SUMMARY
Venoms consist of toxic components that are delivered to their victims via bites or stings. Venoms also represent a major class of allergens in humans. Phospholipase A2 (PLA2) is a conserved component of venoms from multiple species and is the major allergen in bee venom. Here we examined how bee venom PLA2 is sensed by the innate immune system and induces a type 2 immune response in mice. We found that bee venom PLA2 induced a T helper type 2 (Th2) cell-type response and group 2 innate lymphoid cell activation via the enzymatic cleavage of membrane phospholipids and release of interleukin-33. Furthermore, we showed that the IgE response to PLA2 could protect mice from future challenge with a near-lethal dose of PLA2. These data suggest that the innate immune system can detect the activity of a conserved component of venoms and induce a protective immune response against a venom toxin.
INTRODUCTION
Viral, bacterial and fungal infections are detected by the innate immune system through the recognition of Pathogen-Associated Molecular Patterns (PAMPs) by Pattern Recognition Receptors (PRRs), such as the Toll-like receptors. PAMPs consist of microbial molecules that are unique to microbial non-self, essential for microbial fitness, and conserved within a class of microbes. These features of PAMPs allow PRRs to distinguish self from infectious non-self and to target appropriate immune responses towards foreign antigens. Recognition of PAMPs by PRRs leads to the induction of anti-microbial T helper type 1 (Th1), Th17 and associated IgG2 responses (Palm and Medzhitov, 2009).
In contrast to infections with bacteria, viruses and fungi, parasitic worms (helminths) and allergens induce Th2 and IgE responses. The mechanisms by which the innate immune system recognizes helminths and allergens and instructs Th2 and IgE responses remain largely unknown, but appear to be independent of PRRs (Palm et al., 2012). Instead, it has been proposed that allergens and helminths are detected through sensing of the outcomes of their unique activities (Donnelly et al., 2006; Palm et al., 2012; Pulendran and Artis, 2012). For example, proteases excreted by helminths and protease allergens induce type 2 immune responses in a manner that is dependent on their enzymatic activities (Donnelly et al., 2006). It has also been suggested that the type 2 immune response may be connected to sensing of tissue damage or tissue disruption, and that one purpose of the allergic response is to protect against or repair allergen- or helminth-mediated tissue damage (Palm et al., 2012; Profet, 1991; (Allen and Wynn, 2011). Indeed, while allergens are often thought of as innocuous environmental substances, many allergens clearly have noxious activities that can induce tissue damage (Palm et al., 2012; Profet, 1991).
Recent studies have revealed an important role for the epithelial cell-derived cytokines IL-25, IL-33 and Thymic stromal lymphopoietin (TSLP) in the induction of type 2 responses to helminths and allergens (Pulendran and Artis, 2012). While IL-25 and TSLP act as traditional cytokines and are transcriptionally regulated, IL-33 is constitutively expressed in barrier epithelial cells and lacks a signal sequence. The physiological mechanisms of IL-33 release remain unclear; however, it is thought that IL-33 release is triggered by necrotic cell death or cell lysis (Liew, 2012). IL-33 has long been known as a potent stimulator of type 2 cytokine production by Th2 cells; more recently, IL-33 has been shown to activate Group 2 Innate Lymphoid Cells (ILC2s), which are innate producers of type 2 cytokines such as IL-5 and IL-13 (Walker and McKenzie, 2013).
Venoms consist of a complex mixture of toxic components that are delivered to their victims via bites or stings, and are used by various animal species, including insects, arachnids, cnidaria and reptiles, for defense and predation (Fry et al., 2009). Venoms from various species can induce Th2 and IgE responses and therefore represent a major class of allergens (Bircher, 2005; Habermann, 1972; Madero et al., 2009). Type 2 responses to western honey bee (Apis mellifera) venom are well documented in both mice and humans (Bircher, 2005; Habermann, 1972; Muller, 2010). Bee venom contains a mixture of enzymes, cell-lytic peptides, proteases and bioactive amines. The two major proteinaceous components of bee venom are melittin, a 23 amino acid cationic cell-lytic peptide, and Phospholipase A2 (PLA2), an enzyme that hydrolyzes membrane phospholipids to produce lysophospholipids and arachidonic acid (Habermann, 1972). PLA2 is the major allergen in bee venom (Sobotka et al., 1976), and bvPLA2 enzymatic activity is necessary for its ability to induce an IgE response in mice (Dudler et al., 1995). Furthermore, PLA2s are integral and conserved components of venoms from divergent species, including venomous spiders, scorpions, bees and snakes; in addition, PLA2 is also a common component of hematophageous fluids from some blood-feeding animals, such as mosquitoes and ticks (Fry et al., 2009; Habermann, 1972). Notably, exposure to bvPLA2 leads to damage of cellular membranes and, at high concentrations, necrotic cell death (Ownby et al., 1997).
As venoms represent a major class of noxious allergens, we wished to examine the mechanism by which venoms are sensed by the innate immune system and induce a type 2 immune response. We found that bvPLA2 induced type 2 immune responses in vivo in a manner that was dependent on its enzymatic activity and on the interleukin (IL)-33 receptor component ST2. PLA2 from snake venom also induced a Th2 response, which suggests that PLA2s may represent a type 2-inducing enzymatic activity that is a common component of this class of allergens. Finally, we implicate the IgE response to bvPLA2 in protection against future exposure to this noxious venom component.
RESULTS
Bee venom PLA2 induces a type 2 immune response
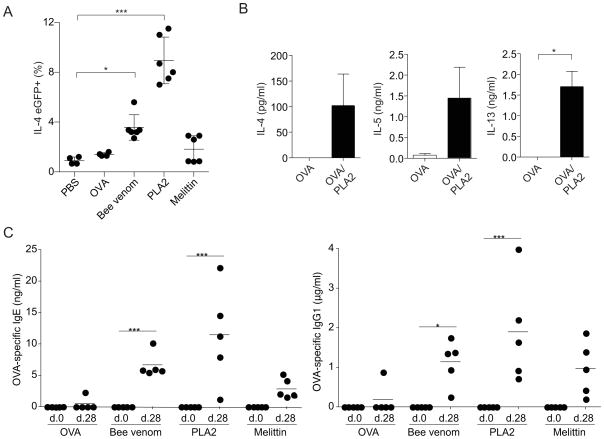
To examine the allergenicity of bee venom and its components, we first tested the ability of bee venom to induce a Th2 response by measuring IL-4 transcription in CD4+ T cells using IL-4-IRES-eGFP (4get) reporter mice (Mohrs et al., 2001). Immunizations with 100μg bee venom (equivalent to approximately 1 bee sting) induced the accumulation of IL-4eGFP expressing CD4+ T cells in the draining lymph node five days after subcutaneous immunization (Figure 1A). Immunization with an equivalent dose of purified bvPLA2 induced even greater Th2 differentiation than whole bee venom, whereas melittin was less efficient at inducing a Th2 response. Immunization with bvPLA2 admixed with endotoxin-free ovalbumin (OVA) also induced an OVA-specific Th2 response that was characterized by secretion of IL-4, IL-5 and IL-13 after restimulation of isolated CD4+ T cells with OVA in vitro (Figure 1B). In contrast, production of IFNγ and IL-17 after restimulation was negligible (data not shown). These results demonstrate that bvPLA2 is a potent inducer of the Th2 response.
Figure 1. Bee venom and bvPLA2 induce Th2 differentiation and antigen-specific IgE and IgG1 production.
(A) Percent IL-4eGFP+ T cells from popliteal LNs of 4get mice 5 days after subcutaneous immunization with PBS, bee venom, bvPLA2, or melittin. (B) Cytokine production after in vitro restimulation of LN CD4+ T cells from BALB/c mice 5 days after subcutaneous immunization with OVA in the absence or presence of bvPLA2. (C) Serum OVA-specific IgE and IgG1 in BALB/c mice after subcutaneous immunization with OVA alone or in the presence of bee venom, bvPLA2, or melittin on day 0 and 21. All error bars show s.e.m. *P<0.05; **P<0.005; ***P<0.001. Data are representative of at least three experiments. See also Figure S1.
We next examined venom-induced IgG1 and IgE production, which requires cognate help from IL-4-producing T cells. Bee venom, melittin and bvPLA2 induced antigen-specific IgG1 and IgE responses to admixed endotoxin-free OVA, while OVA alone did not induce a robust antibody response (Figure 1C). As compared to total bee venom and melittin, bvPLA2 induced the strongest anti-OVA IgG1 and IgE responses. OVA-specific IgG2c production after immunization was undetectable under all immunization conditions (data not shown). Bee venom and bvPLA2, but not melittin, also induced an increase in total serum IgE. The IgE response to bee venom and bvPLA2 remained intact in Toll-like receptor 2 (TLR2) and TLR4-deficient mice, suggesting that the ability of bee venom and bvPLA2 to induce IgE production is not due to contamination with bacterial PAMPs, such as lipopolysaccharide (Supplemental Figure 1A).
The total IgE response induced by bee venom and bvPLA2 was similar in kinetics and magnitude to the IgE response that is induced by the model cysteine protease allergen from papaya, papain (Supplemental Figure 1A and (Sokol et al., 2008)). Papain has been shown to induce recruitment of dendritic cells and basophils to the draining lymph node after subcutaneous immunization. Similarly, bvPLA2 immunization induced the recruitment of dendritic cells and basophils to the draining lymph node 18 hours or 3 days post immunization, respectively (Supplemental Figure 1B, C).
Many allergens can directly activate innate type 2 cells, such as basophils and mast cells, in an IgE-independent manner. For example, human basophils respond to the proteolytic activity of Der p 1 (Phillips et al., 2003), and papain directly stimulates murine basophils to produce IL-4 in a protease-dependent manner (Sokol et al., 2008). Similar to papain, PLA2 was a potent inducer of IL-4 transcription and secretion by in vitro-derived basophils (Supplemental Figure 1D, E). The ability of bvPLA2 to induce IL-4 secretion by basophils also did not require TLR2 or TLR4, again suggesting that bvPLA2 allergenicity is not due to contamination with bacterial products (data not shown).
The T cell response to bvPLA2 is dependent on PLA2 activity
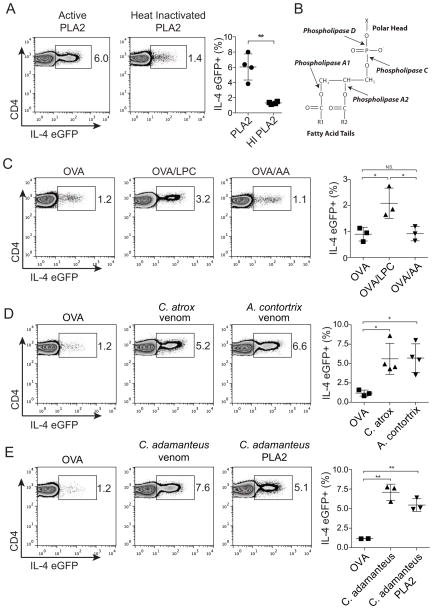
Many known allergens are enzymes, and the enzymatic activities of many of these allergens are required to induce Th2 and IgE responses (Palm et al., 2012; Pulendran and Artis, 2012). A previous study using enzymatically inactive mutants of bvPLA2 found that the enzymatic activity of bvPLA2 is necessary for its ability to induce anti-PLA2 IgE production in mice (Dudler et al., 1995). We therefore tested whether PLA2 activity is also critical for its ability to induce Th2 responses. We found that heat inactivation of bvPLA2 eliminates its ability to induce a Th2 response in 4get mice (Figure 2A), which suggests that the enzymatic activity of bvPLA2 is necessary to induce the Th2 response.
Figure 2. PLA2 induces a Th2 response through cleavage of membrane phospholipids to produce lysophospholipids.
(A–E) CD4+ GFP+ T cells from popliteal LNs of 4get mice 5 days after subcutaneous immunization with (A) active bvPLA2 or heat-inactivated bvPLA2 (C) OVA in the presence of LPC or AA (D) OVA, Crotalus atrox venom or Agkistrodon contortrix venom (E) OVA, Crotalus adamanteus venom, or Crotalus adamanteus PLA2. (B) PLA2 hydrolyzes phospholipids into arachidonic acid (AA) and lysophospholipids (e.g., LPC). All error bars show s.e.m. *P<0.05; **P<0.005. Data are representative of at least three experiments. See also Figure S2.
PLA2s hydrolyze membrane phospholipids to produce lysophospholipids, such as lysophosphatidylcholine (LPC) and arachidonic acid (AA), which is the precursor of eicosanoids (Figure 2B) (Burke and Dennis, 2009). As the allergenicity of bvPLA2 is dependent on its enzymatic activity, we sought to test which product of bvPLA2 activity is responsible for its ability to induce a Th2 response. We found that LPC, but not AA, induced a Th2 response in 4get mice when admixed with OVA (Figure 2C). LPC, but not AA, also induced IL-4 production by in vitro-derived basophils (Supplemental Figure 2A). LPC has been shown to activate the G protein-coupled receptor 132 (Gpr132, also referred to as G2A), which led us to test the role of G2A in the Th2 response to PLA2 (Kabarowski et al., 2001). Surprisingly, Gpr132−/− mice showed intact Th2 cell differentiation in response to bvPLA2 immunization (Supplemental Figure 2B). Thus the Th2-inducing activity of LPC may be mediated by a different receptor, or its effect may be receptor independent - for example, LPC also acts as a detergent and can induce cell lysis. In this regard, it is notable that Phospholipase D, which cleaves membrane phospholipids at a different site from PLA2, also induced a Th2 response (data not shown). These data suggest that PLA2 induces the Th2 response by hydrolyzing membrane phospholipids to produce lysophospholipids, such as LPC.
The immune system senses PLA2s from multiple venomous species
As PLA2s are a conserved component of venoms from divergent animal species, we hypothesized that detection of PLA2 activity may be used by the innate immune system as a way to detect multiple venoms. Consistent with this possibility, venoms from the Western Diamondback rattlesnake (Crotalus atrox) and Northern Copperhead (Agkistrodon contortrix mokasen) efficiently induced Th2 responses (Figure 2D) and induced IL-4 production by in vitro-derived basophils (Supplemental Figure 2C). Furthermore, similar to bvPLA2, purified PLA2 from the Eastern Diamondback rattlesnake (Crotalus adamanteus) was sufficient to induce a Th2 response that was comparable in magnitude to the response induced by total C. adamanteus venom (Figure 2E). These data suggest that PLA2, a conserved component of multiple venoms, can be used by the immune system to detect envenomation, which results in the induction of a Th2 response.
Th2 responses induced by bvPLA2 are dependent on MyD88 and the IL-33 receptor (ST2)
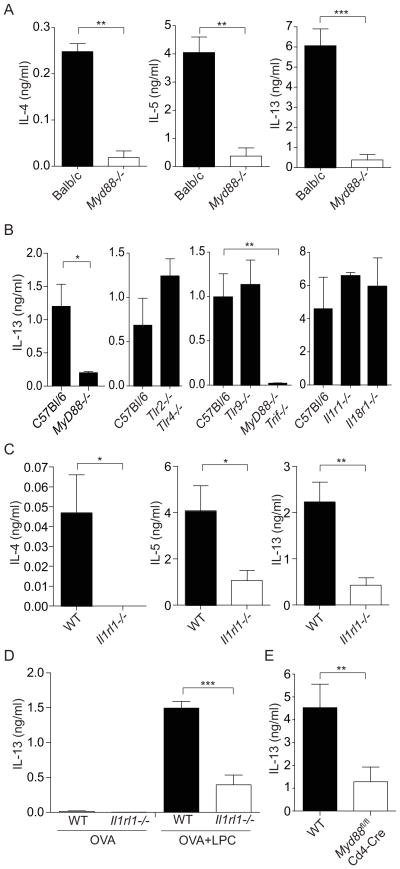
We next asked which innate immune pathways are involved in the induction of Th2 responses to bvPLA2. MyD88 is the central signaling adaptor for the TLRs and for receptors that recognize the IL-1 family of cytokines, including IL-1, IL-18 and IL-33 (Dinarello, 2009). MyD88 is also critical for the initiation of T cell responses to many bacterial and viral pathogens (Palm and Medzhitov, 2009). We therefore tested the role of MyD88 in the bvPLA2-induced Th2 response. We found that Th2 responses induced by bvPLA2 were largely dependent on MyD88 (Figure 3A, B). In contrast, bvPLA2-induced Th2 responses were independent of TLR2, TLR4, TLR9, TRIF, IL-1R and IL-18R (Figure 3B), thus ruling out the contribution of TLRs, IL-1 and IL-18 in the response to bvPLA2.
Figure 3. bvPLA2-induced Th2 responses are dependent upon MyD88 and the IL-33-receptor (ST2).
(A–C) Cytokine production after in vitro restimulation of LN CD4+ T cells from (A) BALB/c and Myd88−/− BALB/c (B) C57Bl/6, Myd88−/− C57Bl/6, Tlr2−/−;Tlr4−/−, Tlr9−/−, Myd88−/−;Trif−/−, Il1r1−/−, and Il18r1−/−, and (C) C57Bl/6 and Il1rl1−/− (ST2-deficient) mice 5 days after immunization with OVA and bvPLA2. (D) Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and Il1rl1−/− mice 5 days after immunization with OVA with or without LPC. (E) Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and MyD88fl/fl;Cd4-Cre mice 5 days after immunization with OVA and bvPLA2. All error bars show s.e.m. N.S. P>.05; *P<0.05; ***P<0.001. Data are representative of at least three experiments.
TLRs and the cytokines IL-1 and IL-18 are critical for the initiation and coordination of Th1 and Th17 responses. In contrast, IL-33 is a potent inducer of type 2 cytokine production by T cells, basophils, and ILC2s (Schmitz et al., 2005). We tested the role of IL-33 in the Th2 response to bvPLA2 by examining mice lacking ST2, which is a component of the receptor for IL-33 (Townsend et al., 2000). We found that ST2-deficient mice showed reduced Th2 responses to bvPLA2 (Figure 3C). Furthermore, LPC-induced Th2 responses were also dependent on ST2 (Figure 3D). This suggests that bvPLA2 induces a Th2 response by stimulating production of LPC, which then leads to the induction of a Th2 response through activation of ST2.
IL-33 can induce Th2 responses through its actions on dendritic cells, or through a direct effect on T cells themselves (Besnard et al., 2011; Humphreys et al., 2008; Liew, 2012). We were interested in identifying which cell type IL-33 acted upon to induce Th2 differentiation in response to bvPLA2. Using conditional knockout mice that lack MyD88 specifically in CD4+ T cells, we found that Th2 responses to bvPLA2 were largely dependent on MyD88 expression in T cells (Figure 3E). In contrast, mice lacking MyD88 specifically in dendritic cells showed normal Th2 responses to bvPLA2 immunization (data not shown). These data demonstrate that IL-33 controls the Th2 response to bvPLA2 by acting directly on CD4+ T cells, whereas IL-33 signaling in dendritic cells is dispensable for this response.
bvPLA2 induces IL-33 release and ST2-dependent activation of ILC2s
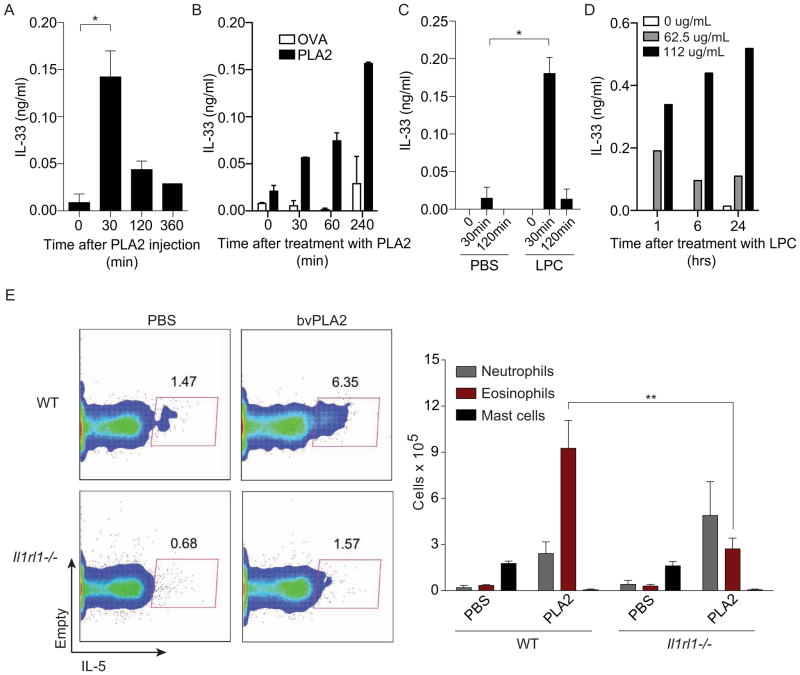
Considering that ST2 expression was required for activation of bvPLA2-induced Th2 responses, we were interested in determining whether bvPLA2 could induce the release of IL-33. IL-33 lacks a signal sequence and its physiological mechanism of release remains unclear. However, it has been suggested that IL-33 release may be triggered by cell damage and/or necrotic cell death (Liew, 2012). As PLA2s cause membrane damage and can trigger cell death at high concentrations, we tested whether bvPLA2 could trigger IL-33 release. We found that bvPLA2 could induce IL-33 production in vivo after intraperitoneal injection (Figure 4A) and in vitro using a cell line that overexpresses IL-33 (Figure 4B).
Figure 4. bvPLA2 and LPC induce IL-33 release and activation of ILC2s.
(A and C) IL-33 concentration in peritoneal fluid after (A) bvPLA2 and (C) LPC intraperitoneal immunizations. (B and D) IL-33 concentration in supernatants of cells overexpressing IL-33 after stimulation with (B) bvPLA2 and (D) LPC. (E) Peritoneal exudate collected from WT and Il1rl1−/− (ST2-deficient) mice on day 4 after daily intraperitoneal injections with PLA2 for 3 days. FACS plots show group 2 innate lymphoid cells, as defined by CD45+Lin-CD90.2+, after ex vivo stimulation with PMA and ionomycin to induce cytokine production. Eosinophils, mast cells and neutrophils were defined by morphology (n=5 mice/group). All error bars show s.e.m. *P<0.05; **P<0.005; ***P<0.001. Data are representative of at least two experiments. See also Figure S3.
Similarly, LPC could also induce IL-33 release both in vivo and in vitro (Figure 4C, D), suggesting that bvPLA2 may act by producing LPC, which then leads to IL-33 release. These data suggest that the ability of PLA2 to induce the type 2 immune response may be related to its ability to induce membrane damage and, therefore, IL-33 release.
Since IL-33 is released from necrotic cells, we also examined the role of regulated necrosis, or necroptosis, in the Th2 response to PLA2 (Kaczmarek et al., 2013). However, mice deficient in RIP3, which is required to induce programmed necrosis (Vandenabeele et al., 2010), exhibited intact Th2 responses after immunization with bvPLA2 (Supplemental Figure 3).
IL-33 has recently become well known as a potent stimulator of ILC2s, which are innate producers of type 2 cytokines such as IL-5 and IL-13 and thereby drive eosinophilia and goblet cell hyperplasia (Walker and McKenzie, 2013). As we found that bvPLA2 induces IL-33 release in vivo, we wondered whether PLA2 might also induce the activation of ILC2s. Intraperitoneal injection with bvPLA2 led to the appearance of IL-5 expressing ILC2s in the peritoneal cavity and eosinophilia (Figure 4E). Furthermore, both ILC2 activation and the accompanying eosinophilia were reduced in ST2-deficient mice. These data suggest that bvPLA2 induces ILC2 activation by triggering the release of IL-33.
The bvPLA2-induced IgE response is independent of ST2
As we found that IL-33 is critical for inducing both Th2 and ILC2 responses to PLA2, we next examined the requirement for IL-33 in the induction of IgE responses. As cognate help from Th2 cells is required for the IgE response, we expected that the PLA2-induced IgE response would be reduced in ST2-deficient mice. However, PLA2-induced IgE responses were normal in ST2-deficient mice (Supplemental Figure 4A). One possible reason for the differential requirement for ST2 in Th2 versus IgE responses is that we examine the Th2 response after a primary immunization, while the IgE response is only detectable after a secondary immunization. Therefore, we could not compare the requirement for ST2 during primary Th2 and IgE responses. However, when we examined the secondary Th2 response induced by bvPLA2, we found that, like the IgE response, it remained largely intact even in the absence of ST2 (Supplemental Figure 4A). It is notable that a similar discrepancy between Th2 and IgE responses also exists in response to Schistosoma mansoni eggs (Townsend et al., 2000) and the protease allergen papain: Th2 responses induced by papain are MyD88-dependent (Supplemental Figure 4B), while IgE responses are MyD88-independent (Sokol et al., 2008).
An FcεR1α-dependent immune response to bvPLA2 helps to protect against bvPLA2-mediated toxicity
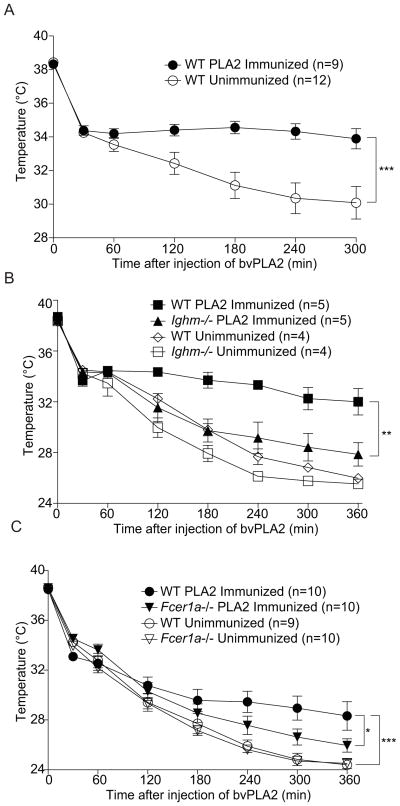
We were next interested in testing the potential role of the immune response to venoms in defense against the noxious effects of envenomation. As anti-venoms function via transfer of neutralizing anti-venom IgGs (Chippaux and Goyffon, 1998) and neutralizing anti-PLA2 antibodies have been observed in humans (Mohammed and El-Karemi, 1961), we examined whether repeated immunizations with PLA2 in mice could provide protection from the noxious effects of envenomation through induction of an antibody response. We found that weekly intraperitoneal immunizations with low dose bvPLA2 for 6 weeks could indeed protect against challenge with a near-lethal dose of bvPLA2 (Figure 5A) and that B cell-deficient mice failed to acquire protection (Figure 5B). We initially expected to find that neutralizing IgGs were responsible for this protection; however, transfer of serum from mice that were resistant to PLA2 challenge due to repeated PLA2 administration failed to provide any measurable protection from high-dose PLA2 exposure (data not shown).
Figure 5. Immunization with bvPLA2 protects from subsequent challenge with a near-lethal dose of bvPLA2 in a B cell- and FcεR-dependent manner.
(A) Rectal temperature measurements of naïve or immunized C57Bl/6 mice challenged with a near-lethal dose of bvPLA2. Mice were immunized intraperitoneally for 6 consecutive weeks with 50μg bvPLA2 before challenge. (B) Rectal temperatures of naïve or immunized C57Bl/6 and B cell-deficient muMT mice treated as in (A). (C) Rectal temperatures of naïve or immunized C57Bl/6 and Fcer1a−/− mice treated as in (A). *P<0.05; **<0.005; ***P<0.001. All error bars show s.e.m. Data are representative of at least three experiments. See also Figure S4.
While the concentration of IgG in the serum is high in immunized animals, IgE is mostly bound to the Fc ε R on mast cells and basophils and only very low amounts of free IgE are present in the serum. We therefore next considered the possibility that IgE rather than IgG1 might provide protection from envenomation. Notably, mast cells have been shown to directly detoxify venom components and to protect from venom-mediated toxicity and death (Akahoshi et al., 2011; Higginbotham, 1965; Higginbotham and Karnella, 1971; Metz et al., 2006). This raises the possibility that IgE-dependent responses to venoms may also provide protection against venom toxins. We sought to test this by examining whether the IgE response to bvPLA2 was critical for protection against bvPLA2 toxicity. We found that protection from bvPLA2 challenge was partially dependent on the FcεR, as mice deficient for FcεR1α (Dombrowicz et al., 1993) did not acquire full protection against PLA2 toxicity as compared to immunized wild type mice (Figure 5C). Importantly, mast cells from FcεR1α-deficient mice appear to develop and function normally (Dombrowicz et al., 1993) and FcεR1α-deficiency had no effect on the sensitivity of unimmunized mice to PLA2 toxicity (Figure 5C).
Although our earlier studies suggested that the IgE response induced by PLA2 is independent of MyD88 and ST2, we wished to test directly whether deficiency in IL-33 signaling might still affect development of protection against envenomation. However, as expected from our earlier finding that antibody responses to repeated immunization with PLA2 are ST2-independent, MyD88-deficient mice developed protective responses equivalent to those seen in wild type mice (Supplemental Figure 4C).
Finally, as previous studies have suggested that lower doses of antigen can bias antibody production towards IgE in mice (Dudler et al. JI, 1995), we were also interested in determining whether immunizations with lower doses of bvPLA2 would lead to either hypersensitivity or enhanced protection. However, we observed that protection was reduced when using lower doses of bvPLA2 for immunization (Supplemental Figure 4D), and there was no evidence of systemic anaphylaxis in these mice. Therefore, it appears that using a lower antigen dose for immunizations does not lead to hypersensitivity under these conditions.
DISCUSSION
We show here that bvPLA2 is a potent inducer of the type 2 immune response, including Th2 responses, ILC2 responses and antigen-specific IgE and IgG1 production in mice. We confirm that the ability of bvPLA2 to induce the Th2 response is dependent on its enzymatic activity (Dudler et al., 1995), and show that bvPLA2 induces the Th2 response through the cleavage of membrane phospholipids and production of lysophospholipids, such as LPC. These data suggest that the immune system senses envenomation through detection of a molecular activity of a venom component and support the hypothesis that the detection of enzymatic activities of allergens by the innate immune system can lead to the initiation of Th2 and IgE responses (Shakib et al., 2008; Wills-Karp et al., 2010). We find that a PLA2 from snake venom can also induce a Th2 response. Since PLA2 is a common component of venoms from diverse species, sensing of PLA2 activity may allow the immune system to detect venoms from multiple species using a single sensing mechanism. This feature of PLA2 detection is reminiscent of pattern recognition of conserved PAMPs (Janeway, 1989) except that conserved enzymatic activities are detected instead of conserved molecular patterns. Based on these data, we propose that the phospholipase activity of PLA2s represent a Th2-inducing activity that is conserved among a class of allergens, similar to protease activity found in another major class of allergens.
The molecular mechanisms involved in innate sensing of allergens and instruction of Th2 responses remain largely unknown. We examined the role of innate immune receptors and adaptors in the activation of bvPLA2-induced Th2 responses. We found a critical role for the TLR and IL-1R family adaptor protein MyD88 in response to bvPLA2 immunization. Surprisingly, although we previously found that total IgE production in response to papain is MyD88-independent (Sokol et al., 2008), we found that papain-induced antigen-specific Th2 responses are MyD88-dependent.
Recent studies have highlighted the critical role of IL-33/ST2 in type 2 inflammation and allergic disease states. IL-33 plays a significant role in innate type 2 responses by inducing the expansion of type 2 innate lymphoid cells, which produce IL-5 and IL-13, and contribute to respiratory and gastrointestinal inflammation and anti-helminth responses (Oliphant et al., 2011). Furthermore, a role for MyD88 and ST2 in driving the Th2 response to Trichinella spiralis has recently been demonstrated (Scalfone et al., 2013). We find here that ST2 is required for the induction of bvPLA2- and LPC-induced Th2 responses. Furthermore, we show that MyD88 expression in T cells is required for optimal Th2 responses to bvPLA2. This suggests that the Th2-inducing effects of IL-33 in response to bvPLA2 are largely dependent on the effect of IL-33 on T cells themselves.
Our findings suggest a model in which bvPLA2 hydrolyzes membrane phospholipids to produce lysophospholipids (such as LPC), which then induce the release of IL-33 through disruption of cellular membranes and induction of cell death. IL-33 then supports Th2 differentiation by signaling through ST2 on T cells. IL-33 can be released from damaged, mechanically disturbed, and necrotic cells and, thus, can act as an ‘alarmin’ (Zhao and Hu, 2010);(Kakkar et al., 2012). Immunization with bvPLA2 or LPC likely leads to cell death in affected tissues. Our data thus support the hypothesis that bvPLA2-induced cell death may be responsible for its ability to induce Th2 responses. Notably, we found that Phospholipase D, which also cleaves membrane phospholipids, is also a potent inducer of the Th2 response. This raises the possibility that cell death resulting from disruption of cellular membranes maybe a shared feature of some of the Th2 inducing stimuli. Importantly, however, not all triggers of cell death are equally potent inducers of the Th2 response. For example, while melittin is an effective inducer of cell death, we found that it was less efficient than PLA2 at inducing the type 2 immune response. This suggests that only certain forms of cell death result in induction of a Th2 response.
Interestingly, LPC is also found in the saliva of some hematophagous insects, such as the ‘kissing bug’ Rhodnius prolixus and plays an important role in the modulation of the host immune response (Mesquita et al., 2008). It would be interesting to investigate whether LPC present in the saliva of blood-sucking insects contributes to type 2 immune responses elicited by their bites.
While we observed that bvPLA2-induced ILC2 and primary Th2 responses were dependent on ST2, the IgE response appeared to be largely unaffected by ST2-deficiency. A likely explanation for this difference is that ST2 is required for primary but not secondary T cell responses. Because the IgE response is undetectable after primary immunization (data not shown), it was not possible to examine whether it was ST2 dependent. When we examined the secondary Th2 response induced by bvPLA2 we found that it was also largely independent of ST2. This suggests that the Th2 response in ST2-deficient mice may recover to normal levels upon repeated immunization. It is thus possible that the primary IgE response is defective in ST2-deficient mice, but falls below the limit of detection. Alternatively, the IgE response may simply not require ST2.
Because allergens are generally thought of as innocuous, immune responses to allergens (that is, allergic responses) are considered to be accidental and pathological. However, many allergens, including venoms, are clearly noxious. This observation has lead to the hypothesis that one purpose of the type 2 immune response to allergens is to protect the host against toxins and other noxious environmental substances (Palm et al., 2012; Profet, 1991). The type 2 immune response may play beneficial roles in response to envenomation by leading to detoxification of venom components (Higginbotham and Karnella, 1971), enhancing clearance of toxins (Palm et al., 2012), activating tissue repair mechanisms (Allen and Wynn, 2011), and inducing avoidance to prevent future exposure (Profet, 1991). We found that immunization of mice with bvPLA2 leads to protection from subsequent challenge with a near-lethal dose of bvPLA2, and that this protection is partially dependent on FcεR1α expression and B cells. Taken together, our data suggest that the IgE response can provide protection from the noxious effects of envenomation, presumably through bvPLA2-specific IgE-mediated mast cell degranulation.
What determines whether the IgE response leads to protective immunity or pathological anaphylaxis in mice remains to be elucidated. Although PLA2 immunization induces protection in C57Bl/6 background mice, we noted that repeated immunizations with bvPLA2 occasionally resulted in development of hypersensitivity to bvPLA2 in Balb/c mice. Thus, genetic factors may play an important role in determining whether the IgE response to envenomation leads to protective or anaphylactic responses. How our findings translate to the human immune response to venom allergens remains to be determined. For example, it is thought that repeated immunizations with high doses of antigen are effective at preventing anaphylaxis in humans in the setting of venom immunotherapy because they lead to the induction of IgG responses, which compete with IgE for allergen binding (Ozdemir et al., 2011). Furthermore, while we could not find a major role for protective IgG responses in mice, bvPLA2-neutralizing IgG antibodies have been observed in humans (Mohammed and El-Karemi, 1961). Thus, murine and human immune responses to bee venom may differ substantially, perhaps reflecting the distinct role of venomous snakes in the ecology of rodents and primates.
Taken together, our data suggest that the immune system has evolved a mechanism to detect a conserved, noxious component of venoms and that sensing of the noxious effects of venoms leads to the induction of a type 2 immune response that can protect against future venom exposure. IL-33 release after cell death may represent one mechanism by which noxious xenobiotics, such as venoms, are sensed by the immune system and induce a type 2 immune response. Thus, these studies reveal a potential mechanistic link between noxious activities and the induction of a type 2 immune response and support the emerging connection between type 2 immunity and sensing of tissue damage (Allen and Wynn, 2011; Palm et al., 2012).
EXPERIMENTAL PROCEDURES
Mice
Mice were bred at the Yale Animal Resources Center at Yale University in specific pathogen-free conditions and all experiments were done in accordance with approved guidelines, regulations, and protocols as determined by the Institutional Animal Care and Use Committee at Yale. BALB/c and C57BL/6 mice were purchased from NCI or Jackson Laboratories; Fcer1a−/−, Ighm−/− (muMT), Il1r1−/−, and Il18r1−/− mice were from Jackson. Il1rl1−/− mice were kindly provided by Andrew McKenzie (University of Cambridge) via Paul Bryce (Northwestern University); Myd88−/− Balb/c mice were from Mark Shlomchik (Yale University); Ripk3−/− mice were from Vishva Dixit (Genentech); and Gpr132−/− mice were from Janusz Kabarowski (University of Alabama). 4get mice (Mohrs et al., 2001), Myd88−/−;Trif−/−, Myd88−/− C57BL/6, Tlr2−/−;Tlr4−/−, and Tlr9−/− mice were bred in our colony. MyD88fl/fl;Cd4-Cre mice were constructed by D.D.S. (manuscript in preparation).
Reagents and antibodies
Bee venom phospholipase A2 (bvPLA2), bee venom, lysophosphatidylcholine (LPC), melittin, arachidonic acid (AA), Crotalus atrox venom, Agkistrodon contortrix venom, Crotalus adamanteus venom, and o-phenylenediamine dihydrochloride (OPD) were purchased from Sigma. Low endotoxin (endograde) OVA was from Biosource. Papain was purchased from Calbiochem. The following reagents are from the indicated sources: DX5 beads (Miltenyi biotec), Ack lysing buffer (Lonza), FCS (Benchmark), recombinant IL-4, IL-5, IL-13, IL-33 (R&D Systems), recombinant IL-3 (Peprotech), RNA-Bee (Tel-TesT), SMART MMLV RT reagents (Clontech), SYBR Green QPCR mix (Quanta), endograde OVA (Biovendor, LLC), Crotalus PLA2 (Worthington Biochemical), Lipofectamine 2000 and pcDNA3.1/V5-His C (Invitrogen), mIL-33 ELISA antibody pairs (R&D Systems). (DX5-APC, CD4-APC, Streptavidin-HRP, ELISA antibody pairs (mIL-4, mIL-13), biotinylated anti-mouse IL-5, anti-CD16/32 from ebioscience and IgE-bio, Streptavidin-PerCP, purified mouse IgE, purified rat anti-mouse IL-5 from BD Pharmingen. IgG1 and total-IgE ELISA antibody pairs were from Southern Biotech.
T cell assays
For in vitro restimulation of T cells, mice were immunized subcutaneously in both hind footpads with 50ul PBS/site containing 50ug endograde OVA alone or in combination with 50ug bvPLA2 or 500nmol LPC. Inguinal and popliteal lymph nodes were isolated 6 days after immunization. Total lymph node CD4+ T cells were isolated by incubating cells with anti-CD4 magnetic beads followed by positive selection by AutoMACS (Miltenyi biotec). Purified CD4+ T cells (1.5×105) were co-cultured with irradiated (850 rads) splenocytes (3×105) and titrating doses of OVA (Sigma; 900ug/mL-100ug/mL) in round bottom 96 well plates for 5–6 days before analysis of secreted cytokines.
For analysis of GFP+ T cells from 4get mice, 50ug OVA, 50ug bvPLA2, 50ug melittin, 50ug bee venom, 125ug Crotalus PLA2, 500nmol LPC, or 500nmol AA were injected in both hind footpads, while 25ug C. atrox or A. conto venom was injected in only one footpad. LPC and AA were injected in the presence of 50ug OVA. Heat-inactivation of bvPLA2 was achieved by incubating at 100°C for 3 hours. Popliteal lymph nodes were isolated and stained 4–5 days after immunization. Lymph node single cell suspensions were incubated with anti-CD16/32 Fc block before staining with CD4-APC to detect GFP+ T cells.
Antibody production
For analysis of antibody production, mice were immunized subcutaneously as above with 50ug/site of bvPLA2, melittin, bee venom, HSA, or papain on day 0 and day 21. For analysis of OVA-specific antibody production, mice were immunized as above in the presence of 50ug endograde OVA. Sera were analyzed as indicated by ELISA.
Protection assays
For protection assays, mice were immunized intraperitoneally (IP) with 50ug bvPLA2 weekly for 6 weeks and challenged on day 37–40. Mice were challenged IP with bvPLA2 with a dose adjusted to mouse weight (150ug PLA2/250ul PBS/25 gram mouse). Rectal temperatures were followed for 5–6 hours and then daily for 72 hours after immunization. At rectal temperatures below 26.5°C mice were euthanized and considered to have had a lethal response.
IL-33 release assays
For analysis of intraperitoneal IL-33 release, C57Bl/6 mice were immunized with bvPLA2(100ug at 1mg/mL) or LPC (100ug at 1mg/ml) intraperitoneally. At the specified timepoints after immunization, mice were euthanized and peritoneal cytokines were isolated by washing the peritoneum with PBS. Peritoneal fluid IL-33 was analyzed by ELISA.
To analyze IL-33 release in vitro, a stable cell line overexpressing full-length mouse IL-33 (Mus musculus IL33, CCDS ID CCDS29740) was utilized. This cell line was generated by cloning mil-33 into pcDNA3.1/V5-His C with subsequent transfection of the construct into 293 cells (Lipofectamine transfection) and clonal selection with G418. Cells were stimulated with OVA, bvPLA2, or LPC (all at 125ug/ml or 62.5ug/ml) for the specified time points. Supernatants were collected from cell cultures and IL-33 release was analyzed by ELISA.
Dendritic cell activation and basophil migration
For analysis of lymph node DCs and basophils, mice were immunized subcutaneously as above with the addition of papain (100ug/ms) and LPS (20ug/ms). Popliteal lymph node single cell suspensions were stained after 18 hours with CD11c-APC, CD80-PE, and CD86-FITC to detect DCs and after 3 days with DX5-APC and IgE-FITC to detect basophils.
Enzyme-linked immunosorbent assays
Supernatants were collected from T cell co-cultures 4–5 days after plating and were added to Nunc Maxisorp 96-well plates, which had been coated with purified anti-mouse IL-4 antibody (2ug/mL), IL-5 (2ug/mL), or IL-13 (4ug/mL) and subsequently blocked with 1% BSA. Cytokines were detected with biotinylated anti-mouse IL-4 (.5ug/mL), IL-5 (1ug/mL), IL-13 (.5ug/mL). For detection of IL-33, cell supernatants or intraperitoneal fluid samples were isolated at the specified timepoints and plated on Maxisorp plates that had been coated with purified anti-mouse IL-33 (.8ug/mL); cytokine was detected with biotinylated anti-mouse IL-33 (.5ug/mL). Antibody binding was detected with streptavidin-HRP and developed with OPD.
For serum OVA-specific IgG1 antibody detection, plates were coated with OVA, blocked, and eight 3-fold serial dilutions of sera were added (1:50 starting dilution). Detection was achieved with biotinylated anti-IgG1 (Southern Biotech). Antibody binding was detected as above. OVA-specific IgE was detected using a mouse anti-OVA IgE kit (Chondrex) as directed by the manufacturer. Total IgE was detected similarly to above coating with anti-mouse IgE (Southern Biotech) and detecting with biotinylated anti-IgE (Southern Biotech).
Bone marrow-derived basophil stimulations
Mixed mast cell and basophil cultures were derived by incubating total bone marrow cells, that had undergone RBC lysis, in the presence of RPMI supplemented by 30ng/mL mIL-3 and 10% FCS, as described (Sokol et al., 2008). Briefly, cells were plated at 5×106 cells/mL, and subsequently replated on day 3 and day 7 at 1×106 cells/mL. DX5+ basophils were sorted from these cultures on day 10–11 by DX5 positive selection by AutoMACS. DX5+ basophils were resuspended at 2 million cells/mL and stimulated with titrating doses of HSA (250ug/ml), OVA (250ug/ml), total bee venom (12.5ug/ml), melittin (12.5ug/ml), bvPLA2 (250ug/mL), papain (100ug/mL), C. atrox venom (312.5ug/ml), A. conto venom (156.25ug/ml), lysophosphatidylcholine (200uM), and arachidonic acid (200uM). Doses that induced the greatest amount of IL-4 production (as measured by ELISA) are shown in parentheses above and are presented in the figures. IL-4 production was analyzed by ELISA 6 hours after stimulation, as described above, or qPCR analysis of RNA induction at 1 and 3 hours after stimulation. Briefly, total RNA was extracted with RNA-Bee and reverse transcribed with an oligo (dT) primer. cDNA was analyzed by qPCR amplification using SYBR Green QPCR master Mix on an MX300P QPCR System (Stratagene) with analysis by comparative quantification using MXPro software. Unstimulated samples were used as calibrators and samples were normalized to β-actin expression. Primers used for RT-PCR analysis were as follows: il-4, 5′-AGATGGATGTGCCAAACGTCC-3′, 5′-GAAAAGCCCGAAAGAGTCTCTGC-3′; β-actin, 5′-GAAGTCCCTCACCCTCCCAA-3′, 5′-GGCATGGACGCGACCA-3′.
bvPLA2-induced inflammation and activation of ILC2s
Mice were injected i.p. with PBS, 50 ug or 100 ug bvPLA2 for 3 consecutive days. Cells were harvested on day 4 by injecting 2 mL of PBS into the peritoneal cavity. Total and differential cell counts were determined by flow cytometry (BD Accuri C6) and cytospin preparations stained with eosin/haematoxylin (Diff-quick protocol). Peritoneal cells were resuspended in RPMI containing 10% FBS and incubated overnight with 50 ng/mL PMA and 500 ng/mL ionomycin or 10 ng/mL of murine recombinant IL-7 and IL-33 (both cytokines from Biolegend). Single-cell suspensions were stained with fluorochrome-conjugated antibodies against the following surface molecules: lineage markers (CD3e, CD19, CD11b, CD11c, Ly-6G, NK1.1, FceRI), CD90.2 (Thy-1.2), CD45, ICOS and c-kit in staining buffer. Cells were then fixed and permeabilized for intracellular staining of IL-5 and IL-13 according to the manufacturer’s instructions (BD Cytofix/cytoperm protocol). All antibodies used were from eBioscience or BD Biosciences. Cell acquisition was performed on LSRII instruments (BD) and data were analyzed using FlowJo software (TreeStar).
Statistical analysis
Statistical analyses were performed using ANOVA or t-test, as appropriate.
Supplementary Material
Supplemental Figure 1: Bee venom induces total IgE production, dendritic cell and basophil migration, and bone marrow-derived basophil activation.
(A) Total IgE production in BALB/c, C57Bl/6, TLR2−/−;TLR4−/− mice after subcutaneous immunization with HSA, bee venom, bvPLA2, melittin, or papain on both day 0 and 21. (B) Activated CD11c+ DCs (CD86+/CD80+) in the popliteal LNs of BALB/c mice 18 hours after subcutaneous immunization with PBS, OVA, bee venom, papain, or LPS. (C) DX5+ IgE+ basophils in the popliteal LNs of 4get mice 3 days after subcutaneous immunization with PBS, OVA, LPS, bee venom, bvPLA2 or papain. (D) Secreted IL-4 and IL-6 from BM-derived BALB/c basophils treated for 6 hours with HSA, OVA, bee venom, melittin, bvPLA2, or papain. (E) relative quantity of IL-4 induction from BM-derived BALB/c basophils treated with papain or bvPLA2 for 1 or 3 hours. Error bars show s.e.m. Data are representative of at least three experiments. Related to Figure 1.
Supplemental Figure 2: Snake venoms and LPC activate bone marrow-derived basophils, but Th2 responses to bvPLA2 are G2A-independent.
(A and B) Secreted IL-4 from BM-derived BALB/c basophils treated for 6 hours with bvPLA2, LPC, AA, or papain (A), or papain, bee venom, C. atrox venom, or A. contortrix venom (B). (C) Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and Gpr132−/− (G2A) mice 5 days after subcutaneous immunization with OVA and bvPLA2. Error bars show s.e.m. Data are representative of at least three experiments. Related to Figure 2.
Supplemental Figure 3: The bvPLA2-induced Th2 response is independent of the cell death-associated molecule, RIP3.
Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and Ripk3−/− (RIP3) mice 5 days after subcutaneous immunization with OVA in the presence of bvPLA2. Error bars show s.e.m. Data are representative of three experiments. Related to Figure 4.
Supplemental Figure 4: PLA2-induced IgE, secondary Th2 cell responses and immunization-mediated protection are ST2/MyD88-independent.
(A) Anti-OVA IgE production and T cell IL-13 production upon restimulation in wild type and Il1rl1−/− (ST2-deficient) mice on day 28 after immunization with bvPLA2 and OVA on day 0 and day 21. (B) IL-13 production after in vitro re-stimulation of LN CD4+ T cells from C57Bl/6 and MyD88−/− mice 5 days after subcutaneous immunization with OVA in the presence of papain. Error bars show s.e.m. ***P<0.001. Related to Figure 5. (C) Rectal temperature measurements of WT and MyD88−/− mice immunized 6 times with low dose PLA2 (50ug) and then challenged with a near lethal dose of PLA2. (D) Rectal temperature measurements of WT C57Bl/6 mice that had been immunized 6 times with 50μg bvPLA2, 1μg, or 0.1μg of PLA2 and then challenged with a near-lethal dose of PLA2. Error bars show s.e.m. Related to Figure 5.
HIGHLIGHTS.
PLA2 induces IL-33 release, and Th2 and ILC2 activation
PLA2 from both bee and snake venoms induces Th2 cell-type responses
ST2-deficient mice exhibit diminished Th2 cell and ILC2 responses to bvPLA2
FcεR1α contributes to protection from bvPLA2 toxicity
Acknowledgments
We would like to thank members of the Medzhitov lab for helpful discussions, C. Annicelli and S. Cronin for animal care and technical help, O. Colegio for helpful comments on the manuscript, and Andrew McKenzie, Mark Shlomchik, Janusz Kabarowski, and Vishva Dixit for making available the St2−/−, MyD88−/−;BALB/c, Gpr132−/−, Ripk3−/− mice, respectively. This study was supported by grants from the National Institutes of Health (RO1 AI08977, 5RO1 AI055502, and R37 AI046688) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, Schlenner SM, Feyerabend TB, Rodewald HR, Pejler G, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- Bircher AJ. Systemic immediate allergic reactions to arthropod stings and bites. Dermatology. 2005;210:119–127. doi: 10.1159/000082567. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP, Goyffon M. Venoms, antivenoms and immunotherapy. Toxicon. 1998;36:823–846. doi: 10.1016/s0041-0101(97)00160-8. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen- induced inflammatory responses. Chem Immunol Allergy. 2006;90:45–64. doi: 10.1159/000088880. [DOI] [PubMed] [Google Scholar]
- Dudler T, Machado DC, Kolbe L, Annand RR, Rhodes N, Gelb MH, Koelsch K, Suter M, Helm BA. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J Immunol. 1995;155:2605–2613. [PubMed] [Google Scholar]
- Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, Nevalainen TJ, Norman JA, Lewis RJ, Norton RS, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Higginbotham RD. Mast cells and local resistance to Russell’s viper venom. J Immunol. 1965;95:867–875. [PubMed] [Google Scholar]
- Higginbotham RD, Karnella S. The significance of the mast cell response to bee venom. J Immunol. 1971;106:233–240. [PubMed] [Google Scholar]
- Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012 doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY. IL-33: a Janus cytokine. Ann Rheum Dis. 2012;71(Suppl 2):i101–104. doi: 10.1136/annrheumdis-2011-200589. [DOI] [PubMed] [Google Scholar]
- Madero MF, Gamez C, Madero MA, Fernandez-Nieto M, Sastre J, del Pozo V. Characterization of allergens in four South American snake species. Int Arch Allergy Immunol. 2009;150:307–310. doi: 10.1159/000222684. [DOI] [PubMed] [Google Scholar]
- Mesquita RD, Carneiro AB, Bafica A, Gazos-Lopes F, Takiya CM, Souto-Padron T, Vieira DP, Ferreira-Pereira A, Almeida IC, Figueiredo RT, et al. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect Immun. 2008;76:5543–5552. doi: 10.1128/IAI.00683-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- Mohammed AH, El-Karemi MM. Immunity of bee keepers to some constituents of bee venom: phospholipase-A antibodies. Nature. 1961;189:837–838. doi: 10.1038/189837b0. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Muller UR. Insect venoms. Chem Immunol Allergy. 2010;95:141–156. doi: 10.1159/000315948. [DOI] [PubMed] [Google Scholar]
- Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby CL, Powell JR, Jiang MS, Fletcher JE. Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon. 1997;35:67–80. doi: 10.1016/s0041-0101(96)00078-5. [DOI] [PubMed] [Google Scholar]
- Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2011;41:1226–1234. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–171. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalfone LK, Nel HJ, Gagliardo LF, Cameron JL, Al-Shokri S, Leifer CA, Fallon PG, Appleton JA. Participation of MyD88 and IL-33 as innate drivers of Th2 immunity to Trichinella spiralis. Infect Immun. 2013 doi: 10.1128/IAI.01307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shakib F, Ghaemmaghami AM, Sewell HF. The molecular basis of allergenicity. Trends Immunol. 2008;29:633–642. doi: 10.1016/j.it.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Sobotka AK, Franklin RM, Adkinson NF, Jr, Valentine M, Baer H, Lichtenstein LM. Allergy to insect stings. II. Phospholipase A: the major allergen in honeybee venom. J Allergy Clin Immunol. 1976;57:29–40. doi: 10.1016/0091-6749(76)90076-2. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. The Journal of experimental medicine. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Current opinion in immunology. 2013;25:148–155. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–110. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010;7:260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Bee venom induces total IgE production, dendritic cell and basophil migration, and bone marrow-derived basophil activation.
(A) Total IgE production in BALB/c, C57Bl/6, TLR2−/−;TLR4−/− mice after subcutaneous immunization with HSA, bee venom, bvPLA2, melittin, or papain on both day 0 and 21. (B) Activated CD11c+ DCs (CD86+/CD80+) in the popliteal LNs of BALB/c mice 18 hours after subcutaneous immunization with PBS, OVA, bee venom, papain, or LPS. (C) DX5+ IgE+ basophils in the popliteal LNs of 4get mice 3 days after subcutaneous immunization with PBS, OVA, LPS, bee venom, bvPLA2 or papain. (D) Secreted IL-4 and IL-6 from BM-derived BALB/c basophils treated for 6 hours with HSA, OVA, bee venom, melittin, bvPLA2, or papain. (E) relative quantity of IL-4 induction from BM-derived BALB/c basophils treated with papain or bvPLA2 for 1 or 3 hours. Error bars show s.e.m. Data are representative of at least three experiments. Related to Figure 1.
Supplemental Figure 2: Snake venoms and LPC activate bone marrow-derived basophils, but Th2 responses to bvPLA2 are G2A-independent.
(A and B) Secreted IL-4 from BM-derived BALB/c basophils treated for 6 hours with bvPLA2, LPC, AA, or papain (A), or papain, bee venom, C. atrox venom, or A. contortrix venom (B). (C) Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and Gpr132−/− (G2A) mice 5 days after subcutaneous immunization with OVA and bvPLA2. Error bars show s.e.m. Data are representative of at least three experiments. Related to Figure 2.
Supplemental Figure 3: The bvPLA2-induced Th2 response is independent of the cell death-associated molecule, RIP3.
Cytokine production after in vitro restimulation of LN CD4+ T cells from C57Bl/6 and Ripk3−/− (RIP3) mice 5 days after subcutaneous immunization with OVA in the presence of bvPLA2. Error bars show s.e.m. Data are representative of three experiments. Related to Figure 4.
Supplemental Figure 4: PLA2-induced IgE, secondary Th2 cell responses and immunization-mediated protection are ST2/MyD88-independent.
(A) Anti-OVA IgE production and T cell IL-13 production upon restimulation in wild type and Il1rl1−/− (ST2-deficient) mice on day 28 after immunization with bvPLA2 and OVA on day 0 and day 21. (B) IL-13 production after in vitro re-stimulation of LN CD4+ T cells from C57Bl/6 and MyD88−/− mice 5 days after subcutaneous immunization with OVA in the presence of papain. Error bars show s.e.m. ***P<0.001. Related to Figure 5. (C) Rectal temperature measurements of WT and MyD88−/− mice immunized 6 times with low dose PLA2 (50ug) and then challenged with a near lethal dose of PLA2. (D) Rectal temperature measurements of WT C57Bl/6 mice that had been immunized 6 times with 50μg bvPLA2, 1μg, or 0.1μg of PLA2 and then challenged with a near-lethal dose of PLA2. Error bars show s.e.m. Related to Figure 5.