time-to-event analyses were performed to identify univariate predictors of SCD. Multivariate time-to-event analyses were performed using Cox proportional hazards regression to identify independent predictors of SCD. A stepwise parsimonious selection technique constructed a model with the possible parameters of age, sex, body-mass index, obesity (body-mass index ≥ 30 kg/m2), smoking history, hypertension, diabetes, coronary artery disease, cardiomyopathy or heart failure, and ventricular arrhythmias. To assess the risk of SCD posed by OSA, the following parameters were included into the model independently of one another: OSA status, AHI, awake oxygen saturation, lowest nocturnal oxygen saturation, and mean nocturnal oxygen saturation.
We also performed classification and regression tree (CART) analyses to identify thresholds of relevant continuous variables that had the best discriminative power for predicting SCD. These analyses found the following thresholds: AHI 20, and lowest nocturnal oxygenation saturation 78%. These dichotomized variables were introduced into the univariate and multivariate models. A predetermined subgroup of patients with OSA was created to assess the effects of continuous positive airway pressure therapy (baseline characteristics shown in Table 5).
Table 5. Baseline characteristics of the subgroup of patients with obstructive sleep apnea (N = 8,327).
| Number of primary outcomes (SCD or resuscitated SCD) | 98 (1.2%) |
| Age, yrs | 54 (+/- 17) |
| Male sex | 74% |
| Body-mass index, kg/m2 | 35 (+/- 9) |
| Smoking history | 59% |
| Diabetes mellitus | 15% |
| Hypertension | 45% |
| Stroke or transient ischemic attack | 5% |
| Coronary artery disease | 15% |
| Cardiomyopathy or heart failure | 6% |
| Ventricular ectopy or nonsustained VT | 0.4% |
| Implanted ICD | 0.1% |
| Obstructive sleep apnea (AHI ≥ 5) | 100% |
| Apnea-hypopnea index | 38 (+/- 32) |
| Awake O2 sat | 95% (+/- 4%) |
| Mean nocturnal O2 sat | 93% (+/- 3%) |
| Lowest nocturnal O2 sat | 80% (+/- 11%) |
Results
The study sample consisted of 10,701 adult residents of Minnesota, whose baseline characteristics are described in Table 1. Results of subjects' polysomnography are described in Table 2. Follow-up occurred up to 15 years and averaged 5.3 years. During this time, 142 subjects had fatal or resuscitated SCD, which reflects an averaged annual risk of SCD of 0.27% for the study population. SCD was due to an unidentifiable or non-definitive cause (due to out-of-hospital death and lack of autopsy) in 58 subjects, definite ventricular arrhythmia in 44 subjects, acute myocardial infarction in 18 subjects, massive pulmonary embolism in 1 subject, and resuscitated SCD (either via ICD therapy or advanced cardiac life support) in 21 subjects.
Table 1. Baseline characteristics of study population.
| Age, yrs | 53 (+/- 14) |
| Male sex | 68% |
| Body-mass index, kg/m2 | 34 (+/- 9) |
| Smoking history | 57% |
| Diabetes mellitus | 14% |
| Hypertension | 41% |
| Stroke or transient ischemic attack | 5% |
| Coronary artery disease | 14% |
| Cardiomyopathy or heart failure | 6% |
| Ventricular ectopy or nonsustained ventricular tachycardia | 0.4% |
| Implanted ICD | 0.1% |
Table 2. Results of diagnostic polysomnography in the study population.
| Obstructive sleep apnea | 78% |
| Central sleep apnea | 0.03% |
| Sleep efficiency (mean +/- SD) | 74% (+/- 17%) |
| Arousal index, events/hour (mean +/- SD) | 38 (+/- 28) |
| Apnea-hypopnea index, events/hour (mean +/- SD) | 31 (+/- 32) |
| Awake oxygen saturation (mean +/- SD) | 95% (+/- 4%) |
| Mean nocturnal oxygen saturation (mean +/- SD) | 93% (+/- 3%) |
| Lowest nocturnal oxygen saturation (mean +/- SD) | 82% (+/- 11%) |
Clinical predictors and sleep parameters that predicted SCD in univariate analyses are shown in Table 3. CART analyses found that SCD was best predicted by these thresholds for the following continuous variables: age 60 years (HR 5.53, 95% CI 3.84 – 7.94, P<0.0001), AHI 20 (HR 1.60, 95% CI 1.14 – 2.24, P=0.007; Figure 1), mean nocturnal oxygen saturation 93% (HR 2.93, 95% CI 1.98-4.33, P<0.0001), and lowest oxygen saturation 78% (HR 2.60, 95% CI 1.85-3.65, P<0.0001; Figure 2).
Table 3. Univariate predictors of sudden cardiac death.
| HR | 95% CI | P value | |
|---|---|---|---|
| Age (per 1 year) | 1.07 | (1.05,1.08) | <0.001 |
| Male sex | 1.78 | (1.18, 2.70) | 0.006 |
| Body-mass index (per 1 kg/m2) | 1.00 | (0.98,1.02) | 0.845 |
| Obesity (body-mass index ≥ 30 kg/m2) | 1.02 | (0.72, 1.45) | 0.903 |
| Smoking history | 2.19 | (1.06, 4.5) | 0.034 |
| Diabetes mellitus | 4.10 | (2.9,5.8) | <0.001 |
| Hypertension | 3.63 | (2.5,5.2) | <0.001 |
| Stroke or transient ischemic attack | 4.65 | (3.03, 7.1) | <0.001 |
| Coronary artery disease | 14.53 | (10.22, 20.66) | <0.001 |
| Cardiomyopathy or heart failure | 22.62 | (16.23, 31.51) | <0.001 |
| Ventricular ectopy or NSVT | 18.63 | (9.79, 35.45) | <0.001 |
| Implanted ICD | 59.09 | (18.70,186.68) | <0.001 |
| Sleep parameters | |||
| Sleep efficiency (per 10% decrease) | 1.20 | (1.09,1.30) | <0.001 |
| Arousal index (per 10 events/hour) | 1.09 | (1.05,1.14) | <0.001 |
| Apnea-hypopnea index (per 10 events/hour) | 1.05 | (1.0,1.09) | 0.038 |
| Awake O2 saturation (per 10% decrease) | 1.33 | (1.13,1.55) | <0.001 |
| Mean nocturnal O2 saturation (per 10% decrease) | 2.33 | (1.72,3.14) | <0.001 |
| Lowest nocturnal O2 saturation (per 10% decrease) | 1.30 | (1.16,1.44) | <0.001 |
ICD, implantable cardioverter-defibrillator. NSVT, nonsustained ventricular tachycardia. O2, oxygen.
Figure 1. Survival based on apnea hypopnea index.
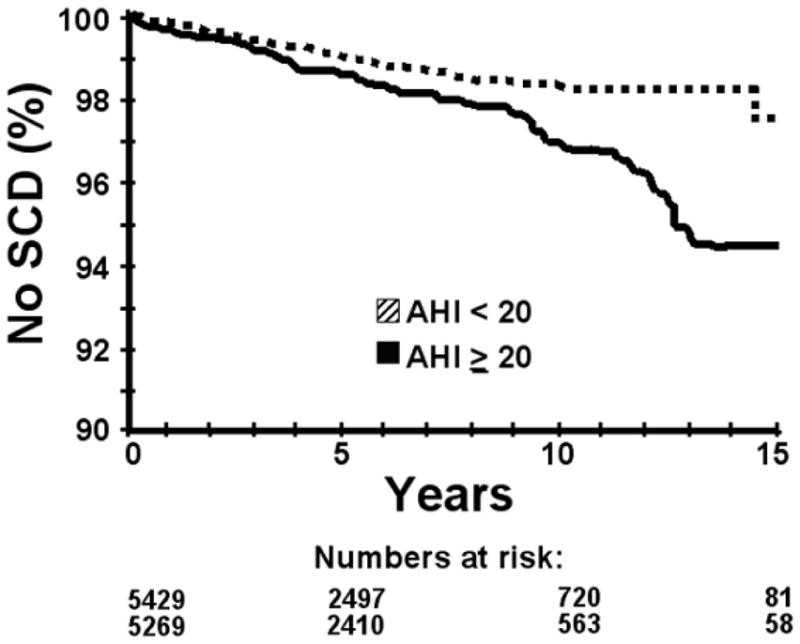
Survival free of fatal or resuscitated sudden cardiac death (SCD) in the total study population, based on the apnea hypopnea index (AHI) threshold determined by classification and regression tree analysis (AHI < 20 vs. AHI ≥ 20). Hazard ratio = 1.60, 95% CI 1.14 – 2.24, P = 0.007.
Figure 2. Survival based on oxygen saturation.
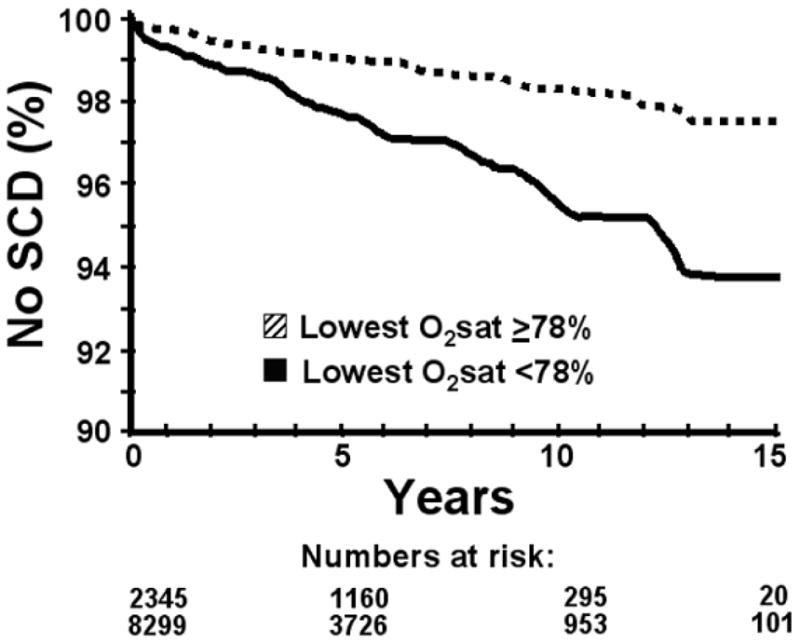
Survival free of fatal or resuscitated sudden cardiac death (SCD) in the total study population, based on the lowest nocturnal oxygen saturation (O2sat) threshold determined by classification and regression tree analysis (≥ 78% vs. < 78%). In multivariate analysis, hazard ratio = 1.81, 95% CI 1.28 – 2.56, p=0.0008.
Risk factors for SCD in the multivariate analyses are shown in Table 4. In multivariate analysis, the CART-determined lowest nocturnal oxygen saturation of 78% had a HR for SCD of 1.81 (95% CI 1.28-2.56, P=0.0008). The baseline characteristics of the predetermined subgroup of OSA patients is shown in Table 5, and the results of multivariate analyses in this subgroup are shown in Table 6.
Table 4. Independent predictors of sudden cardiac death in the total study population (results of multivariate regression).
| HR | 95% CI | P value | |
|---|---|---|---|
| Age, yrs | 1.02 | (1.01,1.04) | 0.005 |
| Hypertension | 1.48 | (1.02,2.15) | 0.04 |
| Coronary artery disease | 4.76 | (3.14,7.22) | <0.001 |
| Cardiomyopathy or heart failure | 7.32 | (4.99,10.73) | <0.001 |
| Ventricular ectopy or NSVT | 3.34 | (1.73, 6.43) | <0.001 |
|
| |||
| Sleep parameters | |||
|
| |||
| Apnea-hypopnea index (per 10) | 1.03 | (0.98,1.08) | 0.281 |
| Mean nocturnal O2 saturation (per 10%) | 1.49 | (0.96,2.28) | 0.073 |
| Lowest nocturnal O2 saturation (per 10%) | 1.14 | (1.01,1.27) | 0.029 |
Table 6. Independent predictors of sudden cardiac death in subjects with obstructive sleep apnea (results of multivariate regression).
| HR | 95% CI | P value | |
|---|---|---|---|
| Age, yrs | 1.02 | (1.002,1.04) | 0.03 |
| Hypertension | 1.57 | (1.01,2.44) | 0.04 |
| Coronary artery disease | 5.88 | (3.64,9.48) | <0.001 |
| Cardiomyopathy or heart failure | 7.20 | (4.71,11.0) | <0.001 |
| Ventricular ectopy or NSVT | 4.08 | (1.64,10.14) | 0.002 |
|
| |||
| Sleep parameters | |||
|
| |||
| Apnea-hypopnea index (per 10) | 1.06 | (1.0,1.13) | 0.05 |
| Lowest nocturnal O2 saturation (per 10%) | 1.13 | (1.0,1.29) | 0.05 |
Discussion
The principal and novel findings of the present study are that in a population of 10,701 adults referred for sleep studies, the presence of OSA predicted incident SCD and the magnitude of risk was predicted by multiple parameters that characterize OSA severity, including the AHI and nocturnal oxygen desaturation. Notably, the severity of nocturnal hypoxemia, which is an important pathophysiological feature of OSA, strongly predicted SCD independently of other well-established risk factors. Our analysis showed that for the lowest nocturnal oxygen saturation, the best discriminating threshold of 78% predicts an 81% increase in the risk of SCD.
Biological plausibility
There is a cascade of possible pathophysiological mechanisms linking OSA to SCD during the daytime and during sleep (6). Obstructive apneic events cause systemic hypoxemia, which is sometimes severe and prolonged. These repetitive oxygen desaturations in OSA patients may cause ventricular ectopy. Hypoxemia, with associated hypercapnia, also activates the chemoreflex, which increases vascular sympathetic nerve activity and serum catecholamines. Tachycardia and surges in blood pressure at the end of apneas result in increased myocardial oxygen demand at a time when oxygen saturation is at its lowest, a situation that may lead to myocardial ischemia and potentially dysrhythmic consequences. Individuals with OSA also have a paradoxical increase in coagulability during the night. Platelet activation and aggregation are increased, fibrinogen levels are increased, and fibrinolytic activity is decreased during sleep in patients with OSA.
An increased risk of SCD in individuals with OSA may also be explained by cardiac autonomic dysfunction. OSA affects mechanisms mediating heart rate variability, including central nervous system coupling between cardiac and ventilatory parasympathetic inputs, the arterial baroreflex, and feedback from pulmonary stretch receptors (13). As a result, heart rate variability is decreased in patients with OSA (14). In addition, the electrocardiographic QTc interval, which represents the duration of ventricular repolarization, and QTc interval dispersion, which reflects the heterogeneity of repolarization, are abnormal in patients with OSA. QTc interval dispersion correlates directly with the AHI and the duration of nocturnal hypoxemia (15). Furthermore, the increase in sympathetic drive persists during the awake daytime period in individuals with OSA (16). While the precise link between autonomic function and SCD remain largely unknown (17), chronic sympathetic overdrive has been identified as a risk marker for SCD (18). Also, OSA is present in a large proportion of patients with heart failure, and has been implicated in chronic left ventricular dysfunction (19,20), thus contributing to neurohumoral activation and myocardial remodeling that produce the substrate for SCD.
Previous studies
A controlled, multicenter study showed that during sleep nonsustained ventricular tachycardia occurred in 5.3% and complex ventricular ectopy occurred in 25% of patients with sleep-disordered breathing, which included OSA as well as central sleep apnea (21). After adjustment for comorbidities, patients with sleep-disordered breathing had a 3.4-fold risk of non-sustained ventricular tachycardia and a 1.7-fold risk of complex ventricular activity compared to patients with normal sleep. The only previous controlled longitudinal study assessing the risk of SCD in patients with OSA compared the rate of SCD after an average of 7.5 years in 107 OSA patients who were compliant with continuous positive airway pressure therapy and 61 OSA patients who had discontinued therapy. SCD occurred in 4 patients (7%) with untreated OSA and in no patients (0%) with treated OSA (there was one arrhythmic death in a treated patient during coronary bypass surgery) (22). Additional observations supporting the influence of OSA on the occurrence of SCD are the strikingly different day-night patterns of SCD in patients with OSA compared to the general population. We had previously shown that individuals with OSA had an increased risk of SCD from 10 PM to 6 AM and that individuals without OSA had a diurnal pattern of sudden death that was similar to that expected in the general population, with a peak between 6 AM and noon (9). In that study, individuals with OSA had a 2.6-fold risk of nocturnal SCD, and the severity of OSA correlated with the magnitude of this risk. Our present findings suggest that this increase of SCD during the night may represent “excess” deaths, rather than simply a shift of SCD from other times of the day to the night.
Causes of SCD
The mechanism of SCD is usually considered to be a ventricular arrhythmia. However, a number of other processes can mimic the unexpected and rapidly fatal presentation required by the definition of SCD. In our study sample, 13% of SCD were attributed directly to myocardial infarction, which may be explained by fatal sequelae such as cardiogenic shock or mechanical complications, as well as potential misclassification due to unrecognized ventricular arrhythmias. SCD may also result from other cardiovascular events, such as massive pulmonary embolism (1 subject in our study), aortic dissection, and subarachnoid hemorrhage. Another less recognized but potentially important cause of sudden death in patients with OSA is apnea itself. An obstructive apnea may not terminate due to ineffective arousal mechanisms related to impaired chemosensitivity, which leads to profound cerebral hypoxemia and death. Three such cases, with polysomnographic and electrocardiographic monitoring, have been described (23,24).
Limitations
Limitations of the present study should be considered. To mitigate referral bias, we restricted the study sample to state residents. The study sample was drawn from individuals referred to the sleep disorder center at our institution, which may limit generalization of our findings to non-selected community residents. The average annual rate of SCD in the study population was 0.27%, higher than the estimated annual risk of SCD in the general population of 0.1-0.2% (12), thus reflecting the referral pattern to our sleep disorders center.
The limits of a retrospective analysis are inherent to this study, including the one-time assessment of subject comorbidities at the time of their sleep studies and use of dichotomous variables to classify the presence or absence of specific conditions that exist on a spectrum of risk, such as cholesterol levels or blood pressure. Also, other relevant comorbidities may have existed. The retrospective analysis limits the interpretation of the results of continuous positive airway pressure therapy in the subgroup analysis of individuals with OSA. The medical records rarely contained data regarding the frequency, duration, or effectiveness of continuous positive airway pressure therapy in our subjects, and continuous positive airway pressure machines that assess compliance were generally not used during the study years.
Another limitation relates to the challenges of classifying SCD. We used standardized methods using the best available data and current definitions to confirm or exclude diagnoses of SCD in our study sample. The use of state records and death certificate data has been performed and validated in prior epidemiological studies of SCD, including our regional population (25-27). We improved on past methods by directly reviewing every available death record from the larger population to confirm the diagnosis based on current definitions of SCD (12).
Last, a possible limitation is related to the interpretation of ICD therapies for SCD classification. A sub-analysis of an ICD trial has questioned the basis for using ICD therapy as a surrogate for SCD (28). Our methods are in accordance with the vast majority of ICD trials, in which ICD therapy has been considered a resuscitated SCD.
Conclusion
In summary, in this cohort study of 10,701 adults referred to a sleep disorders clinic and undergoing diagnostic polysomnography for the first time, the risk of incident SCD after an average of 5 years was significantly and independently associated with OSA, based both on the frequency of apneas and hypopneas, and the severity of nocturnal oxygen desaturations. These findings should encourage ongoing research of the mechanisms of SCD in individuals with OSA, as well as the development of clinical trials of OSA therapy in select populations at risk for SCD.
Acknowledgments
Relationship with Industry and Financial Disclosure: Dr. Somers has served as a consultant for ResMed, Medtronics, and NeuPro; has received support from a gift from the Phillips-Respironics Foundation to the Mayo Foundation; and is working on intellectual property related to sleep and heart disease with Mayo Health Solutions. Dr. Gami has served as a consultant for Medtronic, Boston Scientific, and St. Jude Medical. This research was supported by grants HL65176 and NIH 1 UL1 RR024150 from the National Institutes of Health.
Abbreviations
- AHI
apnea hypopnea index
- CART
classification and regression tree
- ICD
implantable cardioverter-defibrillator
- OSA
obstructive sleep apnea
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anonymous. Preventing heart disease and stroke: addressing the nation's leading killers. Atlanta: Centers for Disease Control and Prevention, Department of Health And Human Services; 2003. [Google Scholar]
- 2.Myerburg RJ. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992;85(1 Suppl):I2–10. [PubMed] [Google Scholar]
- 3.Spooner PM, Albert C, Benjamin EJ, Boineau R, Elston RC, George AL, Jr, Jouven X, Kuller LH, MacCluer JW, Marban E, Muller JE, Schwartz PJ, Siscovick DS, Tracy RP, Zareba W, Zipes DP. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a national heart, lung, and blood institute workshop, part I. Circulation. 2001;103(19):2361–2364. doi: 10.1161/01.cir.103.19.2361. [DOI] [PubMed] [Google Scholar]
- 4.Spooner PM, Albert C, Benjamin EJ, Boineau R, Elston RC, George AL, Jr, Jouven X, Kuller LH, MacCluer JW, Marban E, Muller JE, Schwartz PJ, Siscovick DS, Tracy RP, Zareba W, Zipes DP. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a National Heart, Lung, and Blood Institute workshop, Part II. Circulation. 2001;103(20):2447–2452. doi: 10.1161/01.cir.103.20.2447. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Somers VK. Sudden death and obstructive sleep apnea. In: Antzelevitch Gusaak, Friedman Wilde, Ackerman Shen., editors. Electrical Diseases of the Heart: Genetics, Mechanisms, Treatment, Prevention. London: Springer; 2007. [Google Scholar]
- 6.Gami AS, Somers VK. Sleep apnea and cardiovascular disease. In: Zipes DP, editor. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 8. Philadelphia: Saunders; 2007. [Google Scholar]
- 7.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 9.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 11.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of the American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 12.Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P, Camm AJ, Cappato R, Cobbe SM, Di Mario C, Maron BJ, McKenna WJ, Pedersen AK, Ravens U, Schwartz PJ, Trusz-Gluza M, Vardas P, Wellens HJ, Zipes DP. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J. 2001;22(16):1374–1450. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- 13.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension. 1998;32(6):1039–1043. doi: 10.1161/01.hyp.32.6.1039. [DOI] [PubMed] [Google Scholar]
- 14.Roche F, Xuong AN, Court-Fortune I, Costes F, Pichot V, Duverney D, Vergnon JM, Gaspoz JM, Barthelemy JC. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol. 2003;26(3):669–677. doi: 10.1046/j.1460-9592.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Chin K, Hosokawa R, Takahashi K, Sumi K, Ohi M, Mishima M. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125(6):2107–2114. doi: 10.1378/chest.125.6.2107. [DOI] [PubMed] [Google Scholar]
- 16.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3(1):108–113. doi: 10.1016/j.hrthm.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103(16):2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 19.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98(21):2269–2275. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 21.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 23.Dyken ME, Yamada T, Glenn CL, Berger HA. Obstructive sleep apnea associated with cerebral hypoxemia and death. Neurology. 2004;62(3):491–493. doi: 10.1212/01.wnl.0000106952.84223.f3. [DOI] [PubMed] [Google Scholar]
- 24.Pearce S, Saunders P. Obstructive sleep apnoea can directly cause death. Thorax. 2003;58(4):369. doi: 10.1136/thorax.58.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folsom AR, Gomez-Marin O, Gillum RF, Kottke TE, Lohman W, Jacobs DR., Jr Out-of-hospital coronary death in an urban population--validation of death certificate diagnosis. The Minnesota Heart Survey. Am J Epidemiol. 1987;125(6):1012–1018. doi: 10.1093/oxfordjournals.aje.a114617. [DOI] [PubMed] [Google Scholar]
- 26.Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75(7):681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 27.Goraya TY, Jacobsen SJ, Kottke TE, Frye RL, Weston SA, Roger VL. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am J Epidemiol. 2003;157(9):763–770. doi: 10.1093/aje/kwg057. [DOI] [PubMed] [Google Scholar]
- 28.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113(6):776–782. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]


