Introduction and Epidemiology
In the United States, prostate cancer is the most commonly diagnosed non-cutaneous malignancy and second leading cause of cancer related death in men. Nearly 240,000 new cases and 30,000 prostate cancer specific deaths are estimated for 2013.1 Age is one of the most significant risk factors for prostate cancer. A large autopsy study revealed clinically latent prostate cancer in up to 11% of men in their twenties, which increased to more than 40% of men by age 50.2 As defined by the SEER database, the likelihood of developing invasive prostate cancer also increases from 1 in 8,000 in men less than forty to 1 in 8 in men greater than seventy years old.1 The exact cause behind the progression from indolent to clinically significant prostate cancer in the later decades of life remains unclear. However, it is likely multifactorial and includes race, family history, and environment.2
The incidence of identified prostate cancer in the U.S. population has varied significantly over the past three decades. The advent of PSA testing led to a rapid increase in prostate cancer detection. This peaked in 1992 and was followed by an equally impressive decline in incidence through 1995. This then stabilized for a few years until an increase in reported prostate cancer cases occurred from 1998–2002, followed by another decrease.1 The reason behind this temporal increase remains unclear, however there was a similar increase in the Canadian population during this same time.3
Internationally, the incidence of prostate cancer varies greatly, with the highest incidence seen in the U.S. population and some of the lowest in Asia,3 suggesting strong associations with genetics and the environment, in addition to differences in screening practices. Interestingly, there has been a reported increase in the incidence of prostate cancer in some Asian populations adopting western-style diets,4,5 suggesting a role for lifestyle factors, including nutrition, in prostate carcinogenesis.
Dietary Studies and Prostate Cancer
The link between nutrition and prostate cancer risk has been investigated in numerous studies. The National Cancer Institute Physician Data Query (PDQ) on Prostate Cancer Prevention provides a review of the current recommendations for certain nutrients and their association with either prevention or promotion of clinically significant prostate cancer.6 Some of these include dietary fat, dairy and calcium products, multivitamin use, selenium, Vitamin E, lycopene, and folate. Interestingly, folate is listed as a possible protective factor that may decrease the risk of prostate cancer, while folic acid, the synthetic version of folate used to fortify foods and contained in supplements, is listed as a nutrient that may increase the risk of prostate cancer.6 This conclusion mainly stems from the results of a placebo-controlled randomized trial of aspirin and folic acid supplementation for the chemoprevention of colorectal adenomas, in which 643 men had been assigned to either 1mg of folic acid supplementation or placebo.7 Median follow up was 7 years. The 10 year probability of being diagnosed with prostate cancer was 9.7% (95% CI = 6.5 to 14.5) in the folic acid group and 3.3% (95% CI = 1.7 to 6.4) in the placebo group, with an age-adjusted hazard ratio of 2.63 (95% CI = 1.23 to 5.65, Wald test P=0.01).7 Conversely, in men not taking folic acid supplements, there was a non-significant trend towards an inverse association between prostate cancer risk and both dietary folate intake (HR=0.65, 95% CI = 0.35 to 1.2) and baseline plasma folate level (HR=0.42, 95% CI = 0.17 to 1.04). These findings are therefore potentially contradictory, and offer little insight into recommendations for patients already diagnosed with prostate cancer. For these reasons, and in order to further understand the complex role folate likely plays in prostate cancer carcinogenesis and progression, we review the current literature herein.
Biology of Folate, Folic Acid, and Carcinogenesis
Folate is a naturally occurring water soluble B vitamin that is found as various polyglutamated forms in fruits, vegetables, and liver products. Folic acid is the synthetic, fully oxidized, monoglutamyl form of folate that is more stable and therefore used in dietary supplements and fortification of whole grains and cereals.7–9 Polyglutamated folates from natural food sources are hydrolyzed into monoglutamates in the small intestine, absorbed, and circulate as the monoglutamated form. Monoglutamated folates are then transported into cells through various mechanisms. Although the term folate is often used interchangeably to refer to both natural food folates and folic acid, the two have markedly different bioavailabilities.9 There is limited research into their separate biologic roles however, and so we will include both in this review, just as they appear in the literature.
Once intracellular, folates are polyglutamated, increasing cellular retention and affinity for most folate metabolizing enzymes. As seen in Figure 1, folate-mediated one-carbon metabolism then catalyzes the de novo synthesis of the purine nucleotides and thymidylate, as well as the remethylation of homocysteine to methionine.8 Methionine is then used to synthesize s-adenosylmethionine (S-AdoMet), which is the universal methyl group donor for the methylation of RNA, cytosine bases in DNA, histones, and other small molecules.8 These methylation reactions are important for regulating various cellular functions such as chromatin remodeling, gene transcription, mRNA translation, and cell signaling.8
Figure 1.
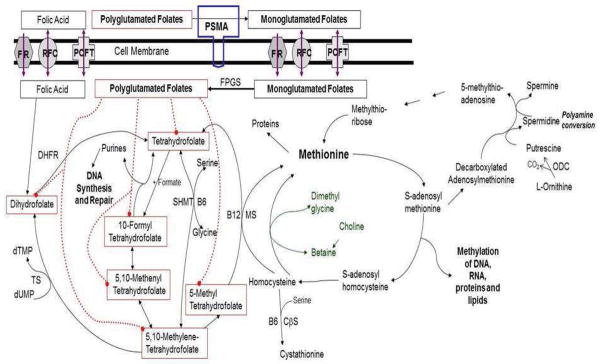
Folate and One-carbon metabolism. Folate plays an integral part in nucleotide synthesis, methionine synthesis and all methylation reactions, and polyamine synthesis. Prostate specific membrane antigen (PSMA), Proton Coupled Folate Transporter (PCFT), Reduced Folate Carrier (RFC), Folate Receptor (FR), Folypolyglutamate synthetase (FPGS), Dihydrofolate Reductase (DHFR), Thymidine monophosphate (dTMP), Thymidylate Synthetase (TS), Uridine monophosphate (dUMP)
It follows that folate deficiency can result in alterations in the nucleotide pool for DNA synthesis, leading to misincorporation of uracil into DNA, decondensed chromosomes, double stranded breaks, and translocations.10,11 Additionally, folate deficiency may reduce or alter DNA methylation, interfering with gene regulation and leading to carcinogenesis. As reviewed by Choi, et al, folate deficiency has been implicated in the etiology of colorectal, cervical, breast, pancreatic, esophageal, and gastric cancers.12 Due to its vital roles in cell growth and division, availability of either exogenous folate or intracellular polyglutamyl folate can directly regulate the rate of cancer cell replication.13 It has been postulated then that folate may play a dual role in tumorigenesis, depending on the amount of available folate and whether or not the cell has already become neoplastic. Indeed, animal studies have shown that folate supplementation is protective prior to initiation of carcinogenesis. After neoplastic transformation, however, folate depletion inhibits tumor growth.14,15 These findings are consistent with both a protective role for folate via maintenance of nucleotide pools and proper epigenetic regulation, as well as a detrimental role via enhanced cellular proliferation post-transformation.
Although prostate cancer cells do not divide as rapidly as many other tumors, the prostate relies heavily on the folate one-carbon metabolism pathway for the production of polyamines, which are derived from s-AdoMet.16 Polyamines are small organic molecules that occur ubiquitously in cells and are thought to be involved in numerous physiologic processes related to cell proliferation and growth.17 Prostate cells are the richest source of the polyamine spermine, which is found in seminal plasma at concentrations between 50 to 350 mg/dL.17 A recent study demonstrated that normal prostate and prostate cancer cells display a priority in maintaining polyamine synthesis over the other methylation and nucleotide synthesis processes needed for genomic stability, thus making prostate cells more sensitive to conditions of relative folate deprivation at otherwise normal physiologic levels and potentially leading to carcinogenesis.16 The growth rates of these cells are necessarily limited by low folate levels, however if these cells are then exposed to higher folate concentrations, as can occur with high levels of supplementation, transformed cells may have a proliferative advantage.16
With multiple functions and varying effects on prostate carcinogenesis, it becomes imperative to know the level of serum folate in the population and whether there is any association between folate intake and prostate cancer incidence or progression.
NHANES Review
The National Health and Nutrition Examination Survey (NHANES) is a complex survey that began in 1959 and combines interviews and physical examinations to determine the health and nutritional status of the United States population.18 The second and third installments of NHANES, 1976–1980 and 1988–1994 respectively, demonstrated significant folate deficiencies (serum folate < 6.81nM) in some populations.19 Due to the findings that folate supplementation could help reduce fetal neural tube defects,20 fortification of U.S. cereal-grain products began in the mid 1990s and became mandatory in 1998. Pfeiffer, et al19 reviewed serum folate levels in the pre- (1988–1994) and post-fortification (1999–2004) NHANES databases. For the purposes of this review, we will focus on changes in the male population. In males of all ages, serum folate prior to fortification had a median of 12nM. In post-fortification 1999–2000, the median serum folate more than doubled to 30.14nM. By the 2003–2004 survey, the median had decreased slightly to 26.06nM, but remained significantly increased compared to pre-fortification subjects.
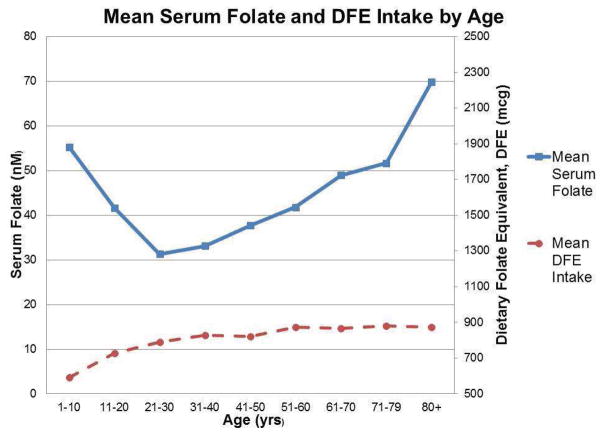
We analyzed the data from the 2007–2010 NHANES using a subpopulation of males of all ages, who provided 2 days of dietary recall and had fasted for at least 4 hours prior to measurement of serum folate. This represented approximately 69 million men in the U.S. population. Mean serum folate in this population was 42nM (95% CI 39.2 to 44.8). Of note, starting in 2007, folate levels were measured with the more accurate microbiologic assay rather than the Quantaphase II radioassay, which likely accounts for the increase in levels compared to 2001–2004 analysis.18 As seen in Figure 2 (currently unpublished), the now well documented U-shaped curve of serum folate versus age distribution18,19 was once again observed, with mean serum folate at its lowest level of 31.3nM (95% CI 29.5 to 33.1) between ages 21–30 and increasing significantly with each subsequent decade up to a mean of 69.9 nM (95% CI 57.96 to 81.95) in those men 80 years of age and older. This increase was seen despite statistically equal intake of dietary folate equivalents (DFE) across the age groups.
Figure 2.
Graph comparing mean serum folate (nM) across age groups (top line) and mean dietary folate equivalent (DFE) intake (bottom line) in the NHANES 2007–2010 subpopulation of males who fasted for at least 4 hours prior to serum folate measurement and who completed two days of dietary recall. There is a significant increase in serum folate after the 3rd decade of life despite statistically equal intake of folate.
It is also important to mention that current recommendations for folate intake are based on a lower estimated bioavailability for food folate (50%), compared to folic acid, than the recently reported level of 80%,9,21 likely leading to higher levels of intake than were intended through the recommended daily allowances (RDAs) set forth by the FDA. With such an increase in folate ingestion in the post-fortification era, and a now well-established increase in serum folate levels as men age, it has become important to determine if too much folate poses a health risk to men.
Folate and Prostate Carcinogenesis
Epidemiologic Evidence
There have been a limited number of epidemiological studies examining the effect of dietary folate or folic acid on the incidence of prostate cancer. The results have varied between four showing a positive correlation,7,22–24 three finding a negative correlation,25–27 and seven showing a null association.28–34 Additionally, there are two published meta-analyses that pooled different combinations of these studies, with one finding a positive overall correlation for prostate cancer incidence in patients taking at least 0.4mg/day of folic acid supplementation, RR of 1.24 (95% CI 1.03 to 1.49),35 and the other showing a positive fixed-effects pooled estimate for prostate cancer per 10nM of serum folate increase, OR of 1.19 (95% CI 1.03 to 1.37).34
Several challenges arise when analyzing the results among these studies. One of the most important problems is variation of the populations, in that they were from many different countries as well as different study periods, with some that began in the 1980s and early 90’s versus others that began in the 2000’s. This is significant because dietary habits and timing of any folic acid fortification, and thus serum folate levels, vary greatly between countries. For example, in one of the studies,26 90% of Swedish subjects had serum folate levels less than 11.1nM, while in a Finnish study,33 75% of cases had serum folate levels less than 10.8nM. By comparison, only 2.5% of U.S. men had serum folate levels less than 10.4nM19 in the post-fortification era up to 2004.
Another important problem with these studies is the large variation in screening methods for prostate cancer. As discussed previously, the incidence of prostate cancer is strongly dependent on overall screening practices, which vary greatly between countries.1–3 Unfortunately, no standard screening process was defined for five studies,22,24,28–30 with only a review of various cancer registries being used for four of them.23,28–30 Two of the studies mentioned defined PSA screening criteria in their populations,27,34 while only one had strict guidelines for scheduled DRE and PSA testing, as well as prostate biopsy protocols.31
A third category of variation between these studies is the definition of the intervention variable, with some groups estimating folate and folic acid intake from dietary questionnaires, and others using quantified serum folate levels. Six studies measured serum folate levels22,26,28–30,34 while six others reported various levels of folate or folic acid intake.24,25,27,31,32,35 Only one study reported both variables.33 As we have determined from review of the most recent NHANES data (Figure 2), there can be significant deviations in serum folate levels with equal intake of DFEs between individuals and across age groups. Therefore, it may be difficult to conclude the effect of either folic acid intake or serum folate levels on prostate cancer incidence without the complimentary variable. Additionally, many of the studies measured either serum folate or folate intake only once at study enrollment. Since they spanned anywhere from 4 to 10 years of follow up, it is difficult to assume that folate intake and serum folate levels remained constant.
Taking the inconsistency of study designs into consideration, it comes as no surprise that their conclusions vary so dramatically. We therefore turn to experimental models to glean any clues for the role of folate in prostate carcinogenesis.
Prostate Carcinogenesis in vitro
There have been few studies looking into the effect of either folate deficiency or folate saturation on prostate carcinogenesis. Bistufli et al36 recently published the effects of relative folate deficiency on prostate cancer cell lines in vitro. When comparing prostate cancer cells in an environment with 100nM folic acid to one with a supraphysiologic level of 2μM folic acid, there was significant genetic and epigenetic instability as well as phenotypic changes seen in the cells grown in the lower concentration.36 More specifically, there were chromosomal rearrangements in 24–37% of cells and greater CpG island hypermethylation. Compared to the supraphysiologic control group, there were 14 new hypermethylated regions and altered global histone hypermethylation. They also reported significant increases in the dUTP:dTTP ratio, uracil misincorporation into DNA, and in the number of DNA single strand breaks.36
In order to examine the effect of physiologic levels of folate variation, Bistulfi et al37 investigated the effect of folate deficient, folate adequate, and high folate diets in the transgenic adenoma of the mouse prostate (TRAMP) model on prostate cancer tumorigenesis. The different diets did have a significant effect on serum and prostate tissue folate levels. Prostates in the folate deficient diet mice had a significantly lower cellular proliferation rate, as measured by Ki67 staining, compared to the normal and high folate diets.37 Additionally, the folate deficient mice had significantly fewer prostate lesions that progressed beyond HGPIN before 22 weeks (1/23), whereas the control and high folate diet groups had 10/22 and 7/21 mice that progressed to cancer (p=0.02), respectively. There were also significantly fewer mice that developed lymph node metastases in the folate deficient group compared to the control and high folate groups, while E-cadherin staining, which is generally lost during progression towards malignancy, was significantly retained in the folate deficient group compared to the two other groups.37 Therefore, while folate supplementation did not seem to enhance prostate cancer progression in this model, these findings do suggest that relative deficiency of folate blocks prostate tumorigenesis and progression to metastasis.
Another study performed by Petersen et al38 investigated the effect of common physiologic levels of folic acid on cultured human prostate cancer cell lines. The PC-3, LNCaP, and DU145 cell lines were exposed to 4nM, 20nM, or 100nM of folic acid. When compared to the U.S. population, these three levels of folic acid concentrations match well to folate deficiency, normal, and high serum folate, respectively. Both PC-3 and LNCaP cells showed significant increases in growth rates when they were grown in higher folate levels, however the DU145 cells did not show a difference.38 The same study also investigated the relative invasiveness of the cell lines between the folate groups; interestingly, a significantly greater proportion of cells in all three lines invaded across a matrigel matrix when grown in 100nM folic acid. These results suggest increased levels of folic acid are able to confer increased invasiveness, a measure of tumorigenicity, in prostate cancer cells.38
Prostate Cancer and Folate in vivo
Experimental models can reveal many novel discoveries; however the question remains as to whether the findings will translate to humans. Tomaszewski et al39 examined the relationship between patient serum folate levels and the proliferation rate of prostate tumor cells in Gleason Grade 7 radical prostatectomy specimens. When comparing tumors from the patients within the highest quintile of serum folate (117 +/− 15nM) to those from patients in the lowest quintile (18 +/− 9nM), tumors from the highest quintile group had an increased proliferative index, as measured by Ki67 staining, of 6.17 +/−3.2% vs 0.86 +/−0.92% (p<0.0001).39 Additionally, between both groups there was no significant difference in the proliferation index of the normal glands adjacent to tumor, which is likely reflective of their maintenance of normal cell cycle regulation. This study therefore supported the findings by Petersen et al,38 that increasing levels of serum folate lead to increased prostate cancer cell proliferation.
As reviewed by Mason et al,40 there was an increase in colorectal cancer incidence in both the U.S. and Canada that coincided with mandatory folic acid fortification in the mid 90’s. It has been postulated that the increased folate levels seen at this time may have allowed previously existing, however otherwise clinically indolent, tumors to proliferate. With the evidence just discussed regarding increasing tumorigenicity and proliferation rates of prostate cancer cells occurring in high folate environments,37–39 it is tantalizing to suggest the same phenomenon could explain the increase in prostate cancer incidence seen in North America from 1998–2002.1,3 In a recent case report, a patient with GS 3+4=7 prostate cancer had been managed successfully, PSA < 3ng/mL, for 10 years with intermittent androgen deprivation therapy using leuprolide, flutamide, and finasteride. He subsequently developed biochemical progression with a rising PSA to 21.3ng/mL, despite attempts at anti-androgen withdrawal, adding other anti-androgens, and eventually continuing leuprolide while adding docetaxel for over 18 weeks.41 It was then discovered that the patient had begun taking high dose supplements containing a total of 8mg of mixed folates and 5mg of Vitamin B12 (a folate coenzyme) at the beginning of his PSA rise. His serum folate at the time of his PSA peak was 303.6 nM. After stopping the supplementation and discontinuing his consumption of fortified foods, his serum folate level dropped to 9.06 nM. Remarkably, his PSA started to decline within two weeks, nadiring at 2.08 ng/mL.41
Folate and Prostate Specific Membrane Antigen
In addition to the increasing evidence that folate plays an important role in prostate carcinogenesis and progression, there are a growing number of reports that prostate specific membrane antigen (PSMA) is also key to this process. PSMA is a type II membrane protein with glutamate carboxypeptidase activity that removes gamma-linked glutamates from various substrates, such as polyglutamated folate and methotrexate.42 PSMA is highly expressed in LNCaP prostate cancer cells, however not in the PC-3 or DU145 cell lines. However when PSMA was ectopically expressed in PC-3 cells, it sequentially removed glutamates from polyglutamated methotrexate, suggesting a role for PSMA in methotrexate resistance as this so-called “antifolate” chemotherapeutic, requires glutamylation for maximum activity.42
In a study designed to localize PSMA throughout normal and malignant human tissue, PSMA was found in normal prostatic epithelium, duodenal mucosa, and proximal tubules in the kidney.43 Additionally, PSMA was seen in 33/35 primary prostatic adenocarcinomas, 7/8 prostate cancer metastases to the lymph nodes, and 8/18 prostate cancer metastases to bone. In a separate study, PSMA immunostaining intensity was confirmed to be increased in prostate adenocarcinoma when compared to benign prostate epithelium and prostatic intraepithelial neoplasia.44 Lapidus et al45 later established that there is also increased PSMA enzymatic activity in prostate cancer cells when compared to BPH and normal prostate tissues.
Intense staining for PSMA in the peritumoral and endotumoral capillary endothelial cells in 8/17 renal cell carcinomas, 7/13 urothelial carcinomas, and 3/19 colon carcinomas has also been documented.43 Due to the high expression of PSMA in the vasculature of many solid tumors, Conway et al46 investigated and confirmed that PSMA-null mice have severely impaired angiogenesis and that PSMA activity is necessary for endothelial cell invasion in vitro. However this study did not consider the potential effect of a folate/PSMA interaction.
Yao et al47 explored the effect of PSMA expression specifically on prostate carcinogenesis in a tissue recombinant mouse model. They found that 30% and 47% of PSMA-transgenic prostate specimens at 16 and 24 weeks demonstrated adenocarcinoma, while no adenocarcinoma was seen in the wild-type tissues (p=0.046 and p=0.012 respectively). Additionally, Yao et al demonstrated that PC-3 cells expressing PSMA have increased invasiveness and growth advantage in low (<1nM) and physiologic (25nM) folate environments when compared to PSMA absent PC-3 cells (p<0.05).47
In a subsequent study to further elicit the interaction of folate and PSMA, Yao et al48 separately confirmed that PSMA increases prostate cancer cellular folic acid uptake and increases cellular proliferation in physiologic relevant environments of folate. By comparing PC-3 cells expressing PSMA to PC-3 vector-alone cells, they found a significantly higher proliferation rate in the presence of poly-gamma-glutamated folate for PSMA expressing cells. This confirmed the role of PSMA as a folate hydrolase, which subsequently provided a growth advantage for these cells. Interestingly though, PSMA was also associated with a nearly 2-fold increase in the uptake and retention of tritiated folic acid (p<0.001), which is the monoglutamyl and fully bioavailable form of folate, suggesting a novel role for PSMA as a folate transporter.48 In a physiologic range of the monoglutamyl folate, this increase in uptake related to PSMA expression manifested as significantly higher growth rates for these cells (p<0.001). Therefore, at physiologic levels of both polyglutamyl and monoglutamyl forms of folate, PSMA provided a growth advantage.
The evidence for the role of PSMA in prostate carcinogenesis and progression is not confined to the laboratory. Ross et al49 performed immunohistochemical staining on 136 prostatectomy specimens and found that high PSMA expression was correlated with pathologic stage (p=0.029), tumor grade (p=0.030), aneuploidy (p=0.010) and biochemical recurrence (p=0.001). On multivariate analysis, PSMA was also found to be an independent predictor of biochemical recurrence (p=0.002).49 Another study investigated if PSMA expression was changed by androgen deprivation and found it was upregulated in 55% of post-treatment samples.50 They also examined the response of LNCaP cells in vitro to increased levels of testosterone and found a decrease in PSMA expression, further suggesting negative regulation of PSMA expression by androgens.50 Since the storage organ for folate is the liver, which expresses both PSMA and androgen receptors, it is possible that castration affects folate metabolism, potentially resulting in higher serum folate levels and causing a negative impact on disease progression, though these ideas are yet to be tested.
Taken together, the evidence demonstrates an important pathway for how folate can directly impact prostate carcinogenesis, and suggests a mechanism for how androgen resistant prostate cancer cells may be affected by folate as well.
Conclusions
Folate likely plays a dual role in prostate carcinogenesis; protective of DNA damage prior to neoplastic transformation and then acts as a promoter of tumor progression via increased cellular proliferation and invasion. There continues to be conflicting epidemiologic evidence regarding the effect folate has on prostate cancer risk, but this can likely be explained by vast differences in both folate and supplemental folic acid intake. In the post-fortification era, serum folate levels in the U.S. male population have significantly increased, with many older men now having levels that correlate with increased prostate cancer proliferation rates. Therefore, with growing experimental evidence that folate and PSMA can contribute to prostate tumorigenesis and progression, continued research is required to further delineate these complex relationships. At this time though, it seems prudent to recommend against folic acid supplementation in men diagnosed with prostate cancer, with any further recommendations requiring additional confirmatory research.
Acknowledgments
Support/Financial Disclosures
Research Support – The project described was supported by the National Institutes of Health through Grant Numbers UL1 RR024153 and UL1TR000005 (University of Pittsburgh Clinical and Translational Science Institute), and by RO1 138444 (DOK & DJB).
We would like to thank Ben Ristau, M.D. for helpful discussions and proof-reading the manuscript.
Footnotes
Conflict of Interest – none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–6. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53:171–84. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 4.Park SK, Sakoda LC, Kang D, et al. Rising prostate cancer rates in South Korea. Prostate. 2006;66:1285–91. doi: 10.1002/pros.20419. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu H, Ross R, Bernstein L, et al. Cancers of the Prostate and Breast Among Japanese and White Immigrants in Los Angeles County. British Journal of Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. PDQ Prostate Cancer Prevention. Bethesda, MD: National Cancer Institute; [Accessed 09/24/2012]. Date last modified 03/29/2012. Available at: http://www.cancer.gov/cancertopics/pdq/prevention/prostate/healthprofessional. [Google Scholar]
- 7.Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–5. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stover PJ, Field MS. Trafficking of intracellular folates. Adv Nutr. 2011;2:325–31. doi: 10.3945/an.111.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkels RM, Brouwer IA, Siebelink E, et al. Bioavailability of food folates is 80% of that of folic acid. Am J Clin Nutr. 2007;85:465–473. doi: 10.1093/ajcn/85.2.465. [DOI] [PubMed] [Google Scholar]
- 10.Blount BC, Ames BN. Analysis of uracil in DNA by gas chromatography-mass spectrometry. Anal Biochem. 1994;219:195–200. doi: 10.1006/abio.1994.1257. [DOI] [PubMed] [Google Scholar]
- 11.Krumdiek C, Howard-Peebles P. On the nature of folic acid-sensitive fragile sites in human chromosome: an hypothesis. American Journal of Medical Genetics. 1983;16:23–28. doi: 10.1002/ajmg.1320160105. [DOI] [PubMed] [Google Scholar]
- 12.Choi SW, Mason JB. Folate and Carcinogenesis: An Integrated Scheme. Journal of Nutrition. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 13.Bertino JR, O’Brien P, McCullough JL. Inhibition of growth of leukemia cells by enzymic folate depletion. Science. 1971;172:161–2. doi: 10.1126/science.172.3979.161. [DOI] [PubMed] [Google Scholar]
- 14.Baggott JE, Vaughn WH, Juliana MM, et al. Effects of folate deficiency and supplementation on methylnitrosourea-induced rat mammary tumors. J Natl Cancer Inst. 1992;84:1740–4. doi: 10.1093/jnci/84.22.1740. [DOI] [PubMed] [Google Scholar]
- 15.Song J, Medline A, Mason JB, et al. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–40. [PubMed] [Google Scholar]
- 16.Bistulfi G, Diegelman P, Foster BA, et al. Polyamine biosynthesis impacts cellular folate requirements necessary to maintain S-adenosylmethionine and nucleotide pools. Faseb J. 2009 doi: 10.1096/fj.09-130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wein, Kavoussi, Novick, et al. Campbell-Walsh Urology. Philadelphia, PA: Saunders, an imprint of Elsevier Inc; 2012. [Google Scholar]
- 18.Hyattsville, MD. Date last modified 09/01/2012. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). Accessed 07/20/2012.
- 19.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr. 2007;86:718–27. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 21.Intakes ARotSCotSEoDR, its Panel on Folate OBV, Choline et al. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Press; 1998. [PubMed] [Google Scholar]
- 22.Hultdin J, Van Guelpen B, Bergh A, et al. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113:819–24. doi: 10.1002/ijc.20646. [DOI] [PubMed] [Google Scholar]
- 23.Lawson KA, Wright ME, Subar A, et al. Multivitamin Use and Risk of Prostate Cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 24.Vlajinac H, Marinkovic J, Ilic M, et al. Diet and Prostate Cancer: A Case-Control Study. European Journal of Cancer. 1997;33:101–107. doi: 10.1016/s0959-8049(96)00373-5. [DOI] [PubMed] [Google Scholar]
- 25.Pelucchi C, Galeone C, Talamini R, et al. Dietary folate and risk of prostate cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2005;14:944–8. doi: 10.1158/1055-9965.EPI-04-0787. [DOI] [PubMed] [Google Scholar]
- 26.Rossi E, Hung J, Beilby JP, et al. Folate levels and cancer morbidity and mortality: prospective cohort study from Busselton, Western Australia. Ann Epidemiol. 2006;16:206–12. doi: 10.1016/j.annepidem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Shannon J, Phoutrides E, Palma A, et al. Folate intake and prostate cancer risk: a case-control study. Nutr Cancer. 2009;61:617–28. doi: 10.1080/01635580902846593. [DOI] [PubMed] [Google Scholar]
- 28.Beilby J, Ambrosini GL, Rossi E, et al. Serum levels of folate, lycopene, beta-carotene, retinol and vitamin E and prostate cancer risk. Eur J Clin Nutr. 2010;64:1235–8. doi: 10.1038/ejcn.2010.124. [DOI] [PubMed] [Google Scholar]
- 29.Ebbing M, Bønaa KH, Nygård O, et al. Cancer Incidence and Mortality After Treatment With Folic Acid and Vitamin B12. JAMA. 2009;302:2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 30.Johansson M, Appleby PN, Allen NE, et al. Circulating Concentrations of Folate and Vitamin B12 in Relation to Prostate Cancer Risk: Results from the European Prospective Investigation into Cancer and Nutrition Study. Cancer Epidemiol Biomarkers Prev. 2008;17:279–285. doi: 10.1158/1055-9965.EPI-07-0657. [DOI] [PubMed] [Google Scholar]
- 31.Kristal AR, Arnold KB, Neuhouser ML, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2010;172:566–77. doi: 10.1093/aje/kwq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens VL, Rodriguez C, Pavluck AL, et al. Folate nutrition and prostate cancer incidence in a large cohort of US men. Am J Epidemiol. 2006;163:989–96. doi: 10.1093/aje/kwj126. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R, et al. Null association between prostate cancer and serum folate, vitamin B(6), vitamin B(12), and homocysteine. Cancer Epidemiol Biomarkers Prev. 2003;12:1271–2. [PubMed] [Google Scholar]
- 34.Collin SM, Metcalfe C, Refsum H, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1632–42. doi: 10.1158/1055-9965.EPI-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wien TN, Pike E, Wisloff T, et al. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open. 2012;2:e000653. doi: 10.1136/bmjopen-2011-000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bistulfi G, Vandette E, Matsui S, et al. Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biol. 2010;8:6. doi: 10.1186/1741-7007-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bistulfi G, Foster BA, Karasik E, et al. Dietary folate deficiency blocks prostate cancer progression in the TRAMP model. Cancer Prev Res (Phila) 2011;4:1825–34. doi: 10.1158/1940-6207.CAPR-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen LF, Brockton NT, Bakkar A, et al. Elevated physiological levels of folic acid can increase in vitro growth and invasiveness of prostate cancer cells. BJU Int. 2012;109:788–95. doi: 10.1111/j.1464-410X.2011.10437.x. [DOI] [PubMed] [Google Scholar]
- 39.Tomaszewski JJ, Cummings JL, Parwani AV, et al. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011;71:1287–93. doi: 10.1002/pros.21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason JB, Kim SJ. Revisiting the goldilocks phenomenon: folate and colorectal cancer risk. Am J Gastroenterol. 2010;105:1914–6. doi: 10.1038/ajg.2010.189. [DOI] [PubMed] [Google Scholar]
- 41.Tisman G, Garcia A. Control of prostate cancer associated with withdrawal of a supplement containing folic acid, L-methyltetrahydrofolate and vitamin B12: a case report. J Med Case Rep. 2011;5:413. doi: 10.1186/1752-1947-5-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto J, Suffoletto B, Berzin T, et al. Prostate-specific Membrane Antigen: A Novel Folate Hydrolase in Human Prostatic Carcinoma Cells. Clinical Cancer Research. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 43.Silver D, Pellicer I, Fair W, et al. Prostate-specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clinical Cancer Research. 1997;3:81–85. [PubMed] [Google Scholar]
- 44.Bostwick D, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adneocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 45.Lapidus R, Tiffany C, Isaacs J, et al. Prostate-Specific Membrane Antigen (PSMA) Enzyme Activity Is Elevated in Prostate Cancer Cells. Prostate. 2000;45:350–354. doi: 10.1002/1097-0045(20001201)45:4<350::aid-pros10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310–24. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao V, Parwani A, Maier C, et al. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68:9070–7. doi: 10.1158/0008-5472.CAN-08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao V, Berkman CE, Choi JK, et al. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate. 2010;70:305–16. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 49.Ross J, Sheehan C, Fisher H, et al. Correlation of Primary Tumor Prostate-Specific Membrane Antigen Expression with Disease Recurrence in Prostate Cancer. Clinical Cancer Research. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 50.Wright G, Grob M, Haley C, et al. Upregulation of Prostate-Specific Membrane Antigen After Androgen Deprivation Therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]