Abstract
The primary site of urocortin 1 (Ucn1) expression in the brain is the centrally projecting Edinger-Westphal nucleus. The EWcp is innervated by dopaminergic neurons of the ventral tegmental area (VTA). To investigate whether activity of EWcp is regulated by the VTA, we investigated the effects of local pharmacological inhibition of VTA activity on the induction of Fos immunoreactivity in the EWcp of male C57BL/6J mice. A unilateral intracranial administration of the GABA agonist muscimol aimed at the VTA resulted in increased number of Fos-positive cells in the EWcp. This induction was lower than that produced by an intraperitoneal injection of 2.5 g/kg of ethanol. To investigate whether inhibition of dopaminergic neurons was responsible for induction of Fos, a second experiment was performed where the dopamine agonist quinpirole was unilaterally injected targeting the VTA. Injections of quinpirole also significantly induced Fos in EWcp neurons. The induction occurred only on the side of EWcp ipsilateral to the VTA injection. These results indicate that activity of EWcp is inhibited by tonic activity of dopaminergic VTA neurons, and that unilateral projections of VTA onto Ucn1-containing EWcp neurons provide a link between systems regulating approach and avoidance behaviors.
Keywords: Edinger-Westphal nucleus, urocortin, stress, dopamine, tonic inhibition
1.1 Introduction
The discoveries of CRF and CRF-related peptides urocortins (Ucns) led by Wylie Vale were truly seminal events. While CRF acting on CRF1 receptor is the major mediator of the hypothalamic-pituitary-adrenal response to stress and contributes to the regulation of anxiety-related behaviors, Ucns (i.e., peptides Ucn1, Ucn2 and Ucn3) are thought to optimize the organism’s response to life-threatening situations (Bale and Vale, 2004; Lewis et al., 2001; Neufeld-Cohen et al., 2010; Reyes et al., 2001; Vale et al., 1981; Vaughan et al., 1995). Adaptation to diverse environments requires an organism to adjust their approach behavior to rewards in accordance with their need to avoid the potential environmental harm, and vice versa (Alcaro and Panksepp, 2011; Korte et al., 2005). This suggests that the CRF/Ucn system, regulating adaptation to stress should be able to interact with the reward system. Dysregulation of such interactions could lead to pathological conditions, such as depression or alcohol and drug addiction (Ryabinin et al., 2012; Schank et al., 2012).
Multiple examples showing influences of the CRF/Ucn system on reward pathways have been reported (Kalivas et al., 1987; Korotkova et al., 2006; Rodaros et al., 2007; Ungless et al., 2003; Wang et al., 2005). These influences adjust activity of the reward system in anticipation of, during or following stress. On the other hand, although neurocircuits regulating reward should also be able to influence sensitivity to stress, examples of such influences are hard to come by.
It is notable that Ucn1-containing neurocircuits are well placed to be regulated by the brain regions associated with reward. For example, the main brain source of Ucn1 in the brain is the centrally projecting Edinger-Westphal nucleus (EWcp) (Bittencourt et al., 1999; Kozicz et al., 2011; Kozicz et al., 1998; Vaughan et al., 1995; Weitemier et al., 2005). The EWcp is a midbrain region located in the proximity to the ventral tegmental area (VTA), a key part of the reward pathway. Besides Ucn1, EWcp also contains several other peptides important for regulation of food consumption, such as cocaine- and amphetamine-regulated transcript, cholecystokinin and nesfatin-1 (Giardino et al., 2012). EWcp has been reported to be sensitive to stress (Gaszner et al., 2004; Kozicz, 2003; Kozicz et al., 2001). It is also sensitive to administration and self-administration of alcohol and addictive substances, and the response to alcohol occurs in the absence of response to stress (Spangler et al., 2009; Turek and Ryabinin, 2005). This suggests that although EWcp is the main source of Ucn1, presumably a stress-related peptide, it is not only involved in regulation of avoidance behaviors, but also behaviors associated with reward and approach. In agreement with this idea, lesions of this nucleus decreased food consumption without affecting anxiety-like behaviors (Weitemier and Ryabinin, 2005).
It has been shown that dopaminergic fibers from the VTA, as well as from the rostral linear nucleus of raphe, extend into the EWcp and impinge on Ucn1-containing neurons (Bachtell et al., 2002; Gaszner and Kozicz, 2003). These fibers could be in the position to regulate activity of the Ucn1-containing neurons, and provide a link between the reward and stress-regulating systems. To test this possibility, we investigated whether inhibition of the VTA by site-specific injections of the GABA agonist muscimol would modulate Fos-immunoreactivity (Fos-ir) in the EWcp. After we observed an induction of Fos in the EWcp following muscimol injections, we tested whether it was mediated specifically by inhibition of dopaminergic neurons following injections of quinpirole, a dopaminergic agonist capable of acting on inhibitory D2 autoreceptors located on these neurons (Congar et al., 2002; Jeziorski and White, 1989).
2. Materials and Methods
2.1 Animals
All animal procedures were approved by the Oregon Health & Science University animal care and use committee and were in accordance with National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Seven to eight week old male C57BL/6J mice were obtained from Jackson Laboratories (Sacramento, CA) and housed four per cage on a 12:12 light/dark cycle with water and food available ad libitum. One to two weeks following arrival animals underwent stereotaxic surgery for unilateral guide cannula implantation according to previously described protocols (Ryabinin et al., 2008; Weitemier and Ryabinin, 2006). The tip of a unilateral cannulae targeted VTA with following coordinates: −3.3 mm (AP), ± 0.5 mm (ML) and: 4.7 mm (DV) with a guide depth of 2 mm. Three size-matched C57BL/6J mice did not undergo surgery and were used as positive controls (see below).
2.2 Microinjections
Experiments were performed 4–7 days following surgery. In Experiment 1, saline (0.2 microliter) or the GABAA agonist, muscimol, (Sigma-Aldrich, 20 ng in 0.2 microliter) was infused via injector cannulae into the VTA (rate: 0.2 microliter/min). Three C57BL/6J mice were injected intraperitoneally with 2.5 g/kg ethanol (20% v/v in normal saline) as positive controls for Fos induction. This dose of ethanol was selected as the minimal dose eliciting maximal Fos response (Bachtell et al., 2002). In Experiment 2, saline (0.2 microliters) or the dopamine D2 agonist, quinpirole hydrochloride (Sigma-Aldrich, 1 microgram in 0.2 microliters) was infused via injector cannulae into the VTA (rate: 0.2 microliter/min). In both experiments, injectors were left in place for an additional minute to allow for diffusion. Ninety minutes post-injection, the animals were euthanized by an overdose of CO2 gas and the brains removed and stored overnight in 2% formaldehyde made with 10 mM phosphate-buffered saline (PBS). The brains were then cryoprotected in 30% sucrose/PBS until they were sliced on a cryostat.
2.3 Histochemistry
Thirty micron-thick floating coronal sections encompassing the entire EWcp region were sliced on a cryostat. Since the VTA is located within the same coronal plane as EWcp, half of the sections were stained with thionin to verify correct placement of the cannulae (Figure 1A). The other 7–8 sections across the entire sagittal extent of the EWcp underwent immunohistochemistry to detect the Fos protein according to previously established protocols (Giardino et al., 2011; Ryabinin et al., 1997; Spangler et al., 2009). Briefly, seven-nine slices per animal were included in each staining. Endogenous peroxidase activity was quenched with 0.3% peroxide in PBS and blocked with 4.5% normal goat serum (Vector Laboratory) in PBS and 0.3% Triton X-100. Slices were incubated overnight in a 1:2000 dilution of rabbit polyclonal antibody against Fos (Santa Cruz Biotechnology) in PBS/Triton X-100 and 0.1% bovine serum albumin. Biotinylated anti-rabbit secondary antibody was used to detect the primary antibody. This reaction was revealed using a Vectastain ABC kit (Vector) and a metal enhanced DAB kit (Thermo Scientific). Slices were mounted, dehydrated and coverslipped. The number of Fos-positive cells was counted manually using a Leica DM4000 microscope by an individual unaware of the group assignment of the animals. Cells in focus showing dark nuclear staining within the landmarks of EWcp were counted as positive.
Figure 1.
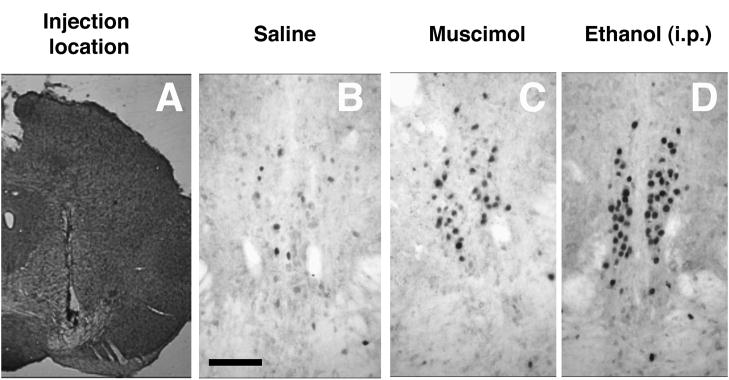
Representative images showing verification intra-VTA location of cannula tracks (A), Fos immunoreactivity in EWcp following intra-VTA injections of saline (B), Fos immunoreactivity in EWcp following intra-VTA injections of muscimol (C), Fos immunoreactivity in EWcp following intraperitoneal injection of 2.5 g/kg of ethanol (D). Scale bar for panels B–D indicates 100 microns.
2.4. Data analysis
Only animals with verified locations of injections were included in the analyses. A single value was calculated by averaging the cell counts from the EWcp across 6–8 slices encompassing rostral, intermediate and caudal portions of the brain region. Data were analyzed by one-way or two-way ANOVA. Significant effects were followed by Fisher PLSD post-hoc analysis.
3. Results
3.1. Intra-VTA muscimol induces Fos-ir in the EWcp
To investigate whether pharmacological treatment inhibiting activity of the VTA would affect activity of the EWcp, we analyzed Fos-ir in the EWcp following muscimol injection into the VTA. The injection site was located primarily in the VTA, partially encroaching on the substantia nigra pars compacta (Figure 1A). Mice injected with saline into the VTA served as a negative control, while mice injected with 2.5 g/kg ethanol intraperitoneally served as a positive control in accordance with previous studies demonstrating sensitivity of EWcp to ethanol (Bachtell et al., 1999; Bachtell et al., 2003; Chang et al., 1995; Topple et al., 1998).
Mice injected with saline into the VTA exhibited a very low number of Fos-positive neurons in EWcp. In contrast, animals injected with muscimol into the VTA showed a substantial increase in the number of Fos-positive cells in the EWcp. An even greater increased in Fos-ir was observed in animals injected intraperitoneally with ethanol. The location of the Fos-positive cells corresponded to landmarks of the mouse EWcp described previously (Spangler et al., 2009; Weitemier et al., 2005). The surrounding periaqueductal grey did not exhibit any Fos response, making the identification of Fos-positive cells as belonging to the EWcp obvious (Figure 1).
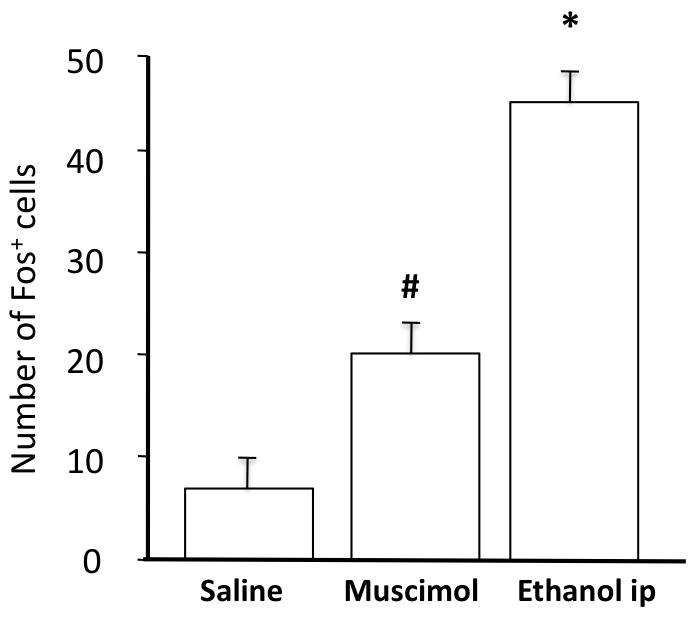
Statistical analyses confirmed this observation. There was a significant effect of treatment on the number of Fos-positive cells: F2,21=26.3, p<0.0001. Post-hoc Fisher PLSD confirmed that all three treatment groups showed significant differences in Fos immunoreactivity between each other (p<0.05, Figure 2).
Figure 2.
Number of Fos-positive neurons of EWcp following either intra-VTA injection of saline (N=7), intra-VTA injection of muscimol (N=14) or intraperitoneal injection of 2.5 g/kg of ethanol (N=3). Fisher PLSD: * - significantly different from all other groups (p<0.05), # - significantly different from Saline (p<0.05).
3.2. Intra-VTA quinpirole induces Fos-ir in the EWcp
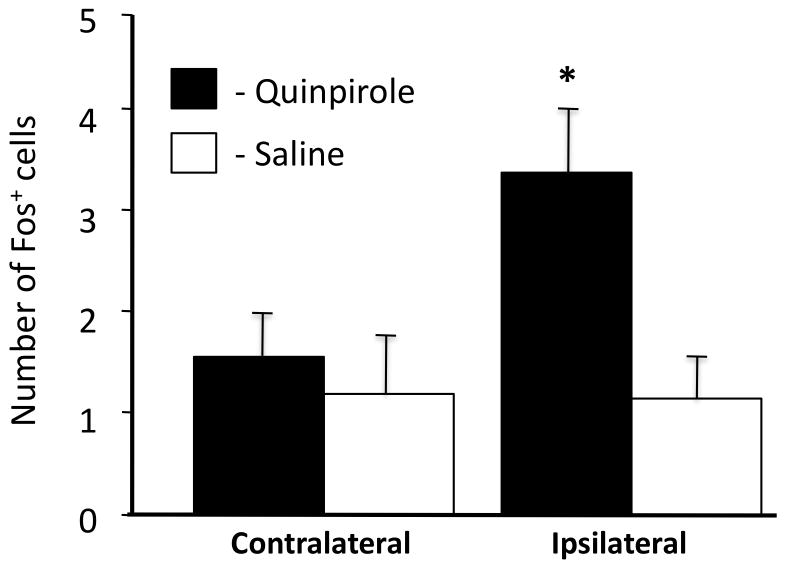
To investigate whether induction of Fos after injection of muscimol into the VTA could be mediated by dopaminergic neurons, we investigated whether the dopamine agonist quinpirole, acting on dopamine D2 receptors to inhibit dopaminergic activity, would also activate EWcp neurons. The number of Fos-positive cells was evaluated separately for each side of the brain to assess whether the innervation of EWcp is unilateral or bilateral. As in the previous experiment, saline-injected mice exhibited a very low basal level of Fos-ir in EWcp. Intra-VTA quinpirole administration resulted in a substantial increase in the number of Fos-positive cells on the side of the brain ipsilateral to the injection. No increase in Fos numbers was observed on the contralateral side of the brain indicating that the innervation of EWcp from VTA is unilateral. The overall numbers of Fos-positive cells observed here were substantially lower than in the previous experiment, most likely an artifact of the variability in levels of immunoreactivity between individual immunohistochemical stainings, which were performed more than two years apart. Statistical analysis confirmed these observations. Specifically, ANOVA indicated that the effect of treatment did not reach significance (F1,11=3.87, p=0.075), but the there was a significant effect of side (F1,11=6.21, p=0.030) and a significant treatment by side interaction (F3,11=4.98, p=0.047). Post-hoc Fisher PLSD tests found a significant difference in the number of Fos-positive cells in the side of EWcp ipsilateral to VTA injection of quinpirole versus all other treatments (Figure 3, p<0.05).
Figure 3.
Number of Fos-positive neurons in the contralateral and ipsilateral side of EWcp following either intra-VTA injection of saline (N=5) or intra-VTA injection of quinpirole (N=8). Fischer PLSD: * - significantly different from all other groups (p<0.05).
4. Discussion
Our results show that site-specific administration of substances inhibiting neuronal activity of the VTA results in induction of Fos in the EWcp. This is observed after both general inhibition of VTA activity by muscimol and after presumably cell-specific inhibition of dopaminergic neurons by quinpirole.
These findings agree with our previous finding showing that EWcp neurons are contacted by tyrosine hydroxylase-positive fibers originating from the VTA (Bachtell et al., 2002). Innervation of EWcp by dopaminergic neurons has also been demonstrated in rats (Gaszner and Kozicz, 2003). While our histological assessment does not allow distinguishing whether the injected drugs also acted on neurons of the substantia nigra pars compacta, the morphological evidence indicates that EWcp is innervated by VTA. Therefore, we assume that activation of EWcp resulted from inhibition of dopaminergic neurons of VTA, and not substantia nigra.
The previous study demonstrating innervation of EWcp by dopaminergic neurons in rats also showed that depletion of dopaminergic fibers by 6-hydroxydopamine had no effect on Fos immunoreactivity induced in the EWcp by an immunological challenge (Gaszner and Kozicz, 2003). However, this previous study did not examine basal levels of Fos in the EWcp of unstressed animals. Dopaminergic neurons are known to exhibit tonic firing and depolarization-induced phasic burst firing (Dreher and Burnod, 2002; Grace, 1991; Grace, 2000). While we have not tested whether burst firing exhibited by dopaminergic neurons has an effect on the EWcp, our finding of increased Fos-ir in the EWcp following administration of inhibitory substances into the VTA, suggests that the EWcp is under constant inhibitory control by tonically firing dopaminergic neurons.
As a consequence, our results suggest presence of inhibitory D2 or D4 dopamine receptors on EWcp neurons. To date, no studies have directly assessed presence of these receptors on EWcp neurons. While mRNA encoding D2 receptors is clearly present in the EWcp (Allen Institute for Brain Science, 2004; Gong et al., 2003), it is possible that it is mostly present on the few dopaminergic neurons that intermingle withinthe “proper” EWcp neurons (Bachtell et al., 2002; Fonareva et al., 2009; Spangler et al., 2009). On the other hand, in situ hybridization mapping studies of D4 receptors have been hampered by technical difficulties, whereas studies on transgenic mice carrying the green fluorescence marker under the D4 receptor gene promoter did not report detection of this marker in EWcp (Noain et al., 2006). Our previous experiments using systemic approaches found that intraperitonal injection of dopamine D2 receptor antagonist haloperidol completely blocked ethanol-induced Fos-ir, while intraperitoneal injection of dopamine agonist apomorphine failed to induce Fos in mouse EWcp (Bachtell et al., 2002). These findings are in agreement with the notion that other brain regions expressing D2 receptors, rather than direct actions of dopamine in EWcp, mediate induction of Fos in EWcp region following systemic administration of ethanol. Indeed, the number of Fos-positive cells following the intra-VTA administration of muscimol was significantly smaller that the number of Fos-positive cells induced by systemic ethanol, indicating that other neurotransmitter mechanisms, besides dopamine, regulate activity of the EWcp. In agreement with this explanation, a large number of neurotransmitters and peptides were shown to regulate activation of Fos in the EWcp (Bachtell et al., 2002; Gaszner et al., 2007; Kaur and Ryabinin, 2010; Linden et al., 2004; Trinh et al., 2003). On the other hand, our studies indicate that the innervation of EWcp by VTA is unilateral. Unilateral drug injections into the VTA were unlikely to have elicited a maximal EWcp response. A bilateral inhibition of the VTA would have elicited a more robust response than observed here after unilateral muscimol or quinpirole.
Although the number of Fos-positive cells in the present study was higher in ethanol than in intra-VTA muscimol-injected animals, the location of Fos-positive cells were identical after both treatments. Our previous studies indicated that ethanol-mediated induction of Fos in EWcp occurs exclusively in Ucn1-containing neurons (Bachtell et al., 2003; Ryabinin et al., 2003; Spangler et al., 2009). Therefore, the Fos-positive cells of the EWcp activated by muscimol or quinpirole into VTA are most likely Ucn1-containing neurons.
There is abundant evidence that the VTA is sensitive to the activity of CRF-like peptides, and that this influence is in large part mediated by CRF2 receptors (Kalivas et al., 1987; Korotkova et al., 2006; Rodaros et al., 2007; Ungless et al., 2003; Wang et al., 2005). The presumed function of such influence is to regulate the reward approach and seeking behaviors in relation to experienced or anticipated stressful situations. Our study for the first time shows that the relationship between systems regulating reward and anxiety also works in the opposite direction: basal activity of the VTA, a reward-related area, inhibits activity of the EWcp, the major brain source of a stress-related peptide Ucn1. It is concluded that the interactions between the VTA and the CRF/Ucn peptide system appear to be bidirectional.
It has been proposed that Ucn1, acting on both CRF1 and CRF2 receptors is involved in production of an optimal response to stress (Ryabinin et al., 2012). An optimal response to stress should take into account environmental conditions and state of the animal. It is tempting to speculate that under basal conditions low activity of the VTA inhibits Ucn1 neurons to prevent the development of high anxiety states. This behavioral part of this hypothesis awaits experimental confirmation.
5.1. Conclusions
Our experiments show that administration of inhibitory agents targeting dopaminergic neurons of the VTA leads to induction of Fos in the EWcp. Together with existing literature, they indicate that the dopaminergic VTA innervation of the Ucn1-containing EWcp neurons is functional, and that the EWcp is under tonic inhibitory influence of the VTA. This existence of a functional projection from neurons of the reward pathway to neurons attributed to stress pathways adds another important detail to our understanding of reciprocal reward-stress connections.
Highlights.
Intra-VTA muscimol increases Fos immunoreactivity in the centrally projecting Edinger-Westphal nucleus.
Intra-VTA quinpirole increases Fos immunoreactivity in the centrally projecting Edinger-Westphal nucleus.
These results indicate that VTA tonically inhibits the centrally projecting Edinger-Westphal nucleus.
The VTA - Edinger-Westphal nucleus connection could serve as a link between reward-and stress-regulating systems.
Acknowledgments
These studies were supported by NIH grants AA013738, AA016647 and AA010760.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrey E. Ryabinin, Email: ryabinin@ohsu.edu.
Davelle L. Cocking, Email: cocking@ohsu.edu.
Simranjit Kaur, Email: siimraan@gmail.com.
References
- Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011;35:1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Allen Institute for Brain Science. Allen Brain Atlas. 2004 from http://www.brain-map.org.
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Congar P, Bergevin A, Trudeau LE. D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of K+ channels. J Neurophysiol. 2002;87:1046–1056. doi: 10.1152/jn.00459.2001. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Burnod Y. An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw. 2002;15:583–602. doi: 10.1016/s0893-6080(02)00051-5. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res. 2009;33:1956–1965. doi: 10.1111/j.1530-0277.2009.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Korosi A, Palkovits M, Roubos EW, Kozicz T. Neuropeptide Y activates urocortin 1 neurons in the nonpreganglionic Edinger-Westphal nucleus. J Comp Neurol. 2007;500:708–719. doi: 10.1002/cne.21177. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Kozicz T. Interaction between catecholaminergic terminals and urocortinergic neurons in the Edinger-Westphal nucleus in the rat. Brain Res. 2003;989:117–121. doi: 10.1016/s0006-8993(03)03367-5. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Cote DM, Li J, Ryabinin AE. Characterization of Genetic Differences within the Centrally Projecting Edinger-Westphal Nucleus of C57BL/6J and DBA/2J Mice by Expression Profiling. Front Neuroanat. 2012;6:5. doi: 10.3389/fnana.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Jeziorski M, White FJ. Dopamine agonists at repeated “autoreceptor-selective” doses: effects upon the sensitivity of A10 dopamine autoreceptors. Synapse. 1989;4:267–280. doi: 10.1002/syn.890040403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J Comp Neurol. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Li M, Arimura A. The activation of urocortin immunoreactive neurons in the Einger-Westphal nucleus following stress in rats. Stress. 2001;4:85–90. doi: 10.3109/10253890109115724. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Greene SJ, Bergeron M, Schoepp DD. Anxiolytic activity of the MGLU2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology. 2004;29:502–513. doi: 10.1038/sj.npp.1300321. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci U S A. 2010;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci. 2006;24:2429–2438. doi: 10.1111/j.1460-9568.2006.05148.x. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsoory MM, Kozicz T, Thiele TE, Neufeld-Cohen A, Chen A, Lowery-Gionta EG, Giardino WJ, Kaur S. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46:349–357. doi: 10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151:780–790. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Expression of c-Fos in the mouse Edinger-Westphal nucleus following ethanol administration is not secondary to hypothermia or stress. Brain Res. 2005;1063:132–139. doi: 10.1016/j.brainres.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005;119:1235–1243. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]