Abstract
In the present study, we investigated the possible development of tolerance to the antihyperalgesic effect of μ-opioid receptor (MOR) agonists under a neuropathic pain-like state. Repeated treatment with fentanyl, but not morphine or oxycodone, produced a rapid development of tolerance to its antihyperalgesic effect in mice with sciatic nerve ligation. Like the behavioral study, G-protein activation induced by fentanyl was significantly reduced in membranes obtained from the spinal cord of nerve-ligated mice with in vivo repeated injection of fentanyl. In β-endorphin-knockout mice with nerve ligation, developed tolerance to the antihyperalgesic effect of fentanyl was abolished, and reduced G-protein activation by fentanyl after nerve ligation with fentanyl was reversed to the normal level. The present findings indicate that released β-endorphin within the spinal cord may be implicated in the rapid development of tolerance to fentanyl under a neuropathic pain-like state.
Keywords: Fentanyl, mouse, neuropathic pain, opioid tolerance, μ-opioid receptor, spinal cord
INTRODUCTION
Although drugs that act on μ-opioid receptor (MOR), such as morphine, fentanyl and oxycodone, have been used clinically as analgesics, these MOR agonists also have undesirable effects, such as tolerance, and physical and psychological dependence (Ventafridda and De Conno, 1981; Raynor et al. 1994). It has been considered that opioid tolerance is, in part, the end result of a coordinated balance between processes that govern the desensitization, internalization and resensitization of MORs (Claing et al. 2002; Gainetdinov et al. 2004). The initial process in these events is the phosphorylation of intracellular domains of MORs. Phosphorylated MORs are mostly internalized via clathrin-coated pits into early endosomes and subsequently dephosphorylated by intracellular protein phosphatases. The dephosphorylated MORs may either be recycled to the plasma membrane or transported to lysosomes for degradation. Previous biochemical studies on cultured enteric neurons have indicated that fentanyl induces either the functional desensitization or internalization of MORs (Minnis et al. 2003). In contrast, under the same condition, morphine does not promote the detectable internalization of MORs in cultured cells after prolonged or acute treatment in healthy animals, although it has been well-established that morphine causes the development of tolerance to its pharmacological actions (Minnis et al. 2003). On the other hand, recent studies have demonstrated that morphine activates MORs with promoting internalization of MORs via β-arrestin-2-dependent mechanisms in striatal neurons (Haberstock-Debic et al. 2005). Thus, the mechanisms that underlie the development of analgesic tolerance to MOR agonists are very much complicated. To further understand properties of analgesic tolerance to MOR agonists, it has been necessary to investigate possible changes in analgesic efficacy following repeated treatment with MOR agonists at optimum doses just for the relief of chronic pain associated with physiological changes in the endogenous MOR system.
In a previous study, we demonstrated that repeated treatment with fentanyl caused a rapid desensitization to its ability to block hyperalgesia under an inflammatory pain state, whereas morphine did not have a similar effect (Imai et al. 2006). In addition, repeated treatment with fentanyl, but not morphine, resulted in the attenuation of MOR resensitization, and a subsequent increase in the levels of phosphorylated-MOR in the spinal cord of mice with inflammatory pain. These findings raise the possibility that chronic treatment with fentanyl may cause a different modulation of either the desensitization, internalization or resensitization of MORs in the spinal cord under a pain-like state compared with chronic treatment with morphine.
One mechanism for the MOR desnsitization or attenuation of MOR resensitization by fentanyl in the spinal cord under chronic pain could be a sustained increase in release of the endogenous μ-opioid neuropeptide β-endorphin after sciatic nerve ligation. In fact, it has been reported that β-endorphin is released within some brain regions during pain state (Zangen et al. 1998; Zubieta et al. 2001). In their reports, they mentioned that the extracellular levels of β-endorphin in the arcuate nucleus increased by 88% under pain-like state. Based on these findings, we assumed that β-endorphin might be released within the spinal cord, as well as brain regions, under pain-like state, as compensatory mechanism for the inhibition of pain transmisson. As sustained exposure to β-endorphin could results in receptor phosphorylation and uncoupling of receptors from effector systems, and thus desensitization, neuropathic pain associated with release of β-endorphin may interfere MOR resensitization by fentanyl.
To further understand the mechanisms that underlie the development of tolerance to this opioid analgesic-induced antihyperalgesic effect under chronic pain, we evaluated the effect of repeated administration of morphine, fentanyl or oxycodone on neuropathic pain-like hyperalgesia and the possible development of tolerance following sciatic nerve ligation. As in the mouse model of inflammatory pain, we demonstrated that repeated treatment with fentanyl, but not morphine or oxycodone, caused a rapid desensitization to its antihyperalgesic effect in nerve-ligated mice. Furthermore, we found that β-endorphin could be a key modulator for the high degree of antinociceptive tolerance to fentanyl caused by sciatic nerve injury. Based on this phenomenon, the present study was performed to investigate the effects of fentanyl on antihyperalgesic effect in β-endorphin knockout (KO) mice.
MATERIALS AND METHODS
The present study was conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals of Hoshi University, as adopted by the Committee on Animal Research of Hoshi University. Every effort was made to minimize the numbers and any suffering of animals used in the following experiments.
Animals
Male and female β-endorphin derived from proopiomelanocortin (POMC) gene-KO mice (8–13 weeks old, 22–30 g) (The Jackson Laboratory, Bar Harbor, ME, USA), which had a C57BL/6J and 129S2/SvPas mixed genetic background as described previously (Niikura et al. 2008), their wild-type (WT) male and female C57BL/6J mice (8–13 weeks old, 22–30 g) (The Jackson Laboratory, Bar Harbor, ME, USA) and male ICR mice (7–9 weeks old, 20–25 g) (Tokyo Laboratory Animals Science Co., Ltd., Tokyo, Japan) were used in the present study. Animals were housed in a room maintained at 23 ± 1°C with a 12-hour light–dark cycle. Food and water were available ad libitum. Each animal was used only once.
Drugs
The drugs used in the present study were fentanyl citrate (Hisamitsu Pharmaceutical Co., Inc., Tokyo, Japan), morphine hydrochloride (Daiichi-Sankyo Co., Tokyo, Japan), oxycodone hydrochloride (a kind gift from Shionogi Pharmaceutical Co. Inc., Osaka, Japan) and β-endorphin (Sigma-Aldrich Co., St. Louis, MO, USA), which were dissolved in 0.9% physiological saline (Otsuka Pharmaceutical Co. Inc., Tokyo, Japan) for in vivo experiments or assay buffer for in vitro experiments.
Neuropathic pain model
Mice were anesthetized with 3% isoflurane. We produced a partial sciatic nerve injury by tying a tight ligature with a 8–0 silk suture around approximately one-third to one-half the diameter of the sciatic nerve on the right side (ipsilateral side) under a light microscope (SD30, Olympus, Tokyo, Japan), as described previously (Seltzer et al. 1990; Malmberg and Basbaum 1998). In sham-operated mice, the nerve was exposed without ligation.
Guanosine-5′-o-(3-thio) triphosphate ([35S]GTPγS) binding assay
For membrane preparation, the mouse spinal cord was quickly removed after decapitation and rapidly transferred to a tube filled with ice-cold bufferh. The membrane homogenate (3–8 βg protein/assay) was prepared as described previously (Narita et al. 2001) and incubated at 25°C for 2 hours in 1 ml of assay buffer with various concentrations of each agonist, 30 μM guanosine-5′-diphosphate and 50 pM [35S]GTPγS (specific activity, 1000 Ci/mmol; Amersham, Arlington Heights, IL, USA). The reaction was terminated by filtration using Whatman GF/B glass filters (Brandel, Gaithersburg, MD, USA) that had been presoaked in 50 μM Tris-HCl, pH 7.4, and 5 μM MgCl2 at 4°C for 2 hours. The filters were washed three times with 5 ml of ice-cold Tris-HCl buffer, pH 7.4, and then transferred to scintillation-counting vials. Next, 4 ml of clear-sol 2 (Nacalai Tesque, Inc., Kyoto, Japan) was added to the vials and equilibrated for 12 hours. The radioactivity in the samples was determined with a liquid scintillation analyzer. Nonspecific binding was measured in the presence of 10 μM unlabeled GTPγS.
Measurement of thermal hyperalgesia and tactile stimulus
To assess the sensitivity to thermal stimulation, each of the hind paws of mice was tested individually using a thermal stimulus apparatus (UGO-BASILE, Biological Research Apparatus, Varese, Italy). The intensity of the thermal stimulus was adjusted to achieve an average baseline paw-withdrawal latency of approximately 9 to 12 seconds in naive mice. Only quick hind-paw movements (with or without licking of the hind paws) away from the stimulus were considered to be a withdrawal response. Paw movements associated with locomotion or weight-shifting were not counted as a response. The paws were measured alternating between the left and right with an interval of more than 3 minutes between measurements. The latency of paw withdrawal after the thermal stimulus was determined as the average of three measurements per paw.
Statistical analysis
The data from the [35S]GTPγS binding assay are expressed as the mean ± standard error of the mean (SEM) of % Stimulation. The data regarding hyperalgesic responses are shown as the mean ± SEM of the paw-withdrawal latency. Receptor binding curves were fitted using Graph-Pad Prism 4.0 (Graph-Pad Software Inc., La Jolla, CA, USA). The statistical significance of differences between groups was assessed by two-way analysis of variance followed by the Bonferroni/Dunn multiple comparison test or Student's t-test.
RESULTS
Effect of single or repeated subcutaneous (s.c.) injections of morphine, fentanyl or oxycodone on the neuropathic pain-like state induced by nerve injury in mice
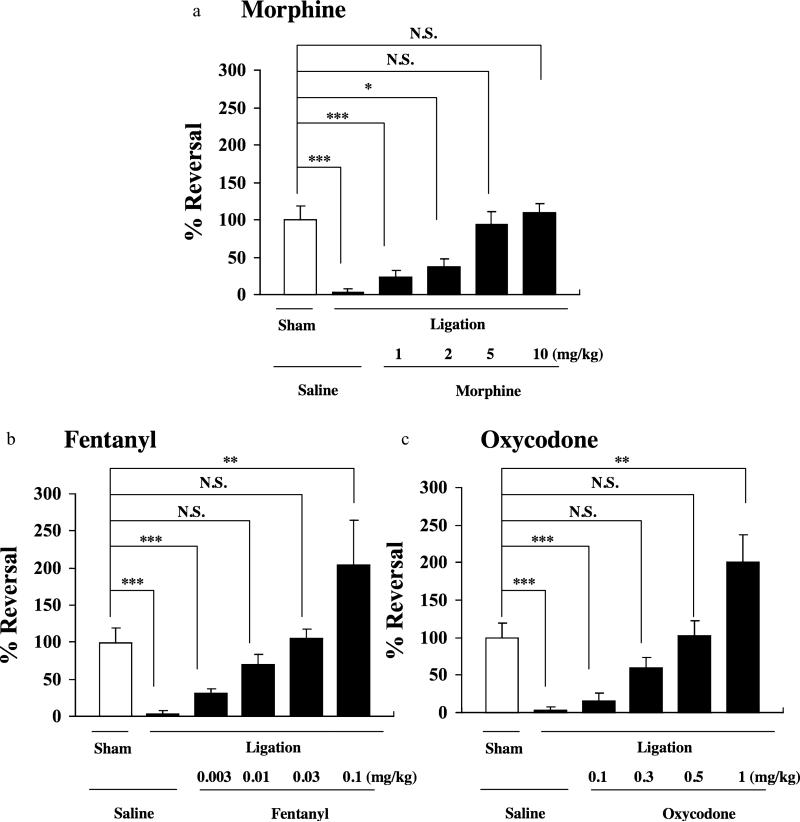
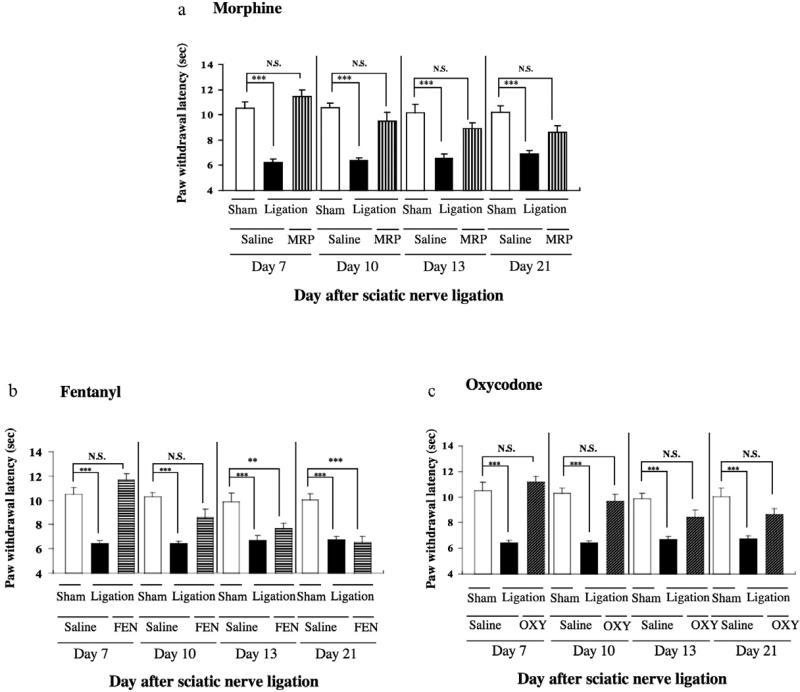
In the present study, mice with partial sciatic nerve ligation exhibited marked neuropathic pain-like behavior only for the ipsilateral side at 7 days after nerve ligation (***P < 0.001 versus sham-saline group, Fig. 1). The persistent painful state caused by sciatic nerve ligation lasted for more than 21 days after surgery in mice (Fig. 2). A single s.c. injection of either morphine (1–10 mg/kg), fentanyl (0.003–0.01 mg/kg) or oxycodone (0.1–1 mg/kg) at 7 days after sciatic nerve ligation recovered the decreased thermal threshold observed on the ipsilateral side in sciatic nerve-ligated mice in a dose-dependent manner, and maximal antihyperalgesic responses were seen at 30, 15 or 15 minutes after the injection of morphine, fentanyl or oxycodone, respectively (*P < 0.05, **P < 0.01 or ***P < 0.001 versus sham-saline group, Fig. 1). At a dose of 5.0 mg/kg, 0.03 mg/kg or 0.5 mg/kg, s.c. administration of morphine, fentanyl or oxycodone almost completely reversed the decrease in the thermal threshold without excessive effects in sciatic nerve-ligated mice.Therefore, we proposed that the optimal doses for the morphine-, fentanyl- or oxycodone-induced antihyperalgesiceffectinnerve-ligatedmicewere5.0,0.03or0.5 mg/kg, respectively. As shown in Fig. 2a and c, the thermal hyperalgesia observed on the ipsilateral side after nerve ligation was clearly reversed by each repeated s.c. injection of morphine (5 mg/kg) or oxycodone (0.5 mg/kg) once a day for 14 consecutive days from 7 days after nerve ligation. In contrast, the antihyperalgesic effect following repeated treatment with fentanyl (0.03 mg/kg) was gradually tolerated (**P < 0.01 or ***P < 0.001 versus sham-saline group; Fig. 2b).
Figure 1.
Effect of s.c. injection of morphine, fentanyl or oxycodone on the latency of paw withdrawal in response to a thermal stimulus on the ipsilateral side in sham-operated or sciatic nerve-ligated ICR mice. The thermal threshold was measured just before and 30, 15 or 15 minutes after s.c. injection of morphine, fentanyl or oxycodone, respectively. Groups of mice were treated s.c. with morphine (1–10 mg/kg) (a), oxycodone (0.003–0.1 mg/kg) (b) or fentanyl (0.003–0.01 mg/kg) (c) 7 days after the operation. Each column represents the mean ± standard error of the mean of 8–10 mice. *P < 0.05, **P < 0.01 and ***P < 0.001 versus sham-saline group. N.S. = not significant
Figure 2.
Effect of repeated s.c. injection of morphine (MRP) (a), fentanyl (FEN) (b) or oxycodone (OXY) (c) on the latency of paw withdrawal in response to a thermal stimulus on the ipsilateral side in sciatic nerve-ligated ICR mice. Repeated s.c. injection of saline, morphine (5 mg/kg), fentanyl (0.03 mg/kg) or oxycodone (0.5 mg/kg) was started 7 days after sciatic nerve ligation. ICR mice were repeatedly injected with saline, morphine, fentanyl or oxycodone once a day for 14 consecutive days. During the first 6 days after surgery, mice were not treated with saline, morphine, fentanyl or oxycodone.The thermal threshold was measured 7, 10, 13 and 20 days after ligation. Each column represents the mean ± standard error of the mean of 8–10 mice. **P < 0.01 and ***P < 0.001 versus Sham-saline group on day 1. N.S. = not significant
Changes in G-protein activation induced by repeated subcutaneous (s.c.) injection of morphine, fentanyl or oxycodone in the spinal cord of mice with nerve ligation
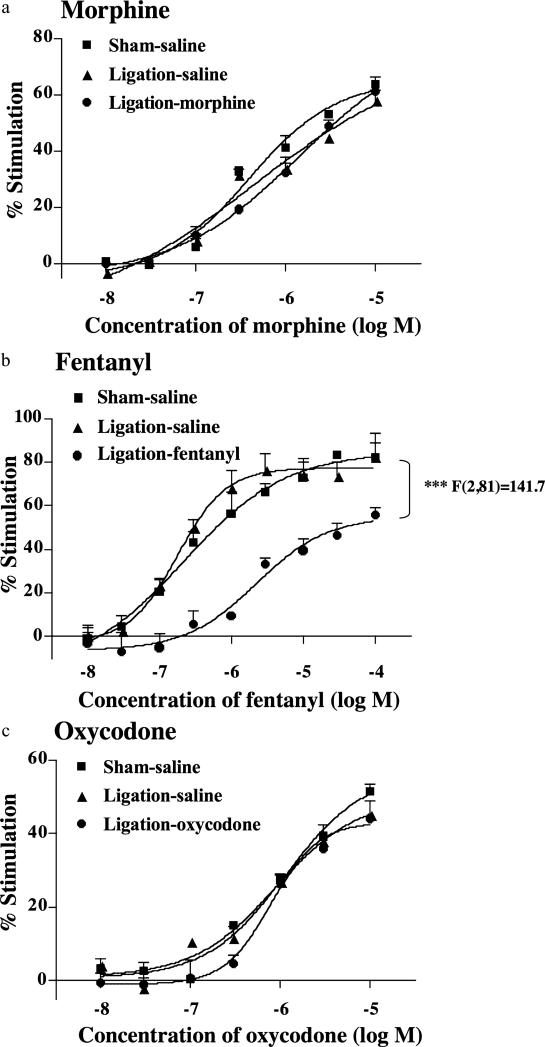
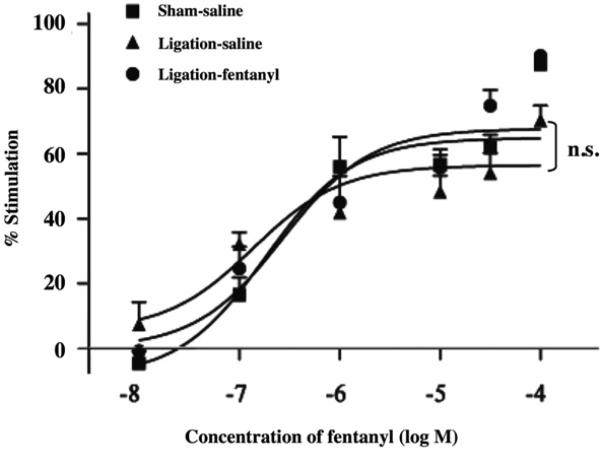
We investigated the ability of morphine, fentanyl or oxycodone to activate G-proteins through the stimulation of MOR in membranes of the ipsilateral side of the spinal cord obtained from mice treated with saline, morphine, fentanyl or oxycodone once a day for 14 consecutive days from 7 days after sham operation or nerve ligation (Fig. 3). The activation of G-proteins induced by morphine (0.001–10 μM), fentanyl (0.001–100 μM) or oxycodone (0.001–10 μM) on the ipsilateral side of the spinal cord was examined by monitoring the binding of [35S]GTPγS to membranes. Morphine, fentanyl and oxycodone each produced a concentration-dependent increase in the binding of [35S]GTPγS to spinal cord membranes obtained from sham-operated mice (Fig. 3). In sciatic nerve-ligated mice following repeated injection of saline, the levels of [35S]GTPγS binding stimulated by fentanyl, morphine or oxycodone were similar to that found in sham-operated mice (Fig. 3a-c). The binding of [ 35S]GTPγS stimulated by fentanyl was significantly decreased in nerve-ligated mice by the repeated s.c. injection of an optimal dose of fentanyl compared with the findings in sham-operated mice [F(2,81) = 141.7; P < 0.001 versus sham-saline group, Fig. 3c]. In contrast, there was no difference in G-protein activation in the spinal cord between sham-operated and nerve-ligated mice with the repeated s.c. injection of an optimal dose of morphine or oxycodone (Fig. 3a or c). Furthermore, the maximal G-protein stimulation by fentanyl was significantly decreased in nerve-ligated mice with the repeated s.c. injection of an optimal dose of fentanyl (***P < 0.001 versus sham-saline group, Fig. 3b). This reduction was not observed in the nerve-ligated β-endorphin KO mice treated with the optimum dose of fentanyl for 14 days (Fig. 4).
Figure 3.
Effect of repeated injection of morphine (a), fentanyl (b) or oxycodone (c) on the morphine-, fentanyl- or oxycodone-induced increase in [35S] GTPgS binding to membranes of the ipsilateral side of the spinal cord obtained from sham-operated and sciatic nerve-ligated ICR mice. Repeated s.c. injection of saline, morphine, fentanyl or oxycodone was started 7 days after sciatic nerve ligation. ICR mice were repeatedly injected with saline, morphine, fentanyl or oxycodone once a day for 14 consecutive days. During the first 6 days after surgery, mice were not treated with saline, morphine, fentanyl or oxycodone. Membranes were prepared at 21 days after nerve ligation. Each value represents the mean ± standard error of the mean of four samples
Figure 4.
Effect of repeated injection of fentanyl on the fentanyl-induced increase in [35S] GTPgS binding to membranes of the ipsilateral side of the spinal cord obtained from sham-operated and sciatic nerve-ligated β-endorphin knockout (KO) mice. Repeated s.c. injection of fentanyl was started 7 days after sciatic nerve ligation. Mice were repeatedly injected fentanyl once a day for 14 consecutive days. During the first 6 days after surgery, mice were not treated with fentanyl. Membranes were prepared at 21 days after nerve ligation. Each value represents the mean ± standard error of the mean of six samples. Each group was consisted of four males and two females. n.s. = not significant
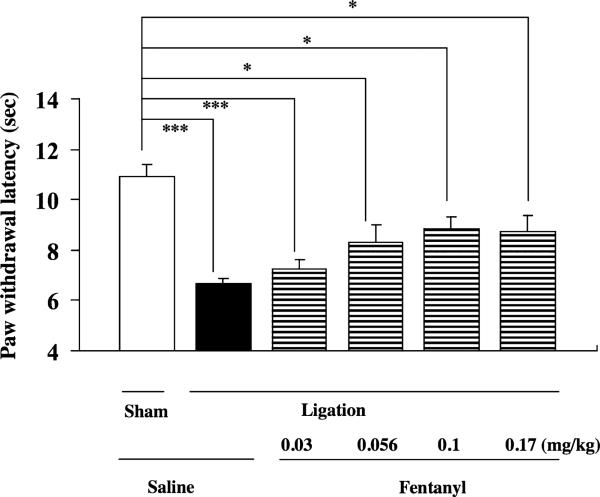
We further examined whether a single s.c. injection of fentanyl at relatively higher doses (0.03–0.17 mg/kg) could produce an antihyperalgesic effect in mice by using repeated treatment with an optimal dose of fentanyl under a neuropathic pain-like state (Fig. 5). Mice were repeatedly injected with saline or an optimal dose of fentanyl (0.03 mg/kg) for 14 consecutive days beginning at 7 days after nerve ligation. One day after the last injection of fentanyl, mice were challenged with fentanyl (0.03–0.17 mg/kg, Fig. 5). Fentanyl (0.056–0.17 mg/kg) failed to recover the decreased thermal threshold in nerve- ligated mice following the repeated injection of an optimal dose of fentanyl (*P < 0.05 versus sham-saline group, Fig. 5).
Figure 5.
Effect of s.c. injection of fentanyl (0.03–0.17 mg/kg) on the latency of paw withdrawal in response to a thermal stimulus on the ipsilateral side in sciatic nerve-ligated ICR mice with the administration of fentanyl (0.03 mg/kg) for 14 consecutive days. The thermal threshold was measured 15 minutes after the s.c. injection of fentanyl. Each column represents the mean ± standard error of the mean of eight mice. *P < 0.05, and ***P < 0.001 versus Sham-saline group
Involvement of β-endorphin in the tolerance to fentanyl-induced antihyperalgesia under a pain-like state
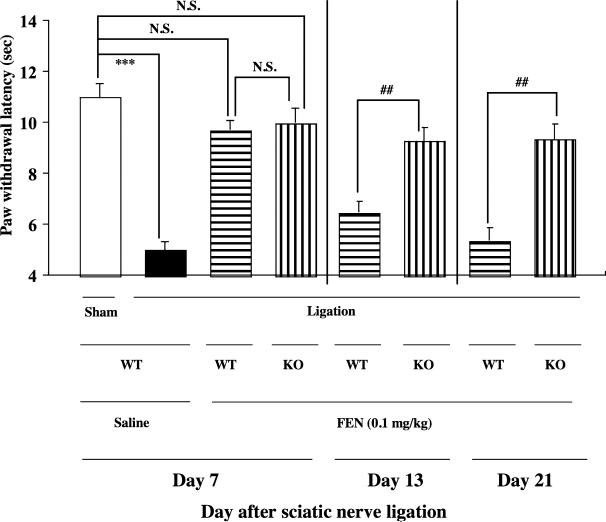
We compared the potency of the antihyperalgesic effect induced by the repeated injection of fentanyl between nerve-ligated WT and β-endorphin KO mice (Fig. 6). In the present study, both WT and β-endorphin KO mice with partial sciatic nerve ligation exhibited a marked neuropathic pain-like behavior to almost the same degree (***P < 0.001 versus sham-saline group Fig. 6). Under these conditions, the single s.c. injection of fentanyl (0.1 mg/kg) 7 days after nerve ligation almost completely reversed the decrease in the thermal threshold without excessive effects in sciatic nerve-ligated WT and β-endorphin KO mice, and maximal antihyperalgesic responses were seen at 15 minutes after fentanyl injection (Fig. 6). The antihyperalgesic effect following repeated treatment with fentanyl (0.1 mg/kg) was gradually tolerated from 14 days after sciatic nerve ligation in WT mice. In contrast, the potency of the antihyperalgesic effect of fentanyl was preserved in nerve-ligated β-endorphin KO mice under repeated s.c. treatment with fentanyl (##P < 0.01 versus knockout-ligation-fentanyl group; Fig. 6).
Figure 6.
Effect of the repeated s.c. injection of fentanyl (0.1 mg/kg) on the latency of paw withdrawal in response to a thermal stimulus on the ipsilateral side in sciatic nerve-ligated, wild-type (WT) or β-endorphin knockout (KO) mice. Repeated s.c. injection of fentanyl was started 7 days after sciatic nerve ligation. Mice were repeatedly injected with fentanyl once a day for 14 consecutive days. During the first 6 days after surgery, mice were not treated with fentanyl.The thermal threshold was measured 7, 13 or 20 days after nerve ligation at 15 minutes after s.c. injection of fentanyl. Each column represents the mean ± standard error of the mean of five to six mice (consisting of two to three males and three females). ***P < 0.001 versus WT-sham-saline group. ##P < 0.01 versus β-endorphin KO-ligation-fentanyl group. N.S. = not significant
DISCUSSION
In the present study, a neuropathic pain-like state induced by partial sciatic nerve ligation was suppressed by the single s.c. injection of morphine, fentanyl or oxycodone in a dose-dependent manner. At doses of 5.0, 0.5 and 0.03 mg/kg, s.c. administration of morphine, oxycodone and fentanyl, respectively, completely reversed the decreased thermal threshold without excessive effects in nerve-ligated mice. Based on the present findings, we proposed that the optimal doses for the morphine-, oxycodone- and fentanyl-induced antihyperalgesic effects in sciatic nerve-ligated mice were 5 mg/kg, 0.5 mg/kg and 0.03 mg/kg, respectively. If we combine this result with our previous findings, the optimal dose for a morphine-induced antihyperalgesic effect in sciatic nerve-ligated mice was higher than that under inflammatory pain, whereas the optimal doses for fentanyl and oxycodone under a neuropathic pain-like state and an inflammatory pain-like state were similar. Under these conditions, the antihyperalgesic effect induced by fentanyl in mice with sciatic nerve ligation rapidly disappeared during the consecutive administration of fentanyl (0.03 mg/kg), whereas the potencies of morphine (3 mg/ kg) and oxycodone (0.5 mg/kg) with regard to their anti-hyperalgesic effects were preserved in nerve-ligated mice even after repeated s.c. treatment with morphine or oxycodone. Furthermore, even relatively higher doses of fentanyl (0.056–0.17 mg/kg) failed to reverse the hyperalgesia in sciatic nerve-ligated mice under the consecutive administration of fentanyl (0.03 mg/kg). Consistent with these results, the dose-response curve for G-protein activation induced by fentanyl was significantly shifted to the right and its maximal response was dramatically decreased in membranes of the spinal cord of nerve-ligated mice following the repeated injection of fentanyl (ligation-fentanyl group) compared with those in the sham-fentanyl and ligation-saline group. In contrast, these phenomena were not observed in nerve-ligated mice with the repeated administration of morphine or oxycodone. These findings provide evidence that the consecutive injection of fentanyl, unlike morphine and oxycodone, may extensively induce the development of tolerance to its antihyperalgesic effect under a persistent pain state. This event could be associated with the repeated administration of fentanyl-induced functional desensitization of MORs under a neuropathic pain-like state.
Several lines of evidence indicated that, in response to a pain stimulus, endogenous β-endorphin is released within some brain regions (Zubieta et al. 2001). We previously reported that β-endorphin released in the ventral tegmental area is a key factor in regulating the dysfunction of MOR to negatively modulate opioid reward under a neuropathic pain-like state (Niikura et al. 2008). Therefore, we next examined using β-endorphin KO mice whether a lack of β-endorphin expression could affect fentanyl-induced tolerance to antinociception under a neuropathic pain-like state. These β-endorphin KO mice showed no changes in the expression of other peptide products (e.g. ACTH and MSH) from the POMC gene (Rubinstein et al. 1996). With β-endorphin KO mice, we began by investigating whether a deletion of the β-endorphin gene could influence the development of a neuropathic pain-like state induced by sciatic nerve ligation in mice. As a result, there were no differences in decreased thermal hyperalgesia or increased tactile allodynia between β-endorphin KO and WT mice. Under these conditions, the fentanyl-induced antihyperalgesic tolerance under sciatic nerve ligation was abolished in β-endorphin KO mice. In addition, the reduced activation of G-proteins by fentanyl observed in the spinal cord of nerve-ligated mice after the repeated s.c. injection of fentanyl was dramatically suppressed in the spinal cord of nerve-ligated β-endorphin KO mice treated with the optimum dose of fentanyl for 14 days. These results suggest that released endogenous β-endorphin, in response to long-lasting pain, may play a critical role in the fentanyl-induced antihyperalgesic tolerance under a neuropathic pain-like state.
It has been widely accepted that receptor desensitization appear to play a key role in the development of opioid tolerance (Bohn et al. 2000; Gainetdinov et al. 2004; Walwyn et al. 2004). Furthermore, it has been considered that opioid tolerance is, in part, the end result of internalized MORs (Whistler & von Zastrow, 1998, 1999; Claing et al. 2002; Kieffer & Evans 2002; Koch et al. 2005; Zollner et al. 2008). The initial process in these events is the phosphorylation of intracellular domains of MOR. Phosphorylated MORs are mostly internalized via clathrin-coated pits into early endosomes and subsequently dephosphorylated by intracellular protein phosphatases. The dephosphorylated MORs might either be recycled to the plasma membrane or transported to lysosomes for degradation. A growing body of evidence suggests that among diverse serine (Ser)/threonine (Thr) residues of the intracellular domain of MOR, the phosphorylation of Ser 375 in the mouse MOR is essential for the internalization of MORs (Schulz et al. 2004). In a previous study, we found that repeated treatment with fentanyl, but not morphine, resulted in an increase in the levels of phosphorylated-MOR (Ser 375) associated with the enhanced inactivation of protein phosphatase 2A and a reduction in Rab4-dependent MOR resensitization in the spinal cord of mice that showed inflammatory pain (Imai et al. 2006). Althoug further studies are still needed, the present study raise the possibility that released β-endorphin within the spinal cord may result in a loss of the coordinated balance between processes that govern the desensitization, internalization and resensitization of MORs. This phenomenon could be associated with the mechanism that underlies the rapid development of tolerance to fentanyl under a neuropathic pain-like state.
CONCLUSION
We have demonstrated that repeated treatment with fentanyl at an excessive dose causes a rapid antihyperalgesic tolerance in sciatic nerve-ligated mice, whereas morphine and oxycodone do not produce this phenomenon. This condition may reflect the clinical observation that tolerance to morphine analgesia is not a major concern when patients suffer from severe pain. In addition, the discrepancy between the present findings and classical basic understanding that chronic morphine treatment is believed to lead to severe analgesic tolerance may result from the fact that most previous studies concerning molecular events in opioid tolerance have been performed using an excessive dose of MOR agonists in naive rodents. Furthermore, the present findings strongly indicate that β-endorphin within the spinal cord may be involved in the prolongation of the fentanyl-induced desensitization of MORs. This phenomenon may explain the high degree of tolerance to fentanyl-induced antihyperalgesia under a neuropathic pain-like state in rodents.
Footnotes
Authors Contribution
NM, HM, UY, DLA, ST designed the study. NM, IS wrote the manuscript. IS, NA, OA, AM, RM, SY, KN performed the experiments. IS, NA, OA, AM, RM, SY, KN performed the data analysis. All authors have critically reviewed content and approved final version submitted for publication.
References
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Narita M, Hashimoto S, Nakamura A, Miyoshi K, Nozaki H, Hareyama N, Takagi T, Suzuki M, Suzuki T. Differences in tolerance to anti-hyperalgesic effects between chronic treatment with morphine and fentanyl under a state of pain. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006;26:183–192. [PubMed] [Google Scholar]
- Kieffer BL, Evans CJ. Opioid tolerance-in search of the holy grail. Cell. 2002;108:587–590. doi: 10.1016/s0092-8674(02)00666-9. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Minnis JG, Patierno S, Kohlmeier SE, Brecha NC, Tonini M, Sternini C. Ligand-induced mu opioid receptor endocytosis and recycling in enteric neurons. Neuroscience. 2003;119:33–42. doi: 10.1016/s0306-4522(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Suzuki T, Dun NJ, Imai S, Yajima Y, Nagase H, Tseng LF. Enhanced mu-opioid responses in the spinal cord of mice lacking protein kinase Cgamma isoform. J Biol Chem. 2001;276:15409–15414. doi: 10.1074/jbc.M009716200. [DOI] [PubMed] [Google Scholar]
- Niikura K, Narita M, Nakamura A, Okutsu D, Ozeki A, Kurahashi K, Kobayashi Y, Suzuki M, Suzuki T. Direct evidence for the involvement of endogenous beta-endorphin in the suppression of the morphine-induced rewarding effect under a neuropathic pain-like state. Neurosci Lett. 2008;435:257–262. doi: 10.1016/j.neulet.2008.02.059. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Ventafridda V, De Conno F. Organizing pain control and rehabilitation service in a cancer centre. Int Rehabil Med. 1981;3:149–154. doi: 10.3109/03790798109166794. [DOI] [PubMed] [Google Scholar]
- Walwyn WM, Keith DE, Jr, Wei W, Tan AM, Xie CW, Evans CJ, Kieffer BL, Maidment NT. Functional coupling, desensitization and internalization of virally expressed mu opioid receptors in cultured dorsal root ganglion neurons from mu opioid receptor knockout mice. Neuroscience. 2004;123:111–121. doi: 10.1016/j.neuroscience.2003.08.060. [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J Biol Chem. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- Zangen A, Herzberg U, Vogel Z, Yadid G. Nociceptive stimulus induces release of endogenous beta-endorphin in the rat brain. Neuroscience. 1998;85:659–662. doi: 10.1016/s0306-4522(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]