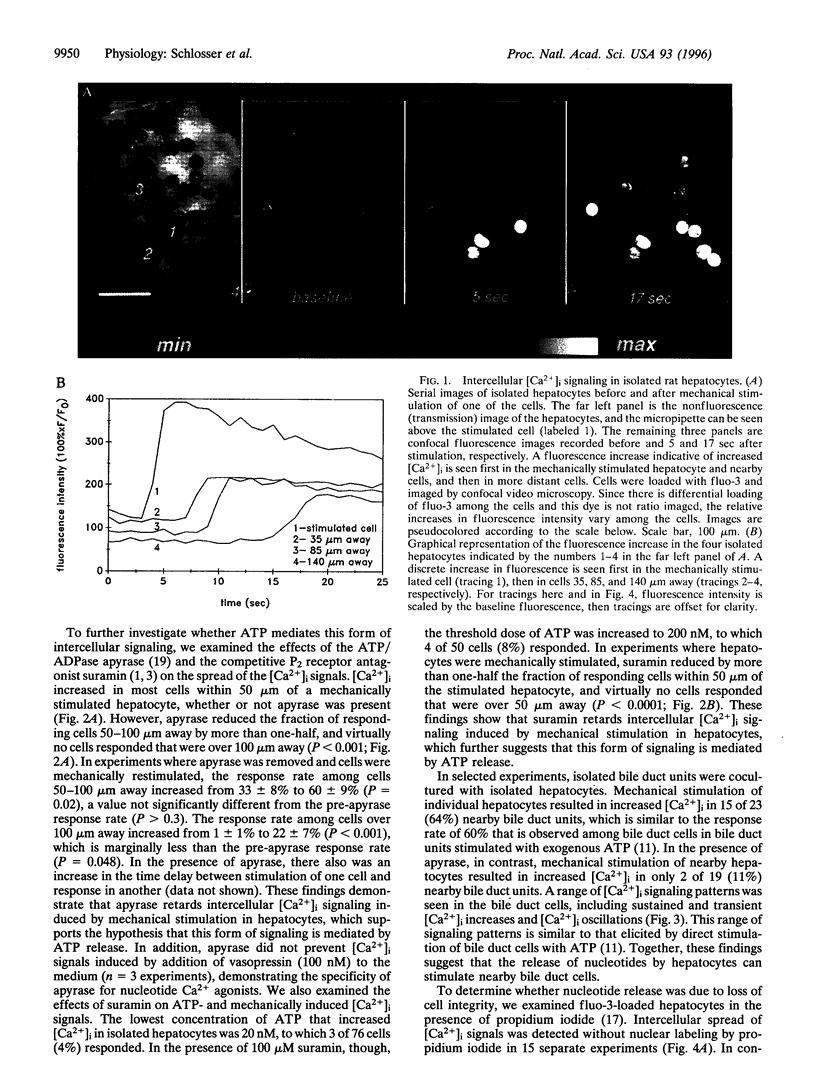
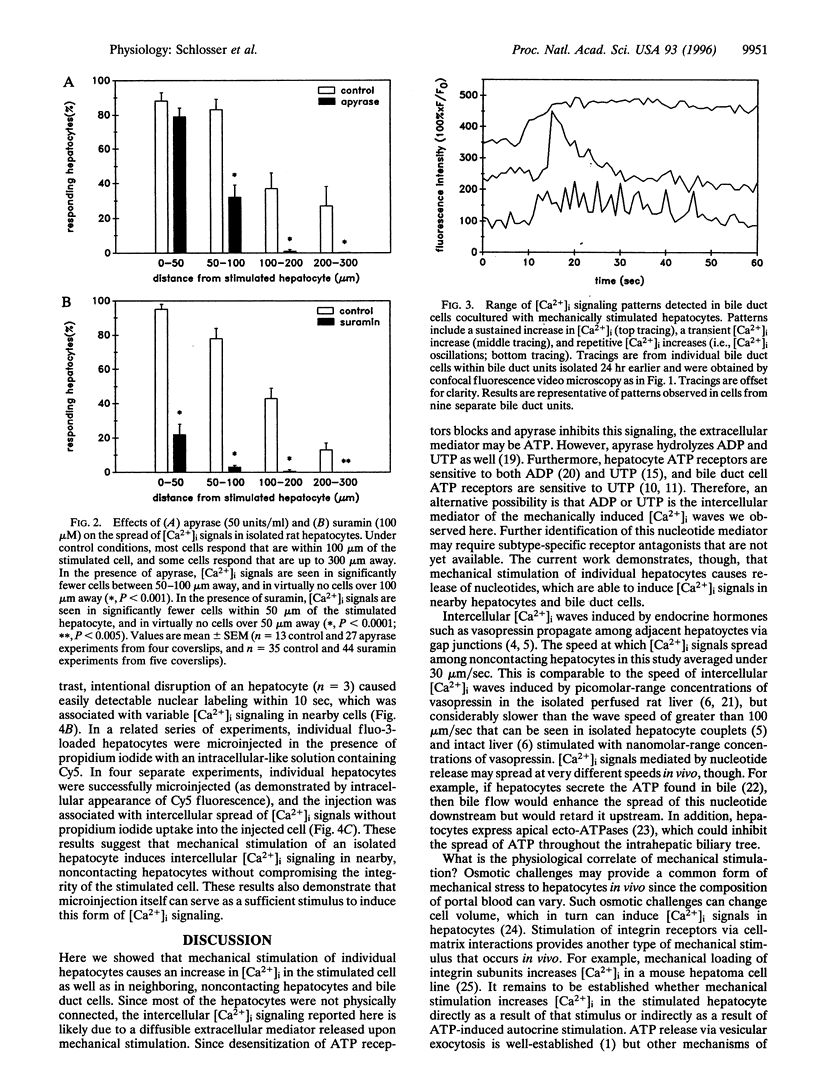
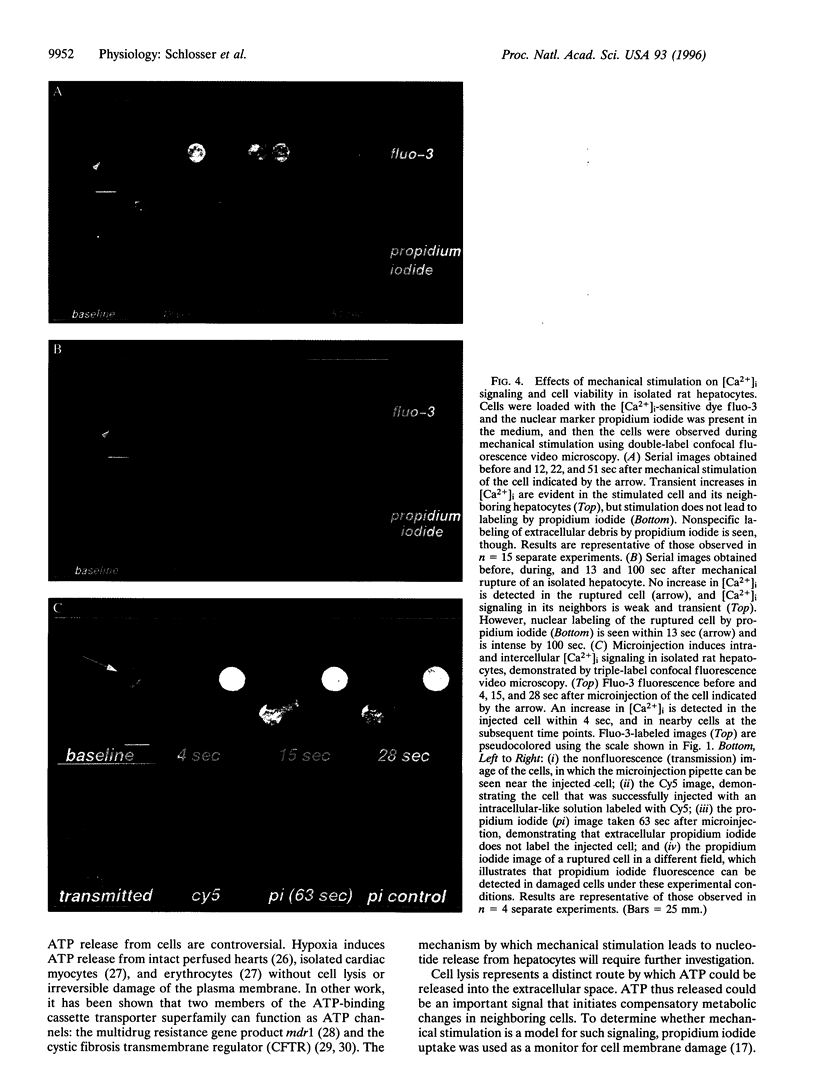
Abstract
Intercellular communication among certain cell types can occur via ATP secretion, which leads to stimulation of nucleotide receptors on target cells. In epithelial cells, however, intercellular communication is thought to occur instead via gap junctions. Here we examined whether one epithelial cell type, hepatocytes, can also communicate via nucleotide secretion. The effects on cytosolic Ca2+ ([Ca2+]i) of mechanical stimulation, including microinjection, were examined in isolated rat hepatocytes and in isolated bile duct units using confocal fluorescence video microscopy. Mechanical stimulation of a single hepatocyte evoked an increase in [Ca2+]i in the stimulated cell plus an unexpected [Ca2+]i rise in neighboring noncontacting hepatocytes. Perifusion with ATP before mechanical stimulation suppressed the [Ca2+]i increase, but pretreatment with phenylephrine did not. The P2 receptor antagonist suramin inhibited these intercellular [Ca2+]i signals. The ATP/ADPase apyrase reversibly inhibited the [Ca2+]i rise induced by mechanical stimulation, and did not block vasopressin-induced [Ca2+]i signals. Mechanical stimulation of hepatocytes also induced a [Ca2+]i increase in cocultured isolated bile duct units, and this [Ca2+]i increase was inhibited by apyrase as well. Finally, this form of [Ca2+]i signaling could be elicited in the presence of propidium iodide without nuclear labeling by that dye, indicating that this phenomenon does not depend on disruption of the stimulated cell. Thus, mechanical stimulation of isolated hepatocytes, including by microinjection, can evoke [Ca2+]i signals in the stimulated cell as well as in neighboring noncontacting hepatocytes and bile duct epithelia. This signaling is mediated by release of ATP or other nucleotides into the extracellular space. This is an important technical consideration given the widespread use of microinjection techniques for examining mechanisms of signal transduction. Moreover, the evidence provided suggests a novel paracrine signaling pathway for epithelia, which previously were thought to communicate exclusively via gap junctions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. H., Prat A. G., Gerweck L., Seneveratne T., Arceci R. J., Kramer R., Guidotti G., Cantiello H. F. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M. Multidrug resistance genes, p-glycoprotein and the liver. Hepatology. 1990 Jul;12(1):159–165. doi: 10.1002/hep.1840120125. [DOI] [PubMed] [Google Scholar]
- Baquet A., Meijer A. J., Hue L. Hepatocyte swelling increases inositol 1,4,5-trisphosphate, calcium and cyclic AMP concentration but antagonizes phosphorylase activation by Ca2(+)-dependent hormones. FEBS Lett. 1991 Jan 14;278(1):103–106. doi: 10.1016/0014-5793(91)80094-j. [DOI] [PubMed] [Google Scholar]
- Berven L. A., Hughes B. P., Barritt G. J. A slowly ADP-ribosylated pertussis-toxin-sensitive GTP-binding regulatory protein is required for vasopressin-stimulated Ca2+ inflow in hepatocytes. Biochem J. 1994 Apr 15;299(Pt 2):399–407. doi: 10.1042/bj2990399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G. S., Burgess G. M., Putney J. W., Jr Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J Biol Chem. 1993 Aug 25;268(24):17917–17923. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Chari R. S., Schutz S. M., Haebig J. E., Shimokura G. H., Cotton P. B., Fitz J. G., Meyers W. C. Adenosine nucleotides in bile. Am J Physiol. 1996 Feb;270(2 Pt 1):G246–G252. doi: 10.1152/ajpgi.1996.270.2.G246. [DOI] [PubMed] [Google Scholar]
- Clemens M. G., Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar;312:143–158. doi: 10.1113/jphysiol.1981.sp013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori O., Traverso-Cori A., Tetas M., Chaimovich H. Substrate specificity and inhibition studies on potato apyrase. Biochem Z. 1965 Aug 6;342(3):345–358. [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. Evidence for two Ca2(+)-mobilizing purinoceptors on rat hepatocytes. Biochem J. 1990 Jul 15;269(2):499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of phosphoinositides in the regulation of liver function. Hepatology. 1988 Jan-Feb;8(1):152–166. doi: 10.1002/hep.1840080129. [DOI] [PubMed] [Google Scholar]
- Forrester T. Release of ATP from heart. Presentation of a release model using human erythrocyte. Ann N Y Acad Sci. 1990;603:335–352. doi: 10.1111/j.1749-6632.1990.tb37684.x. [DOI] [PubMed] [Google Scholar]
- Gaisano H. Y., Wong D., Sheu L., Foskett J. K. Calcium release by cholecystokinin analogue OPE is IP3 dependent in single rat pancreatic acinar cells. Am J Physiol. 1994 Jul;267(1 Pt 1):C220–C228. doi: 10.1152/ajpcell.1994.267.1.C220. [DOI] [PubMed] [Google Scholar]
- Graf J., Boyer J. L. The use of isolated rat hepatocyte couplets in hepatobiliary physiology. J Hepatol. 1990 May;10(3):387–394. doi: 10.1016/0168-8278(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Grierson J. P., Meldolesi J. Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995 Mar 3;270(9):4451–4456. doi: 10.1074/jbc.270.9.4451. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Yang L. J., Williamson J. R. Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem. 1991 Oct 5;266(28):18573–18579. [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Brauneis U., Gatmaitan Z., Arias I. M. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991 Oct;14(4 Pt 1):640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- Koike M., Kashiwagura T., Takeguchi N. Gluconeogenesis stimulated by extracellular ATP is triggered by the initial increase in the intracellular Ca2+ concentration of the periphery of hepatocytes. Biochem J. 1992 Apr 1;283(Pt 1):265–272. doi: 10.1042/bj2830265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Russell W. E. Two Ca2+-dependent ATPases in rat liver plasma membrane. The previously purified (Ca2+-Mg2+)-ATPase is not a Ca2+-pump but an ecto-ATPase. J Biol Chem. 1988 Sep 5;263(25):12253–12258. [PubMed] [Google Scholar]
- McGill J. M., Basavappa S., Mangel A. W., Shimokura G. H., Middleton J. P., Fitz J. G. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology. 1994 Jul;107(1):236–243. doi: 10.1016/0016-5085(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Mennone A., Alvaro D., Cho W., Boyer J. L. Isolation of small polarized bile duct units. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6527–6531. doi: 10.1073/pnas.92.14.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P., Bodin P., Loesch A., Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990 Jul 31;170(2):649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Burgstahler A. D. Coordination of hormone-induced calcium signals in isolated rat hepatocyte couplets: demonstration with confocal microscopy. Mol Biol Cell. 1992 Jan;3(1):113–121. doi: 10.1091/mbc.3.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. H., Burgstahler A. D., Mennone A., Boyer J. L. Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol. 1996 Jul;271(1 Pt 1):G86–G96. doi: 10.1152/ajpgi.1996.271.1.G86. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Burgstahler A. D., Mennone A., Fallon M. B., Gonzalez C. B., Saez J. C. Ca2+ waves are organized among hepatocytes in the intact organ. Am J Physiol. 1995 Jul;269(1 Pt 1):G167–G171. doi: 10.1152/ajpgi.1995.269.1.G167. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H. Cellular and subcellular calcium signaling in gastrointestinal epithelium. Gastroenterology. 1994 May;106(5):1349–1364. doi: 10.1016/0016-5085(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Gautam A., Bruck R., Isales C. M., Boyer J. L. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992 Jan;15(1):107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Gautam A., Ng O. C., Bruck R., Boyer J. L. Hormonal regulation of paracellular permeability in isolated rat hepatocyte couplets. Am J Physiol. 1992 Jun;262(6 Pt 1):G1079–G1086. doi: 10.1152/ajpgi.1992.262.6.G1079. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Mariwalla K. Characterization and function of ATP receptors on hepatocytes from the little skate Raja erinacea. Am J Physiol. 1996 Mar;270(3 Pt 2):R561–R570. doi: 10.1152/ajpregu.1996.270.3.R561. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Moyer M. S., Burgstahler A. D., O'Carroll A. M., Brownstein M. J., Lolait S. J. Mechanisms of subcellular cytosolic Ca2+ signaling evoked by stimulation of the vasopressin V1a receptor. J Biol Chem. 1992 Nov 15;267(32):23282–23289. [PubMed] [Google Scholar]
- Nebe B., Rychly J., Knopp A., Bohn W. Mechanical induction of beta 1-integrin-mediated calcium signaling in a hepatocyte cell line. Exp Cell Res. 1995 Jun;218(2):479–484. doi: 10.1006/excr.1995.1181. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y., Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992 Sep 17;359(6392):241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Reisin I. L., Prat A. G., Abraham E. H., Amara J. F., Gregory R. J., Ausiello D. A., Cantiello H. F. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994 Aug 12;269(32):20584–20591. [PubMed] [Google Scholar]
- Robb-Gaspers L. D., Thomas A. P. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995 Apr 7;270(14):8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Egan M. E., Hwang T. H., Fulmer S. B., Allen S. S., Cutting G. R., Guggino W. B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995 Jun 30;81(7):1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Stauffer P. L., Zhao H., Luby-Phelps K., Moss R. L., Star R. A., Muallem S. Gap junction communication modulates [Ca2+]i oscillations and enzyme secretion in pancreatic acini. J Biol Chem. 1993 Sep 15;268(26):19769–19775. [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993 Aug 27;74(4):661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Phillips M. J. Ca2+ causes active contraction of bile canaliculi: direct evidence from microinjection studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6164–6168. doi: 10.1073/pnas.81.19.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]