Abstract
Objective
Vascular remodeling due to smooth muscle cell (SMC) proliferation and neointima formation is a major medical challenge in cardiovascular intervention. However, anti-neointima drugs often indistinguishably block re-endothelialization, an essential step toward successful vascular repair, due to their non-specific effect on endothelial cells (EC). The objective of this study was to identify a therapeutic target that differentially regulates SMC and EC proliferation.
Approach and Results
By using both rat balloon-injury and mouse wire-injury models, we identified CTP synthase (CTPS) as one of the potential targets that may be used for developing therapeutics for treating neointima-related disorders. CTPS1 was induced in proliferative SMCs in vitro and neointima SMCs in vivo. Blockade of CTPS1 expression by small hairpin RNA or activity by cyclopentenyl cytosine suppressed SMC proliferation and neointima formation. Surprisingly, cyclopentenyl cytosine had much less effect on EC proliferation. Of importance, blockade of CTPS1 in vivo sustained the re-endothelialization due to induction of CTP synthesis salvage pathway enzymes nucleoside diphosphate kinase A and B in ECs. Diphosphate kinase B appeared to preserve EC proliferation via utilization of extracellular cytidine to synthesize CTP. Indeed, blockade of both CTPS1 and diphosphate kinase B suppressed EC proliferation in vitro and the re-endothelization in vivo.
Conclusions
Our study uncovered a fundamental difference in CTP biosynthesis between SMCs and ECs during vascular remodeling, which provided a novel strategy by using cyclopentenyl cytosine or other CTPS1 inhibitors to selectively block SMC proliferation without disturbing or even promoting re-endothelialization for effective vascular repair following injury.
Keywords: CTP synthase, vascular remodeling, smooth muscle proliferation, re-endothelialization, nucleoside diphosphate kinase
Introduction
Neointimal hyperplasia is one of the major obstacles limiting the long-term clinical efficiency of cardiovascular intervention including angioplasty, bypass, and transplantation arteriopathy, etc.1 Neointima formation also contributes to the development and progression of several proliferative cardiovascular diseases such as hypertension and diabetic vascular complications.2 Under pathological conditions, vascular injury causes denudation of endothelial layer, which triggers a series of acute and chronic inflammatory responses characterized by the production of various different growth factors or inflammatory cytokines.3, 4 Media layer smooth muscle cell (SMC) proliferation and migration in response to the injury-induced factors such as platelet-derived growth factor-BB (PDGF-BB) are essential events contributing to subsequent neointimal thickening,5, 6 which eventually leads to vessel narrowing. Re-endothelialization halts neointima formation and initiates the successful vascular repair.7, 8 However, currently available anti-neointimal drugs indiscriminately block the proliferation of both SMCs and ECs, leading to impaired re-endothelialzation and prolonged wound healing process. Therefore, it is critical to develop a novel anti-proliferation strategy that is SMC-sensitive.
CTP synthase (CTPS) is a metabolic enzyme that catalyzes CTP biosynthesis from UTP, ATP and glutamine, an essential step for DNA and RNA synthesis during cell proliferation.9,10 Interestingly, in our study of SMC differentiation from mesenchymal progenitor cells, CTPS1 was upregulated during the differentiation process (data not shown), which led us to hypothesize that CTPS1 may modulate SMC phenotype. In the present study, we found that CTPS1 was induced by PDGF-BB in primary cultured proliferating SMCs in vitro and neointimal SMCs following vascular injury in vivo. Blocking CTPS function suppressed SMC proliferation and neointima formation with sustained re-endothelialization in injured vessels. Our study identified CTPS1 as a novel target for developing anti-neointima drugs that are SMC-sensitive but have little side-effect on re-endothelialization during vascular repair.
Results
CTPS1 was up-regulated in cultured SMCs in vitro and neointimal SMCs in vivo
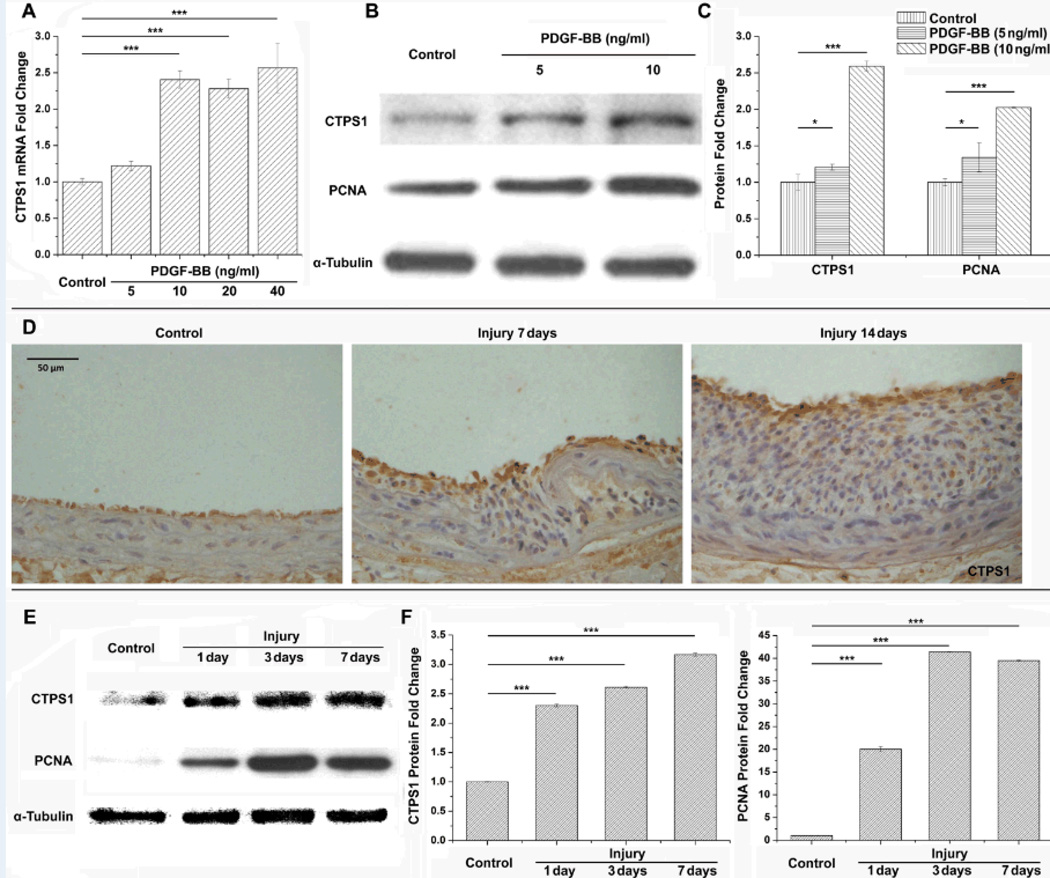
It is well established that CTPS is involved in the proliferation of various different cells.11–13 Although two different CTPS isotypes (CTPS1 and CTPS2) are identified in mammals, we found that CTPS1, but not CTPS2, was markedly up-regulated in SMCs by PDGF-BB (Figure 1A–1C, and Supplemental Figure I). PDGF-BB is a well-known SMC mitogen that induces SMC proliferation and neointima formation following vascular injury.14 PDGF-BB-mediated cell proliferation was confirmed by the up-regulation of proliferating cell nuclear antigen (PCNA) (Figure 1B–1C). Since SMC proliferation is one of the major events in neointima formation under pathological conditions,15 we examined CTPS1 expression in rat carotid arteries undergoing vascular remodeling after balloon-injury. As shown in Figure 1D, CTPS1 was expressed at a low level in the media SMCs, but was highly induced in neointimal SMCs. A low level of CTPS1 expression was also observed in ECs. Quantitative analysis showed that CTPS1 was significantly up-regulated as early as 1 day after vascular injury. The expression was gradually increased during neointimal formation (Figure 1E–1F). PCNA expression correlated with the CTPS1 expression (Figure 1F).
Figure 1. CTPS1 was up-regulated in proliferative SMCs both in vitro and in vivo.
(A) CTPS1 mRNA expression was up-regulated by PDGF-BB in proliferating SMCs. (B) CTPS1 and PCNA protein expression was induced by PDGF-BB in SMCs. (C) Quantification of CTPS1 and PCNA protein expression shown in B by normalizing to α-tubulin. (D) CTPS1 was expressed in neointima SMCs following vascular injury as shown by immunohistochemistry staining. (E) Time dependent protein expression of CTPS1 and PCNA in rat carotid arteries following injury. (F) Quantification of CTPS1 and PCNA protein expression shown in E by normalizing to α-tubulin. *P<0.05, **P<0.01, ***P<0.001 (n=3).
Blocking CTPS activity inhibited SMC proliferation
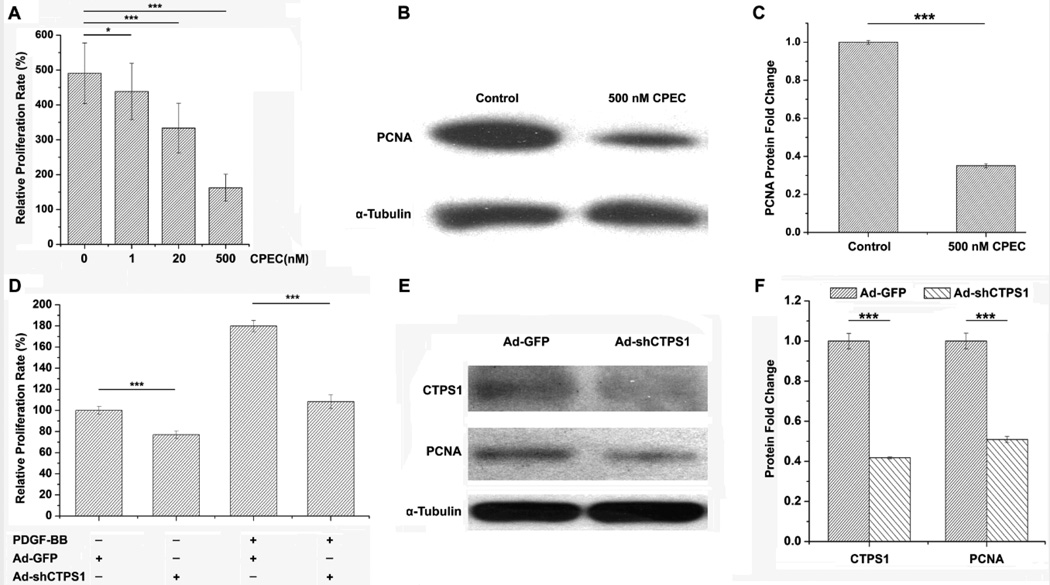
To determine if CTPS1 plays a role in SMC proliferation, we blocked CTPS activity using cyclopentenyl cytosine (CPEC), an effective and specific inhibitor for CTPS.16, 17 As shown in Figure 2A, blockade of CTPS activity significantly suppressed SMC proliferation in a dose dependent manner. CPEC treatment also dramatically suppressed PCNA expression (Figure 2B–2C). To confirm the specificity of CTPS1 function, we blocked CTPS1 expression by small hairpin RNA (shRNA) and found that knockdown of CTPS1 (Figure 2E–2F) effectively suppressed PDGF-BB-induced SMC proliferation (Figure 2D). CTPS1 shRNA appeared to inhibit vehicle-treated SMC growth as well (Figure 2D). Consistently, CTPS1 knockdown also inhibited PCNA expression (Figure 2E–2F), further demonstrating the essential role of CTPS1 in SMC proliferation.
Figure 2. Blockade of CTPS1 activity or expression impaired SMC proliferation.
(A) CTPS inhibitor CPEC blocked SMC proliferation in a dose-dependent manner. (B) 500 nM CPEC treatment for 24 h decreased PCNA protein expression in SMC. (C) Quantification of PCNA protein expression shown in B by normalizing to α-tubulin. (D) Knockdown of CTPS1 blocked PDGF-BB-induced SMC proliferation. (E) Knockdown of CTPS1 by adenovirus-expressed shRNA (Ad-shCTPS1) attenuated PCNA expression. (F) Quantification of CTPS1 and PCNA expression shown in E by normalizing to α-tubulin. *P<0.05, **P<0.01, ***P<0.001 (n=4).
Blockade of CTPS1 activity or expression did not induce SMC apoptosis but impaired cell cycle progression
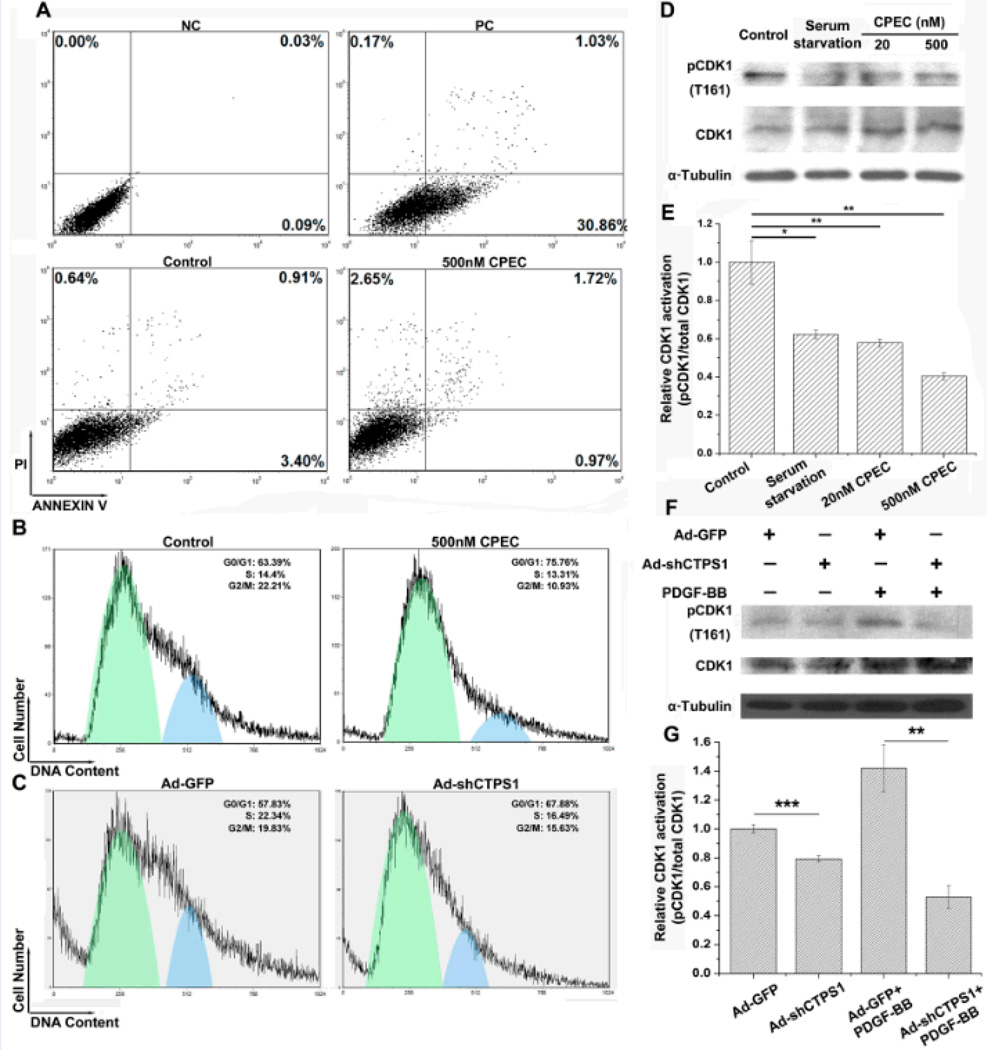
Since CPEC is a chemical with potential cytotoxicity, we sought to determine if CPEC inhibited SMC proliferation through a non-specific toxic effect. Apoptosis analysis using propidium iodide/Annexin V double staining and flow cytometry indicated that 24 h treatment with 500 nM CPEC (the highest dosage used in this study) did not induce SMC apoptosis, comparable with the treatment with vehicle (Figure 3A). The serum-starved cells were used as a positive control, which caused a significant cell apoptosis (30.86%) (Figure 3A). However, CPEC treatment or CTPS1 knockdown significantly blocked cell cycle progression, as indicated by the accumulation of cells in G0/G1 phase (Figure 3B–3C). It appeared that CPEC treatment (Figure 3D–3E) or CTPS1 knockdown by shRNA (Figure 3F–3G) dramatically inhibited the activation of cell cycle regulator cyclin-dependent kinase 1 as evidenced by suppression of its phosphorylation at T161.
Figure 3. Blocking CTPS1 activity or expression impaired cell cycle progression without inducing apoptosis in SMCs.
(A) Flow Cytometry analysis indicated that SMC apoptosis was not evident when treated with 500 nM CPEC, comparable to vehicle-treated cells (Control). NC: negative control; PC: positive control. (B) 500 nM CPEC treatment induced SMC cell cycle arrest at G0/G1 stage. (C) Knockdown of CTPS1 by shRNA caused SMC cell cycle arrest at G0/G1 stage. (D) Both the low or high dosage of CPEC blocked cyclin-dependent kinase 1 (CDK1) (T161) phosphorylation in SMCs. (E) CDK1 activation (phosphorylation) relative to the total CDK1 expression showed in D. (F) Knockdown of CTPS1 by shRNA blocked CDK1 (T161) phosphorylation in SMCs. (G) CDK1 activation (phosphorylation) relative to the total CDK1 expression showed in D. *P<0.05, **P<0.01, ***P<0.001 (n=4).
CTPS1 played an essential role in injury-induced vascular remodeling
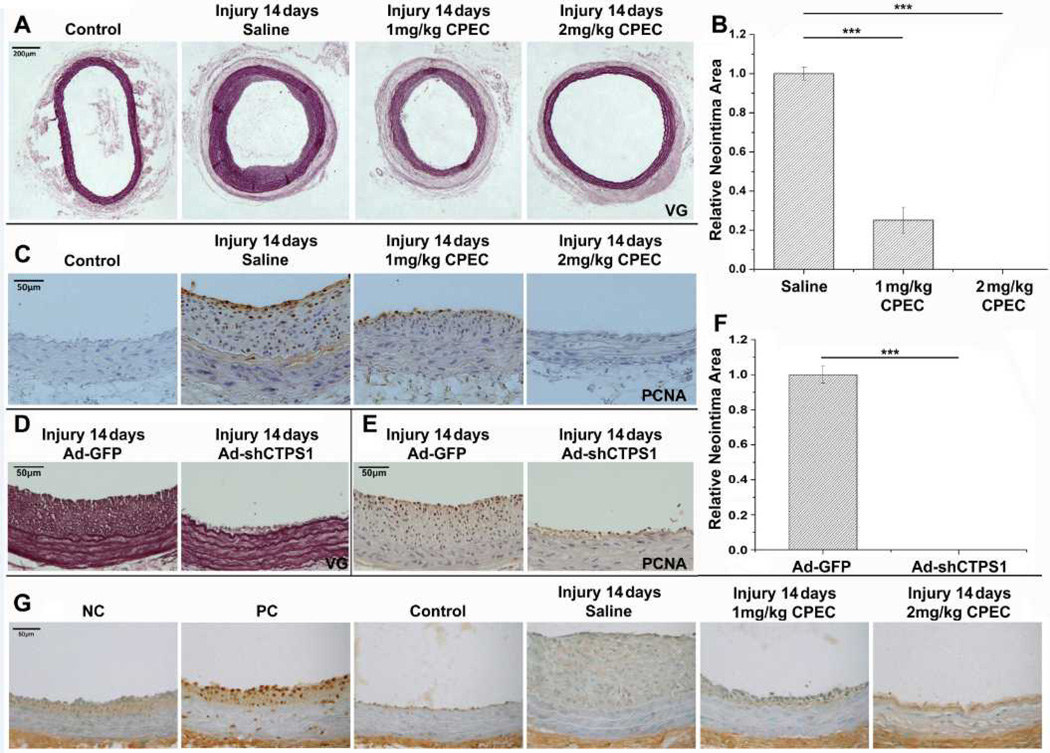
Since CTPS1 played an essential role in SMC proliferation in vitro and was induced in neointima SMCs following balloon injury, we hypothesized that CTPS1 plays an important role in injury-induced neointima formation in vivo. To test this hypothesis, we injured rat carotid arteries with balloon catheter to induce neointima formation and administered CPEC via mini-osmotic pumps to block CTPS1 activity. As expected, a thick layer of neointima was formed 14 days after injury. However, the neointima was dramatically blocked by a low dosage (1 mg/kg body weight/day), and completely blocked by a higher dosage of CPEC (2 mg/kg b.w./day) (Figure 4A–4B). The PCNA expression was also dramatically blocked by the low dosage of CPEC and completely blocked by the higher dosage of CPEC (Figure 4C). To confirm the specificity of CTPS1 function, we knocked down CTPS1 using adenoviral-mediated shRNA delivery in injured arteries. As shown in Figure 4D–4F, CTPS1 shRNA dramatically blocked neointima formation (Figure 4D & 4F) as well as PCNA expression in neointima SMCs (Figure 4E). To determine whether or not CPEC had a toxic effect on neointima SMCs, the in vivo cell apoptosis was detected by TUNEL assay. Consistent with the in vitro results (Figure 3A), no apoptotic cells were observed in the vessel sections from CPEC-treated arteries even with the higher dosage of CPEC (2 mg/kg b.w./day) (Figure 4G). These results demonstrate that CTPS1 is a novel drug target for blocking injury-induced neointima formation/vascular remodeling.
Figure 4. Blockade of CTPS1 activity or expression suppressed injury-induced neointima formation without induction of cell apoptosis.
(A) CPEC blocked balloon injury-induced neointima formation in a dose-dependent manner, as shown by elastin (VG) staining. (B) Quantification of CPEC effects on neointima formation. Neointima areas for saline or CPEC-treated arteries in A were measured. (C) CPEC inhibited PCNA expression in a dose-dependent manner, as shown by immunohistochemistry staining. (D) Knockdown of CTPS1 by shRNA (Ad-shCTPS1) blocked neointima formation following injury, as shown by elastin staining. (E) Knockdown of CTPS1 by Ad-shCTPS1 blocked injury-induced PCNA expression. (F) Quantification of the neointima areas observed in D. (G) In vivo apoptosis analysis via TUNEL assay indicated that both the low or high dosage of CPEC did not induce cell apoptosis in neointima cells. NC: negative control; PC: positive control. Both PC and NC used artery sections treated with 1 mg/kg of CPEC, while PC was prepared by additional treatment with DNase I. *P<0.05, **P<0.01, ***P<0.001 (n=5).
Blockade of CTPS1 activity or expression impacted ECs differently from SMCs in vitro and sustained re-endothelialization in vivo
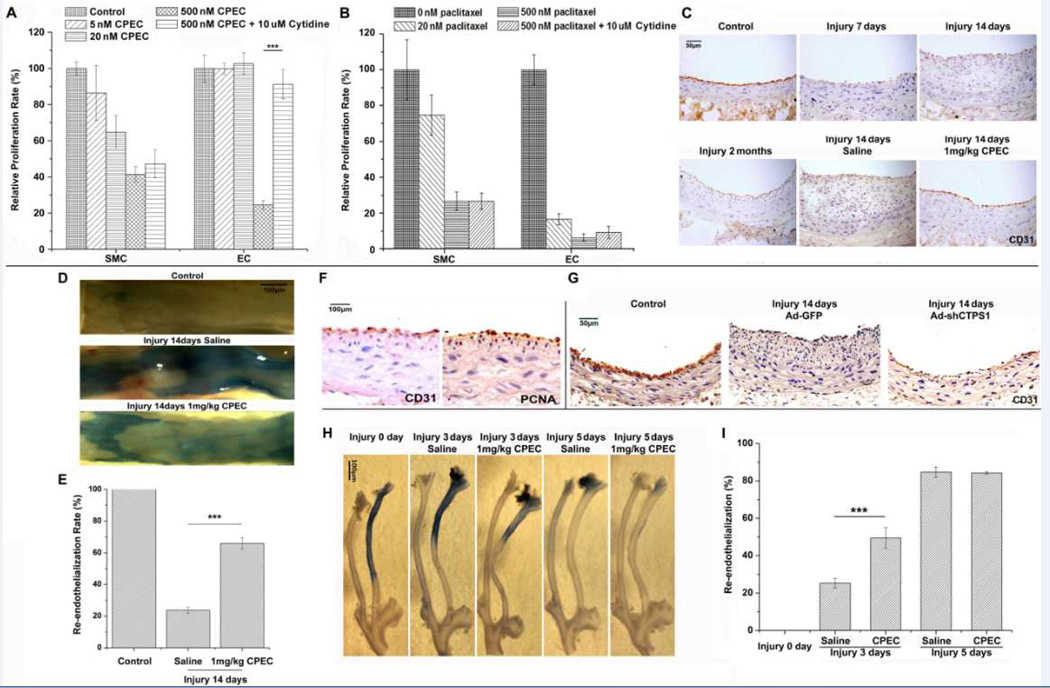
Re-endothelialization is an essential step toward successful vascular repair in injury-induced vessel wall remodeling. Current anti-neointima strategies targeting genes or signaling pathways for SMC proliferation also block re-endothelialization and cause thrombosis as a side effect.18, 19 It is important, therefore, to identify drug targets that do not block re-endothelialization. Since CTPS1 played a very important role in neointima formation, we sought to determine if blocking CTPS1 activity or expression has any effect on ECs. We first tested if CPEC has any effect on EC proliferation in vitro. As shown in Figure 5A, EC proliferation was not affected by low dosages of CPEC treatments (≤20nM), in which SMC proliferation was attenuated, indicating that SMCs are more sensitive than ECs to CPEC challenge. A higher dosage of CPEC (500 nM) significantly blocked the proliferation of both SMCs and ECs, indicating that a certain level of CTPS activity is required for EC proliferation. Indeed, CTPS1 was induced in ECs by different growth stimuli (Supplemental Figure II). Interestingly, addition of extracellular cytidine restored EC, but not SMC, proliferation blocked by the high dosage of CPEC (Figure 5A), suggesting that SMCs and ECs may use different pathways to regulate their CTP synthesis, and consequently their proliferation. Although cytidine is important for EC proliferation, abundant CTP alone appeared not to be able to stimulate either EC or SMC proliferation (Supplemental Figure III) probably because the proliferation also requires many other factors including CDK activity.
Figure 5. The effect of blocking CTPS1 on EC proliferation in vitro and re-endothelialization during vascular repair in vivo.
(A) CPEC had less effect on the proliferation of ECs as compared to SMCs. C166 (EC), but not SMC proliferation was sustained in a low dose of CPEC (5–20 nM) treatment although high dose of CPEC (500 nM) blocked the proliferation of both cells. ECs, but not SMCs, were able to utilize extracellular cytidine (10 µM) to restore their proliferation blocked by 500 nM CPEC. (B) Paclitaxel suppressed both EC and SMC proliferation. Cytine did not restore SMC or EC proliferation blocked by paclitaxel. (C) Blockade of CTPS activity by CPEC accelerated re-endothelialization during vascular repair. ECs were indicated by CD31 staining. CD31-positive cells appeared in 1 mg/kg CPEC, but not saline-treated arteries 14 days after the injury. Control is the CD31 staining of the uninjured artery. CD31-positive cells were also observed in arteries 2 months after injury. (D) Re-endothelialization in injured rat artery was accelerated by CPEC treatment. The area without re-endothelialization was stained by Evans Blue. (E) The extent of re-endothelialization in control or injured rat arteries was quantified by measuring the Evans Blue-unstained area as shown in D. CPEC treatment promoted re-endothelialization. (F) PCNA expressed in CD31-positive cells in 1 mg/kg CPEC-treated arteries with 14 days of injury, indicative of EC proliferation. (G) Knockdown of CTPS1 by shRNA (Ad-shCTPS1) promoted re-endothelization in arteries with 14 days of balloon injury. ECs were stained with CD31. (H) Blockade of CTPS activity promoted re-endothelialization in wire-injured mouse carotid arteries as shown by Even Blue staining of the whole arteries. 3 days following injury, CPEC markedly accelerated the re-endothelialization as compared to the saline-treated group. (I) Quantification of re-endothelialization in wire-injured mouse arteries observed in H. Evans Blue-unstained areas were measured. *P<0.05, **P<0.01, ***P<0.001 (n=5).
Paclitaxel-(Taxus), one of drugs currently used for coating stents and treating coronary artery diseases, effectively blocks SMC proliferation and restenosis following angioplasty. However, paclitaxel suppressed both EC and SMC proliferation at a lower (20 nM) or higher dosage (500 nM) (Figure 5B), consistent with previous reports. Unlike blockade of CTPS1, paclitaxel-mediated inhibitory effect could not be restored by cytidine (Figure 5B). In addition, ECs appeared to be more sensitive to paclitaxel than SMCs. These results suggest that blocking CTPS1 may be a better strategy to block restenosis during cardiovascular intervention.
To determine if CTPS1 plays a role in re-endothelialization during vascular remodeling, we systematically administered CPEC via osmotic pump into rats with balloon-injury. As shown in Figure 5C, a weak re-endothelialization was found in the carotid arteries after 2 months of vascular injury as indicated by the expression of EC marker CD31, suggesting a slow and weak proliferation of ECs during the natural vascular repair process. However, CPEC administration (1 mg/kg/day) induced a strong re-endothelialization 14 days after the injury while significantly inhibiting the neointima formation as compared to saline-treated arteries (Figure 5C, the last two images). Quantification of the re-endothelialization areas in Evans Blue stained vessel (Figure 5D) showed that CPEC treatment increased the re-endothelialization area to 66% of the injured vessels as compared to 24% in saline-treated vessels (Figure 5E). Consistently, PCNA was strongly expressed in CD31-positive cells (Figure 5F), suggesting that CPEC treatment preserved or promoted EC proliferation in injured vessel. The specificity of CPEC effect on CTPS1 activity was confirmed by adenovirus-mediated CTPS1 shRNA. As shown in Figure 5G, knockdown of CTPS1 induced re-endothelialization while blocking neointima formation, similar to the effect observed in CPEC treatment (Figure 5C). To further establish CTPS function in re-endothelialization after vascular injury, we observed the endothelial denudation and re-endothelialization in a different model, i.e., mouse wire-injury model. The re-endothelialization was observed in intact carotid arteries using Evans Blue staining of the injured areas. Systematic administration of CPEC was achieved via osmotic pump (1 mg/kg/day). As shown in Figure 5H–5I, re-endothelialization in mouse arteries occurred rapidly following injury. 5 days after injury, re-endothelialization was completed, consistent with the previous reports.20 CEPC treatment did not delay the rapid re-endothelialization. Importantly, at 3 days after the injury, CPEC-treated arteries appeared to have a larger area that was re-endothelialized compared to the saline-treated arteries (Figure 5H–5I), indicating that CPEC accelerated the re-endothelialization process. Taken together, these data indicate that blockade of CTPS activity or expression sustains EC proliferation, which may improve the re-endothelialization during vascular repair following injury.
Induction of CTP synthesis salvage pathway sustained the proliferation of CPEC-treated ECs in vitro and re-endothelialization of the injured vessel in vivo
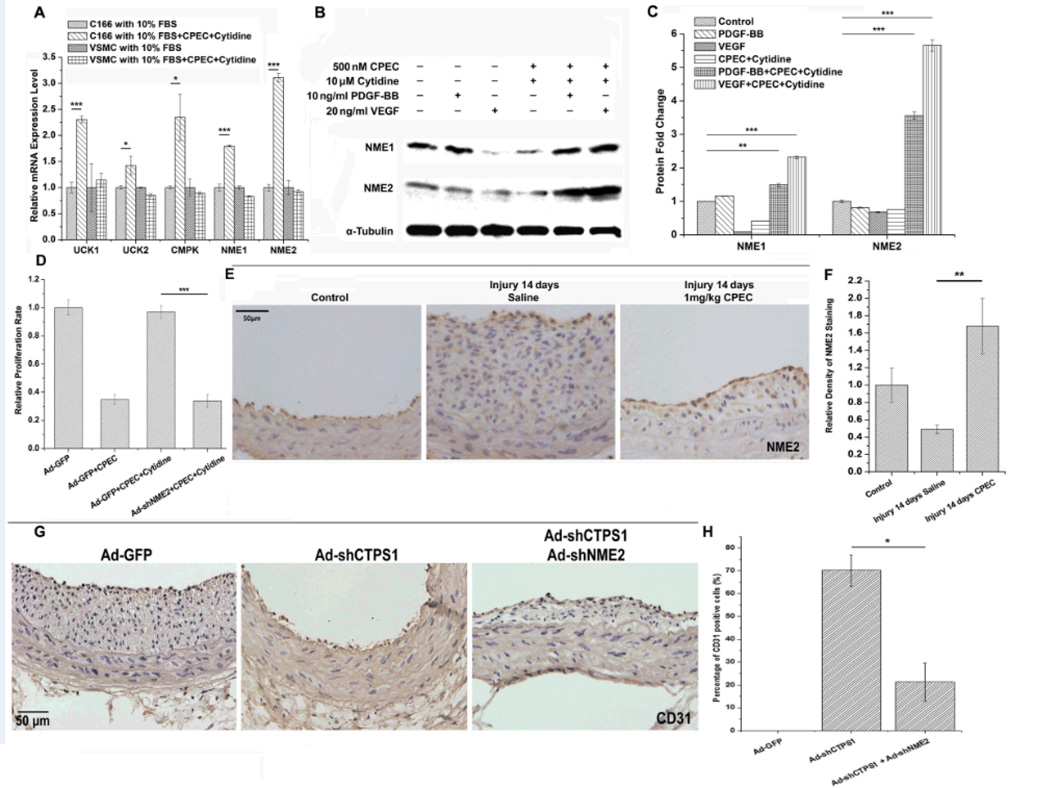
Since ECs, but not SMCs, used extracellular cytidine to restore cell proliferation in the high-dosage of CPEC treatment (Figure 5A), we sought to determine the alternative pathway that preserved the EC proliferation. CTPS synthesizes CTP via a so-called “neo-synthesis pathway” using UTP, ATP and glutamine as substrates.21 However, there is a "salvage pathway" that utilizing cytidine as substrate when the neo-synthesis pathway is deficient.22, 23 Cytidine is utilized by several salvage pathway enzymes, including UdK1/2, CMPK, and nucleoside diphosphate kinase A and B (NME1 and NME2).24, 25 As shown in Figure 6A, CPEC significantly induced mRNA expression of all the salvage pathway enzymes in the presence of cytidine in ECs, but not SMCs, suggesting that ECs, but not SMCs, are able to use the salvage pathway when CTPS pathway is blocked. Since NME1/2 play similar roles in the salvage pathway as CTPS in the neo-synthesis pathway, and the end product of both NMEs and CTPS is CTP, NME1 or NME2 are most likely to be responsible for preserving the EC proliferation when CTPS was blocked. CPEC appeared to induce NEM2 expression more than NME1 in proliferative ECs (Figure 6A). Interestingly, PDGF-BB, VEGF, or CPEC treatment alone did not upregulate the expression of NME1 or NME2 in ECs. However, combination of CPEC with either one of the growth factors (VEGF or PDGF) in the presence of cytidine dramatically up-regulated the NME especially the NME2 expression (Figure 6B–6C). These data suggest that an elevated level of the salvage pathway activities may be required for EC proliferation when the neo-synthesis pathway is blocked.
Figure 6. Blockade of CTPS1 activity or expression triggered the induction of enzymes for CTPS synthesis salvage pathway in ECs, but not SMCs, which sustained EC proliferation under CPEC treatment.
(A) mRNA expression of salvage pathway-related genes in ECs (C166) and SMCs with or without the addition of CPEC and cytidine. CPEC induced the expression of salvage pathway-related genes only in ECs, but not SMCs. (B) NME1 and NME2 protein expression was induced in ECs by CPEC in the presence of both cytidine and growth factors (PDGF-BB or VEGF). (C) Quantification of NME1 and NME2 expression observed in B by normalizing to α-Tubulin. (D) Knockdown of NME2 by shRNA (Ad-shNME2) blocked cytidine function in restoring CPEC-blocked EC proliferation. NME2 knockdown disabled ECs in utilizing cytidine to promote EC proliferation. (E) CPEC induced a strong expression of NME2 in ECs, but not SMCs, of arteries undergoing re-endothelialization. (F) Quantification of relative NME2 expression shown in E by normalizing the NME2 staining density to the control (set as 1). (G) Knockdown of both CTPS1 and NME2 suppressed re-endothelialization in arteries with 14 days of balloon injury. ECs were stained with CD31. (H) Relative percentage of CD31 positive cells in injured arteries from G. *P<0.05, **P<0.01, ***P<0.001 (n=5).
Although both NME1 and NME2 are important for the nucleoside diphosphate kinase activity in the salvage CTP synthesis pathway,26 NME2 overexpression in SMCs (Supplemental Figure IV) displayed a stronger activity than NME1 in utilizing cytidine to restore CPEC-attenuated proliferation (Supplemental Figure V). Consistently, knockdown of NME2 by adenovirus-expressed shRNA dramatically decreased cytidine-mediated restoration of EC proliferation that was blocked by a high dosage of CPEC (Figure 6D), demonstrating an essential role of NME2 in utilizing cytidine to mediate EC proliferation. These results indicate that NME2 protects ECs from CPEC challenge through utilizing cytidine as the substrate for CTP salvage synthesis pathway.
To test if NME2 is involved in the re-endothelialization in vivo, we examined if NME2 is expressed in arteries undergoing vascular repair following injury-induced vascular remodeling. As shown in Figure 6E–6F, NME2 was expressed mainly in ECs but not SMCs in arteries. CPEC treatment induced a strong NME2 expression in ECs of the arteries with enhanced re-endothelialization (Figure 6E vs. 5C-5D), suggesting that NME2 may be critical for the re-endothelialization. To test this, we knocked down both CTPS1 and NME2 using shRNAs in injured rat carotid arteries. As shown in Figure 6G–H, knockdown of CTPS1 and NME2 simultaneously suppressed re-endothelialization as indicated by the loss of CD31 positive cells as compared to CTPS1 knockdown alone. These data demonstrate that NME2 is essential for the proliferation of ECs when the cells are treated with CPEC, which facilitates the re-endothelialization in vivo.
Discussion
Using both pharmacological and loss of function approaches, we demonstrate that targeting CTPS1 can effectively suppress neointima formation following vascular injury. Importantly, low dosages of CPEC inhibit SMC, but not EC growth, suggesting that ECs are less sensitive to CPEC treatment. High dosage of CPEC suppresses both SMC and EC proliferation, indicating that a relatively low level of CTPS activity is required for EC proliferation. Interestingly, the inhibitory effect of high dosage of CPEC can be reversed by addition of cytidine, a substrate for CTP synthesis salvage pathway, indicating that salvage pathway is very important for EC proliferation. Thus, our study demonstrates for the first time that SMCs and ECs have a distinct preference in utilizing CTP synthesis pathways for their proliferation. SMCs appear to mainly utilize CTPS-mediated de novo synthesis pathway. Although CTPS-mediated pathway is much less essential for ECs, ECs appear to use both the de novo and the salvage pathways to synthesize CTP.
We have identified a novel strategy to potentially overcome a long-standing medical challenge, i.e., the impaired re-endothelialization in anti-neointima therapy following cardiovascular interventions. Drug-eluting stents are a common treatment for coronary artery diseases. However, drugs currently used in clinic such as sirolimus and paclitaxel have side effects causing defective re-endothelialization and increasing risk of late thrombosis.27 Our exciting finding is that blockade of CTPS1 function accelerates re-endothelialization in two injury models, which is likely due to the reduction of proliferating SMCs as well as the induction of NME-mediated salvage pathways. Proliferative SMCs are known to produce various paracrine factors to inhibit EC proliferation.28–30 Reduction of proliferating SMCs benefits re-endothelialization as long as EC proliferation is not inhibited, which can be achieved via the activation of CTP salvage pathway. Salvage pathway enzymes NME2 appears to be expressed at a relatively low level in normal EC growth condition. However, when CTPS activity is blocked by CPEC, NME2 is dramatically induced in ECs while neointima proliferating SMCs are significantly reduced. Although both NME1 and NME2 are important for CTP synthesis, NME2 appears to have a stronger capability in utilizing cytidine. Indeed, knockdown of NME2 significantly diminishes EC proliferation in the presence of CPEC and cytidine. These results demonstrate that the salvage pathway is up-regulated in order to compensate for CPEC-blocked CTP synthesis, which sustains the EC proliferation. The combined effect of blocking CTPS1 on inhibiting SMC proliferation while sustaining EC proliferation results in the accelerated re-endothelialization in EC-denuded vessels.
It is interesting that ECs, but not SMCs, utilize salvage pathways to synthesize CTP, which is likely due to the accessibility of ECs to the salvage pathway-specific substrate cytidine. Cytidine is circulating in the blood stream, which consistently interacts with ECs. Therefore, ECs may adopt a unique mechanism by which cytidine is used to synthesize CTP even under normal growing or quiescent states. This specialized ability is obviously enhanced when the de novo pathway is impaired.
One limitation of this study is that the effect of the systematic blockade of CTPS on inflammatory cells such as leukocyte infiltration or the differentiation of bone marrow-derived endothelial progenitor cells is not studied. These cells are also involved in neointima formation and re-endothelialization in vivo, which may be investigated in the future. Nevertheless, our study discovered the divergent mechanisms in CTP synthesis between SMCs and ECs, which lays a foundation for developing SMC-sensitive therapeutics in preventing neointima formation while promoting re-endothelialization. Thus, targeting CTPS is likely to result in significantly-improved vascular repair. In addition to CPEC, several other inhibitors including 3-deazauridine,31 carbodine32, and 6-diazo-5-oxonorleucine33 also effectively block CTPS activity, which further make CTPS as a promising target in curing proliferative vascular diseases including those observed in cardiovascular interventions.
Materials and Methods
Reagents and cell culture
Rat aortic smooth muscle cells (SMCs) were cultured by enzyme-digestion method from rat thoracic aorta as described previously1. SMC phenotype of the cultured cells was confirmed by the expression of smooth muscle α-actin and SM22α. Endothelial cell C166 was purchased from ATCC and grown at 37°C in a humidified atmosphere of 5% CO2 in DMEM (Invitrogen) supplemented with 10% FBS.
CPEC (compound 375575) was obtained from the Open Chemical Repository of National Cancer Institute Developmental Therapeutics Program (DTP). CTPS1 (sc-131474), PCNA (sc-56), CDK1 (sc-137034) and phospho-CDK1 (T161) (sc-101654) antibodies were from Santa Cruz Biotechnology (CA, USA). NME1 (#3345S) and NME2 (SAB1400187) antibodies were purchased from Cell Signaling (Danvers, MA, USA), and Sigma-Aldrich (St. Louis, MO, USA), respectively.
Animals
Male Sprague-Dawley rats weighing 450 to 500 g and male FVB mice (13 weeks; mean weight 24 g) were purchased from Harlan. FVB mice were chosen in this study because FVB mice respond better to wire injury and produce thicker neointima compared to C57BL6 mice.2 All animals were housed under conventional conditions in the animal care facilities and received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals. Animal surgical procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Construction of adenoviral vectors
NME1 and NME2 cDNA were individually subcloned into the XhoI site of pShuttle-IREShrGFP-1 (Agilent) and was confirmed by sequencing. Adenoviral vectors expressing CTPS1 and NME2 short hairpin RNA (shRNA) (shCTPS1 and shNME2) were constructed and the viruses were purified as described previously1. The shRNA sequences were as follows: shCTPS1 top strand: 5’-CGC GTC GCG CTA GAG CAC TCT GCA TTG GCC ATT AAT TCA AGA GAT TAA TGG CCA ATG CAG AGT GCT CTA GCG CTT TTT TCC AAA-3’; shCTPS1 bottom strand: 5’-AGC TTT TGG AAA AAA GCG CTA GAG CAC TCT GCA TTG GCC ATT AAT CTC TTG AAT TAA TGG CCA ATG CAG AGT GCT CTA GCG CGA-3’; shNME2 top strand: 5’-CGC GTC GAG ATC CAT CTG TGG TTT AAG CCC GAA GAT 2 TCA AGA GAT CTT CGG GCT TAA ACC ACA GAT GGA TCT CTT TTT TCC AAA-3’; shNME2 bottom strand: 5’-AGC TTT TGG AAA AAA GAG ATC CAT CTG TGG TTT AAG CCC GAA GAT CTC TTG AAT CTT CGG GCT TAA ACC ACA GAT GGA TCT CGA-3’. Green fluorescent protein (GFP)-expressing adenovirus (Ad-GFP) was used as a control.
Rat carotid artery injury model and adenoviral gene transfer
Rat carotid artery balloon injury was performed using 2F Fogarty arterial embolectomy balloon catheter (Baxter Edwards Healthcare) as described previously3. Adenovirus gene transfer was achieved by incubation of 5×109 pfu of adenovirus in balloon-injured carotid arteries for 20 min as described previously4. 7, 14 or 60 days later, the balloon-injured arteries were perfused with saline, fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned. Subsequent morphometric analyses were performed in a double-blinded manner. Ten sections that were evenly distributed in the vessel segments were collected for analysis. The sections were stained with modified hematoxylin and eosin or elastica van Gieson (VG) staining. Cross-sectional images were captured with a Nikon microscope (Nikon America Inc). The circumference of the lumen, internal elastic lamina, and external elastic lamina were measured using Image-pro Plus Software. For immunohistochemistry (IHC) staining, sections were rehydrated, blocked with 5% goat serum, permeabilized with 0.01% Triton X-100 in PBS, and incubated with primary antibodies overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody. The sections were counterstained with hematoxylin.
Mouse carotid artery wire injury model
Mice were anesthetized with ketamine hydrochloride (80 mg/kg IP) and xylazine (5 mg/kg IP), a 0.38-mm flexible angioplasty guidewire was advanced by 1 cm via a transverse arteriotomy of the external carotid artery, and endothelial denudation of the common carotid artery was achieved by 3 rotational passes5.
Cell proliferation assay
Cell proliferation was evaluated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay using a TACS MTT Cell Proliferation Assay Kit (Trivegen). The optical density at 570 nm was measured.
Quantitative RT-PCR (qPCR) and western blot
Total RNA was extracted from cells or tissues using Trizol reagent (Invitrogen) and reverse transcribed to cDNA using M-MLV reverse transcriptase (Promega). qPCR was performed on a Stratagene Mx3005 qPCR thermocycler (Agilent Technologies, La Jolla, CA). Primer sequences used in this study were listed in Supplementary Table 1. Western blot was performed as described previously6.
TUNEL assay
In vivo cell apoptosis was evaluated by detecting DNA fragmentation using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP–digoxigenin nick end-labeling method (TUNEL kit, Roche, USA). Apoptotic cells were observed under a fluorescent microscope. In vitro cell apoptosis was measured by Flow Cytometry. Cells were stained with both Annexin V-FITC (BD Biosciences) and propidium iodide (PI) and analyzed on a FACSCalibur™ (Becton Dickinson). The percentages of positive-stained cells were quantified using CELLQuest™ software (Becton Dickinson). Non-stained cells served as controls.
Cell cycle flow cytometry analysis
1 × 106 cells were harvested and resuspended in 500 µl of reaction buffer containing 1 µl of Nuclear-ID™ Red dye (Nuclear-ID™ Red Cell Cycle Analysis Kit, Enzo Life Sciences, USA). After mixing, cells were incubated for 15 min in the dark. Cell cycle analysis was performed on a FACSCalibur™ (Becton Dickinson) and analyzed by the CELLQuest™ software (Becton Dickinson).
Statistical analysis
Each experiment was repeated for more than three times. All values are presented as means ± SEM. Comparisons of parameters between two groups were made by t test. Comparisons of parameters among more than two groups were made by one-way analysis of variance, and comparisons of different parameters between each group were made by a post hoc analysis using a Bonferroni test. P values < 0.05 were considered to be statistically significant.
Supplementary Material
Significance.
Drug-eluting stents are commonly used for treating coronary artery diseases. However, drugs currently used in clinic such as sirolimus and paclitaxel have side effects causing defective re-endothelialization and increasing risk of late thrombosis because of their non-specific effect on inhibiting proliferation of both smooth muscle and endothelial cells. Our study demonstrates for the first time that smooth muscles and endothelial cells have distinct preferences in utilizing CTP synthesis pathways for their proliferation, which makes CTP synthase an ideal target for treating proliferative vascular diseases including those observed in cardiovascular interventions. The underlying mechanism is that blockade of CTP synthase induces CTP synthesis salvage pathway in endothelial cells, but not in smooth muscles, which sustains endothelial cell proliferation while blocking smooth muscle proliferation.
Acknowledgements
We would like to acknowledge the Developmental Therapeutics Program of NCI for providing CPEC.
Funding sources:
This work was supported by grants from National Institutes of Health (HL093429 and HL107526 to S.-Y.C.).
Non-standard Abbreviations and Acronyms
- SMC
smooth muscle cell
- EC
endothelial cells
- CTPS
CTP synthase
- CPEC
cyclopentenyl cytosine
- NME
nucleoside diphosphate kinase
- shRNA
small hairpin RNA
- PDGF
platelet-derived growth factor
- PCNA
proliferating cell nuclear antigen
- TdT
deoxynucleotidyl transferase
- CDK1
cyclin-dependent kinase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Degertekin M, Serruys PW, Foley DP, Tanabe K, Regar E, Vos J, Smits PC, van der Giessen WJ, van den Brand M, de Feyter P. Persistent inhibition of neointimal hyperplasia after sirolimus-eluting stent implantation. Circulation. 2002;106:1610–1613. doi: 10.1161/01.cir.0000034447.02535.d5. [DOI] [PubMed] [Google Scholar]
- 2.Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr. Opin. Lipidol. 2004;15:523. doi: 10.1097/00041433-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am J Physiol Lung Cell Mol Physiol. 1997;272:L197–L202. doi: 10.1152/ajplung.1997.272.2.L197. [DOI] [PubMed] [Google Scholar]
- 4.Cotran RS, Pober JS. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J Am Soc Nephrol. 1990;1:225–235. doi: 10.1681/ASN.V13225. [DOI] [PubMed] [Google Scholar]
- 5.Fingerle J, Johnson R, Clowes A, Majesky M, Reidy M. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci. 1989;86:8412. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Bauters C, Isner JM. The biology of restenosis. Prog Cardiovasc Dis. 1997;40:107–116. doi: 10.1016/s0033-0620(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 8.Kinlay S, Libby P, Ganz P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001;12:383. doi: 10.1097/00041433-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ostrander DB, O’Brien DJ, Gorman JA, Carman GM. Effect of CTP synthetase regulation by CTP on phospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:18992–19001. doi: 10.1074/jbc.273.30.18992. [DOI] [PubMed] [Google Scholar]
- 10.Long CW, Levitzki A, Koshland D., Jr The subunit structure and subunit interactions of cytidine triphosphate synthetase. J. Biol. Chem. 1970;245:80–87. [PubMed] [Google Scholar]
- 11.Hofer A, Steverding D, Chabes A, Brun R, Thelander L. Trypanosoma brucei CTP synthetase: a target for the treatment of African sleeping sickness. Proc. Natl. Acad. Sci. 2001;98:6412. doi: 10.1073/pnas.111139498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Clercq E, Murase J, Marquez VE. Broad-spectrum antiviral and cytocidal activity of cyclopentenylcytosine, a carbocyclic nucleoside targeted at CTP synthetase. Biochem. Pharmacol. 1991;41:1821–1829. doi: 10.1016/0006-2952(91)90120-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg AA, Lenthe H, Busch S, Korte D, Roos D, Kuilenburg ABP, Gennip AH. Evidence for transformation-related increase in CTP synthetase activity in situ in human lymphoblastic leukemia. Eur. J. Biochem. 1993;216:161–167. doi: 10.1111/j.1432-1033.1993.tb18128.x. [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB–binding protein and regulates growth factor–induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 15.Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl. Acad. Sci. 1995;92:5855. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyer JD, Malinowski NM, Treanor SP, Marquez VE. Antitumor activity and biochemical effects of cyclopentenyl cytosine in mice. Cancer Res. 1986;46:3325–3329. [PubMed] [Google Scholar]
- 17.Politi PM, Xie F, Dahut W, Ford H, Kelley JA, Bastian A, Setser A, Allegra CJ, Chen AP, Hamilton JM. Phase I clinical trial of continuous infusion cyclopentenyl cytosine. Cancer Chemother. Pharmacol. 1995;36:513–523. doi: 10.1007/BF00685802. [DOI] [PubMed] [Google Scholar]
- 18.Hofma SH, Van Der Giessen WJ, Van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JMR, Van Essen D, De Feyter PJ, Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Suh SY, Choi CU, Na JO, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ. Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent versus paclitaxel-eluting stent. JACC: Cardiovasc Interve. 2008;1:65–71. doi: 10.1016/j.jcin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2012;93:223–231. doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar A, Bearne SL. Aspartate-107 and leucine-109 facilitate efficient coupling of glutamine hydrolysis to CTP synthesis by Escherichia coli CTP synthase. Biochem. J. 2003;369:497. doi: 10.1042/BJ20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson CM, Parkinson FE. Potential signalling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. Trends Pharmacol. Sci. 1997;18:387–392. doi: 10.1016/s0165-6147(97)01106-1. [DOI] [PubMed] [Google Scholar]
- 23.Schimmel K, Gelderblom H, Guchelaar HJ. Cyclopentenyl cytosine (CPEC): an overview of its in vitro and in vivo activity. Curr. Cancer Drug Targets. 2007;7:504–509. doi: 10.2174/156800907781386579. [DOI] [PubMed] [Google Scholar]
- 24.Payne R, Cheng N, Traut T. Uridine kinase from Ehrlich ascites carcinoma. Purification and properties of homogeneous enzyme. J. Biol. Chem. 1985;260:10242–10247. [PubMed] [Google Scholar]
- 25.Sandeck H, Haaverstad R, Lundgren S, Larsson E. Genome-Wide Profile of Pleural Mesothelioma versus Parietal and Visceral Pleura: The Emerging Gene Portrait of the Mesothelioma Phenotype. PLoS One. 2009;4(8):e6554. doi: 10.1371/journal.pone.0006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postel EH, Zou X, Notterman DA, La Perle KMD. Double knockout Nme1/Nme2 mouse model suggests a critical role for NDP kinases in erythroid development. Mol. Cell. Biochem. 2009;329:45–50. doi: 10.1007/s11010-009-0110-9. [DOI] [PubMed] [Google Scholar]
- 27.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 28.Dhanabal M, Ramchandran R, Waterman MJF, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 29.Stouffer GA, Hu Z, Sajid M, Li H, Jin G, Nakada MT, Hanson SR, Runge MS. β3 integrins are upregulated after vascular injury and modulate thrombospondin-and thrombin-induced proliferation of cultured smooth muscle cells. Circulation. 1998;97:907–915. doi: 10.1161/01.cir.97.9.907. [DOI] [PubMed] [Google Scholar]
- 30.Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao WY, Johns DG, Mitsuya H. Potentiation of the anti-HIV activity of zalcitabine and lamivudine by a CTP synthase inhibitor, 3-deazauridine. Nucleosides, Nucleotides Nucleic Acids. 2000;19:371–377. doi: 10.1080/15257770008033015. [DOI] [PubMed] [Google Scholar]
- 32.Georges-Courbot M, Contamin H, Faure C, Loth P, Baize S, Leyssen P, Neyts J, Deubel V. Poly (I)-poly (C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob. Agents Chemother. 2006;50:1768–1772. doi: 10.1128/AAC.50.5.1768-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bearne SL, Hekmat O, Macdonnell JE. Inhibition of Escherichia coli CTP synthase by glutamate gamma-semialdehyde and the role of the allosteric effector GTP in glutamine hydrolysis. Biochem. J. 2001;356:223. doi: 10.1042/0264-6021:3560223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- 1.Wang JN, Shi N, Xie WB, Guo X, Chen SY. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:e19–e26. doi: 10.1161/ATVBAHA.111.230706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooley BC. Mouse strain differential neointimal response in vein grafts and wire-injured arteries. Circ J. 2007;71:1649–1652. doi: 10.1253/circj.71.1649. [DOI] [PubMed] [Google Scholar]
- 3.Tulis DA. Rat carotid artery balloon injury model. Methods Mol Med. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dollery CM, Humphries SE, McClelland A, Latchman DS, McEWAN J. In vivo adenoviral gene transfer of timp - 1 after vascular injury reduces neointimal formation. Annals of the New York Academy of Sciences. 1999;878:742–743. doi: 10.1111/j.1749-6632.1999.tb07778.x. [DOI] [PubMed] [Google Scholar]
- 5.Werner N, Junk S, Laufs U, Link A, Walenta K, Böhm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circulation research. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 6.Shi N, Xie WB, Chen SY. Cell division cycle 7 is a novel regulator of transforming growth factor-β-induced smooth muscle cell differentiation. Journal of Biological Chemistry. 2012;287:6860–6867. doi: 10.1074/jbc.M111.306209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.