Abstract
We present arguments for an evolution in our understanding of how antioxidants in fruits and vegetables exert their health-protective effects. There is much epidemiological evidence for disease prevention by dietary antioxidants and chemical evidence that such compounds react in one-electron reactions with free radicals in vitro. Nonetheless, kinetic constraints indicate that in vivo scavenging of radicals is ineffective in antioxidant defense. Instead, enzymatic removal of non-radical electrophiles, such as hydroperoxides, in two-electron redox reactions is the major antioxidant mechanism. Furthermore, we propose that a major mechanism of action for nutritional antioxidants is the paradoxical oxidative activation of the Nrf2 (NF-E2-related factor 2) signaling pathway, which maintains protective oxidoreductases and their nucleophilic substrates. This maintenance of ‘Nucleophilic Tone,’ by a mechanism that can be called ‘Para-Hormesis,’ provides a means for regulating physiological non-toxic concentrations of the non-radical oxidant electrophiles that boost antioxidant enzymes, and damage removal and repair systems (for proteins, lipids, and DNA), at the optimal levels consistent with good health.
1. Preface
Here we present arguments for the mechanism of action of nutritional antioxidants that are both a synthesis of evolving ideas that better explain almost all so-called ‘antioxidants,’ and a refutation of the concept that unselective supplementation can be useful. Our thesis is written from an historical perspective in order to enhance the foundations for our proposal of ‘Nucleophilic Tone’ and ‘Para-Hormesis,’ and in an attempt to make these concepts (which are supported by extensive chemical evidence) more accessible to the general reader. We admit to the drawbacks of diminished comprehensiveness and a bias engendered by our involvement for 40 or more years in the field. We also apologize to anyone who feels their work should have been cited here, but note that this applies to thousands of important publications that could not all be included.
2. Introduction
The dawn of agriculture, approximately 10,000 years ago, was a major achievement in human evolution, which resulted in easier availability of metabolic energy from carbohydrates, fats and proteins. In the first half of the last century, studies on metabolism and bioenergetics led to the identification of inorganic and organic compounds, including vitamins, not directly required for energy, but nevertheless indispensable for life. Analysis of deficiency syndromes, by nutritionists, provided the scientific information that today still drives recommendations for prevention of specific diseases directly caused by inadequate intake of specific nutrients. Of course, it was recognized long before the scientific era that the vegetal kingdom also provides a large number of molecules that act as poisons and/or drugs in addition to being a major source of metabolic energy and essential vitamins.
In recent decades, however, a view has emerged about another important impact of nutrition on health. It became clear that many fruits and vegetables contain phytochemicals that may reduce the risk of diseases [1–3], without being related to any specifically defined pharmacological effect or deficiency syndrome. This opinion, first suggested by folk traditions about healthy diets and non-conventional medicine, has frequently been corroborated by epidemiological/statistical evidence of decreased relative risk of various diseases. Animal and in vitro studies of specific phytochemicals have often supported such views.
A major outcome of all this information is the popular recommendation about the importance of a regular intake of fruits and vegetables to minimize the risk of degenerative diseases and cancer [4]. The fact that just a minimal, if any, lowering of risk can be observed in subjects adopting a diet optimized [5] according to the major guidelines, does not limit the relevance of the issue. Instead, such evidence suggests that it is the non-optimal intake that leads to an increased risk of disease. As an example, the concept of cancer prevention, and possibly reversion, by phytochemicals present in fruit and vegetables is usually discussed with regard to the alleged antioxidant effect brought by a plethora of antioxidant compounds present in vegetal foods [6].
In this review, we describe how redox prone ‘antioxidant’ phytochemicals present in fruits and vegetables affect cellular signaling increasing the protective effects of the Nrf2/EpRE pathway that results in a more reductive/electrophilic environment, which we refer to as ‘nucleophilic tone.’ On the basis of available chemical and biological data we propose that ‘antioxidants’ present in fruit and vegetables paradoxically act together to produce an additive increase in electrophilic signaling that results in the induction of protective phase II enzymes and increased nucleophilic substrates, such as glutathione, thioredoxin and NADPH. Furthermore, such nucleophilic substrates are all maintained in a reduced state through increased pentose shunt utilization of glucose. Our ‘Nucleophilic Tone’ concept contrasts markedly with the kinetically unrealistic free radical scavenging proposal that has dominated ‘antioxidant’ discussions for several decades.
3. A brief history of antioxidants
First, we will review how antioxidants became synonymous with free radical scavenging, and how kinetic constraints limit the ability of free radical scavenging to explain dietary antioxidant actions, with the notable exception of vitamin E.
The first semi-empirical use of antioxidants was in the 19th century when several molecules were used to control the process of rubber production and to prevent ‘fatigue’ of the polymers [7]. Soon, the same or similar molecules were introduced in the food industry to prevent rancidity, the most marked outcome of oxidative degradation of stored foods [7]. The chemistry underlying these effects is the quenching of peroxyl radicals and the reduction of hydroperoxides. The most typical examples of compounds acting through these two mechanisms, quenching of free radicals and reduction of electrophiles, are natural or synthetic phenolic compounds and sulfite, respectively.
In the first half of the 20th century, studies on the chemistry of oxidation of organic molecules and the involvement of free radical intermediates led to the generalization by Michaelis (best remembered for his famous description of enzymatic kinetics) that ‘all’ biological oxidations involved free radicals [8]. While this (rather extreme) proposal was subsequently refuted, a consensus was reached that a significant number of biological oxidations (catalyzed by enzymes) do indeed involve the formation of free radical intermediates [9]. Interest in the biological significance of free radical chemistry led Albert Szent-Györgyi (Nobel laureate for the discovery of various Krebs cycle intermediates and vitamin C) to elaborate the concept that incorrect free radical formation or elimination is the ultimate cause of cancer [10]. The free radical in cancer problem, which Szent-Györgyi characterized as “an electronic problem” led him to describe life as having negative entropy or ‘syntropy’ [11]. The concept of ‘syntropy’ is therefore an evolution of the concept of negative entropy introduced by Erwin Schrödinger (also a Nobel laureate), in defining life using quantum physics [12].
During their lifetimes, experimental science did not provide evidence to support the radical hypotheses of Michaelis and Szent-Györgyi. Nevertheless, the concept that free radical chemistry participates in biology was progressively formulated and consolidated. Ultimately, it was concluded that radicals produce cellular damage and that eliminating free radicals must, therefore, be health protective. The free radical theory of aging by Denham Harman, following on observations by Rebecca Gershman in radiation biology [13] (see below) is the most relevant example of this trend in scientific thought [14]. Eventually, as described in the next section, some ‘good’ uses of free radicals were discovered. These contributed to the generally accepted idea of today, that free radicals are part of normal physiology but that poorly controlled production causes damage [15]. Thus, it seemed a perfectly reasonable conclusion that scavenging harmful free radicals with supplemental ‘antioxidants’ would be health protective and promote a healthy, perhaps longer, life. Awkwardly for the field, the concept of free radical scavenging by supplemental antioxidants has been challenged at least in higher animals and in human clinical trials [16–19]. Nonetheless, demonstrations of free radical scavenging in test tube measurements and animal and cell culture experiments of imposed oxidative stress, contributed to the popularity of the unproven concept that free radical scavenging antioxidants protect health and possibly prolong life. The exception appears to be high dose α-tocopherol, at least in prevention of cardiovascular disease [20].
Protection by various antioxidants (or, better, diets containing antioxidants) against different chronic diseases and cancer has been attributed to their antioxidant capacity [21]. But as this does not fit kinetic data, there must be an epistemological error. Our main intent then is to explain how dietary antioxidants, effective in providing health protection, do not, with the exception of vitamin E, act just as free radical scavengers. We will then go on to explain how ‘antioxidant’ phytochemicals paradoxically act through cell signaling to maintain ‘Nucleophilic Tone’ involved in enzymatic antioxidant protection. We define Nucleophilic Tone as the capacity to remove electrophiles through enzyme catalyzed, dynamic flow of reducing equivalents from NADPH, GSH and reduced thioredoxin. In other words, Nucleophilic Tone is the overall potential cellular adaptive response to oxidative challenge brought by electrophiles.
4. Free radicals and oxygen
In the early fifties, the rapid development of nuclear energy was accompanied by a realization of a need for chemoprevention of radiation damage. This seemingly motivated studies by Rebecca Gershman and Dan Gilbert that resulted in a ground breaking achievement [13]. They showed that radiation damage is largely enhanced by oxygen. Ionizing radiation produces free radicals while oxygen, by reacting with free radicals, propagates biological damage through chain reactions.
The mechanism necessitating the involvement of free radicals depends on the peculiar electronic distribution of di-oxygen, which in the ground state has a triplet spin status due to the fact that its two unpaired electrons have the same spin state [22]. The direct interaction of ground state di-oxygen with carbon, which has a singlet status (all electrons paired), is prevented by the extremely high activation energy caused by the need for one of two unpaired electrons to flip its spin in order to become the reactive (excited but non-radical) species, singlet oxygen. The energy to produce singlet oxygen is rarely found in biological systems outside of photo-oxidation catalyzed by exogenous agents.
This ‘spin restriction’ explains the kinetic sluggishness of di-oxygen in oxidizing organic compounds even though such reactions have a high thermodynamic potential. Lowering the activation energy occurs when redox transitions take place by single electrons, as in the reaction of carbon-centered free radicals with triplet di-oxygen [23]. Understanding that radiation involved free radicals further contributed to the wishful thinking that free radical scavengers would protect against oxidative damage. Moreover, the addition of di-oxygen to a free radical produces a hydroperoxyl radical that primes oxidative chain reactions of lipid peroxidation (see below).
Unfortunately, while many organic compounds react with free radicals with high rate constants (see below), a compound that can protect humans against free radical damage from ionizing radiation has never been discovered. Indeed, since almost all biologically relevant molecules can react with the most reactive free radicals at similar rates, it is reasonable to assume that no miraculously effective antioxidant will ever be found. Later in this review, we will deal with so-called hydroxyl radical scavengers, an extreme case of wishful thinking. Regardless, the sole mechanism that has been reported to reduce carcinogenesis in animals exposed to ionizing radiations is previous exposure to low-level radiations, which likely works through enhancing DNA repair and adaptive defense mechanisms [24, 25]. Although still a matter of debate, such protection stresses the relevance of biological adaption (or hormesis) to oxidative stress, where endogenous defenses are activated to provide increased protection against subsequent stress challenges [26–28]
5. Discoveries of superoxide dismutase (SOD), the respiratory burst and production of superoxide by mitochondria. First evidence of in vivo production, and protection against oxidative damage involving a free radical
Appreciation of the relevance of free radicals in biology received a major burst in the early 1970’s with the discoveries of multiple forms of SOD by Irwin Fridovich [29–32], the NADPH oxidase (respiratory burst) by Bernard Babior [33] and the demonstration that production of hydrogen peroxide, first described by Britton Chance, was actually preceded by superoxide production [34, 35]. Knowing that superoxide is physiologically produced by either an enzymatic system for a biological purpose or by a leak of electrons from the respiratory chain, and that enzymes evolved to be competent for its removal, clearly framed the ‘respectability’ of the new field of free radicals in physiology and pathology. Indirectly, these discoveries also contributed support for the paradigm that scavenging free radicals, at least in some conditions, had to be a suitable mechanism for protecting health [36]. Since these pioneering studies were published, it has also become abundantly clear that not all oxidants are actually scavenged in vivo, and that oxidative damage to proteins, lipids, and DNA is a fact of daily life. Of course, we have also learned that cells are protected by secondary and tertiary layers of damage removal and repair systems (e.g. proteinases, lipases, DNA repair enzymes) that help to make life in an oxygen-rich environment possible [37–39]
6. The carbon tetrachloride story (role of hydroperoxyl radicals and vitamin E): First example of an experimental pathology prevented (and cured) by an antioxidant inhibiting lipid peroxidation
In the molecular toxicology area, discovery of the mechanism of carbon tetrachloride toxicity (and halogenated compounds in general) by the teams of Recknagel, Burton, Slater, and Dianzani [40, 41] bridged a relevant gap between toxicity, free radicals and protection by antioxidants. When ingested, halogenated compounds are decomposed by microsomal cytochromes generating carbon centered radicals. These, like radicals produced by radiation, react with oxygen producing hydroperoxyl radicals that initiate and propagate oxidative chain reactions in the lipid phase. This pathological event can be fully prevented, and in some conditions cured, by α-tocopherol [42]. To us, this is the most remarkable evidence that at least one physiological function of vitamin E is the inhibition of lipid peroxidation.
The molecular mechanism of carbon tetrachloride toxicity was rapidly and fully integrated with the growing knowledge on lipid peroxidation. The oxidative degradation of fats exposed to air was first described by De Saussure an the end of 18th century [43] and some chemical clues as to mechanism were then added by Antoine Parmentier [44] the French Pharmacist who studied oxidation of oils and became incorporated into the history of the invention of ‘French fries.’ Rancidity, of course, had been known for centuries but the role of oxygen and detailed understanding of the reactions involved was achieved only in the second part of the 20th century. Semantically, air oxidation of fats is named autoxidation, while a similar event, when catalyzed in living biological material is referred to as lipid peroxidation. In the original formulation by Hochstein and Ernster [45], microsomal lipid peroxidation is initiated by the reduction of a transition metal (usually iron) which in turn is able to form complexes with oxygen that are competent in the extraction of a hydrogen atom from a polyunsaturated lipid in membranes [46]. The alternative, and likely more efficient mechanism of initiation is the decomposition of a lipid hydroperoxide in the presence of a reduced transition metal, forming an alkoxyl radical that is extremely effective in extracting the hydrogen atom from a polyunsaturated lipid [47].
Lipid peroxidation then proceeds through a chain reaction that is seemingly the same as for initiation by radiation or decomposition of halogenated compounds. The carbon centered radical reacts with oxygen producing a lipid hydroperoxyl radical, in turn extracting a hydrogen from another lipid and forming a lipid hydroperoxide and a carbon centered radical. Both autoxidation of lipids and lipid peroxidation are cyclic events where, from a single initiation, a chain reaction starts that is limited only by reagent availability and radical-radical termination reactions. Lipid peroxidation is inhibited by phenolic antioxidants quenching lipid radicals propagating the chain reactions, and by reduction of lipid hydroperoxides from which radicals are continuously generated [47]. It is noteworthy that in biology this concerted interplay between antioxidant scavenging hydroperoxyl radicals and enzymatic reduction of hydroperoxides fully overlaps the synergy between free radical scavengers and ‘peroxidolytic’ compounds in polymer chemistry [7].
7. How the terms ‘free radical scavenger’ and ‘antioxidant’ become synonymous
The scientific achievements briefly summarized above, contributed to substantiation of the syllogism, which states that, since free radicals produce damage and antioxidants scavenge free radicals, antioxidants must be health protective because they quench free radicals. Thus, it was presupposed that the more antioxidants one could pack into cells and tissues, the greater would be the resistance to pathology caused by free radicals. This syllogism subsequently primed the development and implementation of measurements of the capacity of both pure phytochemicals and biological samples to quench free radicals. Such studies have flooded the scientific literature for the past two decades. In these experiments, different radicals produced in vitro are reacted with molecules present in the sample under scrutiny. One can then measure either the extent to which radicals are ‘quenched’ or the kinetics of the oxidation of a reporter molecule, as altered by the ‘antioxidant’ under study. By such methods, both biological samples and thousands of phytochemicals have been shown to have ‘significant’ free radical scavenging antioxidant capacity.
Unfortunately, no major insight into whether radical scavenging actually accounts for a decrease in pathology due to antioxidants (except by SOD and vitamin E) has emerged from the enormous amount of data generated to date. The most reasonable reasons for this apparent failure are: (1) The analytical systems used by researchers were markedly different from the conditions actually extant in vivo. Free radicals have generally been produced for in vitro studies at much greater rates than would be observed in realistic physiological or even pathological conditions (aside from exposure to lethal radiation); (2) As illustrated by examples in the next section, the most reactive free radical will react with approximately the same rate constant with many low molecular weight antioxidants and the biological molecules (proteins, lipids, DNA, etc.) to be protected. Thus, most candidate antioxidant compounds have no advantage over any others in terms of rate constant. Concentration is, of course, the other contributing factor to the rate of radical reactions but, with the exception of vitamin E and the endogenous antioxidant glutathione, the concentrations that can be achieved in vivo cannot overcome the kinetic limitation; (3) Quenching a given free radical in vitro does not necessarily translate into having an antioxidant effect in vivo. Although concentration and rate constant figure into this issue as well, the location of free radical production versus where antioxidants may ultimately localize within cells and tissues can also be a major factor in limiting in vivo effectiveness.
8. Can free radicals be efficiently scavenged under biological conditions?
a. Hydroxyl and alkoxyl radicals – Why we need enzymes instead of free radical scavengers
The rate of an antioxidant-oxidant reaction, which would be a second order reaction, is determined by the equation, Reaction rate = k[A][B], where k is a second order rate constant, [A] is the concentration of the antioxidant, and [B] is the concentration of the reactive species.
The hydroxyl radical is produced by radiolysis of water or decomposition of hydrogen peroxide. This extremely reactive radical reacts with practically all molecules present in a cell with a rate constant approaching the rate of diffusion limitation [48, 49].
| 1) |
It is therefore essentially meaningless to argue that any scavenger could be protective by reacting with hydroxyl radical. Unfortunately, the literature is filled with reports of the latest and greatest hydroxyl radical scavenger, often discovered in an exotic fruit or vegetable.
The same argument against effective scavenging by any particular molecule holds for alkoxyl radicals produced by decomposition of lipid hydroperoxides, which occurs mainly in membranes [50, 51].
| 2) |
For both these similar radicals the only efficient protection mechanism is to prevent their formation rather than trying to scavenge them after they are formed. Such prevention in vivo is by reduction of H2O2 to water, or reduction of lipid hydroperoxides to their corresponding alcohol [47, 52] and these antioxidant reactions are enzyme-catalyzed. While catalase can dismutate H2O2 to H2O and O2, peroxidases and peroxiredoxins reduce hydroperoxides using the endogenous electrophiles, glutathione (GSH) or thioredoxin (Trx) [53]. Reduction of these sulfur-containing substrates is maintained primarily by NADPH derived from the oxidation of glucose in the pentose shunt, which (one could argue) eventually emerges as the ultimate antioxidant.
b. Carbon centered and hydroperoxyl radicals - The special case of vitamin E explained
A carbon-centered radical is produced when a reactive oxidant radical extracts a hydrogen atom from a C-H bond. In the presence of O2 this initiating radical next produces a hydroperoxyl radical [54, 55]:
| 3) |
Reaction 3, which escapes the spin restriction, is also extremely fast and its inhibition can be achieved in extremely anaerobic conditions, such as packages for long-term food storage, but such conditions are hardly pertinent to aerobic living systems.
Once the hydroperoxyl radical is formed from a lipid molecule, a chain reaction is initiated. This event is peculiar to the ordered structure of membrane monolayers [51, 54]. The radical is indeed reduced to a lipid hydroperoxide in a relatively slow reaction by another lipid that is prone to oxidation, such as those containing a polyunsaturated fat having allylic carbons on which a new carbon centered radical is generated.
| 4) |
Repetition of reactions 3 and 4 produces a chain that can be interrupted by consumption of the limiting reagent, or by radical-radical interaction (an ‘annihilation’ reaction).
Lipid hydroperoxides formed can undergo reductive decomposition generating alkoxyl radicals that are competent for the initiation of new chains of reaction, starting from reaction 2 [56].
As mentioned above, reduction of hydroperoxides catalyzed by peroxidases using GSH or Trx prevents reaction 2. A suitable non-enzymatic antioxidant mechanism based on free radical scavenging is only possible with respect to the relatively slow reaction 4 [54].
In fact, hydroperoxyl radicals react with a fast rate constant with phenolic compounds. The most typical, and possibly biologically relevant and specific phenolic ‘antioxidant’ is α-tocopherol (T-OH in reactions 5, 6, and 7), which is transported into the liver by a specific carrier where it is incorporated into HDL that then transfers it to other lipoproteins and then to other tissues.
| 5) |
This reaction is referred to as chain breaking, since the reactivity of the α-tocopherol radical (T-O• in reactions 5, 6, and 7) is much less than that of other radicals (reactions 2 and 4) [51]; i.e., the α-tocopherol radical is ‘ relatively stable’ and the subsequent reaction 6 is very slow [57].
| 6) |
However, a corollary of this evidence is that, under specific conditions, the generation of an α-tocopheroxyl radical; e.g., by electron transfer to a metal ion, can indeed generate a lipid radical and thus slowly initiate lipid peroxidation [57, 58]. Far from being paradoxical, this event is fully consistent with the kinetics of free radical reactions.
The final fate of the α-tocopheroxyl radical is the eventual oxidation to a stable non-radical quinone by a second oxidation or by dismutation of the radical species. Also a reduction back to hydroquinone (recycling) is kinetically favorable and the seemingly most efficient, biologically available, reductant is ascorbate (Vitamin C) [59]:
| 7) |
The rather unreactive ascorbate radical may be eliminated by dismutation or by reduction with NADH catalyzed by ascorbate radical reductases found in the cytosol, or by the membrane-bound NADH-cytochrome b5 reductase activities reported in mitochondria, microsomes and plasma membranes [60–64].
In conclusion, kinetic data indicate that an efficient antioxidant protection is carried out only by the synergistic interplay between: (a) the 2-electron reduction of hydroperoxides and (b) the 1-electron reduction of hydroperoxyl radicals [47].
c. Potential of glutathione as a radical scavenger – sink or sinker?
The nucleophile GSH is the major substrate of peroxidases reducing hydroperoxides but it can also be a direct free radical scavenger. Indeed, the generation of a sulfur-centered radical in the presence of different oxidizing radicals is likely also in light of the high GSH (1–10 mM) concentration in cells. This radical initiates an electron sink pathway proposed by Winterbourn [65] through which electrons are eventually transferred to oxygen forming superoxide, which is very rapidly dismutated by SOD.
| 8) |
| 9) |
This electron sink hypothesis argues for a role of GSH as a valid free radical scavenger and SOD as the final antioxidant enzyme [65]. Although the sink hypothesis is kinetically and thermodynamically feasible [66], the sulfur centered radical can also react rapidly with oxygen producing species that could be competent for other, possibly damaging, reactions [67].
| 11) |
Evidence for either protection or damage through these reactions is however, unavailable. Thus, while the pivotal role of GSH as a nucleophile in enzyme-catalyzed reactions that reduce hydroperoxides is unquestionable, it is still unclear whether it could also play a physiologically relevant role as free radical scavenger.
9. What then, exactly is an antioxidant?
a. Definitions
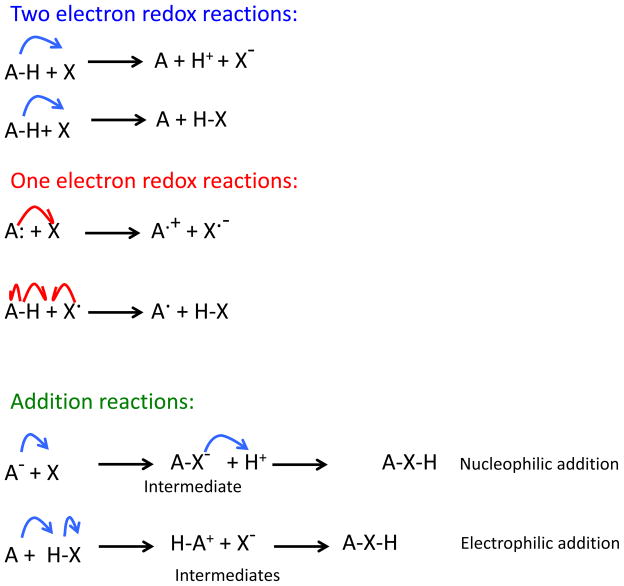
Having discussed the concept of a free radical scavenger as an antioxidant, for the purpose of the forthcoming discussion here, it is useful to define what we mean by ‘antioxidants,’ ‘oxidants,’ ‘electrophiles,’ ‘nucleophiles,’ and ‘reductants.’ Electrophiles are molecules that take electrons from other molecules, called nucleophiles. Oxidants are electrophiles that take one or two electrons from a nucleophile without forming an adduct. Reductants are then nucleophiles that give one or two electrons to an oxidant, without forming an adduct. The transfer of electrons in oxidations and reduction may involve a hydrogen atom (H·) in one-electron reactions or a hydride (H−) in two electron reactions (Fig 1). There are different functional definitions for the word, ‘antioxidant.’ In chemistry, antioxidants are nucleophilic reductants that directly react with oxidants, thus preventing the oxidation of a third molecule.
Fig 1.
Oxidation. Nucleophile give electrons in two electron (blue curved arrows) or one electron (red curved arrows) reactions without forming an adduct. Reductants (HA) are nucleophiles that give one or two electrons to an oxidant (X), without forming an adduct. The transfer of electrons in oxidations and reduction may involve a hydrogen atom (H·) in one-electron reactions or a hydride (H:) in two electron reactions. Nucleophiles can also add to electrophiles thereby forming covalent bonds in either electrophilic or nucleophilic addition reactions.
According to the kinetic constraints discussed above, the antioxidant effect is obtained either by reducing by a single electron (usually by donating a H), or by preventing the formation of the initiating hydroxyl or alkoxyl radical (by reducing the hydroperoxide, from which the initiating radical is generated, to an alcohol). Antioxidants used in polymer chemistry or food preservation (phenolic and hydroperoxide-reducing compounds) are examples of the interplay between these two complementary mechanisms [7]
A large number of synthetic compounds and phytochemicals are, indeed, chain-breaking antioxidants as they form relatively stable radicals, following the extraction of a labile hydrogen atom by a hydroperoxyl radical. This feature derives from the possibility of delocalizing the unpaired electron until the molecule is stabilized by either a second electron extraction or a single electron reduction. This feature most frequently, although not always, is contributed by the common ortho or para hydroquinone moiety present in vegetal polyphenols, while meta hydroquinone moieties cannot produce any antioxidant effect because they cannot form the corresponding quinone. The available evidence, however, argues against such functions being efficient in vivo. Although the rate constant for the reaction with hydroperoxyl radicals is in the same range as vitamin E, the concentration of ortho- or para-quinones reached in cells and in membranes is much lower. Moreover, vitamin E deficiency syndrome cannot be cured by antioxidants other than authentic vitamin E itself. As discussed later, it is possible that when polyphenols are oxidized by reaction with free radicals, the resulting electrophilic quinones are involved in signal transduction pathways to activate cellular antioxidant activities and damage removal/repair systems but this is clearly different from free radical scavenging.
These limitations imposed by bioavailability, concentration and location of phenolic antioxidants in cells are, however, overcome in some specific cases. An example is in the lumen of the digestive tract where ingested foods can undergo oxidative degradation, eventually leading to a post-prandial oxidative stress that is clearly prevented by intake of a large source of phenolic antioxidants, such as wine, with meals [68].
A common misconception is that ‘antioxidants’ can efficiently reduce hydroperoxides (ROOH) by a two electron transition, and thus through a non-radical mechanism. While many compounds, particularly thiols, have reaction rates with ROOH that are fast enough to be significant in vitro, and perhaps also in the extracellular environment, these reactions can only make an insignificant contribution to the reduction of ROOH inside cells. This is because of the slow rate constants of the nucleophilic substitution reaction (SN2). Even if the concentrations of the purported antioxidant that can be reached in cells or plasma are high, the reaction rates are insignificant in comparison with enzymatic reactions catalyzed by various peroxidases and peroxiredoxins [53]. Thus, enzyme-catalyzed reactions using nucleophilic substrates, rather than non-enzymatic free radical scavenging, usually provide for biologically relevant antioxidant defenses.
b. The alternative to free radical scavenging - the paradoxical effect of antioxidants
As a free radical scavenger mechanism for nutritional antioxidants cannot be substantiated on a kinetic basis in vivo, what then accounts for the nutraceutical effect of these compounds? Studies over several decades (only recently markedly helped by conceiving the possible underlying mechanism) demonstrate that many of the so-called ‘antioxidants’ in foods and beverages provide cellular and tissue protection against oxidative damage by inducing endogenous antioxidant defenses. Thus, a variety of compounds, including polyphenols, which can act as chemical antioxidants in vitro, actually induce enzymatic systems in vivo. These enzyme-catalyzed processes in turn alter the steady-state levels of crucial regulatory and/or protective elements. What is now abundantly clear is that, rather than acting as chemical antioxidants in vivo, the chemical properties of these ‘antioxidant’ compounds generate signals for the induction of protective enzymes.
It now appears that the chemically important properties of ‘antioxidants’ in vivo are either pro-oxidant (generating reactive species) and/or electrophilic (having the capacity to form adducts to proteins). Such, properties are typically associated with toxic substances. However, we are fortunate that for most of these compounds it is practically impossible to reach a concentration that is actually toxic. Instead, the concentrations seemingly reached in vivo are sufficient to cause the activation of signaling pathways in cells that have evolved to recognize potential threats by sub-toxic concentrations of electrophiles. In this respect, the limited bioavailability of most ‘antioxidants’ is definitely a fortunate and appropriate characteristic, rather than a limitation that must be overcome in order to provide greater protection.
The explosion in recombinant DNA technology in the last three decades also provided powerful new tools to solve the antioxidant conundrum. Early investigators, having in mind a possible homeostatic equilibrium, expected that by increasing (exogenous) antioxidant capacity, nutritional phenolic antioxidants would cause a decrease in endogenous antioxidant protection through feedback inhibition of regulatory mechanisms. Instead, investigations showed that phenolic compounds, along with isothiocyanates and some other phytochemicals, actually produced an increase endogenous antioxidant protection. This ability to induce antioxidant enzymes and increase their substrates through signal transduction pathways leading to altered gene expression, particularly for the Nrf2 (nuclear factor erythroid 2-related factor 2) pathway, is the focus of the next section.
10. Nrf2/ARE/EpRE (Nuclear factor erythroid 2-related factor 2/Antioxidant Response Element/Electrophile Response Element)
a. Discovery of Nrf2 and EpRE (along with its unfortunate ARE misnomer)
Nrf2 is a member of the Cap’n’collar transcription factor family that includes SKN-1 in C. elegans and CncC in Drosophila. Activation of the Nrf2 transcription factor and the electrophile response element, EpRE (also called the antioxidant response element or ARE) to which Nrf2 binds [69–79] are key in adaptive responses to various oxidative stimuli [80, 81]. Studies demonstrating that phytochemicals present in vegetables acted in cancer chemoprevention by inducing what are called phase II enzymes [82] and how some planar aromatic compounds, initially thought to act as ‘free radical scavenger antioxidants’ also induced these enzymes led to the discovery of the Nrf2/EpRE signaling system. As part of his pioneering work on chemoprevention, Talalay and his coworkers [82] demonstrated that several compounds that induced the Phase II enzymes had to be metabolized to electrophilic compounds in order to function. Over the next few years, the central mechanism through which these electrophilic compounds act was revealed. More than twenty years later, however, this is still an evolving story.
The discovery of Nrf2, the transcription factor regulating expression of most Phase II and some Phase III genes, resulted from a search for the proteins that activated the cis element to which Nrf2 binds. That cis element was first described by Rushmore, Pickett and their colleagues [83] as the antioxidant response element (ARE) because planar aromatic compounds that were identified as antioxidants were able to activate the transcription of genes for Phase II detoxification enzymes. But, at the same time Daniel and coworkers [84] demonstrated that electrophiles and only metabolites of planar aromatic compounds that were metabolized to become electrophiles could activate a cis element they named the electrophile response element (EpRE). Shortly thereafter, Rushmore et al. [85] showed that H2O2 and redox cycling phenolic compounds that generate H2O2 could activate the ARE. Of course phenolic compounds are the metabolic products of the planar aromatic compounds and the quinones formed as part of the redox cycling are electrophiles. The next year, it became clear that the unfortunately named ARE and accurately named EpRE are the same 9 base consensus sequence [86] targeted by Nrf2.
As the EpRE consensus sequence contains tandem TRE or TRE-like sequences, it was initially suggested that the EpRE was activated by the binding of two AP-1 transcription factors composed of c-Jun/c-Fos dimers [86]. But the key transcription factor that binds to EpRE in response to electrophiles, Nrf2, was first described as a transcription factor that bound to tandem repeats of the consensus sequence for the transcription factors AP1 and NF-E2 (activating protein 1 and nuclear factor erythroid 2, respectively) in the β-globin enhancer region although it was clearly not identical to NF-E2 [87]. Nrf2 is therefore also known as NF-E2-related factor 2. In 1996, the first connection was made between Nrf2 and EpRE (called ARE in that work) [88]. This was soon confirmed by others [89].
In their seminal paper, Venopal and Jaiswal also showed that Jun proteins are activating partners of Nrf2 [90] while c-Fos and Fra1 are negative regulators of EpRE activation [88]. There remains controversy concerning the partners of Nrf2 as some suggest that the small Maf proteins are the partners [91] whereas others have suggest that Maf proteins are either inhibitors or place holders that are displaced by Jun proteins when Nrf2 binds to EpRE [78, 92]. In a recent publication, it has been shown that the N-terminal phosphorylation of c-Jun can negatively or positively affect EpRE-dependent transcription in a both a gene- and cell type-dependent manner [93]. This finding contrasts with the well-established requirement for c-Jun N-terminal phosphorylation to be active in promoting transcription when part of the AP-1 complex is bound to the TRE element.
b. Keap1 and its regulation of Nrf2 activation
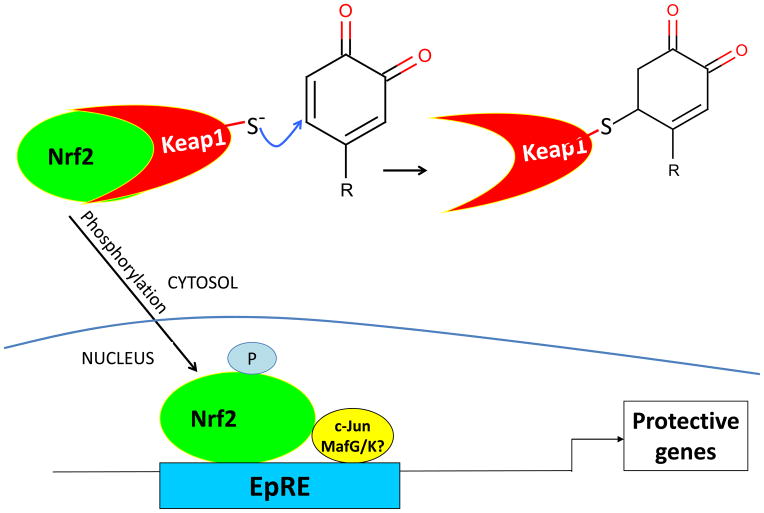
A few years after the discovery of Nrf2, the protein to which it binds, Keap1 (Kelch-like ECH-associated protein 1), which is a homolog of the Drosophila actin-binding protein Kelch, was described by Itoh et al. [94], who proposed that Nrf2 is retained in the cytosol until Keap1 is modified by electrophiles. Keap1 is also called INrf2, for inhibitor of Nrf2, a less common but more descriptive name [95]. While activation of Nrf2 requires modification of Keap1, It was later found that Keap1 does not just simply retain Nrf2. Rather, Keap1 causes the rapid turnover of the Nrf2 transcription factor by assisting in Nrf2 ubiquitinylation (resulting in rapid degradation by the 26S proteasome) [94]. When critical cysteine residues in Keap1 are oxidized or covalently modified, Keap1 is inactivated and the Nrf2 transcription factor half-life is extended [96] (Fig 2).
Fig 2.
Keap1 reacts in a Michael addition with a polyphenol oxidized to the corresponding orthoquinone (or paraquinone) form . The alkylation of Keap1 permits Nrf2 to escape ubiquitinylation and degradation. As described in the text, phosphorylation is also required for Nrf2 translocation to the nucleus and activation of transcription through EpRE.
Further investigation has shown that, although addition of exogenous H2O2 can activate Nrf2 by causing disulfide formation between two Keap1 molecules [97], it is the alkylation of critical cysteine residues on a single Keap1 molecule by non-toxic concentrations of electrophiles that allow Nrf2 to escape degradation. Exactly which of the 27 cysteines in Keap1 are effected by alkylation appears to depend upon both the particular electrophile and its concentration [98–103]. The Keap1 cysteine residue that is most reactive to sulforaphane is Cys489 [99], while Cys273 and Cys288 are the most reactive toward the model alkylating agent, dexamethasone mesylate [98]. Also, as pointed out above, the planar aromatic compounds that were shown to require redox cycling in order to activate Nrf2, form electrophilic quinones during redox cycling. Thus, it may be the quinone rather than the H2O2 formed during redox cycling that is the actual Keap1 modifier.
c. Further aspects of Nrf2 regulation
Although a key aspect of Nrf2 activation is its escape from ubiquitinylation and proteasomal degradation, Nrf2 must also be phosphorylated for translocation to the nucleus where it activates EpRE-regulated genes [104, 105]. The protein kinases, PKCδ, and Akt, involved in the phosphorylation and translocation of Nrf2, are also activated by oxidants and other electrophiles [106–110]. Thus, Keap1 modification, although essential, is not the only reaction involved in the activation of Nrf2 signaling by electrophiles.
While inactivation of Keap1 and Nrf2 phosphorylation are major factors in the activation of EpRE-regulated transcription other studies have indicated the importance of Nrf2/EpRE regulation by transcription factors that partner with Nrf2 in binding to EpRE (Mafs G/F/K [111], JunD [78, 112], c-Maf [92], c-Jun [90], c-Fos and Fra1 [88]) and transcription factors that compete with Nrf2 for binding to EpRE (Bach1 and Nrf1) [113]. Furthermore p21 increases the stability of Nrf2 [114] while c-Myc apparently accelerates Nrf2 degradation and inhibits Nrf2-dependent transcription by binding to it at EpREs [115]. Regulation of Nrf2 is also involved in its dissociation from EpREs and subsequent proteolytic degradation [116]. The potential role of redox signaling or involvement of phytochemicals in these aspects of Nrf2 regulation remains largely unexamined.
11. The endogenous antioxidant system
With the possible exception of the electron sink describe above, and the actual exception of SOD, physiological antioxidant defense does not entail free radical scavenging. Different forms of SOD catalyze, through redox shuttling of Cu or Mn (and Fe in microbes and plants), the dismutation of O2·−, which is constantly produced in cells. This results in production of H2O2, which is then dismutated by catalase to H2O and O2 or reduced to H2O by various peroxidases. SODs are antioxidant enzymes in relation to the removal of O2·−, that is indeed more a reductant rather than an oxidant unless protonated (pKa 4.7) [117]. On the other hand O2·− reacts with ·NO, producing the strong oxidant peroxynitrite [118], or with ferric iron complexes releasing the ferrous iron that reductively decomposes hydroperoxides to generate extremely reactive radicals [7].
Rather than generic free radical scavenging, the reduction of hydroperoxides, and electrophiles in general, emerges as the critical antioxidant mechanism adopted by mammalian cells. Apparently, the synergistic interplay between chain breaking free radical scavengers and peroxidolytic compounds originally defined in polymer chemistry, applies also in living cells, where the nutritionally essential α-tocopherol works as a chain breaker (1 e− transition) and different nucleophiles eliminate, in enzymatic reactions, the electrophilic hydroperoxides (2 e− transition).
Nucleophilic compounds bearing thiol groups (-SH), primarily GSH and thioredoxin reduce electrophiles in SN2 nucleophilic substitution reactions. The oxidized forms of the electron donors (usually disulfides), are then reduced back by hydride (H−) transfer from NADPH. Of course, NADPH is produced primarily in the pentose shunt pathway making glucose the ultimate source of electrons for antioxidant reactions in cells. The reduction of electrophiles is catalyzed by Se/S peroxidases or peroxiredoxins and reductases, again using Se or S as redox moieties linked to a flavin redox center and NADPH as the H− donor. Although heme-iron is used in catalase, the larger part of the physiological antioxidant mechanism is the domain of the redox chemistry of chalcogens (S and Se).
12. Inflammation, oxidative signaling and nucleophilic response
Living organisms are continuously exposed to harmful chemical, physical and biological stimuli challenging cellular, tissue, organ, and organismal homeostasis. According to the concept of milieu intérieur proposed by Claude Bernard, in which both metabolites and macromolecules remain within a physiologically range, ‘disease’ is seen as a permanent alteration of homeostasis [119]. This range is actually very far from thermodynamic equilibrium, which occurs only after death. All organisms evolved mechanisms for counteracting damaging stimuli that alter homeostasis by repairing damage or eliminating irreversibly damaged cells, the survival of which could be risky for the organism.
The reaction to agents that produce injury varies with the ability of particular cells to prevent or repair damage and the amplitude of the response extends over a large range [120]. The basic elements of inflammation consist of an array of reactions addressed to elimination of the stimulus, repair of tissue through cell proliferation and angiogenesis, and elimination of the most damaged cells. Remarkably, practically all of these events are orchestrated by a series of crucial cell signaling pathways, many of which operate through oxidation of peptide/protein sensors that function as transducers or effectors of the signal [121]. Under normal physiological conditions, this oxidation of peptide/protein sensors, which are necessarily by far more sensitive to oxidation than are other proteins, takes place without cell injury. In a general view, response to injury and inflammation evolves with the formation of electrophiles, including oxidants that produce a second level of signaling mimicking the physiological response but also accompanied by a persistent alteration of homeostasis with elevated levels of oxidized metabolites and macromolecules. These deviations from redox homeostasis are often cumulatively referred to as ‘oxidative stress.’
Inappropriate, excessive or prolonged physiological response to a given challenge, a condition comprehensively described as stress, leads to the stable alteration of homeostasis we perceive as disease. In this view, quite different diseases, such as cancer, neurodegeneration, liver and lung fibrosis, etc., can all be seen as examples of inappropriate or poorly controlled activation of the mechanisms of response to injury, irrespective of whether the actual injury exists or not. Uncontrolled proliferation and resistance to apoptosis are hallmarks of cancer [122] while cell death predominates in chronic degenerative diseases [123]. A common element in both groups of diseases, however, is an aberrant activation of defense and healing mechanisms, taking place through uncontrolled oxidative signaling and damage evolving into an ‘oxidative stress’.
The paradigm of a physiological function mimicked in epithelial cell carcinogenesis is wound healing, where inflammation, cell proliferation, angiogenesis, epithelial-mesenchymal transition, and escape from apoptosis are activated. Indeed, these events impressively overlap the hallmarks of cancer where the phenotype is stabilized by mutations of specific genes [122]. In this respect, the shared crucial common motif in cell signaling, is the formation of redox signaling molecules (H2O2, lipid hydroperoxides, α,β-unsaturated carbonyls, and possibly other electrophiles) that activate a series of redox switches through oxidation of specific cysteine residues [121, 123]. Beyond Keap1, which is possibly the most sensitive, these switches are frequently nuclear factors or protein kinases and phosphatases that integrate redox signaling with protein phosphorylation [121]. Other redox signaling includes effects on calcium pumps and phospholipases [124, 125]
13. Nucleophilic Tone & Para-Hormesis versus radical scavenging antioxidants
As a common motif of stress related signaling is oxidative in nature; i.e., formation of electrophiles, it follows that homeostasis for ‘Nucleophilic Tone’ must also entail counteracting mechanisms for switching off the production of (excess) electrophiles. This encompasses the synthesis of nucleophiles, namely GSH and different redoxins, the specific reductases of the oxidized forms, and the supply of reducing equivalents from NADPH by glucose oxidation. But central to the regulation of Nucleophilic Tone is the activation of Nrf2 through the modification of Keap1 by electrophiles.
In the “Omnivore’s Labyrinth,” [3] phytochemicals produced in the vegetal world are exported to animal world where they contribute to maintenance of health. For ‘inadequate’ intake of these compounds there is not a specific deficiency syndrome as there are for vitamins. Rather, there is an increased risk of disease that is much more difficult to demonstrate unequivocally. It follows that, although optimal nutrition minimizes the risk, supplementation to levels that exceed saturation of the Nrf2 activation system will hardly exert any beneficial effect. This concept, however, does not exclude the possibility that innovative more efficient drugs may be designed using bimolecular recognition as the template for interactions of nutraceuticals with other specific biological targets.
This idea of a positive, although dispensable, effect of phytochemicals is elegantly exploited in the concept of xeno-hormesis introduced by David Sinclair: molecules synthesized by plants to counteract stress can work similarly in animals using those plants as food [126]. From a survey of the enormous literature suggesting that natural compounds can have nutraceutical effects, and from studying those compounds recognized as healthy by folk traditions in different areas of the world, a leitmotif comes to light relative to the control of inflammatory reactions and protection against chronic degenerative diseases [2] and cancer [6, 127]. Apparently, these heterogeneous effects are related by control of cell reactivity against inflammatory stimuli [6]. This can be achieved by inhibition of NADPH oxidases from which O2·− is produced, or NF-κB, or by an increase of Nucleophilic Tone primed by modification of the electrophile sensor, Keap1.
This mechanism has been first unraveled for sulforaphane, an electrophile known for its chemopreventive activation of phase II genes through conjugation to Keap1 [128]. Other phytochemicals including curcumin from turmeric [129], diallyl sulfide from garlic [130], and resveratrol from grapes [131], activate Nrf2 through direct conjugation with the reactive cysteine of Keap1.
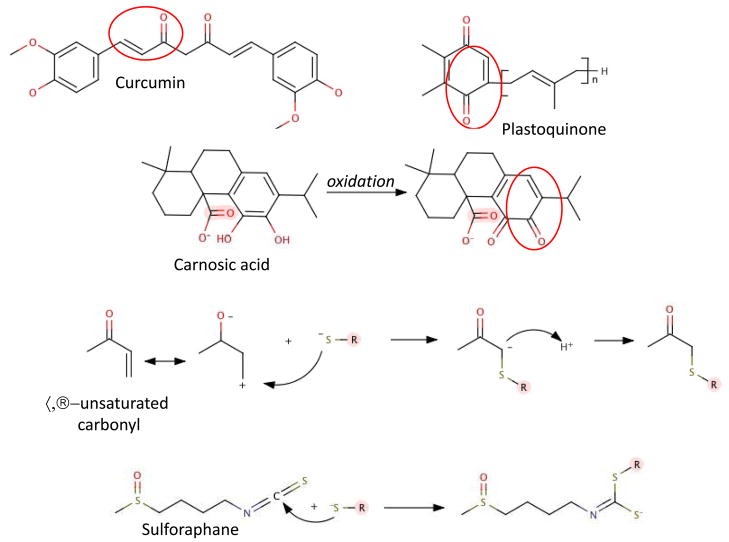
Remarkably, the same phenomenon has been observed for a series of nutritional polyphenols usually referred to as ‘antioxidants’ only because they can react as chemical (free radical scavenging) antioxidants in a test tube. The difference with the former compounds is that the polyphenols, including catechin and epicatechin from cocoa or green tea, carnosic acid from rosemary, hydroxytyrosol from olive leaf, and delphinidin from pomegranates, activate Keap1 upon oxidation [132–137]. As pointed out above, indeed, it is the quinone formed rather than the O2·− and H2O2 that are likely the more effective electrophiles in physiologically relevant Keap1 activation [138]. This seemingly paradoxical effect of antioxidants directly or indirectly modifying Keap1, the sensor for electrophiles, has been repeatedly reported in the past few years. Cumulatively the ortho or para hydroquinone structure emerges as the chemical entity eventually leading to transcriptional activation of genes coding for proteins involved in antioxidant protection and Nucleophilic Tone. Fig 3 illustrates the conversion of a representative dietary antioxidant, carnosic acid to a structure containing an electrophilic α,β-usaturated carbonyl that is present in other representative dietary antioxidants. Fig 3 also shows the Michael addition of a thiolate to electrophiles.
Fig 3.
Michael addition. Some dietary antioxidants contain α,β-unsaturated carbonyls. Others are oxidized to form the similar structures. The electrophilic α,β-unsaturated carbonyl or isocyanate of sulforaphane can react in a Michael addition with the thiolate of the critical cysteine of Keap1.
As the oxidation of a polyphenol to the hydroquinone or quinone appears to be critical to its ability to activate Nrf2 through Keap1 conjugation, the question arises as to how the oxidation occurs. One mechanism is the reaction of the polyphenol with a free radical. Of course, we have spent much time explaining how the elimination of free radicals by scavenging cannot account for the action of polyphenols; however, that is because the concentration of the polyphenols can never be sufficient in vivo to remove a significant portion of the free radicals; i.e, it is not the reactivity but the physiological concentration that is the limitation. On the other hand, polyphenols will be oxidized to electrophilic hydroquinones and quinones during their reaction with free radicals.
Even at low concentrations, quinones are able to activate Keap1. Unlike the competition between polyphenols and enzymes for removal of O2·− and hydroperoxides, the competition here between reaction with Keap1 and enzymatic removal of electrophiles can favor the former. This is because the glutathione S-transferases (GSTs) that remove electrophiles have slow rates of catalysis. An example the second order rate constant for reaction of 1,4-naphthoquinone with a generic thiolate is ~ 6.9 × 104 M−1 s−1 [139] which is reasonably rapid compared to GST reaction rates for most substrates where kcat/KM varies from ~1 to 75 × 104 M−1 s−1 with some exceptions for extremely electrophilic compounds [140]. Indeed, the non-enzymatic conjugation of GSH to GST substrates can be more rapid at the high intracellular GSH concentration than is the GST catalyzed rate [141]. Furthermore, GSTs can be inhibited by quinones and their GSH-conjugates [142, 143].
In summary, we have discussed several issues regarding the physiological mechanisms of action of nutritional antioxidants. Some of these reiterate what several colleagues have pointed out while others are either more obscure or novel. 1). What we hope is achieved by this exercise is a synthesis of these ideas so that the value and limitations of nutritional antioxidants are put into perspective. 2). The measured antioxidant capacity of phytochemicals is simply an index of sensitivity to oxidation rather than an index of protection that these agents can produce in vivo. 3). The principal manner by which nutritional antioxidants act is Nrf2 activation through modification of a specific cysteine in Keap1. 4). This modification of Keap1 requires that the antioxidant be, or be converted to, an electrophile. 5). The consensus chemistry underlying Keap1 modification is Michael addition, which is the reductive addition of a nucleophile (the specifically reactive Keap1 cysteine) to an 3,3-unsaturated carbonyl compound (the active form of the antioxidant). Addition to an isothiocyanate, such as sulforaphane is also a reductive addition reaction. 6). ‘Adequate’ nutritional intake of antioxidants (obviously a controversial subject) provides an adjustable level of nucleophiles that can be regulated to cope with increased electrophiles including oxidants.
From the above chemical, biological, nutritional and epidemiological considerations we propose two new terms. First, we suggest the term, ‘Nucleophilic Tone’ to describe the cellular, tissue, organ, or even organismal level of protection against electrophiles (including many free radicals and/or oxidants) by nucleophiles. Second, we propose the name ‘Para-Hormesis’ to describe the process by which non toxic compounds maintain an adaptive and defense system by mimicking electrophiles and increasing the Nucleophilic Tone; i.e., a hormetic-like response that does not necessarily require an activating or initiating stress (remembering that hormesis refers to the positive adaptational effects of low-dose toxins, toxicants or other stressors). We suggest that the concepts of ‘Nucleophilic Tone and ‘Para-Hormesis’ represent a paradigm shift in our understanding of the physiological mechanisms of action of nutritional antioxidants, from free radical scavengers to stimuli for the regulation of protective defense and repair systems.
Highlights.
Assumptions of free radical scavenging by antioxidants in vivo are kinetically unsound.
Among natural antioxidants, only vitamin E has the potential to act in vivo.
Electrophilic antioxidants and electrophiles derived from polyphenols increase Nucleophilic Tone.
Nucleophilic Tone is the overall potential cellular adaptive response to oxidative challenge brought by electrophiles.
Electrophiles induce Nucleophilic Tone through activation of Nrf2.
Acknowledgments
This work was supported by NIH/NIEHS grants ES020942 (to HJF) and ES003598 (to KJAD) and Strategic Project from the University of Padova (to FU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Craig WJ. Phytochemicals: guardians of our health. J Am Diet Assoc. 1997;97:S199–204. doi: 10.1016/s0002-8223(97)00765-7. [DOI] [PubMed] [Google Scholar]
- 2.Spencer JP. The impact of fruit flavonoids on memory and cognition. The British journal of nutrition. 2010;104(Suppl 3):S40–47. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 3.DeWeerdt S. Food: The omnivore’s labyrinth. Nature. 2011;471:S22–24. doi: 10.1038/471S22a. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. International journal of cancer. Journal international du cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 5.Key TJ. Fruit and vegetables and cancer risk. British journal of cancer. 2011;104:6–11. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 7.Scott G. Antioxidants in science, technology, medicine and nutrition. Chichester, UK: Albion Publishing; 1997. [Google Scholar]
- 8.Michaelis L. Free radicals as intermediate steps of oxidation-reduction. Cold Spring Harb Symp Quant Biol. 1939;7:33–49. [Google Scholar]
- 9.Fisher HF, Conn EE, Vennesland B, Westheimer FH. The enzymatic transfer of hydrogen. I. The reaction catalyzed by alcohol dehydrogenase. The Journal of biological chemistry. 1953;202:687–697. [PubMed] [Google Scholar]
- 10.Moss RW. Free Radical: Albert Szent-Gyorgyi and the Battle over Vitamin C. New York: Paragon House Publishers; 1988. [Google Scholar]
- 11.Szent-Györgyi A. Drive in Living Matter to Perfect Itself. Synthesis. 1977;1:14–26. [PubMed] [Google Scholar]
- 12.Schrödinger E. What is Life - the Physical Aspect of the Living Cell. Cambridge: Cambridge University Press; 1944. [Google Scholar]
- 13.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 15.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 16.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free radical biology & medicine. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Warnholtz A, Munzel T. Why do antioxidants fail to provide clinical benefit? Curr Control Trials Cardiovasc Med. 2000;1:38–40. doi: 10.1186/cvm-1-1-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 19.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nature chemical biology. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 20.Roberts LJ, II, Traber MG, Frei B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radical Biology and Medicine. 2009;46:1558. doi: 10.1016/j.freeradbiomed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taube H. Mechanisms of oxidation with oxygen. J Gen Physiol. 1965;2:S29–52. doi: 10.1085/jgp.49.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cadenas E. Biochemistry of oxygen toxicity. Annual Review of Biochemistry. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421:691–692. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- 25.Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- 26.Wiese AG, Pacifici RE, Davies KJA. Transient adaptation to oxidative stress in mammalian cells. Archives of biochemistry and biophysics. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 27.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. The Journal of biological chemistry. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) Journal of Biological Chemistry. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 30.Keele BB, Jr, McCord JM, Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. The Journal of biological chemistry. 1970;245:6176–6181. [PubMed] [Google Scholar]
- 31.Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. The Journal of biological chemistry. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 32.Yost FJ, Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. Journal of Biological Chemistry. 1973;248:4905. [PubMed] [Google Scholar]
- 33.Babior BM, Kipnes RS, Curnutte JT. The production by leukocytes of superoxide, a potential bactericidal agent. Journal of Clinical Investigation. 1973;52:741. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loschen G, Azzi A, Richter C, Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Letters. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 35.Forman HJ, Kennedy JA. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochemical and Biophysical Research Communications. 1974;60:1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B. Antioxidants in human health and disease. Annual review of nutrition. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 37.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochemical Society symposium. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 38.Davies KJ. An overview of oxidative stress. IUBMB life. 2000;50:241–244. doi: 10.1080/713803723. [DOI] [PubMed] [Google Scholar]
- 39.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 40.Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967;19:145–208. [PubMed] [Google Scholar]
- 41.Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP- iron in isolated rat hepatocytes and rat liver microsomal suspension. Biochemical Journal. 1985;227:629. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–1021. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- 43.Saussure NTd. Recherches chimiques sur la vegetation. Paris: Chez la Ve. Nyon; 1804. [Google Scholar]
- 44.Block BP. Antoine-Augustin Parmentier: pharmacist extraordinaire. Pharm Hist (Lond) 2008;38:6–14. [PubMed] [Google Scholar]
- 45.Hochstein P, Ernster L. ADP-activated lipid peroxidation coupled to the TPNH oxidase system of microsomes. Biochemical and Biophysical Research Communications. 1963;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- 46.Ursini F, Maiorino M, Hochstein P, Ernster L. Microsomal lipid peroxidation: mechanisms of initiation. The role of iron and iron chelators. Free radical biology & medicine. 1989;6:31–36. doi: 10.1016/0891-5849(89)90156-1. [DOI] [PubMed] [Google Scholar]
- 47.Maiorino M, Coassin M, Roveri A, Ursini F. Microsomal lipid peroxidation: effect of vitamin E and its functional interaction with phospholipid hydroperoxide glutathione peroxidase. Lipids. 1989;24:721–726. doi: 10.1007/BF02535211. [DOI] [PubMed] [Google Scholar]
- 48.Dorfman LM, Adams DO. National Standard Reference Data System. US Dept Commerce, Natl. Bureau Standards; 1973. Reactivity of Hydroxyl Radicals in Aquesous Solution; pp. 1–59. [Google Scholar]
- 49.Haag WR, Yao CCD. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ Sci Technol. 1992;26:1005–1013. [Google Scholar]
- 50.Carlsson DJ, Howard JA, Ingold KU. Reactions of alkoxy radicals. II. The absolute rate constant for the combination of t-butoxy radicals. J Am Chem Soc. 1966;88:4725–4726. [Google Scholar]
- 51.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Archives of biochemistry and biophysics. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 52.Ursini F, Maiorino M, Brigelius-Flohe R, Aumann KD, Roveri A, Schomburg D, Flohe L. Diversity of glutathione peroxidases. Methods in enzymology. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 53.Flohe L, Toppo S, Cozza G, Ursini F. A comparison of thiol peroxidase mechanisms. Antioxidants & redox signaling. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 54.Antunes F, Salvador A, Marinho HS, Alves R, Pinto RE. Lipid peroxidation in mitochondrial inner membranes. I. An integrative kinetic model. Free radical biology & medicine. 1996;21:917–943. doi: 10.1016/s0891-5849(96)00185-2. [DOI] [PubMed] [Google Scholar]
- 55.Neta P, Grodkowski J, Ross AB. Rate constants for reactions of aliphatic carbon-centered radicals in aqueous solutions. J Phys Chem Ref Data. 1996;25:709–1050. [Google Scholar]
- 56.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annual review of nutrition. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 57.Bowry VW, Mohr D, Cleary J, Stocker R. Prevention of tocopherol-mediated peroxidation in ubiquinol-10-free human low density lipoprotein. The Journal of biological chemistry. 1995;270:5756–5763. doi: 10.1074/jbc.270.11.5756. [DOI] [PubMed] [Google Scholar]
- 58.Maiorino M, Zamburlini A, Roveri A, Ursini F. Copper-induced lipid peroxidation in liposomes, micelles, and LDL: which is the role of vitamin E? Free radical biology & medicine. 1995;18:67–74. doi: 10.1016/0891-5849(94)00103-q. [DOI] [PubMed] [Google Scholar]
- 59.Scarpa M, Rigo A, Maiorino M, Ursini F, Gregolin C. Formation of alpha-tocopherol radical and recycling of alpha-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes. An electron paramagnetic resonance study. Biochimica et biophysica acta. 1984;801:215–219. doi: 10.1016/0304-4165(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 60.Bando M, Inoue T, Oka M, Nakamura K, Kawai K, Obazawa H, Kobayashi S, Takehana M. Isolation of ascorbate free radical reductase from rabbit lens soluble fraction. Experimental Eye Research. 2004;79:869–873. doi: 10.1016/j.exer.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Ito A, Hayashi S, Yoshida T. Participation of a cytochrome b5-like hemoprotein of outer mitochondrial membrane (OM cytochrome b) in NADH-semidehydroascorbic acid reductase activity of rat liver. Biochemical and Biophysical Research Communications. 1981;101:591–598. doi: 10.1016/0006-291x(81)91300-0. [DOI] [PubMed] [Google Scholar]
- 62.Hara T, Minakami S. On functional role of cytochrome b5. II. NADH-linked ascorbate radical reductase activity in microsomes. J Biochem. 1971;69:325–330. doi: 10.1093/oxfordjournals.jbchem.a129470. [DOI] [PubMed] [Google Scholar]
- 63.Villalba JM, Navarro F, Gomez-Diaz C, Arroyo A, Bello RI, Navas P. Role of cytochrome b5 reductase on the antioxidant function of coenzyme Q in the plasma membrane. Molecular Aspects of Medicine. 1997;18(Suppl):S7–13. doi: 10.1016/s0098-2997(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 64.Coassin M, Tomasi A, Vannini V, Ursini F. Enzymatic recycling of oxidized ascorbate in pig heart: one-electron vs two-electron pathway. Archives of biochemistry and biophysics. 1991;290:458–462. doi: 10.1016/0003-9861(91)90566-2. [DOI] [PubMed] [Google Scholar]
- 65.Winterbourn CC. Superoxide as an intracellular radical sink. Free radical biology & medicine. 1993;14:85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- 66.Koppenol WH. A thermodynamic appraisal of the radical sink hypothesis. Free radical biology & medicine. 1993;14:91–94. doi: 10.1016/0891-5849(93)90513-t. [DOI] [PubMed] [Google Scholar]
- 67.Tamba M, Simone G, Quintiliani M. Interactions of thiyl free radicals with oxygen: a pulse radiolysis study. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;50:595–600. doi: 10.1080/09553008614550991. [DOI] [PubMed] [Google Scholar]
- 68.Ursini F, Sevanian A. Postprandial oxidative stress. Biol Chem. 2002;383:599–605. doi: 10.1515/BC.2002.062. [DOI] [PubMed] [Google Scholar]
- 69.Mulcahy RT, Gipp JJ. Identification of a putative antioxidant response element in the 5′-flanking region of the human γ-glutamylcycteine synthetase heavy subunit gene. Biochemical and Biophysical Research Communications. 1995;209:227–233. doi: 10.1006/bbrc.1995.1493. [DOI] [PubMed] [Google Scholar]
- 70.Moinova HR, Mulcahy RT. An electrophile responsive element (EpRE) regulates β-naphthoflavone induction of the human γ-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. The Journal of biological chemistry. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 71.Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 72.Rahman I, Antonicelli F, MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. The Journal of biological chemistry. 1999;274:5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- 73.Rahman I, Bel A, Mulier B, Lawson MF, Harrison DJ, MacNee W, Smith CAD. Transcriptional regulation of γ-glutamylcysteine synthetase-heavy subunit by oxidants in human aveolar epithelial cells. Biochemical and Biophysical Research Communications. 1996;229:832–837. doi: 10.1006/bbrc.1996.1888. [DOI] [PubMed] [Google Scholar]
- 74.Sekhar KR, Meredith MJ, Kerr LD, Soltaninassab SR, Spitz DR, Xu Z-Q, Freeman ML. Expression of glutathione and γ-glutamylcysteine synthetase mRNA is Jun dependent. Biochemical and Biophysical Research Communications. 1997;234:588–593. doi: 10.1006/bbrc.1997.6697. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka T, Uchiumi T, Kohno K, Tomonari A, Nishio K, Saijo N, Kondo T, Kuwano M. Glutathione homeostasis in human hepatic cells: overexpression of γ-glutamylcysteine synthetase gene in cell lines resistant to buthionine sulfoximine, an inhibitor of glutathione synthesis. Biochemical and Biophysical Research Communications. 1998;246:398–403. doi: 10.1006/bbrc.1998.8631. [DOI] [PubMed] [Google Scholar]
- 76.Tomonari A, Nishio K, Kurokawa H, Arioka H, Ishida T, Fukumoto H, Fukuoka K, Nomoto T, Iwamoto Y, Heike Y, Itakura M, Saijo N. Identification of cis-acting DNA elements of the human γ-glutamylcysteine synthetase heavy subunit gene. Biochemical and Biophysical Research Communications. 1997;232:522–527. doi: 10.1006/bbrc.1997.6319. [DOI] [PubMed] [Google Scholar]
- 77.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free radical biology & medicine. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 78.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. Faseb J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 79.Tu Z, Anders MW. Up-regulation of glutamate-cysteine ligase gene expression by butylated hydroxytoluene is mediated by transcription factor AP-1. Biochem Biophys Res Commun. 1998;244:801–805. doi: 10.1006/bbrc.1998.8345. [DOI] [PubMed] [Google Scholar]
- 80.Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochemical Society symposium. 2004:157–176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- 81.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prochaska HJ, Talalay P. Regulatory mechanisms of monofunctional and biofunctional anticarcinogenic enzyme inducers in murine liver. Cancer Research. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 83.Paulson KE, Darnell JE, Rushmore T, Pickett CB. Analysis of the upstream elements of the xenobiotic compound- inducible and positionally regulated glutathione S-transferase Ya gene. Molecular and Cellular Biology. 1990;10:1841–1852. doi: 10.1128/mcb.10.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-induced expression of murine glutathione S- transferase Ya subunit gene is controlled by an electrophile- responsive element. Proceedings National Academy of Sciences, USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. Journal of Biological Chemistry. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 86.Friling RS, Bergelson S, Daniel V. Two adjacent AP-1 binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proceedings National Academy of Sciences, USA. 1992;89:668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Journal of Clinical Investigation. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 90.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 91.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang H, Ramani K, Xia M, Ko KS, Li TW, Oh P, Li J, Lu SC. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49:1982–1991. doi: 10.1002/hep.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levy S, Jaiswal AK, Forman HJ. The role of c-Jun phosphorylation in EpRE activation of phase II genes. Free radical biology & medicine. 2009;47:1172–1179. doi: 10.1016/j.freeradbiomed.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- 96.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. The Journal of biological chemistry. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. The Journal of biological chemistry. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 99.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chemical research in toxicology. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 100.Hur W, Gray NS. Small molecule modulators of antioxidant response pathway. Current opinion in chemical biology. 2011;15:162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 101.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, van Breemen RB. Sites of alkylation of human Keap1 by natural chemoprevention agents. Journal of the American Society for Mass Spectrometry. 2007;18:2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohnuma T, Nakayama S, Anan E, Nishiyama T, Ogura K, Hiratsuka A. Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicology and applied pharmacology. 2010;244:27–36. doi: 10.1016/j.taap.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 103.Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chemical research in toxicology. 2008;21:705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 104.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaiswal AK. NRF2 signaling in coordinated activation of antioxidant genes expression. Free radical biology & medicine. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 106.Kang KW, Choi SH, Kim SG. Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: the role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric oxide : biology and chemistry/official journal of the Nitric Oxide Society. 2002;7:244–253. doi: 10.1016/s1089-8603(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 107.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. The Journal of biological chemistry. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 108.Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–490. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- 109.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. The Journal of biological chemistry. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 110.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. Journal of cell science. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. The Journal of biological chemistry. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 112.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. The Journal of biological chemistry. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 113.Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. The Journal of biological chemistry. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 114.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levy S, Forman HJ. c-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. The Journal of biological chemistry. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 117.Rao PS, Hayon E. Redox potentials of free radicals. IV Superoxide and hydroperoxy radicals. J Phys Chem. 1975;79:397–402. [Google Scholar]
- 118.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. The American journal of physiology. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 119.Bernard C. Introduction à l’étude de la médecine expérimentale (Paris 1865); English translation. Macmillan & Co., Ltd; 1927. reprinted in 1949. [Google Scholar]
- 120.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 121.Brigelius-Flohe R, Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants & redox signaling. 2011 doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxidants & redox signaling. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Forman HJ, Cadenas E, editors. Oxidative Stress and Signal Transduction. New York: Chapman & Hall; 1997. [Google Scholar]
- 125.Forman HJ, Fukuto J, Torres M. Signal transduction by reactive oxygen and nitrogen species: pathways and chemical principles. Dordrecht ; Boston: Kluwer Academic Publishers; 2003. [Google Scholar]
- 126.Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free radical biology & medicine. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 131.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical pharmacology. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, Berger W, Micksche M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB Journal. 2005;19:807–809. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 133.Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Molecular nutrition & food research. 2011;55:844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mori T, Ishii T, Akagawa M, Nakamura Y, Nakayama T. Covalent binding of tea catechins to protein thiols: the relationship between stability and electrophilic reactivity. Biosci Biotechnol Biochem. 2010;74:2451–2456. doi: 10.1271/bbb.100509. [DOI] [PubMed] [Google Scholar]
- 135.Inoue H, Maeda-Yamamoto M, Nesumi A, Murakami A. Delphinidin-3-O-galactoside protects mouse hepatocytes from (−)-epigallocatechin-3-gallate-induced cytotoxicity via up-regulation of heme oxygenase-1 and heat shock protein 70. Nutr Res. 2012;32:357–364. doi: 10.1016/j.nutres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 136.Cumaoglu A, Ari N, Kartal M, Karasu C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced beta-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011;14:325–334. doi: 10.1089/rej.2010.1111. [DOI] [PubMed] [Google Scholar]
- 137.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, Shimojo Y, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 138.Tu T, Giblin D, Gross ML. Structural determinant of chemical reactivity and potential health effects of quinones from natural products. Chem Res Toxicol. 2011;24:1527–1539. doi: 10.1021/tx200140s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murty VS, Penning TM. Polycyclic aromatic hydrocarbon (PAH) ortho-quinone conjugate chemistry: kinetics of thiol addition to PAH ortho-quinones and structures of thioether adducts of naphthalene-1,2-dione. Chem Biol Interact. 1992;84:169–188. doi: 10.1016/0009-2797(92)90077-x. [DOI] [PubMed] [Google Scholar]
- 140.Van der Aar EM, Bouwman T, Commandeur JN, Vermeulen NP. Structure-activity relationships for chemical and glutathione S-transferase-catalysed glutathione conjugation reactions of a series of 2-substituted 1-chloro-4-nitrobenzenes. Biochem J. 1996;320(Pt 2):531–540. doi: 10.1042/bj3200531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Archives of biochemistry and biophysics. 1988;264:253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- 142.Dierickx PJ. Interaction of benzo- and naphthoquinones with soluble glutathione S-transferases from rat liver. Pharmacol Res Commun. 1983;15:581–591. doi: 10.1016/s0031-6989(83)80029-0. [DOI] [PubMed] [Google Scholar]
- 143.van Ommen B, Ploemen JH, Bogaards JJ, Monks TJ, Gau SS, van Bladeren PJ. Irreversible inhibition of rat glutathione S-transferase 1-1 by quinones and their glutathione conjugates. Structure-activity relationship and mechanism. Biochem J. 1991;276(Pt 3):661–666. doi: 10.1042/bj2760661. [DOI] [PMC free article] [PubMed] [Google Scholar]