Abstract
Assaultive violence exposure during childhood is a significant risk factor for posttraumatic stress disorder (PTSD). The purpose of the present study was to characterize the relationships of assault and PTSD severity with the organization of large-scale networks identified during emotion processing. Adolescent girls aged 12–16 with (N=15) and without (N=15) histories of assault underwent fMRI while engaged in a task that presented images of fearful or neutral facial expressions. Independent component analysis (ICA) identified a frontocingulate network, a frontoparietal network, and a default mode network. Assault exposure was associated with significantly greater activation of the frontocingulate network for fear versus neutral faces. Within the frontocingulate network, PTSD severity was associated with weakened functional connectivity between the left amygdala and the perigenual anterior cingulate. Within the frontoparietal network, assaulted girls demonstrated weakened connectivity of the premotor cortex with the right middle frontal gyrus. Within the default mode network, assault exposure and PTSD severity were associated with strengthening functional connectivity of the parahippocampus with the medial and lateral PFC, respectively. Individual differences in functional connections within the frontocingulate network and frontoparietal network among the assaulted group were strongly associated with caregiver-rated family disengagement. These results demonstrate associations between assault and PTSD symptoms on the functional organization of large-scale frontoparietal, frontocingulate, and default mode networks during emotion processing. The relationship with caregiver-rated family disengagement suggests the impact of family support on the neural processing correlates of assault and PTSD symptoms.
Keywords: Early life trauma, Adolescence, PTSD, Emotion processing, Neuroimaging
1. Introduction
Childhood adversity is a significant risk factor the development of subsequent mental health disorders (Kessler et al., 1997; Kilpatrick et al., 2000; Kilpatrick et al., 2003; Green et al., 2010). Of the various types of childhood adversity, assaultive violence exposure is associated with the greatest risk for mental health disorders (Resnick et al., 1993; Kessler, 2000; Cisler et al., 2012). Assaultive violence exposure, including physical and sexual assault or witnessed violence, is common among adolescents with a national prevalence rate estimated at ~50% (Kilpatrick et al., 2000; Kilpatrick et al., 2003; Cisler et al., 2012). Longitudinal studies indicate that assault among adolescents increases symptoms of PTSD, depression, and substance use assessed three years later (Cisler et al., 2011b, a; Cisler et al., 2012). These studies additionally suggest a dose-dependent relationship between assault exposure severity and degree of risk for subsequent PTSD and other mental health symptoms (Neuner et al., 2004; Kolassa et al., 2010; Cisler et al., 2012). These data demonstrate the strong public health impact associated with assault exposure among adolescents.
Given the potent risk conferred through assault exposure, it is imperative that we develop consistently effective interventions. Understanding the brain mechanisms by which assault confers vulnerability for mental illness, such as PTSD, is a viable means of improving treatments for these victims. To date, there have been numerous investigations of the neurobehavioral consequences and correlates of stressor exposure and PTSD (Hayes et al., 2012). Animal models using rodents have demonstrated persistent structural alterations following stressor exposure in neural regions theorized to mediate emotion processing and cognitive control, including the amygdala, hippocampus, and medial prefrontal cortex (Vyas et al., 2002; Vyas et al., 2004; Arnsten, 2009). Human neuroimaging studies of adult PTSD similarly suggest altered functional activity in regions theorized to mediate emotion processing, emotion regulation, and fear extinction, including amygdala, hippocampus, rostral anterior cingulate cortex (ACC), dorsal ACC, insular cortex, and lateral prefrontral cortex (Shin et al., 2001; Shin et al., 2005; Simmons et al., 2008; Milad et al., 2009; Fonzo et al., 2010; Shin et al., 2011; Hayes et al., 2012). Emerging concepts of stress reactivity include allostasis and allostatic load (McEwen et al., 2011), which refer to on-going neurobiological adaptations to environmental pressures, and these concepts may help understand the link between stress and mental health (Ganzel et al., 2010; McEwen et al., 2011; McEwen and Gianaros, 2011). These prior investigations have furnished data to support inferences regarding brain mechanisms mediating trauma and PTSD and suggest core neural processing deficits.
There are two main limitations of our current knowledge regarding how adolescent assault exposure confers lasting risk for mental illness that the current study sought to address. First, prior human functional imaging studies have focused mostly on functional segregation approaches, in which the activities of neural regions in response to a task are considered in isolation. While functional segregation approaches are useful for understanding the behavior of isolated regions and their relationship with clinical variables, functional segregation approaches are poorly matched to models of human brain function as the product of distributed networks of information processing (Bullmore and Sporns, 2009; Bressler and Menon, 2010; Congdon et al., 2010; Rubinov and Sporns, 2010; Menon, 2011; Smith, 2012). Consistent with this approach, a recent network-level model of psychopathology posits large-scale disruptions within three specific neural networks: a frontoparietal network (also referred to as a central executive network), a frontocingulate network (also referred to as a salience network), and a default mode network (Menon, 2011). The frontoparietal network consists of a distributed set of neural regions including bilateral dorsolateral PFC, medial PFC/dorsal anterior cingulate cortex, and bilateral parietal cortices and is commonly implicated in higher-order cognitive functions, including manipulating information in working memory, problem-solving, and decision-making (Bressler and Menon, 2010; Menon, 2011). The frontocingulate network consists primarily of anterior cingulate and bilateral anterior insular cortices and is involved in detecting and integrating sensory and interoceptive cues (Bressler and Menon, 2010; Menon, 2011). The default mode network consists primarily of the posterior cingulate cortex/precuneus, medial PFC, and lateral parietal cortices and is involved in stimulus-independent thought (e.g., daydreaming) and self-referential mentation (Gusnard and Raichle, 2001; Raichle et al., 2001; Bressler and Menon, 2010; Menon, 2011). These three networks are highly reproducible during both task and rest (Smith et al., 2009; Bressler and Menon, 2010; Menon, 2011) and are theorized to be core neurocognitive networks in which dysfunction may at least partially explain psychopathology (Menon, 2011). Accordingly, to more fully understand the neural processing correlates of assault exposure and risk for subsequent mental health disorders, it seems promising to investigate functional connectivity within these three large-scale neural networks.
The second major limitation of the neural mechanisms by which early life trauma exposure confers subsequent risk for mental health disorders is that most of the prior studies have focused on adults retrospectively recalling childhood maltreatment. This approach is limited by the potential confound of years of individually varying neurodevelopmental adaptation to the trauma, resulting mental health symptoms, and trauma recall bias. We therefore focused on adolescents with a specific and recent trauma type to minimize these confounding factors. While functional brain imaging studies among pediatric PTSD populations are emerging (Carrion et al., 2008; Carrion et al., 2010; Garrett et al., 2012), these studies have focused mostly on functional segregation.
The current study conducted a brain network-analysis of implicit emotion processing among adolescent girls with versus without assault exposure histories. We used a well-validated task in order to provide a valid probe of ecologically relevant neural processing networks mediating implicit emotion processing (Rauch et al., 2000; Bryant et al., 2008; Felmingham et al., 2010). We focused on three specific neural networks, a frontoparietal, frontocingulate, and default mode network, based on research evidence suggesting their importance to neurocognitive function and psychopathology (Bressler and Menon, 2010; Menon, 2011). We focused first on adolescent girls, given the known sex differences in neural correlates of emotion processing and regulation (Domes et al., 2010). We also integrate the neural measure with measures of family functioning in order to understand whether brain function associated with assault is modulated by family characteristics (Hanson et al., 2010). We specifically focused on family disengagement, given that poor social support and family dysfunction are robust risk factors for mental health problems following trauma exposure (Ozer et al., 2003; Trickey et al., 2012). Finally, an important caveat is that our sample was cross-sectional; thus, inferences regarding the causal effects of assault on neural functioning are not possible. Nonetheless, epidemiological research demonstrates that assaulted adolescents are a significantly at risk population, and understanding the neural network processes found among this high-risk sample can greatly improve our theoretical understanding and effective treatment development despite not being able to link causally the identified neural processing deficits to assault exposure.
2. Methods
2.1. Participants
Thirty adolescent girls aged 12–16 years were included in analyses for this study; 15 with assault exposure histories, and 15 control participants. Table 1 displays demographic and clinical variables of this sample. There were two additional control participants scanned whose data were not included in analyses due to excessive head motion. Exclusion criteria for the study included major medical disorders, developmental disorders, current pregnancy, non-removal internal metal objects, and psychotic disorders. Participants were recruited from trauma specialty outpatient clinics as well as from general community advertisements. Community-wide advertisements used language indicating that inclusion criteria were either assaultive violence exposure histories or no assaultive violence exposure histories.
Table 1.
Demographic and clinical characteristics of the sample.
| Measure | Assaulted Girls (n=15) |
Non-Assaulted Girls (n=15) |
P value of group difference |
|---|---|---|---|
| Age | 15.07 (1.10) | 14.27 (1.28) | 0.08 |
| Ethnicity | 56% Caucasian | 60% Caucasian | 0.77 |
| 19% African-American | 33% African-American | ||
| 6% biracial | 7% biracial | ||
| 6% Hispanic | 0% Hispanic | ||
| Direct Assaults | 3.67 (2.61) | 0 (0) | < 0.001* |
| Age at First Assault | 7.27 (3.35) | n/a | |
| Age at Last Assault | 12.13 (2.97) | n/a | |
| Time since Last Assault | 2.93 (3.06) | n/a | |
| Physical Assault from Caregiver | 73% | 0 | < 0.001* |
| Physical Assault from Non-Caregiver | 73% | 0 | < 0.001* |
| Sexual Assault | 53% | 0 | < 0.001* |
| Current PTSD | 20% | 0% | 0.07 |
| Past PTSD | 40% | 0% | 0.01* |
| Current GAD | 20% | 0% | 0.07 |
| Past GAD | 6.7% | 0% | 0.31 |
| Current MDD | 13% | 0% | 0.5 |
| Past MDD | 25% | 0% | 0.3 |
| Current Alcohol Abuse | 13.3% | 0% | 0.10 |
| Past Alcohol Abuse | 26.7% | 0% | 0.017* |
| Current Substance Abuse | 0% | 0% | n.a. |
| Past Substance Abuse | 13.3% | 0% | 0.10 |
| UCLA PTSD Symptoms | 23.73 (20.44) | 4.3 (10.3) | 0.003* |
| Taking Psychotropic Medication | 33% | 0% | .03* |
| CBCL anxious | 5.53 (5.29) | 2.87 (3.2) | 0.11 |
| CBCL depressed | 4.47 (3.76) | 1.2 (2.1) | 0.007* |
| Verbal IQ | 100.80 (11.01) | 109.0 (18.96) | 0.16 |
Note.
indicates significant between-group differences. Values in parentheses indicate SD.
2.2. Assessment
Participants’ current and past mental health was assessed with the K-SADS (Kaufman et al., 1997). Assaultive trauma histories were characterized using the trauma assessment section of the National Survey of Adolescents (Kilpatrick et al., 2000; Kilpatrick et al., 2003), a structured interview used in prior epidemiological studies of assault and mental health functioning among adolescents. Specific assaultive events were assessed with behaviorally specific dichotomous questions and included: 1) sexual assault (i.e., anal penetration, vaginal penetration, oral sex on the perpetrator, oral sex from the perpetrator, digital penetration, fondling of the adolescent, forced fondling of the perpetrator), 2) physical assault (i.e., attacked with a weapon, attacked with a stick, club, or bottle, attacked without a weapon, threatened with a weapon, attacked with fists), and 3) severe abuse from a caregiver (i.e., beaten with fists or an object to the point where bruising or bleeding occurred). The K-SADS and assault assessments were conducted by a trained clinical research coordinator under the supervision of a doctoral-level clinical psychologist. Of the assaulted adolescents (n=15), 10 had been assaulted by both a man and a woman, 4 had been assaulted by only a man, and 1 had been assaulted by only a woman.
A primary caregiver for each participant completed the UCLA PTSD Index (Steinberg et al., 2004) and Child Behavior Checklist (CBCL) (Achenbach, 1991), which provided continuous measures of the severity of PTSD symptoms, general anxiety symptoms (CBCL subscale ‘Anxious-Depressed’), and social functioning (CBCL subscale ‘social problems’). Analyses with PTSD symptom severity were only conducted among the assaulted adolescent girls. Additionally, the caregivers completed the Family Adaptability and Cohesion Scale (FACES) (Rodick et al., 1986; Henggeler et al., 1991; Olson, 1991; Matherne and Thomas, 2001), a self-report measure of family functioning that includes a scale measuring the degree of disengagement in the family (e.g., family members avoid contact with one another). As noted above, social support is a robust predictor of clinical functioning following trauma, so we focused on family disengagement in order to understand the degree to which the support of the immediate family environment modulated brain activity among the assaulted girls. Caregivers for two of the assaulted adolescents did not complete the FACES measures. For two of the assaulted adolescents, the primary caregiver completing the forms had physically abused the child in the past (at least 2 years ago in each case). The caregivers’ ratings in these cases were not discrepant from the child’s ratings nor were they discrepant from the ratings for the other assaulted adolescents (e.g., there was no evidence the caregivers were minimizing symptoms).
2.3. Implicit Emotion Processing Task
Participants were engaged in an emotion processing task, during which they made button presses indicating gender decisions while viewing faces taken from the NimStim facial stimuli set (Tottenham et al., 2009). The faces contained either neutral or fearful expressions, presented either overtly or covertly, in alternating blocks. There were an equal number of female and male faces. Overt faces were presented for 500 ms, with a 1200 ms ISI displaying a blank screen with a fixation cross, in blocks of 8 presentations for a total block length of ~17 s. Covert face blocks used a similar design but were presented for 33 ms followed immediately by a neutral facial expression mask for 166 ms from the same actor depicted in the covert image, and the ISI was 1500 ms. Rest blocks were additionally included that displayed a blank screen with a fixation cross and lasted 10 s. The alternation between each block type (covert, overt, fear, neutral, rest) was additionally separated by 5000 ms of blank screen with a fixation cross. The task was presented in two runs, each lasting ~8 min, during which each block type was presented 5 times. Reaction times and response accuracy were recorded. For the purposes of providing a general test of fear processing per se, we collapsed across covert and overt presentations to create a general contrast of fear versus neutral blocks.
2.4. fMRI acquisition
A Philips 3T Achieva X-series MRI system using an 8-channel head coil (Philips Healthcare, USA) was used to acquire imaging data. Anatomic images were acquired with a MPRAGE sequence (matrix=192×192, 160 sagittal slices, TR/TE/FA=min/min/90°, final resolution=1×1×1mm3 resolution). Echo planar imaging sequences were used to collect the functional images using the following sequence parameters: TR/TE/FA=2000ms/30ms/90°, FOV=240×240mm, matrix=80×80, 37 oblique slices (parallel to AC-PC plane to minimize OFC sinal artifact), slice thickness=3 mm, final resolution 3×3×3 mm3.
2.5. Image preprocessing
Image preprocessing followed standard steps and was completed using AFNI (Cox, 1996) software. In the following order, images underwent despiking, slice timing correction, deobliquing, motion correction using rigid body alignment, alignment to participant’s normalized anatomical images, spatial smoothing using a 5 mm FWHM Gaussian filter, and rescaling into percent signal change. Images were normalized using the MNI 452 template brain. To correct for respiratory and cardiovascular artifacts, fluctuations in white matter voxels and CSF were regressed out of time courses from grey matter voxels following segmentation using FSL (Smith et al., 2004) and using restricted maximum likelihood to account for autocorrelation (Behzadi et al., 2007; Sala-Llonch et al., 2012). This step was implemented directly after motion correction and normalization of the EPI images in the preprocessing stream. Additionally, to correct for residual motion artifacts in the signal that standard motion correction does not correct (Friston et al., 1996; Tohka et al., 2008), Independent Component Analyses (ICA) were completed as a last preprocessing step separately on each participant and each run to identify and remove artifact components from the data (Tohka et al., 2008; Zeng et al., 2009; Kelly et al., 2010).
2.6. Data Analyses
Data underwent a series of planned analyses. First, independent component analysis (ICA) was used to identify neural processing networks engaged during the emotion processing task. Spatial ICA decomposes fMRI data into spatially independent components of temporal coactivation (McKeown et al., 1998; Calhoun et al., 2009), thus identifying distributed functional networks. Voxel-wise coefficient loadings indicate the degree to which each voxel contributes to the overall network, which provides information about the spatial organization of the network. Each network is further characterized by a timecourse, indicating the network’s degree of activation throughout the task. The group ICA back-reconstruction approach (Calhoun et al., 2001a; Erhardt et al., 2011) allows for separately characterizing the functional organization and timecourse of each network in each participant. We conducted ICA using the infomax algorithm within the Group Independent Component Analyses for fMRI Toolbox (GIFT;(Calhoun et al., 2001b)) with the following parameters: group ICA (GICA) using the ICASSO method with 25 iterations to assess component estimation reliability, dimension reduction using singular value decomposition with three reduction steps, and a model order of 20 (i.e., 20 independent components were extracted). Individual spatial component maps and timecourses for each participant were additionally computed in GIFT using the GICA back-reconstruction approach (Calhoun et al., 2001b).
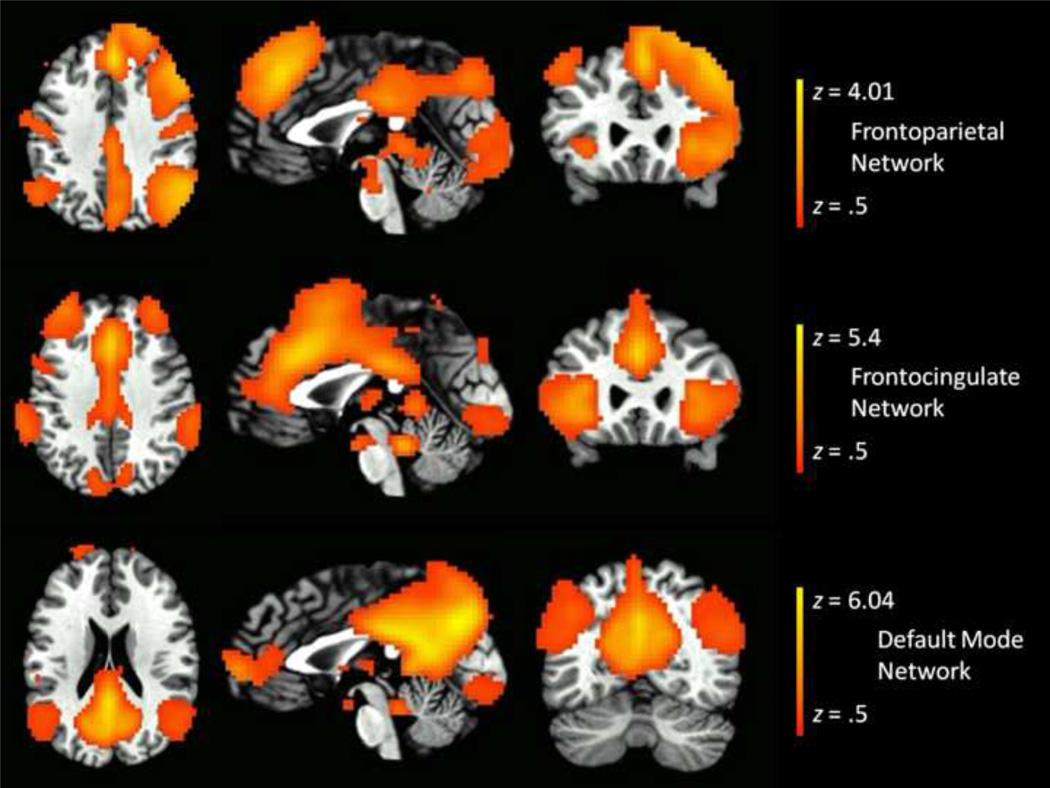
Second, following identification of neural processing networks during the emotion processing task, we then identified three networks that corresponded to a frontocingulate network (also referred to as a salience network), a frontoparietal network (also referred to as a central executive network), and a default mode network. We identified these networks empirically via the following approach. First, we used the automated term-based meta-analysis approach described by (Yarkoni et al., 2011) and publicly available at neurosynth.org to define spatial maps of regions significantly activated in published articles using the terms ‘salience’, ‘frontoparietal’, and ‘default’. These meta-analysis maps are displayed in Supplemental Figure 1. Second, we then correlated each of these meta-analysis spatial maps with the 20 independent components identified from the emotion processing task to identify the component most highly correlated with each meta-analysis spatial map. These spatial correlation analyses identified a unique component most highly correlated with each meta-analysis spatial map: a component visually resembling a frontocingulate network significantly correlated with the meta-analysis fronocingulate spatial map (r = 0.24, p < 0.001), a component visually resembling a frontoparietal network significantly correlated with the meta-analysis frontoparietal spatial map (r = 0.20, p < 0.001), and a component visually resembling a default network significantly correlated with the meta-analysis default spatial map (r = 0.40, p < 0.001). Therefore, we selected these three components as our frontocingulate, frontoparietal, and salience network, respectively (see Figure 1). Supplementary Figures 2, 3, 4 provide detailed depictions of the frontocingulate, frontoparietal, and default mode networks.
Figure 1.
Axial, sagittal, and coronal slices of frontocingulate, frontoparietal, and default mode networks identified through independent component analysis and matched to spatial templates identified through automated term-based meta-analysis (Yarkoni et al., 2011) (see supplemental Figure 1).
Third, following selection of the three networks of interest, we then characterized the degree to which the task design (fear blocks vs neutral blocks) modulated the activation for these three networks. Given the lack of knowledge of the mediating hemodynamic response function (HRF) in a large-scale network, we solved for the shape of the network’s HRF using a finite impulse response model, solving for an 18 s HRF beginning at the block’s onset and including 10 regressors spaced 1 TR apart. This provided an estimate of the HRF’s shape for each block type (supplemental Figure 5 displays the mean estimated HRF across participants for fear and neutral blocks for the default mode network, frontocingulate network, and frontoparietal network). We then calculated the area under the curve (AUC) of these HRF estimates using a numerical integration function, and these AUC estimates were then contrasted between fear and neutral blocks (collapsed across overt and covert presentation types). We then tested the degree to which assault exposure modulated network activity using robust regression (Wager et al., 2005) analyses, in which the AUC contrast for the network was regressed onto these clinical or behavioral variables. False discovery rate (FDR) was used to control for family-wise multiple comparisons.
Fourth, we tested the degree to which functional connectivity between the major nodes comprising the network(s) of interest varied as a function of clinical or behavioral variables. Functional connectivity (FC) was assessed using the beta series correlation method (Rissman et al., 2004). In this approach, each individual block is included in the model as a separate regressor, allowing β estimates to be calculated separately for each individual block. Connectivity unique to a specific task condition is assessed by calculating correlations between the series of β estimates between voxels or regions for one stimulus condition. FC differences between conditions can be calculated by comparing connectivity estimates between stimulus conditions. To define regions-of-interest (ROIs), we placed 6 mm spheres centered on areas of peak activation from the group-level component maps. Specifically, the frontocingulate network was reduced to 15 nodes, the frontoparietal network was reduced to 16 nodes, and the default mode network was reduced to 13 nodes (Supplementary Figures 6, 7, and 8, respectively). Singular value decomposition was used to extract the first principal component of the β estimates from each ROI separately for fear and neutral blocks. Following r-to-z normalization, the correlation estimates were contrasted between conditions. This results in a square matrix for each participant indicating the degree to which FC between each ROI in the network differs between covert fear and covert neutral blocks. The degree to which these FC contrasts varied as a function of assault was tested using univariate robust regression models, with false discovery rate (FDR) used to control for family-wise alpha inflation due to multiple comparisons.
Finally, we tested whether any observed functional connections that differed between groups or were related to PTSD symptom severity were related to external measures of functioning and family characteristics (i.e., disengagement) among just the assaulted group. These analyses test whether the functional connections related to assault exposure or PTSD symptoms severity explain variability in clinical symptom, task performance, and family characteristics among the assaulted group. These analyses entailed multiple regression models using robust regression, in which we entered any functional connection related to assault exposure or PTSD severity at p < 0.05 (uncorrected) simultaneously as predictors of the dependent measures. For connections related to assault exposure or PTSD symptoms, we tested whether these connections explained variability in task performance (RT bias for fear vs neutral blocks) and family disengagement. A separate model was used for each external measure, with FDR used to control for family-wise alpha inflation due to multiple comparisons. The multiple regression model using any functional connection related to assault exposure or PTSD symptoms at p < 0.05 uncorrected is consistent with our focus on a network (i.e., multivariate) approach, in which the multivariate combination of functional connections is more important the univariate contribution of any single functional connection.
3. Results
3.1. Behavioral data
Accuracy on the gender identification task was well above chance for all participants and ranged from 68.3% to 99.8% (M = 88%, SD = 0.08). Reaction time (RT) bias scores, indicative of attentional biases towards threat, were calculated as the difference in RT between fear versus neutral blocks (collapsed across covert and overt presentations to increase power, given that there were no differences in RT bias between covert and overt trials: t = 0.13, p = 0.90). There were no differences in RT bias between the two groups (pcorrected = 0.71). Among the assaulted group, RT bias was significantly positively correlated with general anxiety symptoms (t = 3.44, pcorrected = 0.012) and social problems (t = 3.22, pcorrected = 0.014), but not significantly with PTSD symptoms (pcorrected = 0.14).
3.2. Relationships between behavioral measures and network activation during the task
Percent signal change AUC indices of activation of the networks of interest were regressed separately onto the assault exposure variables. For the frontocingulate network, assaulted adolescents displayed significantly greater activity relative to control adolescents (t = 2.52, pcorrected = .036). PTSD symptom severity among the assaulted group was not associated with frontocingulate activity (pcorrected = 0.43). Assaulted and non-assaulted adolescents did not differ in activation of either the frontoparietal network or default mode network during fear vs neutral blocks (all ps > 0.16).
3.3. Functional connectivity within each neural network as a function of assault exposure and PTSD symptom severity
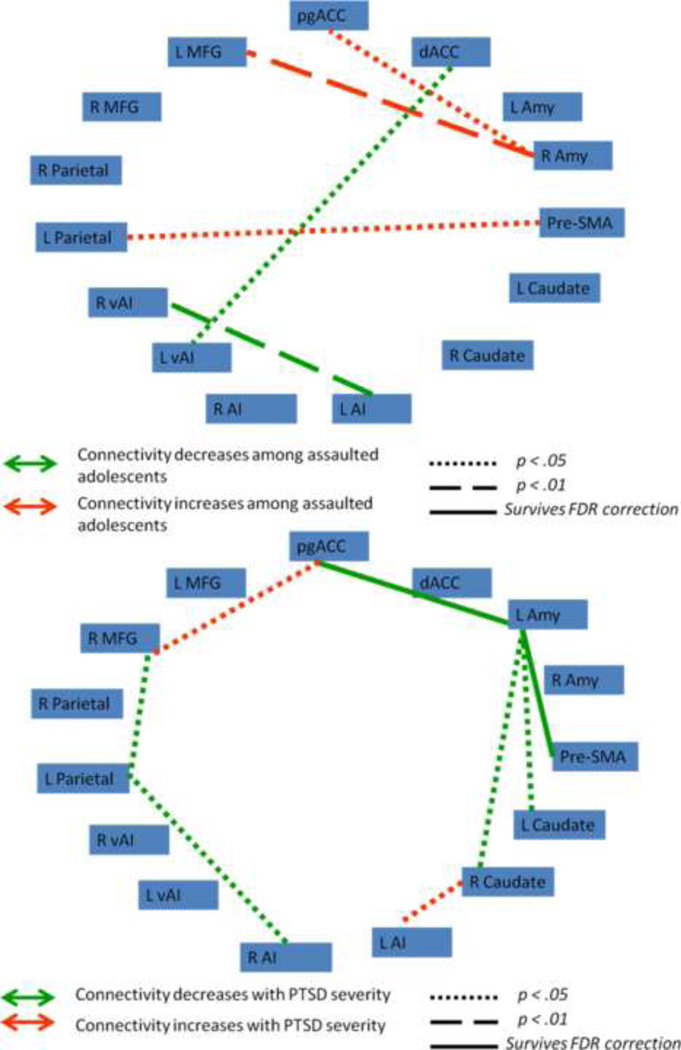
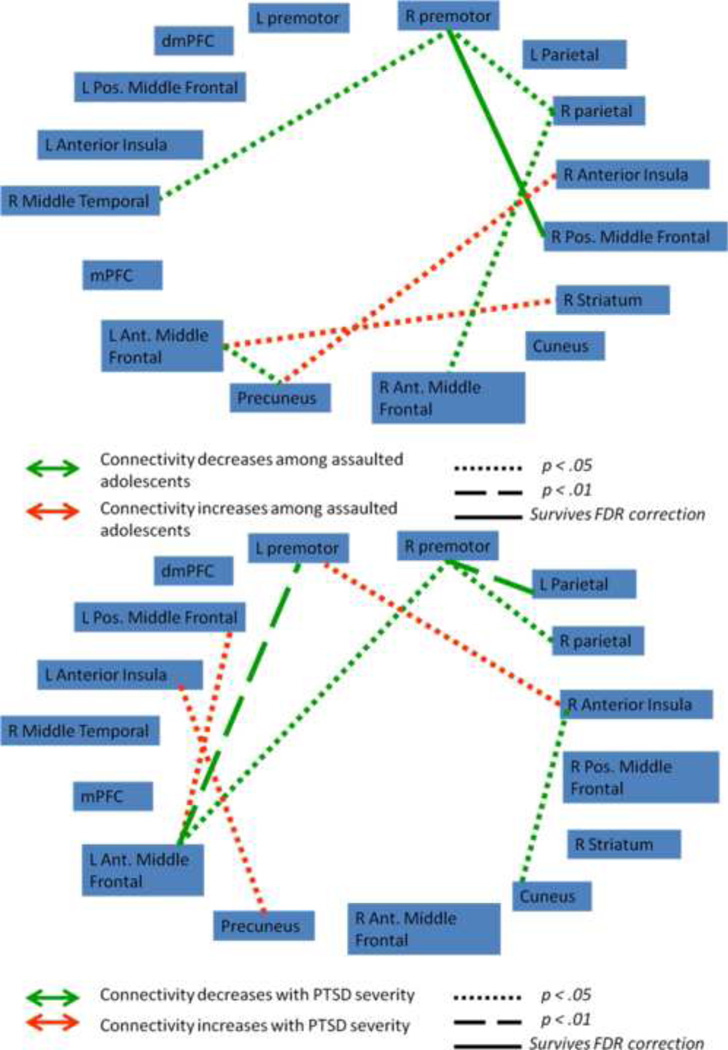
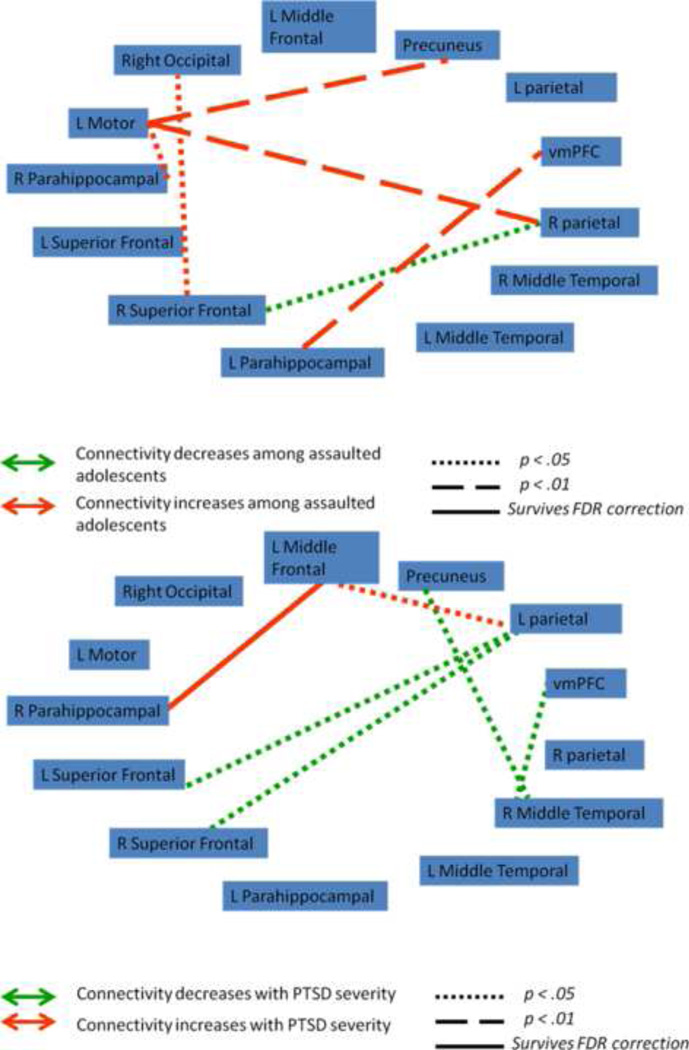
We next tested whether functional connectivity (FC) between nodes within the frontocingulate, frontoparietal, and default mode networks varied as a function of assault exposure. 6 mm spherical regions-of-interest (ROIs) were placed in areas of peak activation from the group-level component maps (Supplemental Figures 6, 7, and 8), with the frontocingulate component reduced to 15 nodes, the frontoparietal component reduced to 16 nodes, and the default mode component reduced to 13 nodes. Contrast matrices were defined for each participant as the difference in FC between fear and neutral connectivity matrices. Figures 2, 3, and 4 illustrate the network functional connections associated with assault exposure and PTSD symptoms for the frontocingulate, frontoparietal, and default mode networks, respectively. For the frontocingulate network, assault exposure was associated with heightened connectivity between right amygdala and middle frontal gyrus; PTSD severity was associated with weakened connectivity between left amygdala and bilateral caudate, perigenual anterior cingulate, and pre-SMA. For the frontoparietal network, assault exposure was associated with weakening of connectivity between the right premotor cortex and middle frontal gyrus; PTSD severity was also associated with weakening of connectivity between the premotor cortex and left parietal cortex and left middle frontal gyrus. For the default mode network, assault exposure was associated with strengthening of connectivity between the parahipocampal gyrus and ventral medial PFC, and the left motor cortex demonstrated heightened connectivity with the precuneus and right parietal cortex among assaulted adolescents; PTSD severity was associated with weakening of connectivity with the bilateral superior frontal gryus, and strengthening of the middle frontal gyrus.
Figure 2.
Functional connectivity indices between nodes in the frontocingulate network that varied as a function of assault exposure (top) and PTSD symptoms severity within the assaulted group (bottom). pgACC = perigenual anterior cingulate; dACC = dorsal anterior cingulate; L Amy = left amygdala; R Amy = right amygdala; Pre-SMA = pre-supplementary area; L Caudate = left caudate; R Caudate = right caudate; L AI = left anterior insula; R AI = right anterior insula; L vAI = left ventral anterior insula; r vAI = right ventral anterior insula; L Parietal = left parietal; R Parietal = right parietal; R MFG = right middle frontal gyrus; L MFG = left middle frontal gyrus.
Figure 3.
Functional connectivity indices between nodes in the frontoparietal network that varied as a function of assault exposure (top) and PTSD symptoms severity within the assaulted group (bottom). R premotor = right premotor; L Parietal = left parietal, R Parietal = right parietal; R Pos Middle Frontal = right posterior middle frontal gyrus; R striatum = right striatum; R Ant Middle Frontal = right anterior middle frontal gyrus; L Ant Middle Frontal = left anterior middle frontal gyrus; mPFC = medial prefrontal cortex; L Pos Middle Frontal = left posterior middle frontal gyrus; dmPFC = dorsomedial prefrontal cortex; L premotor = left premotor.
Figure 4.
Functional connectivity indices between nodes in the default mode network that varied as a function of assault exposure (top) and PTSD symptoms severity within the assaulted group (bottom). vmPFC = ventral medial prefrontal cortex; R superior frontal = right superior frontal gyrus; L superior frontal = left superior frontal gyrus;
3.4. Relationship between functional connectivity within each network and external measures of threat processing and family characteristics
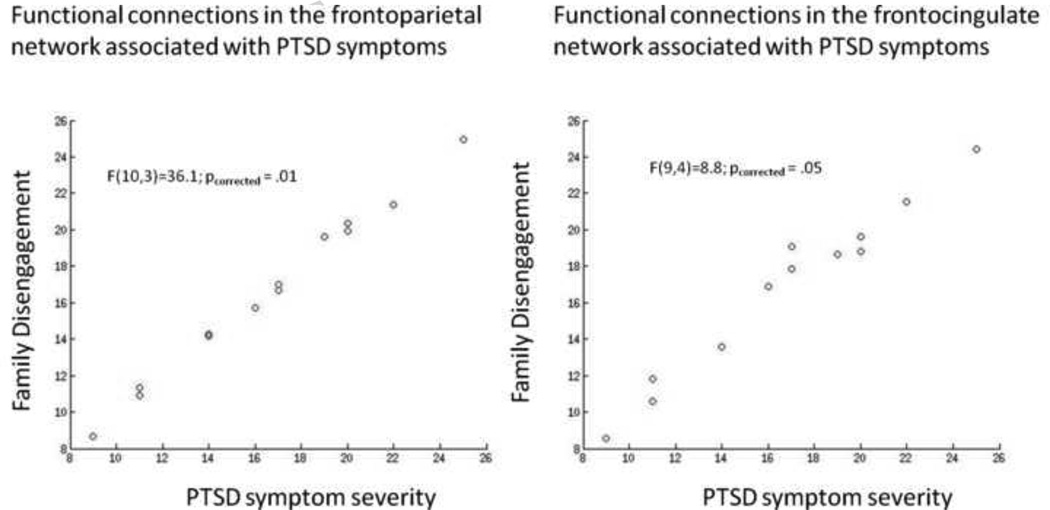
Finally, we tested whether individual differences in threat detection (RT bias towards threat on the emotion processing task) and family disengagement among the assaulted group were related to the functional connectivity patterns within the frontocingulate, frontoparietal, and default mode networks. We conducted multiple robust regression analyses among only the assaulted group in which the functional connections associated with assault exposure (frontocingulate network = 5 FC predictors; frontoparietal network = 7 FC predictors; default mode network = 6 FC predictors) or PTSD symptom severity (frontocingulate network = 9 FC predictors; frontoparietal network = 10 FC predictors; default mode network = 6 FC predictors) at the p < .05 level (uncorrected) were entered simultaneously as predictors of the family disengagement subscale of the FACES-IV measure and the RT bias from the emotion processing task. For the frontoparietal network, the functional connections associated with assault exposure were not significantly related to any of the external measures of functioning (all pcorrected > 0.19). By contrast, the functional connections associated with PTSD symptom severity were significantly related to family disengagement (F(10,3) = 36.11, pcorrected = 0.013; see Figure 5) but not RT bias (pcorrected = 0.39). For the default mode network, the functional connections associated with assault exposure and PTSD symptom severity were both not significantly related to any of the external measures of functioning (all pcorrected > 0.3). For the frontocingulate network, the functional connections associated with assault exposure were not related to either family disengagement or RT bias (pcorrected > .3). The functional connections associated with PTSD symptom severity were significantly related to family disengagement (Figure 5; F(9,4) = 8.82, pcorrected = 0.05) and marginally related to RT bias (F(9,5) = 4.67, pcorrected = 0.052).
Figure 5.
Model performance when using the functional connections associated with PTSD severity in the frontocingulate network to predict family disengagement just among the assaulted participants (left), and when using the functional connections associated with PTSD severity in the frontoparietal network to predict family disengagement just among the assaulted participants (right). The scatter plots depict model-predicted family disengagement (y axis) as a function of observed family disengagement (x axis), with optimal performance indicated via linear fit.
4. Discussion
Based on models positing that the human brain operates via distributed networks of information processing, and that psychopathology can be understood in terms of altered connectivity within large-scale neural networks, we used ICA to define three theoretically-relevant networks: a frontoparietal network (also referred to as a central executive network), theorized to mediate executive function, working memory, and planning; a frontocingulate network (also referred to as a salience network) theorized to mediate the detection and integration of external and internal cues; and a default mode network theorized to mediate self-referential mentation (Bressler and Menon, 2010; Menon, 2011). Following definition of these large-scale networks, we found evidence that assaultive violence exposure was related to heightened activation of the frontocingulate network during fear blocks (vs neutral blocks); neither assault nor PTSD severity were related to activation of the any of the other networks. We found evidence of altered patterns of functional connectivity within each network as a function of assault exposure and PTSD severity. Finally, we found that functional connectivity patterns associated with PTSD severity in the frontocingulate and frontoparietal networks were modulated by family disengagement.
Within the frontocingulate network, assaultive violence exposure was not associated with any single functional connection with sufficient strength to survive correction for multiple comparisons. By contrast, PTSD symptom severity among the assaulted group was associated with significantly weakened functional connectivity between the left amygdala and perigenual anterior cingulate cortex (pgACC) and between the left amygdala and the pre-SMA. The pgACC has been implicated in regulating emotional conflict (Etkin et al., 2006; Egner et al., 2008), while the amygdala has been implicated in detection of biologically-salient stimuli (Whalen et al., 1998; Davis and Whalen, 2001). Thus, weakened functional connectivity between the pgACC and left amygdala during fear processing as a function of PTSD symptoms potentially provides a neurobiological mechanism mediating emotion regulation difficulties noted among individuals with PTSD (Moore et al., 2008; New et al., 2009; Cisler et al., 2010). This explanation of poor integration of salience detection with emotion regulation may similarly explain the observed weakened connectivity between the left amygdala and pre-SMA, given the pre-SMA’s role in response inhibition (Sharp et al., 2010). If one of the functions of the frontocingulate network is indeed to detect and integrate salience internal and external sensory cues (Bressler and Menon, 2010; Menon, 2011), then the weaker connectivity between the left amygdala with the pgACC and pre-SMA as a function of PTSD symptoms may indicate that the ‘brakes’ associated with detection of salient information (i.e., ability to control emotional responses) within this network are weakened as a function of PTSD.
Within the frontocingulate network we observed weakened connectivity of the right premotor cortex with a set of neural regions across the network, including: right parietal cortex, medial PFC, and right middle temporal gyrus. The premotor cortex has been widely implicated in response planning (Hoshi and Tanji, 2000; Cisek and Kalaska, 2005; Nakayama et al., 2008). Accordingly, the weakened functional connectivity between this region and a distributed set of neural regions across the network may indicate weakened ability to integrate action planning with other cognitive processes. For example, weakened connectivity between premotor cortex and parietal cortex may indicate poorer coordination between action planning and working memory. As such, it is possible that poorer integration of the premotor cortex into the frontoparietal network among assault victims operates a neurobiological PTSD sufficiently strong to survive correction for multiple comparisons may indicate that the altered functional connectivity within the frontoparietal network is more closely linked with assault exposure and less strongly linked with clinical symptoms following the assault exposure; however future research is clearly needed to further elucidate the unique effects of assault exposure vs clinical symptoms on functional connectivity within this network.
Within the default mode network, we observed that assault exposure severity was associated with strengthened connectivity of the ventral medial PFC (vmPFC) with the left parahippocampus, while PTSD symptom severity was positively correlated with functional connectivity between the left middle frontal gyrus and right parahippocampus. The default mode network is theorized to mediate self-referential mentation (Raichle et al., 2001; Weissman et al., 2006; Broyd et al., 2009; Spreng et al., 2009). The parahippocampus is implicated both in the default mode network, but also is implicated in prospection, which refers to the ability to imagine ourselves in the future (Spreng et al., 2009). It is difficult to speculate what might be the functional consequences of the heightened connectivity with the parahippocampus among assaulted participants and as a function of PTSD symptoms. Within the framework of the default mode network’s theorized function, a tentative hypothesis is of greater prospective self-directed mentations, which may be manifested clinically in the form of worry (Barlow, 2002).
Finally, though few functional connections were significantly associated with assault or PTSD symptoms when controlling for multiple comparisons, we found that functional connections generally associated with PTSD severity (i.e., p < .05 uncorrected) in the frontocingulate network and frontoparietal networks were strongly related to family disengagement among the assaulted girls. This finding suggests that family characteristics can modulate the same functional connectivity patterns associated with PTSD symptoms. Accordingly, it may be the case that although PTSD severity is associated with altered neural functioning, the degree of family support among assaulted girls appears to be a significant modulator of the effects on neural functioning. Such findings underscore the importance of considering social and contextual factors for understanding clinical and neural responses to early life trauma (Ozer et al., 2003; Davidson and McEwen, 2012; Trickey et al., 2012).
Overall, the pattern of results demonstrates relationships between assault and PTSD severity among adolescent girls on the functional organization of three large-scale networks: a frontocingulate, frontoparietal, and default mode network. While the individual regions (e.g., amygdala, pgACC) and cognitive processes (e.g., emotion detection and regulation) identified here have been the focus of earlier studies (Rauch et al., 2000; Shin et al., 2001; Shin et al., 2011), what the present network analyses may uniquely demonstrate is how a large-scale network is functionally re-organized as a function of trauma, PTSD symptoms, and family characteristics. The present results therefore extend prior knowledge derived from functional segregation approaches into a network-level description of neural processing correlates of trauma and PTSD. Further, it is interesting to note that behavioral performance did not differ as a function of assault exposure, though we found network organization differences. This discrepancy suggests the possibility that comparable behavioral performance is achieved through altered neural processing networks, perhaps raising the possibility that some of the network alterations associated with assault exposures severity are adaptive.
To our knowledge, this is the first investigation focused on network-level neural processing correlates of assault and PTSD symptoms among a high-risk adolescent female sample. However, the study is not without limitations. First, due to the cross-sectional nature of the study, the temporal relationships between assault, PTSD, and the identified neural processing correlates cannot be determined, which precludes any inferences about causality. Second, the study was limited to a female population. While this was necessary for an initial study, given the known sex differences in neural mechanisms of emotion regulation (Domes et al., 2010), the results likely do not generalize to males. Third, only one category of emotional stimuli was used (i.e., fear), which precludes inferences regarding whether the observed effects are specific to fear or common to other emotional stimuli categories (e.g., happy, angry, etc). Fourth, our sample of this high-risk and vulnerable sample was relatively small; thus, replication with larger samples is necessary to strengthen inferences.
Supplementary Material
Acknowledgments
Funding Sources
Portions of this work were supported through grants 1R21MH097784-01, T32 DA022981-02, and UL1RR029884. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no financial conflicts of interest.
References
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, TRF Profiles. Burlington, VT: University of Vermont, Department of Psychology; 1991. [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. New York: Guilford Press; 2002. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Science. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and Biobehavioral Reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001a;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001b;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. Journal of Pediatric Psychology. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. A prospective examination of the relationships between PTSD, exposure to assaultive violence, and cigarette smoking among a national sample of adolescents. Addictive Behaviors. 2011a;36:994–1000. doi: 10.1016/j.addbeh.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. PTSD symptoms, potentially traumatic event exposure, and binge drinking: a prospective study with a national sample of adolescents. Journal of Anxiety Disorders. 2011b;25:978–987. doi: 10.1016/j.janxdis.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: data from the NSA-R. Journal of Traumatic Stress. 2012;25:33–40. doi: 10.1002/jts.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion Regulation and the Anxiety Disorders: An Integrative Review. Journal of Psychopathology and Behavioral Assessment. 2010;32:68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nature Neuroscience. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Human Brain Mapping. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with ptsd symptoms. Depression and Anxiety. 2012;29:449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler SW, Burr-Harris AW, Borduin CM, McCallum G. Use of the Family Adaptability and Cohesion Evaluation Scales in child clinical research. Journal of Abnormal Child Psychology. 1991;19:53–63. doi: 10.1007/BF00910564. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61(Suppl 5):4–12. discussion 13–14. [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of Consulting and Clinical Psychology. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biological Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Matherne MM, Thomas A. Family environment as a predictor of adolescent delinquency. Adolescence. 2001;36:655–664. [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proc Natl Acad Sci U S A. 1998;95:803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Science. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behaviour Research and Therapy. 2008;46:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E. Transformation of a virtual action plan into a motor plan in the premotor cortex. Journal of Neuroscience. 2008;28:10287–10297. doi: 10.1523/JNEUROSCI.2372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry. 2004;4:34. doi: 10.1186/1471-244X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biological Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Olson DH. Three-dimensional (3-D) Circumplex Model and revised scoring of FACES III. Family Process. 1991;30:74–79. doi: 10.1111/j.1545-5300.1991.00074.x. [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rodick JD, Henggeler SW, Hanson CL. An evaluation of the Family Adaptability and Cohesion Evaluation Scales and the Circumplex Model. Journal of Abnormal Child Psychology. 1986;14:77–87. doi: 10.1007/BF00917223. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM, Vidal-Pineiro D, Bargallo N, Junque C, Bartres-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, Macklin ML, Gold AL, Karpf RD, Orr SP, Rauch SL, Pitman RK. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. American Journal of Psychiatry. 2011;168:979–985. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62:1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. Current Psychiatry Reports. 2004;6:96–100. doi: 10.1007/s11920-004-0048-2. [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickey D, Siddaway AP, Meiser-Stedman R, Serpell L, Field AP. A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clinical Psychology Review. 2012;32:122–138. doi: 10.1016/j.cpr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Qiu A, Chodkowski B, Pekar JJ. Spatial and temporal reproducibility-based ranking of the independent components of BOLD fMRI data. Neuroimage. 2009;46:1041–1054. doi: 10.1016/j.neuroimage.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.