Abstract
Viral fusion peptides are short N-terminal regions of type-1 viral fusion proteins that are critical for virus entry. Although the importance of viral fusion peptides in virus-cell membrane fusion is established, little is known about how they function. We report the effects of wild-type (WT) hemagglutinin (HA) fusion peptide and its G1S, G1V, and W14A mutants on the kinetics of poly(ethylene glycol)(PEG)-mediated fusion of small unilamellar vesicles composed of dioleoylphosphatidylcholine, dioleoylphosphatidylethanolamine, sphingomyelin, and cholesterol (molar ratio of 35:30:15:20). Time courses of lipid mixing, content mixing, and content leakage were obtained using fluorescence assays at multiple temperatures and analyzed globally using either a two-step or three-step sequential ensemble model of the fusion process to obtain the rate constant and activation thermodynamics of each step. We also monitored the influence of peptides on bilayer interfacial order, acyl chain order, bilayer free volume, and water penetration. All these data were considered in terms of a recently published mechanistic model for the thermodynamic transition states for each step of the fusion process. We propose that WT peptide catalyzes Step 1 by occupying bilayer regions vacated by acyl chains that protrude into interbilayer space to form the Step 1 transition state. It also uniquely contributes a positive intrinsic curvature to hemi-fused leaflets to eliminate Step 2 and catalyzes Step 3 by destabilizing the highly stressed edges of the hemi-fused microstructures that dominate the ensemble of the intermediate state directly preceding fusion pore formation. Similar arguments explain the catalytic and inhibitory properties of the mutant peptides and support the hypothesis that the membrane-contacting fusion peptide of HA fusion protein is key to its catalytic activity.
Introduction
Membrane fusion is vital to the life of eukaryotic cells. Many processes such as cellular trafficking and compartmentalization, import of nutrients and export of waste, cellular communication, sexual reproduction, and cell division are all dependent on this basic process. It is also an essential step for the entry of viruses into host cells. There is now fairly wide agreement that fusion, whether viral-protein-mediated (1), exocytotic-protein mediated (2), or in model membranes (3,4), proceeds through multiple steps and, at different stages of the process, involves small transient, or large stable pores (1,4,5). Since fusion between membranes with compositions that mimic mammalian membranes (6) is not spontaneous, it is likely that fusion proteins both provide free energy to drive membranes into close contact and catalyze the lipid rearrangements required in biomembrane fusion (7). Our hypothesis has been that membrane-contacting regions of fusion proteins are the key catalytic units of these fusion machines.
A well-studied fusion protein (8–11) is the hemagglutinin (HA) protein of influenza virus. Influenza virus HA has a short N-terminal region termed “fusion peptide” that has been shown by mutational analysis to be critical for fusion (12). Although HA fusion peptide alone does not induce pore formation, it does induce aggregation and membrane rupture at high peptide to lipid ratios (>1:200), and it does promote pore formation in PEG-induced model membrane fusion at lower P/L ratios (13–15). Mutants of the HA fusion protein with reduced physiological or ex-vivo membrane lytic function (G13L, G1E) have fusion peptides that do not promote pore formation, although the mechanistic basis for this remains unknown (15). Structures of N-terminal peptides from three mutants (G1S, G1V, and W14A) of the X31 strain have been proposed based on NMR and spin labeling data (16–18). Phenotypes of these three mutant viruses are also well established. This wealth of information provides an excellent opportunity to examine how viral fusion peptides catalyze fusion.
Several proposals exist for how fusion proteins (specifically fusion peptide regions) might catalyze fusion (15,19–22). Most of these relate to a mechanistic model of fusion as a multiintermediate process (“modified stalk” hypothesis) as developed for planar membranes by Siegel in terms of material properties of mesomorphic lipid phases (23) and extended by us to highly curved membranes (24). These models are not molecular in detail, although the two most critical events in the fusion process (initial intermediate formation and pore formation) are transitions between stable or semistable lipid structures of different topologies and thus must involve dramatic rearrangements of water and lipids that require molecular descriptions (24). The principal difficulties inherent in studying these transitions are that they are very short lived (most estimates indicate pores open in ∼1 to 50 ms) and likely involve only a few lipids in a very small region of interbilayer contact (probably ≲ 2 to 4 nm on edge) (25–27). Methods to experimentally detect distinct molecular rearrangements on this time and length scale do not exist. Nonetheless, recent single-event measurements demonstrate that the multiintermediate model clearly applies to single fusion events (2,28). Unfortunately, even these elegant single-event measurements lack the spatial and time resolution to report microstructural or molecular details of fusion events. Even if it were possible to resolve such structural changes on a relevant time and length scale, membranes do not move in concert through intermediate states, so statistical methods must still be used to interpret even single-event observations.
Our approach to these challenges has been to use an ensemble kinetic model that is consistent with the multiintermediate structural hypothesis (29) and that is uniquely able to provide activation thermodynamics for each step of the multistep fusion process (30). The multiintermediate nature of fusion is widely supported experimentally, and our ensemble kinetic approach has already produced reasonable hypotheses for the nature of microstructures contributing to the initial and final steps of the fusion process, which are consistent with independently performed molecular dynamics (MD) simulations (30). In this paper, we combine activation thermodynamics with measured effects of peptides on membrane structure to propose hypotheses for how WT or mutant HA fusion peptides could alter these microstructural events and thereby catalyze or inhibit fusion of highly curved membranes as triggered by PEG.
Experimental Procedures
We described the methods that are quite routine in our lab for preparing small unilamellar vesicles (SUVs), and for measuring mixing of contents and lipids between them and leakage of contents from them using fluorescence assays in the Supporting Material. In addition, we monitor peptide structure by CD (Circular Dichroism) spectroscopy, peptide binding to membranes by intrinsic fluorescence, and the influence of peptides on SUV structure using a variety of fluorescent probes, as also detailed in the Supporting Material.
Preparation of HA peptides
The native and mutants of X-31 HA peptides were chemically synthesized by standard solid phase method using Fmoc chemistry and purified by the peptide synthesis laboratory at the University of North Carolina-Chapel Hill. The sequences of the peptides are GLFGAIAGFIENGWEGMIDG (X-31 HA, native, WT), SLFGAIAGFIENGWEGMIDG (G1S), VLFGAIAGFIENGWEGMIDG (G1V) and GLFGAIAGFIENGAEGMIDG (W14A). Detailed descriptions of the synthesis and purification of WT peptides are given in previous publication (14). Stock peptide solutions were prepared in DMSO (Dimethyl sulfoxide) solvent, and small aliquots of these solutions were added to vesicle suspensions. DMSO was always less than 0.1% of the buffer volume for fusion and membrane structure experiments and 0.8% for peptide binding experiments. Control experiments in a peptide-free system showed that 0.8 volume % of DMSO had no effect on the structural properties of the bilayers or on the kinetics of fusion.
PEG-triggered SUV fusion
PEG treatment is a widely accepted method for accomplishing close contact of membranes and is thus used to mediate fusion of model membranes (31). Since membrane curvature is known to promote fusion in vitro (32) and is thought to do so in vivo (7), we use highly curved model membranes (SUVs) for our fusion studies. These are routinely prepared as described in the Supporting Material. PEG aggregates SUVs at rates ∼10-fold higher than the rate of any fusion event we have resolved at the concentrations of PEG we use. This results in an initial state of close contact between membranes referred to as “A” (see diagram in Supporting Material) from which our measurements proceed. This avoids the complication of vesicle diffusion and “docking” in our data analysis. For these reasons, PEG-mediated model membrane fusion of SUVs is an excellent mimic of biomembrane fusion (3).
Recording and analyzing the fusion time courses and calculation of activation thermodynamics
In our ensemble kinetic model, a thermodynamic state with aggregated vesicles in close contact (A) transforms to an initial intermediate state (I1) at rate k1; I1 then transforms to a second intermediate state (I2) with rate k2; and I2 on to a fusion pore (FP) state with rate k3. These are thermodynamic states that are associated with average probabilities that lipids (β’s) and contents (α’s) mix as well as average rates of leakage (λ’s). The details of how we analyze kinetic data and obtain activation thermodynamics are given in the Supporting Material and in previous publications (29,30). Briefly, global analysis of time courses of three observables (content mixing, content leakage, lipid mixing) allows us to estimate the three (or two) rate constants for interstate conversion, as well the above mentioned probabilities associated with the different thermodynamic states assumed by the model. A more detailed discussion of these probabilities and their meaning along with a diagram of the ensemble kinetic model is given in the Supporting Material. As well as proposing reasonable models for the microstructural nature of transition states between intermediate states in the fusion process (30), we have successfully utilized these methods to analyze ensemble kinetic data for the effects of peptides (15) and calcium/negatively charged lipids (33) on SUV fusion.
Results
Effect of HA fusion peptide and its mutants on kinetics of PEG-mediated SUV fusion
A low concentration of PEG (5% w/w) had no influence on the Trp fluorescence intensity, membrane binding of the peptide, or depth of peptide membrane penetration (14,15). These three independent measurements indicate that the low concentrations of PEG used in our experiments do not affect the peptide-membrane complex or the physical state of the peptide in solution.
Time courses of lipid mixing (LM), content mixing (CM), and leakage (L) were recorded at five different temperatures (26°C, 30°C, 34°C, 38°C, and 43°C) for DOPC/DOPE/SM/CH SUVs in absence and in presence of peptide (L/P = 200), with fusion induced by PEG. Fig. 1 shows the time courses of LM, CM, and L for control vesicles alone and in the presence of wild-type (WT) and mutant fusion peptides at 30°C. These data were fitted globally for each peptide to either a three-step or two-step (four-state or three-state) sequential ensemble model (15,29,30,33) to obtain the rate constants for each step and probabilities of lipid mixing (β’s) and content mixing (α’s) in each state, as listed in Table 1. Data for WT peptide at 26°C were slightly better described by the four-state model (as reported in Table 1) than by the three-state model (not shown), but either model was judged to be appropriate. Above 26°C, description of data obtained with WT peptide required only a three-state model. We interpret this to mean that either the I1 or I2 state was nearly or completely invisible in the presence of WT peptide. Black lines through the data in Fig. 1 illustrate the ability of a three-state model to account for the data.
Figure 1.

Effect of HA WT fusion peptide (green), G1S (red), G1V (cyan), and W14A (pink) mutants compared with absence of peptide (dark gray), i.e., control for kinetics of lipid mixing, contents mixing and leakage in PC/PE/SM/CH (35/30/15/20 mol%) SUVs, for fusion induced by 5wt% PEG at 30°C. Results (fraction of fluorescence intensity change) are shown for a lipid to peptide ratio 200:1. Measurements were carried out in 10 mM MES, 100 mM NaCl, 1mM CaCl2, and 1 mM EDTA, pH 5.0 at a total lipid concentration of 0.2 mM. The smooth curves through the data represent either four-state (for control, G1S and W14A) or three-state (for WT HA fusion peptide and G1V) sequential model global fit. To see this figure in color, go online.
Table 1.
Parameters defined by nonlinear regression of fusion time courses for the systems mentioned in the text at 30°C
| System | k1 x 103 (sec−1) | k2 x 103 (sec−1) | k3 x 103 (sec−1) | α1 (I1) | Contents mixing |
Lipid mixing |
Leakage |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α2 (I2) | α1 & α2 | α3 (FP) | fCM | β1 (I1) | β2 (I2) | fLM | λ0 x 104 (sec−1) (I0) | λ1 x 104 (sec−1) (I1) | λ2 x 104 (sec−1) (I2) | λ3 x 104 (sec−1) (FP) | |||||
| Control 26°C | 11.4± 0.56 | 2.71± 0.12 | 0.86± 0.09 | 0.12 ± 0.01 | 0.35 ± 0.03 | 0.47 | 0.53± 0.04 | 0.15 | 0.54± 0.07 | 0.46± 0.06 | 0.45 | 0.57 ± 0.02 | 0.33 ± 0.01 | 0.35 ± 0.01 | NA |
| HA WType 26°C | 18.8± 0.65 | 3.42± 0.11 | 0.98± 0.05 | 0.25± 0.01 | 0.40 ± 0.01 | 0.65 | 0.35 ± 0.02 | 0.21 | 0.62± 0.05 | 0.38 ± 0.05 | 0.46 | 1.09± 0.01 | 0.95± 0.05 | 0.50± 0.02 | NA |
| G1S 26° | 14.5± 0.5 | 2.94± 0.10 | 0.16± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.12 | 0.88± 0.02 | 0.12 | 0.59± 0.05 | 0.41± 0.04 | 0.39 | 2.14 ± 0.08 | 0.73 ± 0.04 | 0.26 ± 0.02 | NA |
| G1V 26°C | 17.6± 0.38 | 3.60± 0.13 | † | 0.59± 0.01 | 0.41± 0.01 | 1.0 | † | 0.11 | 0.66± 0.04 | 0.34± 0.02 | 0.39 | 7.76 ± 0.21 | 3.90 ± 0.10 | 0.41 ± 0.08 | ∗ |
| W14A 26°C | 21.6± 0.64 | 4.04± 0.14 | 0.12± 0.05 | 0.30± 0.01 | 0.16± 0.01 | 0.46 | 0.54 ± 0.02 | 0.10 | 0.68± 0.05 | 32± 0.02 | 0.38 | 15.7± 0.11 | 2.81 ± 0.04 | 0.76 ± 0.02 | NA |
| Control 30°C | 12.2± 0.59 | 3.52± 0.17 | 1.03± 0.02 | 0.38 ± 0.01 | 0.20 ± 0.01 | 0.58 | 0.42± 0.02 | 0.21 | 0.63± 0.09 | 0.37± 0.06 | 0.48 | 0.96± 0.06 | 0.94± 0.04 | 0.44± 0.02 | NA |
| HA WType 30°C | 22.3± 0.79 | † | 1.59± 0.04 | 0.44 ± 0.01 | † | 0.44 | 0.56± 0.02 | 0.29 | 0.62± 0.05 | 0.38± 0.04 | 0.46 | 1.91± 0.06 | 1.21± 0.04 | NA | 0.11± 0.03 |
| G1S 30°C | 18.8± 0.59 | 3.82± 0.11 | 0.34± 0.01 | 0.10 ± 0.01 | 0.16 ± 0.01 | 0.27 | 0.74± 0.02 | 0.15 | 0.62± 0.05 | 0.38± 0.03 | 0.41 | 1.39± 0.09 | 1.29± 0.04 | 0.40± 0.01 | NA |
| G1V 30°C | 20.8± 0.56 | 4.18± 0.12 | † | 0.60± 0.01 | 0.40± 0.01 | 1.0 | † | 0.12 | 0.67± 0.06 | 0.33± 0.03 | 0.40 | 11.7± 0.15 | 5.73± 0.08 | 0.60± 0.05 | ∗ |
| W14A 30°C | 24.7± 0.75 | 4.84± 0.18 | 0.25± 0.04 | 0.36± 0.01 | 0.18± 0.01 | 0.54 | 0.46± +0.02 | 0.11 | 0.71± 0.05 | 0.29± 0.02 | 0.41 | 18.4± 0.18 | 4.21± .07 | 1.02± 0.02 | NA |
NA: Value not available.
Only two states were observed in the presence of G1V peptide. In the Discussion, we suggest that this may be because G1V leads to a very leaky I2 state from which no FP state forms (large α2 but α3 = 0).
In the case of WT peptide, the data suggest that k3 is the rate of formation of FP from I1 (see Discussion).
The four peptides produced very different effects on PEG-mediated fusion (Table 1). All the peptides increased k1; the WT peptide did not significantly alter the fraction of lipid mixing (fLM), although G1S, G1V, and W14A all decreased fLM slightly. Although the WT peptide increased the extent of content mixing (fCM), all mutant peptides decreased fCM significantly. As noted, either the I1 or I2 state was not discernible in the presence of WT peptide at 30°C and above, so content mixing occurred in intermediate state (I) with a probability α and in the FP state with probability (1-α). A similar statement can be made about the probability of lipid mixing (β). G1V blocked formation of the FP state (k3 ≈ 0), i.e., blocked fusion; it also greatly increased leakage and triggered a high probability of content mixing early in the fusion process (α1 = 60%). W14A, similar to G1V, also dramatically enhanced the rate of leakage (12- to 20-fold). Although G1S and W14A did not block FP state formation completely, both significantly reduced k3. G1S inhibited content mixing at I1 (i.e., reduced α1 in favor of α3). W14A did not significantly redistribute the mixing of contents relative to the control. To summarize, the mutant peptides, similar to the WT peptide, increased the rate but not extent (β1∗fLM) of I1 (“stalk” (34)) formation, but all three inhibited in some way pore formation and content mixing. These behaviors are consistent with the hemi-fusion phenotype reported for G1S and W14A mutants in HA-expressed-cell– and red-blood-cell–based assays (17,18). G1V mutant inhibited lipid mixing and blocked content mixing (35) in HA-expressed-cell–based assay, again consistent with our observations with model membranes. Only the WT peptide increased the rate of both steps as well as the extent of fusion (fCM).
Binding of HA peptides to PC/PE/SM/CH (35/30/15/20) SUV
We used intrinsic tryptophan fluorescence to monitor binding of HA WT and mutant peptides to PC/PE/SM/CH membranes (13,14). As W14A lacks a tryptophan, we measured the binding of W14A to PC/PE/SM/CH membrane as described in Methods (Fig. S1in the Supporting Material). Experiments were performed at two different concentrations of the peptide and data were fitted globally to a standard surface-binding model (36). The binding parameters (Kd and N [stoichiometry]) for all four peptides are shown in Table S2. These data show that all peptides were more than ∼95% bound to membranes under the conditions of our experiments (20 to 6 μM binding sites, 1 μM peptide). We also collected CD-spectra of the WT and mutant peptides associated with POPC (1-palmitoyl-2-oleoyl-sn-3-phosphocholine) and POPC/CH membranes. The corrected spectra (37) were analyzed (method mentioned in the Supporting Material) using Dichroweb (London, United Kingdom) (38,39). Secondary structures of the WT and mutant peptides on membranes (Table S1 and Fig. S2) generally supported with proposed structures of peptides attached to a solubilizing “host peptide” and incorporated into micelles (16–18), allowing us to adopt these host-peptide models as a starting point for interpreting our data.
Effect of wild-type HA fusion peptide and its mutants on the activation thermodynamics of PEG-mediated SUV fusion
We calculated the activation free energy for the transition states for all steps in the fusion process as described in the Supporting Material. The activation free energy refers to the difference in free energy between initial states (A, I1, I2) and the transition states (TS1, TS2, TS3) for formation of I1, I2, or FP, respectively. Fig. S3 presents a hypothetical reaction diagram illustrating these. Fig. 2 A shows the temperature-dependence of (activation free energy for Step 1) for the control vesicles (no peptide). Activation thermodynamics for control system have been discussed in detail elsewhere (30), but they are reproduced here for reference. The inset to this figure shows the change in caused by WT and mutant peptides. was a nonlinear function of the reciprocal of temperature (not shown) in the presence or absence of peptides, meaning it did not follow the simple Arrhenius equation (constant Van’t Hoff enthalpy) under the conditions examined. Fig. 2, B and C show similar representations of the activation free energies for Step 2 (conversion of I1 to I2) and Step 3 (formation of the FP) for control vesicles. The activation entropies () and enthalpies were calculated and plotted in Fig. 3, A, C, and E for Steps 1, 2, and 3, respectively as described in the Supporting Material. Fig. 3, B, D, and F present calculated and for the WT and the three mutant peptides. The four peptides all altered the activation thermodynamics differently. The kinetic parameters obtained in the presence of WT and mutant peptides are summarized in Table 1. We interpret these differences along with different effects of peptides on bilayer structure and proposed peptide structures (16,17) in the Discussion and Supporting Material in terms of how theses peptides might alter the microstructures associated with initial states (A, I1, and I2) or transition states (TS1, TS2, and TS3) for the three steps of the fusion process (30).
Figure 2.
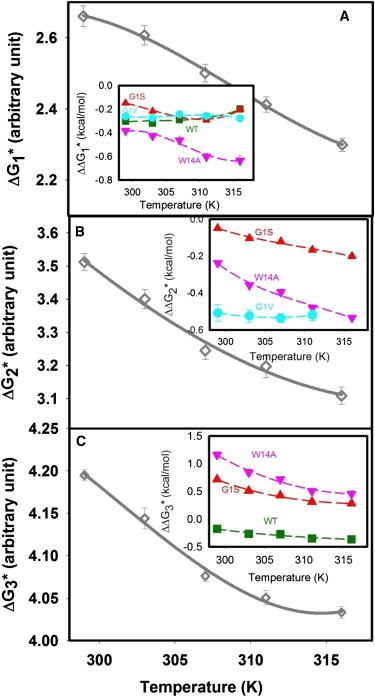
Effect of WT fusion peptide and its mutants on the activation energy barrier for different steps of membrane fusion process. The activation free energy for the formation of (A) I1 state , (B) I2 state , and (C) pore formation is plotted versus temperature for control vesicles. Insets show effect of WT fusion peptide (square), G1S (up triangle), G1V (circle), and W14A (down triangle) mutants on the activation free energies of the control displayed in each frame. To see this figure in color, go online.
Figure 3.

and for the control vesicles (in absence of peptide) are plotted against temperature for the formation of (A) I1 state, (C) I2 state, and (E) FP state. The temperature dependence of the change in () and () induced by the presence of WT and mutant fusion peptides. (solid line) and (dotted line) are plotted for (B) formation of I1 state, (D) formation of I2 state, and (F) pore formation for WT fusion peptide (green), G1S (red), G1V (cyan), and W14A (pink) mutants. To see this figure in color, go online.
Effects of HA fusion peptides on bilayer properties
We examined the effects of WT and mutant fusion peptides on bilayer properties of SUVs using C6NBDPC, DPH, and TMA-DPH, which probe three different bilayer regions and properties. Externally added C6NBDPC partitions between micelles and membranes; its fluorescence lifetime having contributions from the probe in these two environments, from which one may obtain the partition coefficient between these two phases (XC6NBDPC), which offers a measure of free volume within membrane outer leaflets (5). Fig. 4 A shows that all peptides decreased the mole fraction of C6NBDPC in the membrane over nearly the entire temperature range, reflective of a decrease in free volume upon addition of peptide, although the average ΔXC6NBDPC over the entire temperature range for WT peptide was considerably more negative than for any mutant peptide (Fig. 4 A). Because of its polarity, the NBD moiety in C6NBDPC locates in the upper region of the bilayer near the interface (40). We conclude that WT generally filled more upper bilayer space than mutant peptides. In addition, only WT peptide increased the lifetime of C6NBDPC (Fig. 4 B). The lifetime of membrane-associated C6NBDPC provides a measure of thermal motion and polarity (likely because of water penetration) in the neighborhood of the NBD probe, so an increase in lifetime must reflect either a decrease in water penetration to this region or partitioning of the polar NBD moiety further into the membrane (5). TMA-DPH reports the acyl chain ordering near the interface of the bilayer, whereas DPH reports an order parameter for the interior hydrophobic region of the bilayer. We found that only WT peptide increased DPH anisotropy at all temperature, whereas mutants did so only at low temperature (Fig. 4 C). TMA-DPH anisotropy increased at high and low temperatures in the presence of WT but remained unchanged at 308K; it increased at all temperatures for G1S peptide. G1V decreased TMA-DPH anisotropy and thus decreased interfacial order at all temperatures; whereas W14A had different effects at low and high temperatures (Fig. 4 D). In summary, WT peptide was notable compared with all the mutants in its ability to increase DPH anisotropy (Fig. 4 C) and increase C6NBDPC lifetime. It also limited C6NBDPC partitioning into the bilayer as well as or better than all mutant peptides except G1S at 295K.
Figure 4.

Effect of WT fusion peptide and its mutants on the temperature dependence of (A) change in the mole fractions of C6NBD-PC in membranes exposed to different peptides; (B) changes in fluorescent lifetime of C6NBD-PC; (C) DPH anisotropy; and (D) TMA-DPH anisotropy in membranes exposed to different peptides. Peptide-exposed vesicles are compared with control vesicles in all cases: WT fusion peptide (open square); G1S (open up triangle); G1V (open circle); and W14A (open down triangle) mutants. Measurements were carried out in 10 mM MES, 100 mM NaCl, 1 mM CaCl2, and 1 mM EDTA, pH 5.0 at a total lipid concentration of 0.2 mM and lipid/peptide ratio of 200:1 as a function of temperature.
Comparison of HA fusion peptide and 3 mol% hexadecane effects on PEG-mediated SUV fusion
The effects of 3 mol% hexadecane on PEG-mediated fusion of control vesicles have been described previously (30), and the results are summarized here for comparison with results obtained with fusion peptide plus hexadecane (Table S4). Hexadecane increased k1 by ∼29% at 30°C (Table S4, Fig. 5). Subsequent addition of WT fusion peptide increased it by an additional 16%, but to a value that was still less than the effect of WT fusion peptide alone (65%; Tables 1 and S4). The ability of hexadecane to promote I1 formation also increased less rapidly with temperature than did that of WT peptide, and hexadecane increasingly inhibited the ability of WT peptide to promote Step 1 with increasing temperature (Fig. 5 A), suggesting differences in the mechanisms of catalysis by these agents at high temperatures. However, the activation thermodynamics associated with addition of WT peptide (Fig. 3 B) as well as with addition of hexadecane (Fig. 5 B) or with the presence of both indicated enthalpic catalysis by both agents and their combination at low temperatures , suggesting possible similarities in catalytic mechanism at low temperature.
Figure 5.

Temperature dependence the free energy barrier for formation of (A) I1 state (), (B) I2 state (), and (C) pore formation () for control vesicles (open circles), vesicles in the presence of WT HA fusion peptide (open square), vesicles containing 3 mol% hexadecane (solid circle), and vesicles containing 3 mol% hexadecane in presence of WT HA fusion peptide (solid square). (D–F) show (solid lines) and (dotted lines) for the formation of I1, I2, and FP state for hexadecane containing and hexadecane plus WT peptide vesicles. Measurements were carried out in 10 mM MES, 100 mM NaCl, 1mM CaCl2, and 1 mM EDTA, pH 5.0 at a total lipid concentration was 0.2 mM. The lipid/peptide ratio was 200. To see this figure in color, go online.
Perhaps the most notable effect of hexadecane was to restore a second intermediate in the presence of WT peptide and to restore Step 3 (FP formation) in the presence of G1V mutant with a rate comparable with the effect of hexadecane alone (Tables 1 and S4). Similarly, G1S and W14A peptides severely inhibited FP formation (Table 1), and hexadecane reduced this inhibition (see Δki/ki values in Table S4). Of the four peptides, only WT increased k3, and hexadecane reversed this effect (see Δki/ki in Table S4). Since hexadecane is known to fill interstice space in nonlamellar structures, we use the different influences of hexadecane on WT and mutant peptide effects on Step 3 to suggest different mechanisms (see Discussion) by which these two classes of peptides alter Step 3.
Aside from their effects on rates, both hexadecane and the peptides alter the extent of content mixing and in what intermediate state it occurs, with the effect of hexadecane being considerably greater than that of peptides (Tables 1 and S4). Hexadecane significantly increased the extent of content mixing compared with the control, and the increase was mainly because of transient pores in states I1 and I2 (i.e., α3 decreased in favor of α1 and α2, Table S4). Since WT peptide eliminated one intermediate state, content mixing in this state moved to the final pore state (Table 1, 30°C results). Hexadecane-containing vesicles treated with WT peptide reduced the extent of content mixing to roughly that seen with WT peptide alone, and restored a significant fraction of content mixing to an intermediate state from the final pore state (Tables 1 and S4). We discuss this behavior when we address possible mechanisms of action of WT peptide on fusion. None of the peptides and hexadecane significantly altered the extent of lipid mixing.
Discussion
We set out to provide evidence for our overriding hypothesis that membrane-contacting regions of fusion proteins catalyze fusion events. We use an ensemble kinetic analysis that can establish both kinetics and activation thermodynamics of individual steps of the fusion process and show that this analysis is consistent with dwell-time distributions obtained from single-event measurements (28). Knowledge of bilayer structure along with a previously published description of fusion intermediate geometry (24) allow us to propose interpretations of activation thermodynamics. Along with this information, measurements of the influence of WT HA fusion peptide and three nonfusogenic mutants on bilayer structure provide evidence for several unique hypotheses:
-
1.
The average or ensemble structures of these peptides vary with temperature, helping to account for their observed temperature-dependent effects on kinetics and activation thermodynamics of fusion steps.
-
2.
WT HA fusion peptide promotes initial intermediate formation because of its ability to stabilize acyl chain protrusions in transition state microstructures.
-
3.
The unique ability of WT fusion peptide to impart intrinsic positive curvature to exposed leaflets of hemi-fused intermediates explains its ability to eliminate one step of the fusion process and catalyze fusion pore formation.
-
4.
Although the catalytic or inhibitory influences of mutant HA fusion peptides were all peptide specific, these generally catalyze initial intermediate formation by enthalpically destabilizing the initial aggregated state and also entropically stabilizing intermediate transition states because of the peptides’ temperature-dependent microstructural ensembles. Their abilities to impart a negative intrinsic curvature to exposed leaflets and to stabilize interstice space generally account for their inhibitory influences on pore formation.
Peptide structure and bilayer properties
The structural model of the 20-residue WT peptide linked to a polar “host peptide” suggests that it adopts an inverted V-shaped conformation in micelles and lipid bilayers with two helices joined by a “flexible loop” involving Glu11, Asn12, and Gly13 that form a “kink” with an angle of 105° (16). The C-terminal amphipathic helix (residues 16 to 20) occupies the membrane interface, whereas the N-terminal helix (residues 1 to 10) embeds in the hydrophobic interior of the bilayer to form an “inverted V” (18). A more recent NMR-based structure of a 23-residue HA fusion peptide proposes an amphipathic helical hairpin structure that lies at a micelle surface (41). These authors recently proposed that the 23-residue peptide visits several conformations (hairpin, L-shaped, inverted V) on a time scale of microseconds, with low pH favoring the inverted V and L-shaped conformations (42). We observe that WT peptide is unique in its ability to fill space in the vesicle outer leaflet (Fig. 4 A), order the bilayer interior (Fig. 4 C), and to limit water penetration (Fig. 4 B). These observations suggest that WT contributes a positive “intrinsic curvature” to reduce outer leaflet curvature stress in SUVs.
All the mutants are structurally distinct from WT. G1S has an N-terminal Ser but is reported to have a structure and kink angle on a membrane similar to the WT (18), even though our measurements of bilayer properties argue that its structure on the membrane should be significantly different from that of WT. We observed that G1S, because the N-terminal serine will be enthalpically drawn to the more polar bilayer interface, excludes water even more effectively than WT at low temperature (Fig. 4 B) and orders the interface especially at low temperature (Fig. 4 D). We hypothesize that its conformational ensemble favors the previously reported hairpin conformation (41) at low temperatures but likely broadens as temperature increases to include “inverted V” conformations. Based on these observations, G1S may relieve outer leaflet curvature stress at low temperatures but may aggravate it at higher temperatures.
The W14A mutant peptide is reported to have a “kink angle” that is ill defined relative to WT (17). Consistent with this, W14A uniquely switched from slightly producing free volume to dramatically limiting it at high temperature (Fig. 4 A). Similarly, its influence on the interface uniquely switched from ordering at low temperature to disordering at high temperature (Fig. 4 D). Unlike W14A, G1V is proposed to form a rigid helix from G4 to W14 (18), but, similar to W14A, its ability to permit water penetration (Fig. 4 B) and leakage from SUVs (Table 1) also increased dramatically with temperature. However, it was also notably more effective at disordering the interface at high temperatures than was W14A (Fig. 4 D). Thus, although they do fill upper bilayer space with increasing temperature, they likely do so by abandoning and disrupting the interface (Fig. 4 D), thus permitting increasing water penetration and leakage (Table 1). These observations are consistent with both peptides increasing, rather than decreasing, outer leaflet positive curvature stress, i.e., contributing a negative intrinsic curvature to cis leaflets, with G1V likely having the greater effect.
To summarize, although all four of these peptides are distinct, the mutant peptides seem to lack the WT peptide’s ability to contribute a positive “intrinsic curvature” to reduce outer leaflet curvature stress in SUVs or to order acyl chains.
Peptide influences on activation thermodynamics and bilayer structure provide hypotheses for mechanisms of catalysis or inhibition
The activation thermodynamics of the three steps in the fusion process for control vesicles have been published (30) and support structural models for transition states of each step of PEG-mediated fusion: Step 1—chain protrusion into dehydrated space between closely apposed vesicles; Step 2—continuous evolution of structures with a single topology whose energies can be estimated from bulk lipid material properties (see (24)); and Step 3—correlated rearrangements of two or more lipids at stressed edges of single-bilayer intervesicle diaphragms (TMC (Trans-membrane contact) or ETMC geometries in Fig. S3) to reduce both negative curvature stress and interstice stress (30). We now propose that the effects of WT and mutant HA peptides on activation thermodynamics and on bilayer properties can support hypotheses for how these peptides might alter the microstructural ensembles constituting the intermediates and transition states of the fusion process. In doing so, we rely on our knowledge of the bilayer state of the SUV model system as well as on a calculation of the energetics of the free-energy-minimized semistable intermediates in the fusion process (24).
First step: Initial intermediate formation
The activation free energies for each step of the fusion process result from large entropic and enthalpic contributions leading to a relatively small activation free energy (entropy/enthalpy compensation) (30). Thus, each step is said to be enthalpically or entropically allowed . We will refer to the catalytic influence of fusion peptides in similar terms: entropic catalysis versus enthalpic catalysis . Step 1 of the fusion process for PEG-mediated SUV fusion is entropically allowed (30). The activation thermodynamics of Step 1 were proposed to reflect a transition state (TS1 in Fig. S3) involving exposure and movement of lipid acyl chain into the dehydrated interbilayer space between contacting cis leaflets (30). This proposal is in line with a coarse grain simulation of stalk formation between flat bilayers at low hydration and high temperature (43) and with an all atom MD simulation of highly curved bilayers in close contact (44), a system somewhat akin to our experimental system. We address the influence of WT HA and mutant fusion peptides on Step 1 in the context of this proposal.
Only WT peptide enthalpically catalyzed Step 1 over the entire temperature range (Fig. 3 B). WT peptide was distinguished by its ability to fill free volume and to order bilayer acyl chains (Fig. 4, A and C). This should allow it to fill the hydrophobic voids left by acyl chain movement into interbilayer space in TS1 and reduce the enthalpic cost of water entering this space . It will also reduce the entropic gain of removing water from the highly ordered interbilayer space (45) . A change of from positive to negative with temperature (Fig. S4) is consistent with WT peptide promoting acyl chain protrusion that breaks very rigid water structure in the interbilayer space (45) and replaces it with hydrophobic hydration of acyl chains. At higher temperatures, the effect of fewer and increasingly enthalpically favorable states outweighs the hydrophobic effect and becomes negative. This proposal for the catalytic role of WT peptide on Step 1 is consistent with the predictions of a very recent all-atom simulation that predicts that WT peptide should increase the probability of acyl chain protrusions (46).
In summary, our structural and activation thermodynamic observations suggest that WT peptide catalyzes Step1 by filling hydrophobic voids left in TS1 monolayers because of acyl chain protrusion. This contrasts with previous suggestions that WT fusion peptides favor fusion by altering the initial state A (19,20).
A detailed discussion of the roles of mutant peptides in catalyzing Step 1 is given in the Supporting Material. Briefly: 1) G1S’s conformational ensemble makes it ineffective in compensation for acyl chain protrusion but capable of disrupting ordered water in the interbilayer space at low temperature, whereas its apparent ability to contribute a negative intrinsic curvature to exposed SUV leaflets destabilizes state A at high temperatures. 2) Both the G1V and W14A peptides appear to possess broad, temperature-dependent conformational ensembles that destabilize state A by contributing a negative intrinsic curvature to exposed leaflets, but also by entropically stabilizing TS1 by increasing the number of microstructures contributing to it, perhaps by partially entering the interbilayer space.
Second step
The time courses obtained with WT peptide were well described by a model involving only one intermediate. A single intermediate reaction in the presence of WT peptide indicates that either I2 could be destabilized i.e., (I2 free energy is approximately that of TS2), allowing I1 to proceed directly to TS3 without passing through a semistable I2 intermediate; or I1 could evolve without any significant barrier to I2 (i.e., TS2 free energy lowered), which would then proceed via TS3 to FP. For the sake of consistency with results obtained in the absence of fusion peptide, we maintain the nomenclature that k3 is the rate of pore formation and ΔG3∗ the transition state energy for pore formation. As noted, however, it is uncertain at this point whether k3 in the presence of WT peptide is the rate of I1 → FP or I2 → FP.
In the absence of fusion peptides, ΔH2∗ ≳ TΔS2∗ > 0, and ΔCp2∗ < 0 (30), consistent with the increase in positive bending stress and the decrease in interstice energy at the trans-membrane contact (TMC) geometry that roughly defines TS2 (Fig. S3) (24,30). As for Step 1, a detailed discussion of mutant peptides’ influences on Step 2 is reserved to the Supporting Material to save space. The limited catalytic influence of G1S on Step 2 appears to reflect its ability to impart slight intrinsic negative curvature to the exposed cis leaflet of TS2, thereby lowering its free energy (TMC geometry in Fig. S3). The catalytic effect of W14A appears to derive from its ability to contribute a negative intrinsic curvature to the exposed monolayer. Finally, the catalytic influence of G1V is proposed to reflect both a substantial ability to impart both a negative intrinsic curvature to the exposed leaflet in TS2 and to reduce interstice energy in this transition state. These effects of G1V not only should lower the free energy of TS2 but also should allow it to dramatically stabilize I2, thus blocking FP formation (see Step 3).
Third step: WT peptide influence on final pore formation
The stalk must expand to or beyond the TMC structure to transform into a fusion pore (24). Any hemi-fused structure that proceeds beyond the TMC structure has “extended trans-membrane contact” (ETMC) geometry (Fig. S3) and has stressed cis and trans leaflets at its circumference. Both the I2 and the TS3 ensembles have ETMC geometries; the only difference being their different average stalk radii: r2 for I2 and rS∗, where rS∗is a critical stalk radius beyond which TS3 is unlikely to persist and a final fusion pore is very likely (30). In the absence of WT peptide, rS∗ is greater than r2, and the system must pass through I2 to reach rS∗. Fig. S3 illustrates that the TMC and ETMC cross-sections have enormous negative curvature stress in both cis and trans leaflets at the periphery of their single bilayer diaphragms. Expansion of the ETMC diaphragm increases total cis leaflet negative curvature stress, thereby relieving the unfavorable positive curvature stress of SUV outer leaflets. This produces a driving force for stalk radius expansion (24). Opposing this is unfavorable interstice energy reflective of the mismatch between lamellar structures and the nonlamellar ETMC circumference (24). The larger the stalk radius, the larger the ETMC circumference and thus the greater the total negative curvature stress and unfavorable interstice energy. Our hypothesis for pore formation (30) is that both curvature stress and interstice energy at the ETMC circumference can be relieved by coordinated movement of lipids from stressed cis and trans leaflets (Fig. S3) into interstices to create a local, pore-like fluctuation. The presence of such microstructures in the intermediate state ensemble can account for the probabilities αi, but at rS∗, become so numerous or unstable that a final pore (FP state) forms (30).
Our membrane structure measurements show that WT peptide was almost unique in contributing a positive “intrinsic curvature” to the cis monolayer and reducing positive curvature stress in the SUV outer leaflet. This contrasts with the proposal that WT peptide has a negative intrinsic curvature, which is based on the observation that both phosphatidylethanolamine (with a known intrinsic negative curvature) and WT peptide promote hexagonal phase formation (47). However, fusion peptides also decrease bending modulus, which would also lower the free energy barrier to a hexagonal phase (21). Thus, the observation that WT peptide promotes hexagonal phase formation need not imply that it has a negative intrinsic curvature. In support of WT peptide imparting a positive intrinsic curvature to stressed cis leaflets is the observation that it favors highly curved cubic phases that require reduced negative Gaussian curvature more than do mutant peptides (22). We thus submit that an important effect of the WT peptide should be its ability to provide a positive intrinsic curvature to the cis leaflet of fusion intermediates.
The ability of WT peptide to impart a positive intrinsic curvature to exposed cis leaflets can explain the disappearance of the I2 state in the presence of WT peptide (Tables 1 and S3). Small increases in positive intrinsic curvature dramatically increase the barrier to I2 formation and destabilize I2 (24). This appears inconsistent with the aforementioned possibility that I1 decays spontaneously to I2, but it is consistent with the possibility that an evolving ETMC geometry bypasses an unstable I2 to directly reach the rS∗ in TS3. The fact that k2 at 26°C ≳ k3 at 30°C means that the free energy barrier to reach rS∗ directly from I1 is apparently still quite large, and rS∗ may still be larger than r2. However, it could also be the positive intrinsic curvature of WT peptide so destabilizes the ETMC circumference that the frequency or size of pore-like microstructural fluctuations becomes sufficient that rS∗ moves to stalk radii comparable with that of I2, i.e., the I2 state would never be reached. We cannot distinguish between these possibilities, but in both cases the ability of WT peptide to impart a positive intrinsic curvature to exposed cis leaflets is consistent with the loss of the second intermediate and promotion of pore formation. This hypothesis is supported by the ability of hexadecane to restore I2 (Tables 1 and S4) in that the decrease in interstice energy because of hexadecane addition will stabilize I2 and lower the TS2 (TMC) barrier (24).
Work of some researchers has proposed that fusion occurs directly from an unstable “stalk” structure (48), a proposal apparently at odds with our hypothesis for pore formation from an ETMC or at least a TMC geometry. A field theoretical extension of this work using simplified coarse potentials further predicted that the highly stressed edges of stalks stabilize pores form “stalk-pore complexes” (26), but the dimensions of the “stalk” at these complexes are roughly as we would identify for a TMC or ETMC structure (24). Another coarse-grained treatment that included HA fusion peptides predicted what was described as a bicontinuous (interlocking water and hydrophobic regions) “diamond phase” (adjacent pores and stalks) that was stabilized by WT but not mutant peptides (25). Again the dimensions of this peptide-promoted stalk was ∼4 nm, roughly what we would identify as an ETMC (24). Thus, what these authors call “stalks” could well be TMC or ETMC structures by our definition. In addition, what they call “stalk-pore complexes” are consistent with our view that evolution of I1 toward FP proceeds through an ETMC or minimally a TMC geometry, with pore formation because of correlated lipid movements at the strained peripheries of these structures (37).
As for Steps 1 and 2, a discussion of proposed mechanisms for mutant peptides’ inhibition of Step 3 is provided in the Supporting Material. G1V has the greatest ability to contribute intrinsic negative curvature to the merged cis leaflets, promotes ETMC expansion, and lowers the free energy of I2 (37). Thus, we propose that G1V blocks FP state formation by stabilizing I2 and decreasing the driving force (reducing positive cis leaflet curvature stress) for reaching a stalk radius of rS∗. Consistent with this, hexadecane restores FP formation in the presence of G1V peptide (Table S4) and appears to promote ETMC expansion and destabilize I2 sufficiently to overcome the influence of G1V’s negative curvature (24). W14A also contributes intrinsic negative curvature to the merged cis leaflets and inhibits pore formation by stabilizing I2.
The G1S mutant was proposed to impart slight negative intrinsic curvature at high temperature and thus inhibit pore formation in a similar way. At low temperature, it does not fill space (Fig. 5 A) and is unable to promote ETMC expansion by filling interstice space and subsequently inhibit fusion. For mutants, we are led to believe that the introduction of a more negative intrinsic curvature (mainly enthalpic in nature) has a greater influence than the ability to reduce interstice energy (mainly entropic). These two competing influences also likely account the observed > > 0 for mutant peptides. Detailed discussion on the effect of mutant peptides on the pore formation is in the Supporting Material.
Wild-type HA Peptide and interstice energy
We had previously hypothesized that WT HA peptide promotes fusion by occupying hydrophobic space and reducing interstice energy (13,49), although we could not comment until recently on the step(s) at which this influence might occur (15). A more careful examination of kinetics over a temperature range, as presented in this study, discounts this proposal (Tables 1 and S4 and Fig. 5). Fig. 5 A shows that the effects of hexadecane and WT peptide on Step 1 diverge enormously as temperature increases, and even significantly oppose each other. Based on our observations and the discussion so far, we propose that these two agents catalyze Step 1 by different mechanisms, especially at high temperatures: 1) WT peptide specifically orders the bilayer interior and thereby promotes acyl chain excursions into the interbilayer space, whereas 2) hexadecane can align with acyl chains at low temperatures but at high temperatures, it can only occupy hydrophobic free volume and reduce interstice energy (30). This explains why its catalytic ability alone or in the presence of WT peptide is so temperature dependent (Fig. 5 A). The fact that hexadecane increasingly inhibited the catalytic influence of WT peptide on Step 1 with increasing temperature (Fig. 5 A) supports the conclusion that the catalytic mechanism by which WT peptide operates is not primarily because of reducing interstice energy.
The differing influences of WT fusion peptide and hexadecane on Step 2 further argue that WT peptide does not promote fusion by the interstice-filling mechanism of hexadecane. Although WT peptide eliminated Step 2 because of its positive contribution to cis leaflet curvature, hexadecane increased k2 at higher temperatures (Fig. 5 B) because of its interstice-filling ability.
The effects of hexadecane and WT peptide appear to be more similar for Step 3 in that both WT peptide and hexadecane decrease with similar temperature dependencies (Fig. 5 C) and catalyze Step 3 in an entropic fashion (Figs. 3 F and 5 F). However, the catalytic influence of WT peptide on Step 3 was largely reversed by addition of hexadecane, as was the effect of hexadecane reversed by addition of WT peptide (Fig. 5 C). Again, this is consistent with the prediction that increased positive intrinsic curvature and decreased interstice energy have opposing influences (24).
We conclude that WT peptide does not catalyze any step of the fusion process in a manner similar to that of hexadecane, thereby discounting the hypothesis that WT peptide operates only (or even significantly) by filling space and lowering interstice energy (13,49).
Acknowledgments
This work is supported by USPHS grant GM32707 to BRL.
Footnotes
Hirak Chakraborty’s present address is Centre for Cellular and Molecular Biology, Council of Scientific and Industrial Research, Hyderabad 500 007, India.
Supporting Material
References
- 1.Melikyan G.B., Niles W.D., Cohen F.S. Comparison of transient and successful fusion pores connecting influenza hemagglutinin expressing cells to planar membranes. J. Gen. Physiol. 1995;106:803–819. doi: 10.1085/jgp.106.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon T.Y., Okumus B., Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J., Lentz B.R. Secretory and viral fusion may share mechanistic events with fusion between curved lipid bilayers. Proc. Natl. Acad. Sci. USA. 1998;95:9274–9279. doi: 10.1073/pnas.95.16.9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanturiya A., Chernomordik L.V., Zimmerberg J. Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 1997;94:14423–14428. doi: 10.1073/pnas.94.26.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J., Lentz B.R. Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry. 1997;36:6251–6259. doi: 10.1021/bi970404c. [DOI] [PubMed] [Google Scholar]
- 6.Haque M.E., McIntosh T.J., Lentz B.R. Influence of lipid composition on physical properties and peg-mediated fusion of curved and uncurved model membrane vesicles: “nature’s own” fusogenic lipid bilayer. Biochemistry. 2001;40:4340–4348. doi: 10.1021/bi002030k. [DOI] [PubMed] [Google Scholar]
- 7.Lentz B.R., Malinin V., Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr. Opin. Struct. Biol. 2000;10:607–615. doi: 10.1016/s0959-440x(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 8.Leikina E., Markovic I., Kozlov M.M. Delay of influenza hemagglutinin refolding into a fusion-competent conformation by receptor binding: a hypothesis. Biophys. J. 2000;79:1415–1427. doi: 10.1016/S0006-3495(00)76393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markosyan R.M., Melikyan G.B., Cohen F.S. Tension of membranes expressing the hemagglutinin of influenza virus inhibits fusion. Biophys. J. 1999;77:943–952. doi: 10.1016/S0006-3495(99)76945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White J.M. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- 11.Melikyan G.B., Niles W.D., Cohen F.S. The fusion kinetics of influenza hemagglutinin expressing cells to planar bilayer membranes is affected by HA density and host cell surface. J. Gen. Physiol. 1995;106:783–802. doi: 10.1085/jgp.106.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gething M.J., Doms R.W., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J. Cell Biol. 1986;102:11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque M.E., Koppaka V., Lentz B.R. Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion. Biophys. J. 2005;89:3183–3194. doi: 10.1529/biophysj.105.063032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque M.E., McCoy A.J., Lentz B.R. Effects of hemagglutinin fusion peptide on poly(ethylene glycol)-mediated fusion of phosphatidylcholine vesicles. Biochemistry. 2001;40:14243–14251. doi: 10.1021/bi011308l. [DOI] [PubMed] [Google Scholar]
- 15.Haque M.E., Chakraborty H., Lentz B.R. Hemagglutinin fusion peptide mutants in model membranes: structural properties, membrane physical properties, and PEG-mediated fusion. Biophys. J. 2011;101:1095–1104. doi: 10.1016/j.bpj.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X., Bushweller J.H., Tamm L.K. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- 17.Lai A.L., Park H., Tamm L.K. Fusion peptide of influenza hemagglutinin requires a fixed angle boomerang structure for activity. J. Biol. Chem. 2006;281:5760–5770. doi: 10.1074/jbc.M512280200. [DOI] [PubMed] [Google Scholar]
- 18.Li Y.L., Han X., Tamm L.K. Membrane structures of the hemifusion-inducing fusion peptide mutant G1S and the fusion-blocking mutant G1V of influenza virus hemagglutinin suggest a mechanism for pore opening in membrane fusion. J. Virol. 2005;79:12065–12076. doi: 10.1128/JVI.79.18.12065-12076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epand R.F., Martin I., Epand R.M. Membrane orientation of the SIV fusion peptide determines its effect on bilayer stability and ability to promote membrane fusion. Biochem. Biophys. Res. Commun. 1994;205:1938–1943. doi: 10.1006/bbrc.1994.2897. [DOI] [PubMed] [Google Scholar]
- 20.Zemel A., Ben-Shaul A., May S. Modulation of the spontaneous curvature and bending rigidity of lipid membranes by interfacially adsorbed amphipathic peptides. J. Phys. Chem. B. 2008;112:6988–6996. doi: 10.1021/jp711107y. [DOI] [PubMed] [Google Scholar]
- 21.Tristram-Nagle S., Nagle J.F. HIV-1 fusion peptide decreases bending energy and promotes curved fusion intermediates. Biophys. J. 2007;93:2048–2055. doi: 10.1529/biophysj.107.109181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenchov B.G., MacDonald R.C., Lentz B.R. Fusion peptides promote formation of bilayer cubic phases in lipid dispersions. An x-ray diffraction study. Biophys. J. 2013;104:1029–1037. doi: 10.1016/j.bpj.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel D.P. The modified stalk mechanism of lamellar/inverted phase transitions and its implications for membrane fusion. Biophys. J. 1999;76:291–313. doi: 10.1016/S0006-3495(99)77197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinin V.S., Lentz B.R. Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys. J. 2004;86:2951–2964. doi: 10.1016/S0006-3495(04)74346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhrmans M., Marrink S.J. Molecular view of the role of fusion peptides in promoting positive membrane curvature. J. Am. Chem. Soc. 2012;134:1543–1552. doi: 10.1021/ja207290b. [DOI] [PubMed] [Google Scholar]
- 26.Katsov K., Müller M., Schick M. Field theoretic study of bilayer membrane fusion: II. Mechanism of a stalk-hole complex. Biophys. J. 2006;90:915–926. doi: 10.1529/biophysj.105.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malinin V.S., Haque M.E., Lentz B.R. The rate of lipid transfer during fusion depends on the structure of fluorescent lipid probes: a new chain-labeled lipid transfer probe pair. Biochemistry. 2001;40:8292–8299. doi: 10.1021/bi010570r. [DOI] [PubMed] [Google Scholar]
- 28.Kyoung M., Srivastava A., Brunger A.T. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinreb G., Lentz B.R. Analysis of membrane fusion as a two-state sequential process: evaluation of the stalk model. Biophys. J. 2007;92:4012–4029. doi: 10.1529/biophysj.106.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty H., Tarafdar P.K., Lentz B.R. Activation thermodynamics of poly(ethylene glycol)-mediated model membrane fusion support mechanistic models of stalk and pore formation. Biophys. J. 2012;102:2751–2760. doi: 10.1016/j.bpj.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lentz B.R. PEG as a tool to gain insight into membrane fusion. Eur. Biophys. J. 2007;36:315–326. doi: 10.1007/s00249-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 32.Talbot W.A., Zheng L.X., Lentz B.R. Acyl chain unsaturation and vesicle curvature alter outer leaflet packing and promote poly(ethylene glycol)-mediated membrane fusion. Biochemistry. 1997;36:5827–5836. doi: 10.1021/bi962437i. [DOI] [PubMed] [Google Scholar]
- 33.Tarafdar P.K., Chakraborty H., Lentz B.R. Phosphatidylserine inhibits and calcium promotes model membrane fusion. Biophys. J. 2012;103:1880–1889. doi: 10.1016/j.bpj.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlov M.M., Leikin S.L., Chizmadzhev Y.A. Stalk mechanism of vesicle fusion. Intermixing of aqueous contents. Eur. Biophys. J. 1989;17:121–129. doi: 10.1007/BF00254765. [DOI] [PubMed] [Google Scholar]
- 35.Qiao H., Armstrong R.T., White J.M. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol. Biol. Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppaka V., Lentz B.R. Binding of bovine factor Va to phosphatidylcholine membranes. Biophys. J. 1996;70:2930–2937. doi: 10.1016/S0006-3495(96)79863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty H., Lentz B.R. A simple method for correction of circular dichroism spectra obtained from membrane-containing samples. Biochemistry. 2012;51:1005–1008. doi: 10.1021/bi300025c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitmore L., Wallace B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmore L., Wallace B.A. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008;89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 40.Slater S.J., Ho C., Stubbs C.D. Contribution of hydrogen bonding to lipid-lipid interactions in membranes and the role of lipid order: effects of cholesterol, increased phospholipid unsaturation, and ethanol. Biochemistry. 1993;32:3714–3721. doi: 10.1021/bi00065a025. [DOI] [PubMed] [Google Scholar]
- 41.Lorieau J.L., Louis J.M., Bax A. The complete influenza hemagglutinin fusion domain adopts a tight helical hairpin arrangement at the lipid: water interface. Proc. Natl. Acad. Sci. USA. 2010;107:11341–11346. doi: 10.1073/pnas.1006142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorieau J.L., Louis J.M., Bax A. pH-triggered, activated-state conformations of the influenza hemagglutinin fusion peptide revealed by NMR. Proc. Natl. Acad. Sci. USA. 2012;109:19994–19999. doi: 10.1073/pnas.1213801109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smirnova Y.G., Marrink S.J., Knecht V. Solvent-exposed tails as prestalk transition states for membrane fusion at low hydration. J. Am. Chem. Soc. 2010;132:6710–6718. doi: 10.1021/ja910050x. [DOI] [PubMed] [Google Scholar]
- 44.Kasson P.M., Lindahl E., Pande V.S. Atomic-resolution simulations predict a transition state for vesicle fusion defined by contact of a few lipid tails. PLOS Comput. Biol. 2010;6:e1000829. doi: 10.1371/journal.pcbi.1000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasson P.M., Lindahl E., Pande V.S. Water ordering at membrane interfaces controls fusion dynamics. J. Am. Chem. Soc. 2011;133:3812–3815. doi: 10.1021/ja200310d. [DOI] [PubMed] [Google Scholar]
- 46.Larsson P., Kasson P.M. Lipid tail protrusion in simulations predicts fusogenic activity of influenza fusion peptide mutants and conformational models. PLOS Comput. Biol. 2013;9:e1002950. doi: 10.1371/journal.pcbi.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colotto A., Epand R.M. Structural study of the relationship between the rate of membrane fusion and the ability of the fusion peptide of influenza virus to perturb bilayers. Biochemistry. 1997;36:7644–7651. doi: 10.1021/bi970382u. [DOI] [PubMed] [Google Scholar]
- 48.Müller M., Katsov K., Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys. J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haque M.E., Lentz B.R. Influence of gp41 fusion peptide on the kinetics of poly(ethylene glycol)-mediated model membrane fusion. Biochemistry. 2002;41:10866–10876. doi: 10.1021/bi020269q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


