Abstract
Purpose
The expression of genes regulated by estrogen in the uterus was studied in ovariectomized (OVX) rats treated with germinated brown rice (GBR) bioactives, and compared to Remifemin or estrogen at different doses to identify the regulation of these genes in the uterus and their molecular mechanisms.
Methods
Rats were treated orally with GBR bioactives (phenolics), acylated steryl glucosides (ASG), γ-amino butyric acid (GABA), and γ-oryzanol (ORZ) at 100 and 200 mg/kg, Remifemin (REM) at 10 mg/kg and 20 mg/kg, or estrogen (EST) at 0.2 mg/kg. Ribonucleic acid (RNA) was extracted from the uterus, and messenger (m)RNA expression of selected genes encoding estrogen receptor-beta (ER-β), calcium-binding protein (CaBP9k), complement protein (C3), heat shock protein 70 kDa (HSP70), and interleukin (IL)-4 receptor were quantified. Similarly, serum steroid hormone concentration was monitored at 2, 4, and 8 weeks after treatments. ER-β antibody binding to the uterus sections was also studied using immunohistochemistry.
Results
The group treated with EST (0.2 mg/kg) upregulated ER-β, C3, and IL-4 receptor genes compared to other groups (P<0.001). GBR phenolics (200 mg/kg) treatment upregulated the ER-β gene almost to the level of the sham non-treated group. The CaBP9k gene showed upregulation in groups treated with ASG (200 mg/kg), EST (0.2 mg/kg), and ORZ (200 mg/kg) (P<0.05). Estrogen levels increased in groups treated with EST, ASG, and ORZ (200 mg/kg) compared to the OVX untreated group (P<0.05), and there was a slight non-significant decrease (P>0.05) in the progesterone levels in the OVX untreated group compared to the sham and other treated groups. There was a significant increase at 8 weeks in the level of FSH (P<0.05) in the treated groups compared to the OVX untreated group. There was no significant difference (P>0.05) in serum luteinizing hormone (LH) between the OVX untreated group and other groups. The sham and GBR phenolics treated group showed ER-β reactivity at the glandular epithelium, while the group treated with EST showed immunoreactivity at the glandular, luminal, and stromal epithelium.
Conclusion
GBR phenolics moderately regulate the expression of ER-β, HSP70, and IL-4 receptor genes, and gave a positive immunoreaction to ER-β antigen in the uterus. ASG regulates the expression of CaBP9k and IL-4 receptor genes, and ORZ regulates the expression of the CaBP9k gene, while GABA at 100 mg/kg regulates the expression of the HSP70 gene. GBR and its bioactives might have an effect on estrogen-regulated genes in the uterus of rats.
Keywords: estrogen receptor-β gene, GBR-bioactives, serum hormonal level, uterine tissue
Introduction
Targeted therapy is an important aspect of therapy that aims to reduce toxicity and complications. Estrogen is an important hormone that plays a significant role in the modulation of uterine tissue activity. A significant decrease in circulating estrogen due to a decrease in its production by the ovaries triggers many complications ranging from uterine atrophy, urine incontinence, vaginal dryness, and various infections.1–5 Clinical attempts to reverse the effects of low estrogen level in the body by estrogen and replacement of other hormones such as progesterone, have been implicated with the occurrence of different types of cancers and inflammations.5,6 Selective estrogen receptor (ER) agonist/antagonist drugs such as Tamoxifen and Raloxifen serve as a substitute to hormone therapy due to their selective modulatory effects on estrogen-induced tissues such as the uterus. However, these drugs have also been linked to uterine cancer, venous thrombosis, pulmonary embolism, blood clots, teratogenicity, and cataracts.7–9 Different compounds, especially the phytoestrogens and other related bioactives are now the focus of scientific research to replace the conventional hormonal therapy due to the reported side effects of the latter. Some of these compounds have been reported to have a utero-tropic effect, but the molecular mechanisms underlying their estrogenic activity are not clear. A number of studies revealed that the process of germinating brown rice (GBR) increases the level of phenolics and antioxidant activity of GBR which leads to an increase in its bioactivity.10–12 γ-amino butyric acid (GABA), a natural tranquilizer, was also reported to increase after germinating brown rice,13–16 while Britz et al and Usuki et al reported an increase in concentration and bioactivity of γ-oryzanol (ORZ) and acylated steryl glucosides (ASG), respectively.17,18 The steroidal hormones are known for their diverse physiological functions in the body. Female sex steroids, specifically the estrogens and progestins, are synthesized from the ovaries to stimulate the secondary sex and reproductive characteristics. Estradiol (E2) has been reported to stimulate uterine and vaginal epithelial proliferation in vivo, and also plays a role in uterine and vaginal growth and adult function – it is necessary for normal epithelial morphogenesis, cytodifferentiation, and secretory activity in these organs, and the ER, which functions as a ligand-activated transcription factor to turn on target genes in E2-responsive tissues, is the target in which estrogen elicits its main actions.19 Two ER types have been recognized: ER-α and ER-β.20,21 ER-β is a member of the steroid receptor superfamily, and detectable levels of ER-β messenger ribonucleic acid (mRNA) and protein have been detected in the rodent uterus and vagina.22–24 The expression of ER-β was investigated using various procedures such as Northern blotting, reverse transcription polymerase chain reaction (RT-PCR), and in situ hybridization, and a prominent expression was found to exist in tissues such as the prostate, ovary, epididymis, testis, bladder, uterus, lung, thymus, colon, small intestine, pituitary, hypothalamus, cerebellum, and brain cortex.21,25–28 ER-β has been reported to modulate the activities of ER-α in the uterus;29 therefore, studying the activity of bioactive compounds on this particular receptor in the uterus will give more convincing clues to understanding their activity.
Identification and characterization of genes that regulate the action of estrogen serves as the fundamental stage of the study of its mechanism of action in the uterus and other related organs.30 Naciff et al31 reported that not all compounds with estrogenic activity act alike, and the exposure of substances to estrogen sensitive tissue changes their gene expression profile and their analysis could provide a proper understanding of the estrogenic effect of different compounds. Hewitt et al32 studied the expression of genes in rat uteri with the aim of determining whether the expression correlates with the physiological or biological response observed. ER-β, calcium-binding protein (CaBP9k), complement protein (C3), heat shock protein 70 kDa (HSP70), and interleukin (IL)-4 receptor, have been reported to be among the most important estrogen-induced genes in the uterus.30 In our previous study, we observed that treating ovariectomized (OVX) rats with bioactives from GBR modulates the activity of uterine cells.33 The present study was designed to evaluate the expression of genes related to estrogen regulation, steroid hormone concentrations, and ER-β immunohistological expression in the uterus of OVX rats treated with GBR bioactives, Remifemin (REM), or estrogen for 8 weeks.
Materials and methods
Chemicals, drugs, enzyme-linked immunosorbent assay kits, and primers
Rat estradiol enzyme-linked immunosorbent assay (ELISA) kit (catalog No CSB-E05110r) was procured from Cusabio Biotech Ltd (Hubei, People’s Republic of China). Progesterone, follicle stimulating hormone (FSH), and luteinizing hormone (LH) ELISA kits were obtained from ADALTIS EIAgen (Rome, Italy). ER-β primary antibody (NB 120–3577) and goat anti-rabbit immunoglobulin (Ig) G secondary antibody (horseradish peroxidase [HRP]) conjugated, were purchased from Novus Biologicals (Littleton, CO, USA). Cimicifuga racemosa (Remifemin® 20 mg/tab) was purchased from Schaper and Brummer GmbH and Co (Salzgitter, Germany). Conjugated estrogen (Premarin® 0.625 mg/tab) was procured from Wyeth Medica Ireland (Newbridge Co, Kildare, Ireland). Primers were designed using the National Center of Biological Information (NCBI) website and purchased from Biosune (Shanghai, People’s Republic of China), while the internal control (Kanr) was from Beckman Coulter (Miami, FL, USA). All other solvents were of analytical grade and were purchased from Merck KGaA (Darmstadt, Germany).
Brown rice and bioactives extraction
The brown rice was provided by PadiBeras Nasional Berhad (BERNAS; Selangor, Malaysia). GBR phenolics, GABA, ASG, and ORZ were extracted and quantified using high performance liquid chromatography diode array detector (HPLC-DAD; Agilent Technologies, Santa Clara, CA, USA) and GC-MS/MS qqq (gas chromatography–mass spectrometry, triple quadrupole) (Thermo Fischer Scientific, Logan, CA, USA) as previously reported.18,34–36
Treatment groups
A total of 78 Sprague Dawley rats, 12 weeks of age weighing 250–260 g were used in this study. They were acclimatized for 2 weeks before commencement of the experiment. They were randomly assigned into 13 groups of six rats each. The study was carried out according to the guidelines for the use of animals as approved by the Animal Care and Use Committee Faculty of Medicine, University Putra Malaysia. Rats in group 1 were sham operated and served as the control group. Rats in groups 2–13 were OVX under general anesthesia using a combination of xylazine and ketamine. Treatments were administered orally for a period of 8 weeks once daily. Rats in group 2 were OVX without treatment. Groups 3, 4, and 5 were treated with 0.2 mg/kg EST, and 10 mg/kg and 20 mg/kg REM, respectively. Rats in groups 6 and 7 were treated with GBR phenolics at the dose rate of 100 mg/kg and 200 mg/kg, respectively. Groups 8 and 9 were treated with ASG at 100 mg/kg and 200 mg/kg, respectively. Groups 10 and 11 were treated with 100 mg/kg and 200 mg/kg of GABA, respectively, while groups 12 and 13 were treated with ORZ at 100 mg/kg and 200 mg/kg, respectively. Figure 1 summarizes the study design.
Figure 1.
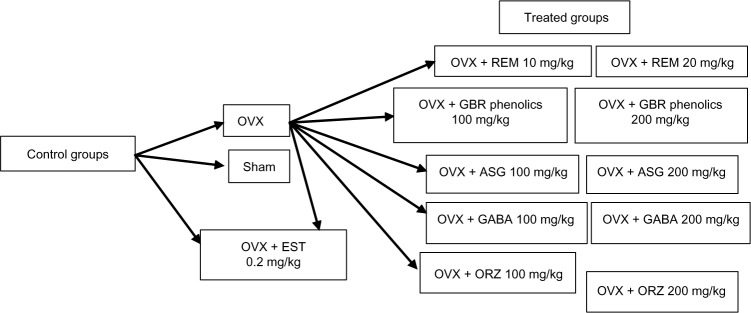
Flow chart of the study design, showing the control and the treatment groups.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin.
Blood sampling
Blood sampling was done under anesthesia at the second, fourth and eighth weeks. Blood was allowed to clot before centrifugation for 15 minutes at 1,000 × g. Serum was obtained and stored at −20°C until analysis.
RNA extraction and gene expression protocols
RNA was extracted from the uterus using the HiYield Total RNA Mini Kit® from Real Biotech (Taipei, Taiwan) following the manufacturer’s instructions. After extraction, yield and purity were measured using a Nanodrop spectrophotometer at 260 nm, and quality was determined using the Agilent 2100 Bioanalyzer (Agilent Technologies).
Primers were prepared according to the manufacturer’s instructions. Stocks were diluted in nuclease-free water to a concentration of 500 nM for the reverse primer, and 200 nM for the forward primer. Each panel contained the five genes of interest plus an internal control gene and normalization genes. Among the normalization genes, cyclophilin A gene gave consistent results and was chosen for normalizing the data for all the genes of interest. Reverse primers, consisting of 20 nucleotides complementary to the target, were tagged to a 19-nucleotide universal reverse sequence, while the forward primers, consisting of 20 nucleotides corresponding to the target gene, were tagged to an 18 nucleotide universal forward sequence as shown in Table 1.
Table 1.
Gene description, accession number, primer sequence, and the product size of the selected genes
| Gene description | Accession number | Primer sequence | Product size | Comment |
|---|---|---|---|---|
| Peptidylprolyl isomerase A | NM_017101 | F: 5′-AGGTGACACTATAGAATATTCTGTAGCTCAGGAGAGCA-3′ R: 5′-GTACGACTCACTATAGGGATTGAAGGGGAATGAGGAAAA-3′ |
145 | House-keeping gene |
| Estrogen receptor-β | NM_012754 | F: 5′-AGGTGACACTATAGAATATGGGTATCATTACGGCGTTT-3′ R: 5′-GTACGACTCACTATAGGGAATAACACTTGCGAAGTCGGC-3′ |
197 | Gene related to uterine proliferation |
| Calcium binding protein | X16635 | F: 5′-AGGTGACACTATAGAATA-3′ R: 5′-TTCAGGAGGCTGGAGAAGTTGGA-3′ |
257 | Gene related to uterine proliferation |
| Complement protein | NM_016994 | F: 5′-AGGTGACACTATAGAATACTGTTGCATAACCCAGCCTT-3′ R: 5′-GTACGACTCACTATAGGGAACGGACAGCCACAGTTTTGT-3′ |
277 | Gene related to uterine proliferation |
| Heat shock 70kD protein 1A | NM_031971 | F: 5′-AGGTGACACTATAGAATATCTTGTCTGCCTCCGATTTC-3′ R: 5′-GTACGACTCACTATAGGGACGAAGGCGTAGAGATTCCAG-3′ |
297 | |
| Interleukin-4 receptor | NM_133380 | F: 5′-AGG TGA CAC TAT AGA ATA TCC GCA CTT CTA CGT GTG AG-3′ R: 5′-GTA CGA CTC ACT ATA GGG ATG GAG TGT GAG GTT GTC TGG-3′ |
317 | Inflammation |
| Kanr | Internal control |
RNA samples (250 ng) were reverse transcribed with multiplex universal reverse primers. Reactions were performed following the protocols stated in the GenomeLab™ Start Kit from Beckman Coulter using a thermal-cycler (Hangzhou Bioer Technology Co., Ltd., Zhejiang, People’s Republic of China) according to the manufacturer’s specifications.
The PCR was carried out in a reaction mixture containing 9.3 μL of the complementary deoxyribonucleic acid (cDNA) from the reverse transcription reaction product, 2 μL of 200 nM forward universal primer set mix, and 4 μL 25 mM MgCl2, 0.7 μL Thermo Start Taq DNA polymerase, and 4 μL of 5× PCR master mix buffer. Amplification was done initially by denaturation at 95°C for 10 minutes, then by 35 two-step cycles of 94°C for 30 seconds and 55°C for 30 seconds, ending in a single extension cycle of 68°C for 1 minute in an XP Thermal Cycler.
PCR products were separated using a GeXPS machine (Beckman Coulter, Brea, CA, USA) and were diluted in water at a ratio of 2:8, and 1 μL of this solution was added to 38.5 μL sample loading solution with 0.5 μL of DNA. The data were analyzed using the fragment analysis module of the gene expression machine, then inserted into profiler software.
Serum hormonal assays (ELISA)
Serum hormonal concentrations were all detected using a spectrophotometer (Thermo Lab Systems ELISA reader OPSYS MR, Thermo Life Sciences, Basingstoke, UK), according to the manufacturer’s protocols. Standards were plotted using the CurveExpert version 1.4.
Estradiol
The assay was performed on a 96-well plate. A blank well was set without any solution, 50 μL of the standard, or the unknown sample was pipetted into the other wells in triplicate, and 50 μL of each HRP-conjugate and antibody were added to the wells and mixed and incubated for 2 hours at 37°C. The wells were washed three times with 200 μL washing buffer, then plates were inverted and blotted on a clean paper towel before adding 50 μL of substrate A and B to each well, and then incubated in the dark for 15 minutes at 37°C. Then 50 μL of the stop solution was added to each well and the optical density was measured at 450 nm, using a spectrophotometer, and the minimal detectable concentration was <15 pg/mL.
Progesterone, FSH, and LH
Enzyme immunoassays were used to quantify the concentration of progesterone, FSH, and LH using a kit and following the manufacturer’s instructions (ADALTIS EIAgen). Briefly, 25 μL of the calibrator, control, or sample were pipetted into the appropriate wells of 96 well plates, then 50 μL of the conjugate was added and the plate was mixed gently for 20–30 seconds, then 50 μL of biotin was added directly into the contents and swirled for 20–30 seconds and incubated at room temperature for an hour. Contents were discarded and plates were inverted and blotted on a clean paper towel and the wells were washed three times with 350 μL of washing buffer before 100 μL of TMB (3,3′, 5,5′-tetramethylbenzidine) substrate was added, and the plates were incubated for 20 minutes at 25°C. Next, 50 μL of the stop solution was added to each well and the optical density was measured using a microplate reader at 450 nm within 30 minutes. The minimum detectable range for progesterone, LH, and FSH was <0.4 ng/mL, 0.15 mIU/mL, and 0.25 mIU/mL, respectively.
Immunohistochemistry
The detection of ER-β immunoreactivity was done following an earlier work by Weihua et al29 with some amendments. Uterine sections (3 μm) were mounted on gelatin coated glass slides, deparaffinized in dilutions of xylol, and rehydrated in graded alcohol, then washed with distilled water. Antigen retrieval was done by placing the sections in 10 mmol citrate buffer, pH 6.0, for 10 minutes at 50 W in a microwave, then cooled at room temperature for 5 minutes. Bovine serum albumin (BSA; 5%) was used on the slide to cover non-specific binding. Sections were then incubated with H2O2 (3%) for 30 minutes to block endogenous peroxidase activity and washed in phosphate-buffered saline (PBS) with 2% Tween 20 (PBST) and distilled water. ER-β was used as the primary antibody, diluted in PBS at a concentration of 2 μg/mL for an hour, then rinsed in PBST and reacted with goat anti-rabbit secondary antibody diluted with PBS containing 0.2% BSA at a ratio of 1:200 for 10 minutes at room temperature, reactions were developed in 3,3 diaminobenzidine (DAB) in chromagen solution (Dako Cytomation, Copenhagen, Denmark) and counterstained with methylene blue for 2 minutes, then sections were cleared in xylene and coverslipped for examination under a light microscope.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Differences were determined by one-way analysis of variance (ANOVA) and mean comparison by Tukey–Kramer post hoc test, using JMP statistical software, version 10.0.0 (SAS Institute Inc, Cary, NC, USA). Differences were considered significant at a level of P<0.05.
Results
Gene expression
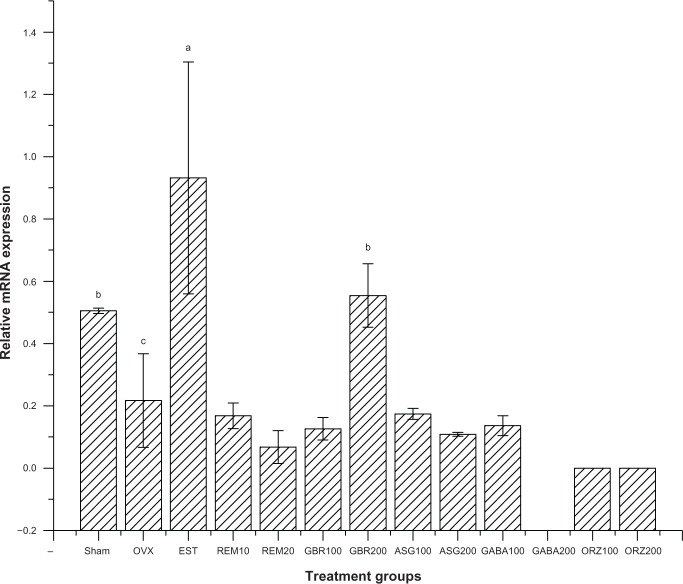
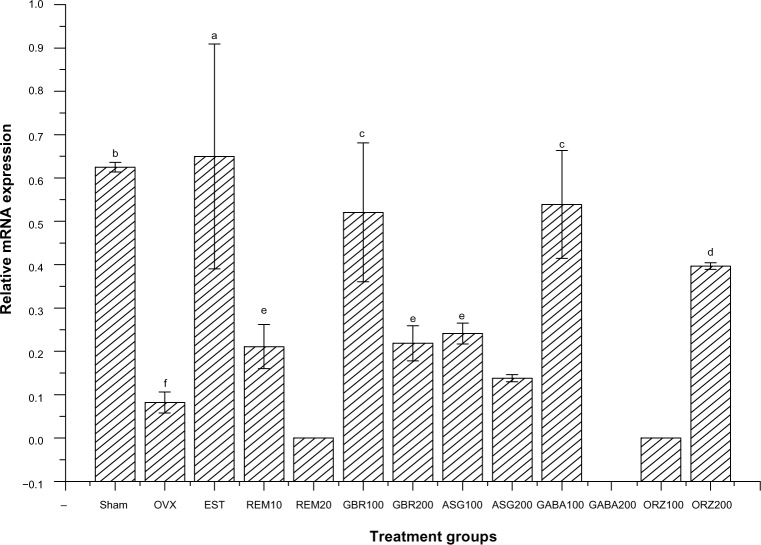
Results for the primer sequence generated from NCBI with the gene descriptions, their accession numbers, and product sizes are described in Table 1. Cyclophilin A served as the housekeeping gene and Kanr as the internal control, while the other five genes are related to estrogen regulation as shown in Table 1. Results for the relative expression of the five genes showed that the group treated with EST (0.2 mg/kg) upregulated ER-β, C3, and IL-4 receptor genes compared to all other treated groups (P<0.001). GBR phenolics (200 mg/kg) treatment upregulated the ER-β gene almost to the level of the sham non-treated group. The CaBP9k gene showed upregulation in groups treated with ASG (200 mg/kg), EST (0.2 mg/kg), and ORZ (200 mg/kg; P<0.05) as described in Figures 2–6.
Figure 2.
Relative messenger RNA expression of ER-β gene in the uterus of OVX rats treated with GBR bioactives, EST, or REM, orally for 8 weeks.
Notes: Levels not connected by the same letters are significantly different (P<0.05); group treated with EST 0.2 mg/kg showed an upregulation of ER-β gene when compared to other treatment groups (P<0.001); group treated with GBR 200 mg/kg also showed an upregulation of ER-β gene compared to other groups (P<0.05).
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; mRNA, messenger ribonucleic acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; ER-β, estrogen receptor-β.
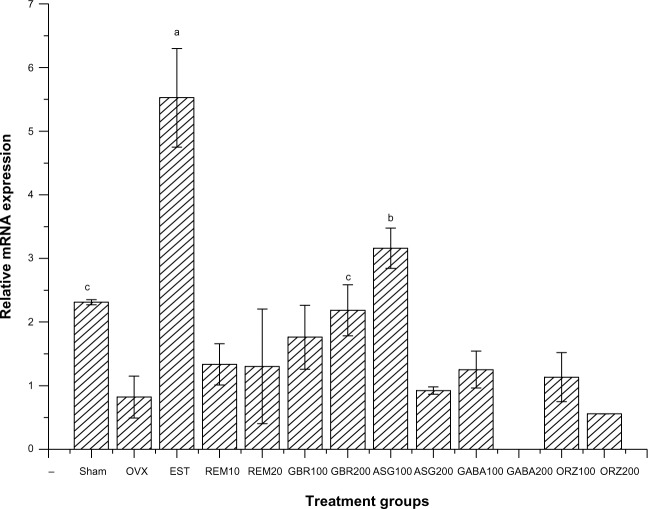
Figure 6.
Relative mRNA expression of the IL-4 receptor gene in the uterus of OVX rats treated with GBR bioactives, EST, or REM, orally for 8 weeks.
Notes: Levels with different letters are significantly different (P<0.05); group treated with EST 0.2 mg/kg showed higher expression of the IL-4 receptor gene compared to all other groups (P<0.05); group treated with ASG100 mg/kg also induces a high upregulation when compared to other treated groups (P<0.05); group treated with GBR 200 mg/kg induces an upregulation of the IL-4 receptor gene similar to the expression exhibits by sham non-treated group.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; IL, interleukin; mRNA, messenger ribonucleic acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin.
Expression of ER-β gene
Groups treated with 0.2 mg/kg EST showed an upregulation of the ER-β gene compared to the OVX untreated group and all other treated groups (P<0.05). The group treated with GBR phenolics showed an upregulation of ER-β gene almost equal to the expression showed by sham untreated group, but significantly higher (P<0.05) when compared with other treated groups (Figure 2).
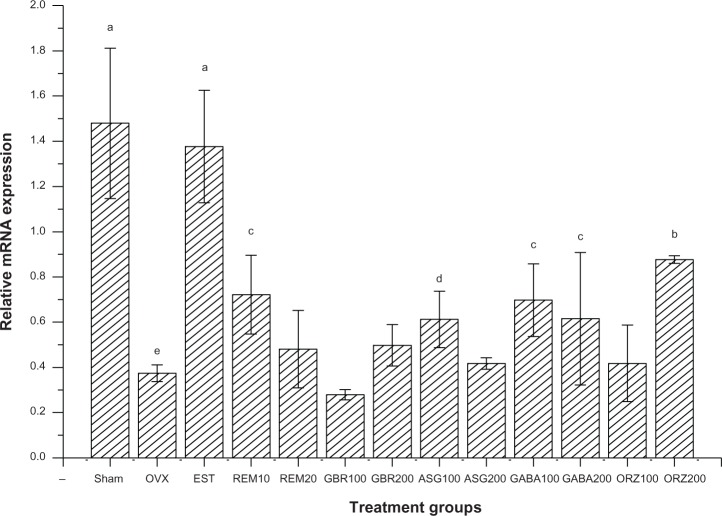
Expression of the CaBP9k gene
Relative mRNA showed an upregulation of the CaBP9k gene in groups treated with 200 mg/kg ASG, 0.2 mg/kg EST, and 200 mg/kg ORZ. Groups treated with 10 mg/kg REM and 200 mg/kg GBR showed an upregulation to the same level with that of sham untreated groups, and greater than the OVX untreated group (P<0.05) as shown in Figure 3.
Figure 3.
Relative mRNA expression of the CaBP9k gene in the uterus of OVX rats treated with GBR bioactives, EST, or REM, orally for 8 weeks.
Notes: Levels not connected by the same letters are significantly different (P<0.05); groups treated with ASG 200 mg/kg, EST 0.2 mg/kg, and ORZ 200 mg/kg showed an upregulation of the CaBP9k gene compared to other treated groups (P<0.05).
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; mRNA, messenger ribonucleic acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; CaBP9k, calcium-binding protein.
Expression of the complement protein gene
Relative mRNA expression in groups treated with 0.2 mg/kg EST showed an upregulation of the C3 gene compared to other treated groups (P<0.05). Groups treated with 10 mg/kg REM, 100 mg/kg ASG, 100 mg/kg and 200 mg/kg GABA, and 200 mg/kg ORZ showed an upregulation of the C3 gene compared to the OVX untreated group (P<0.05; Figure 4).
Figure 4.
Relative mRNA expression of the C3 gene in the uterus of OVX rats treated with GBR bioactives, EST, or REM, orally for 8 weeks.
Notes: Levels not connected by the same letters are significantly different (P<0.05); groups treated with EST 0.2 mg/kg showed an upregulation of the C3 gene compared to other treated groups (P<0.05).
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; mRNA, messenger ribonucleic acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; C3, complement protein.
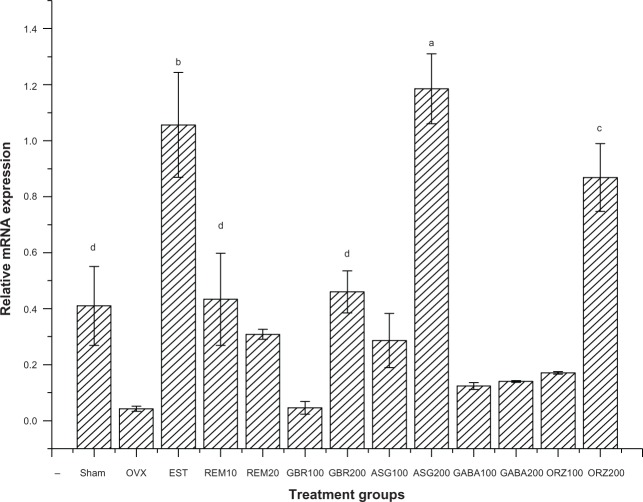
Expression of the HSP70 gene
Relative mRNA expression in groups treated with 0.2 mg/kg EST showed higher expression of HSP70 compared to other groups (P<0.05). Groups treated with GBR and 100 mg/kg GABA also showed higher expression of HSP70 compared to other groups (P<0.05). Groups treated with 10 mg/kg REM, 200 mg/kg GBR, and 100 mg/kg ASG showed an upregulation of HSP70 when compared to the OVX untreated group (P<0.05; Figure 5).
Figure 5.
Relative mRNA expression of the HSP70 gene in the uterus of OVX rats treated with GBR bioactives, EST, or REM, orally for 8 weeks.
Notes: Levels with different letters are significantly different (P<0.05); group treated with EST 0.2 mg/kg showed higher expression of the HSP70 gene compared to other groups (P<0.05); groups treated with GBR and GABA 100 mg/kg also showed higher expression of the HSP70 gene compared to other groups (P<0.05).
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; mRNA, messenger ribonucleic acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; HSP70, heat shock protein 70 kDa.
Expression of the IL-4 receptor gene
Relative mRNA expression in the group treated with 0.2 mg/kg EST showed higher expression of the IL-4 receptor gene compared to other groups (P<0.05). Groups treated with 100 mg/kg ASG also upregulated IL-4 receptor when compared to other treated groups (P<0.05). The group treated with 200 mg/kg GBR phenolics showed an upregulation to the same level as the sham non-OVX, and was higher than the OVX untreated group (P<0.05; Figure 6).
Sex steroid hormones
Estradiol
A significant decrease in serum estrogen concentration was observed in the OVX untreated group when compared to the sham untreated group and the group treated with 0.2 mg/kg EST (P<0.001). Significant increase in serum estrogen levels was also observed when groups treated with ASG and ORZ at the dose of 200 mg/kg were compared to GABA 100 mg/kg and 200 mg/kg, GBR 100 mg/kg and 200 mg/kg, REM 10 mg/kg and 20 mg/kg, or ASG and ORZ 100 mg/kg (P<0.05). Serum estrogen concentration increased but not significant (P>0.05) in groups treated with GABA 100 mg/kg and 200 mg/kg, GBR 100 mg/kg and 200 mg/kg, REM 10 mg/kg and 20 mg/kg, or ASG and ORZ 100 mg/kg compared to OVX untreated group.
Progesterone
There was a slight non-significant decrease (P>0.05) in progesterone levels in the OVX untreated group compared to the sham and all other treated groups (Table 2).
Table 2.
Serum sex steroid hormone concentration in OVX rats after 8 weeks of intervention with GBR phenolics, ASG, GABA, ORZ, EST, and REM at various doses administered orally
| Groups | Estrogen (pg/mL)
|
Progesterone (ng/mL)
|
FSH (mlU/mL)
|
LH (mlU/mL)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2nd week | 4th week | 8th week | 2nd week | 4th week | 8th week | 2nd week | 4th week | 8th week | 2nd week | 4th week | 8th week | |
| SHAM | 38.23±1.43a | 34.87±1.76a | 35.32±1.06a | 0.76±0.14 | 0.64±0.03 | 0.72±0.13 | 1.96±0.04a | 2.64±0.05a | 4.21±0.05b | 0.13±0.03 | 0.13±0.01 | 0.14±0.03 |
| OVX | 17.83±0.43c | 15.31±1.06c | 16.74±0.47c | 0.64±0.21 | 0.51±0.03 | 0.54±0.06 | 2.08±0.07a | 2.10±0.09a | 2.21±0.03a | 0.13±0.03 | 0.13±0.04 | 0.13±0.03 |
| EST0.2 | 32.64±0.37b | 30.39±1.35b | 30.29±0.68b | 0.57±0.17 | 0.65±0.03 | 0.63±0.07 | 2.10±0.02a | 2.88±0.04a | 8.74±0.03b | 0.14±0.03 | 0.13±0.03 | 0.14±0.03 |
| REM10 | 21.52±1.08c | 21.32±0.92c | 22.78±0.54c | 0.57±0.21 | 0.61±0.06 | 0.53±0.04 | 1.85±0.03a | 3.24±0.06b | 3.12±0.11b | 0.13±0.03 | 0.13±0.03 | 0.14±0.04 |
| REM20 | 22.63±0.97c | 21.78±1.04c | 23.29±1.57c | 0.67±0.03 | 0.57±0.12 | 0.65±0.07 | 1.78±0.02a | 2.01±0.02a | 4.22±0.07b | 0.13±0.04 | 0.13±0.03 | 0.14±0.02 |
| GBR100 | 22.75±0.22c | 23.94±1.62b | 21.86±1.24c | 0.56±0.23 | 0.64±0.13 | 0.65±0.03 | 2.19±0.09a | 3.61±0.06b | 4.12±0.11b | 0.13±0.04 | 0.14±0.02 | 0.14±0.01 |
| GBR200 | 23.56±0.57c | 24.11±1.43b | 20.94±0.98c | 0.61±0.25 | 0.58±0.11 | 0.55±0.14 | 2.75±0.0a | 2.61±0.01a | 4.31±0.09b | 0.14±0.03 | 0.13±0.03 | 0.14±0.03 |
| ASG100 | 22.17±0.31c | 24.42±1.35b | 21.08±1.22c | 0.65±0.15 | 0.63±0.10 | 0.74±0.02 | 1.87±0.04a | 2.79±0.06b | 3.79±0.01c | 0.13±0.04 | 0.14±0.04 | 0.13±0.02 |
| ASG200 | 24.90±0.26b | 24.87±0.72b | 24.11±1.51b | 0.67±0.23 | 0.59±0.04 | 0.66±0.06 | 1.83±0.10a | 3.00±0.10b | 4.99±0.02c | 0.13±0.03 | 0.13±0.01 | 0.14±0.03 |
| GABA100 | 21.09±0.47c | 21.40±1.26c | 20.78±0.54c | 0.55±0.26 | 0.57±0.14 | 0.60±0.09 | 1.95±0.03a | 2.27±0.02a | 5.11±0.08b | 0.14±0.03 | 0.13±0.03 | 0.13±0.04 |
| GABA200 | 23.89±0.73c | 21.54±0.93c | 23.25±1.07c | 0.63±0.11 | 0.48±0.17 | 0.64±0.11 | 1.66±0.13a | 2.30±0.03a | 6.03±0.01c | 0.13±0.02 | 0.13±0.04 | 0.13±0.03 |
| ORZ100 | 20.14±0.41c | 21.43±0.81c | 20.78±0.71c | 0.58±0.04 | 0.60±0.12 | 0.681±0.30 | 2.30±0.04a | 3.00±0.08b | 3.98±0.06b | 0.13±0.03 | 0.13±0.03 | 0.14±0.02 |
| ORZ200 | 26.32±0.74b | 27.02±0.76b | 26.85±1.35b | 0.60±0.14 | 0.57±0.14 | 0.64±0.12 | 2.61±0.05a | 2.14±0.04a | 4.76±0.13b | 0.13±0.02 | 0.14±0.02 | 0.14±0.03 |
Notes: Values are expressed as mean ± SD for three readings; levels not denoted by the same superscript letter on the same row are significantly different.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; SD, standard deviation.
FSH
Serum follicular stimulating hormone level showed no significant difference between the 2nd, 4th, and 8th week values in the OVX untreated group (P>0.05). A significant increase was observed (P<0.05) in the other treated groups (P<0.05). There were no significant differences (P>0.05) in FSH levels between the 2nd and 4th week in the sham non OVX group, or the groups treated with EST, 100 mg/kg GABA, 200 mg/kg GBR, 200 mg/kg ORZ, and 20 mg/kg REM. There was a significant increase at the 8th week in the level of FSH (P<0.05) when compared to the values at the 2nd and 4th weeks (Table 2).
LH
There were no significant differences (P>0.05) in serum LH between the OVX untreated group and the sham and other treated groups as shown in Table 2.
Immunohistochemistry
Our immunohistochemical staining showed positive ER-β immunoreactivity in the epithelial cells, glandular, luminal, and stromal epithelium in groups treated with EST. Immunoreactivity was also present in the stroma and glandular cells in the group treated with GBR phenolics, and a slight reactivity was also seen in groups treated with ORZ, GABA, REM, and ASG, as shown in Figure 7.
Figure 7.
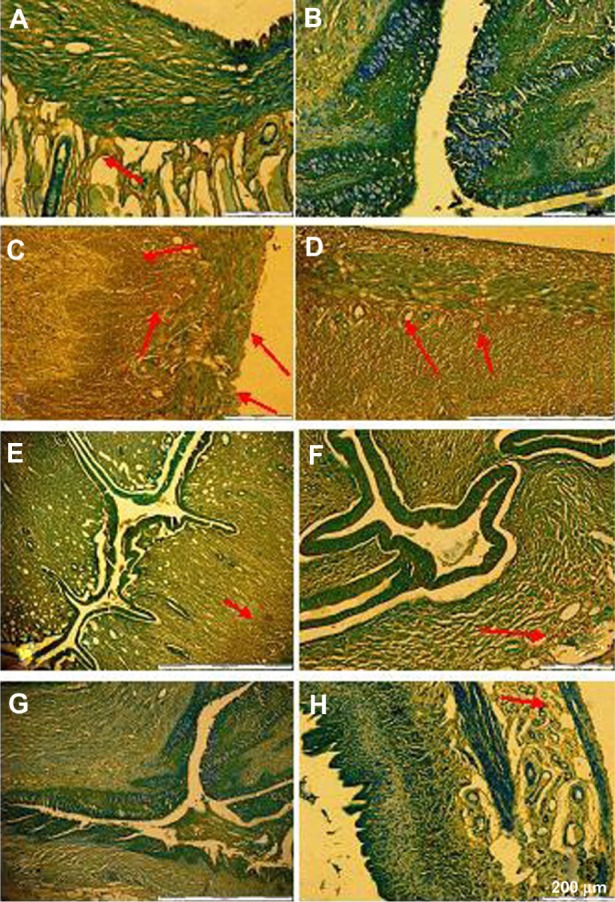
Slides showing immunohistochemical staining of ER-β antigen on uterine tissue of OVX rats. (A) Sham non-treated group; (B) OVX non-treated; (C) EST 0.2 mg/kg; (D) GBR phenolics 200 mg/kg; (E) ASG 200 mg/kg; (F) REM 20 mg/kg; (G) GABA 200 mg/kg; and (H) ORZ 200 mg/kg.
Notes: Red arrows show areas of immunohistochemical staining. The sham (non-OVX group) showed light reactivity on the glandular epithelium, while the group treated with GBR phenolics showed an ER-β reactivity at the glandular and some part of the stroma and the group treated with EST showed an immunoreactivity both at the glandular, luminal, and deep in the stromal cells. Other treated groups showed mild reactivity mostly in the stroma.
Abbreviations: ASG, acylated steryl glucosides; EST, estrogen; GBR, germinated brown rice; GABA, γ-amino butyric acid; ORZ, γ-oryzanol; OVX, ovariectomized; REM, Remifemin; ER-β, estrogen receptor-β.
Discussion
The side effects exhibited by hormonal therapy, and the disadvantages in the clinical use of the synthetic selective ER drugs, argue for research into more natural compounds with selective estrogenic activity. Identification and determination of estrogen-induced genes will surely help in detecting the molecular mechanisms of action of these compounds and their estrogenic affinity towards the uterine tissue. Our choice of doses proved effective with no toxicity to the vital organs. A study on acute and sub-chronic toxicity of GBR extracts reported the use of 75–300 mg/kg without causing toxicity to blood parameters or vital organs such as the kidneys and the liver.37 Ismail et al38 used a dose of 500 mg/kg ORZ without any toxicity or side effects in rats. In the present study, GBR phenolics at 200 mg/kg showed an upregulation of the ER-β gene, and the regulation is around the same level compared to the sham non-OVX group, but EST at 0.2 mg/kg gave a significantly higher expression of this gene compared to GBR, which indicates that although GBR can stimulate ER-β uterine gene expression, this was more towards the normal physiological range.
Traditional Asian diets have been reported to contain multiple plant-derived, nonsteroidal and weakly estrogenic compounds produced either by the plant itself, or phytoe strogens or the fungus fusarium, which infects many cultivated grains.39 This gives a clear indication that, following the germination process, GBR produces some bioactives with weak estrogenic activity, and the EST treated group gives a higher expression than the other treatment groups because of its high affinity to ERs. CaBP9k, C3, and HSP70 genes are regarded as prominent genes regulated by estrogen in the uterus.40–45 Groups treated with 200 mg/kg ASG, 0.2 mg/kg EST, and 200 mg/kg ORZ showed a relatively high expression of CaBP9k, and an increase in serum estrogen and FSH in groups treated with these bioactives. CaBP9k mRNA in the uterus has been reported to fluctuate during the estrous cycle of rats, depending on serum estrogen level, and its level at diestrus was not detectable, but increased only at proestrus, and reached the highest level at estrus, and then decreased with metestrus.46 Nguyen et al47 reported that CaBP9k is mainly regulated by estrogen in the pituitary gland of rats during the estrous cycle. In our previous study,33 we observed an increase in vaginal epithelial cells, an increase in glandular secretory cells, and expression of polyclonal nuclear antigen (PCNA) in the uterus indicating cellular proliferation and regulation of estrus in OVX rat uteri that were treated with GBR bioactives. This gives us a clear indication that these bioactives, at the molecular level, also stimulate the expression of the CaBP9k gene and result in the observed estrogenic effects. Nguyen et al47 reported localization and immunoreactivity of the CaBP9k protein in the stromal cells, the endometrium, luminal epithelial cells, and glandular epithelial cells in maternal uteri following treatment with alkyl phenols. In our work, we observed a localization and immunoreactivity of ER-β in the uterus of OVX rats treated with GBR phenolics. Estrogen stimulates both the mRNA and protein levels of C3 in the uterus, and C3 is an excellent marker for steroid hormone action in the uterus.43,48 Several members of the HSP genes, including the HSP homologous glucose-regulated proteins, are estrogen-regulated and may be indirectly involved in the uterotrophic response.49 Ovariectomy in female rats was highly associated with a decrease in the level of HSP and can be prevented by estrogen replacement therapy.50 The mechanism by which estrogen regulates and increases the level of HSP is through an increase in transcription and translation of heat shock factor (HSF)-1 and HSF-2 in the uterus.49,51 GBR bioactives may also stimulate the expression of HSP through the activation of these two factors. The IL-4 receptor gene was expressed higher in rats treated with EST. Rivera-Gonzalez et al30 observed a large increase in the expression of this gene (about 13-fold) after EST administration to OVX rats. In the present work, GBR bioactives induced expression close to the level obtained in sham non-OVX group. The reason for the downregulation of ER-β, HSP, and IL-4 receptor genes induced by GABA at 200 mg/kg in this study is not known, although it might be due to the fact that GABA at high doses antagonizes the expression of these genes.
Conclusion
Although GBR bioactives regulate the activity of some estrogen-induced genes in the uterus, and our immunohistochemical study showed positive expression of ER-β immunoreactivity in the uterus, studies are still needed to further characterize, and confirm the selective estrogenic effects of these bioactives by in situ hybridization, ER-α-specific binding, and other molecular techniques.
Acknowledgments
We wish to thank the Padiberas National (BERNAS) rice company in Malaysia for funding this research.
Footnotes
Disclosure
The authors report no other conflicts of interest in this work.
References
- 1.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2 Pt 1):230–240. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 2.Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(Suppl 1):S46–S52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353(9152):571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 4.Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(Suppl 1):S74–S76. doi: 10.1086/318842. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Anderson GL, Gass M, et al. WHI Investigators Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol. 2009;14(5):383–391. doi: 10.1007/s10147-009-0935-y. [DOI] [PubMed] [Google Scholar]
- 7.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett-Connor E, Mosca L, Collins P, et al. Raloxifene Use for The Heart (RUTH) Trial Investigators Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 9.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 10.Tian S, Nakamura K, Kayahara H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem. 2004;52(15):4808–4813. doi: 10.1021/jf049446f. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Cho J, Gao T, et al. Increment of physiologically active compounds in germinated brown rice treated with chitosan and its effect on obesity of rat fed a high fat diet. J Korean Soc Food Sci Nutr. 2008;37:985–991. [Google Scholar]
- 12.Sawaddiwong R, Jongjareonrak A, Benjakul S. Phenolic content and antioxidant activity of germinated brown rice as affected by germination temperature and extraction solvent. KMITL Science Journal. 2008;8:45–49. [Google Scholar]
- 13.Charoenthaikij P, Jangchud K, Jangchud A, Prinyawiwatkul W, Tungtrakul P. Germination conditions affect selected quality of composite wheat-germinated brown rice flour and bread formulations. J Food Sci. 2010;75(6):S312–S318. doi: 10.1111/j.1750-3841.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 14.Jannoey P, Niamsup H, Lumyong S, Suzuki T, Katayama T, Chairote G. Comparison of gamma-aminobutyric acid production in Thai rice grains. World J Microb Biot. 2010;26(2):257–263. [Google Scholar]
- 15.Komatsuzaki N, Tsukahara K, Toyoshima H, Suzuki T, Shimizu N, Kimura T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice. J Food Eng. 2007;78(2):556–560. [Google Scholar]
- 16.Karladee D, Suriyong S. γ-Aminobutyric acid (GABA) content in different varieties of brown rice during germination. Science Asia. 2012;38:13–17. [Google Scholar]
- 17.Britz SJ, Prasad PV, Moreau RA, Allen LH, Kremer DF, Boote KJ. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and gamma-oryzanol in brown rice. J Agric Food Chem. 2007;55(18):7559–7565. doi: 10.1021/jf0637729. [DOI] [PubMed] [Google Scholar]
- 18.Usuki S, Ariga T, Dasgupta S, et al. Structural analysis of novel bioactive acylated steryl glucosides in pre-germinated brown rice bran. J Lipid Res. 2008;49(10):2188–2196. doi: 10.1194/jlr.M800257-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse. Biol Reprod. 1998;59(3):470–475. doi: 10.1095/biolreprod59.3.470. [DOI] [PubMed] [Google Scholar]
- 20.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Iafrati MD, Karas RH, Aronovitz M, et al. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3(5):545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 22.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Kupfer D. Enantioselective metabolism of the endocrine disruptor pesticide methoxychlor by human cytochromes P450 (P450s): major differences in selective enantiomer formation by various P450 isoforms. Drug Metab Dispos. 2002;30(12):1329–1336. doi: 10.1124/dmd.30.12.1329. [DOI] [PubMed] [Google Scholar]
- 24.Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J Endocrinol. 1998;156(3):R13–R17. doi: 10.1677/joe.0.156r013. [DOI] [PubMed] [Google Scholar]
- 25.Rosenkranz K, Hinney A, Ziegler A, et al. Systematic mutation screening of the estrogen receptor beta gene in probands of different weight extremes: identification of several genetic variants. J Clin Endocrinol Metab. 1998;83(12):4524–4527. doi: 10.1210/jcem.83.12.5471. [DOI] [PubMed] [Google Scholar]
- 26.Omoto Y, Kobayashi Y, Nishida K, et al. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. 2001;285(2):340–347. doi: 10.1006/bbrc.2001.5158. [DOI] [PubMed] [Google Scholar]
- 27.Enmark E, Pelto-Huikko M, Grandien K, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82(12):4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 29.Weihua Z, Saji S, Mäkinen S, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97(11):5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivera-Gonzalez R, Petersen DN, Tkalcevic G, Thompson DD, Brown TA. Estrogen-induced genes in the uterus of ovariectomized rats and their regulation by droloxifene and tamoxifen. J Steroid Biochem Mol Biol. 1998;64(1–2):13–24. doi: 10.1016/s0960-0760(97)00142-8. [DOI] [PubMed] [Google Scholar]
- 31.Naciff JM, Jump ML, Torontali SM, et al. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68(1):184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt SC, Deroo BJ, Hansen K, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17(10):2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 33.Muhammad SI, Ismail M, Mahmud RB, Salisu AM, Zakaria ZA. Germinated brown rice and its bioactives modulate the activity of uterine cells in oophorectomised rats as evidenced by gross cytohistological and immunohistochemical changes. BMC Complement Altern Med. 2013;13(1):198. doi: 10.1186/1472-6882-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sani IM, Iqbal S, Chan KW, Ismail M. Effect of acid and base catalyzed hydrolysis on the yield of phenolics and antioxidant activity of extracts from germinated brown rice (GBR) Molecules. 2012;17(6):7584–7594. doi: 10.3390/molecules17067584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozan P, Kuo YH, Lambein F. Free amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumers. J Agric Food Chem. 2000;48(3):716–723. doi: 10.1021/jf990729v. [DOI] [PubMed] [Google Scholar]
- 36.Azrina A, Maznah M, Azizah AH. Extraction and determination of oryzanol in rice bran of mixed herbarium UKMB; AZ 6807: MR 185, AZ 6808: MR 211, AZ6809: MR 29. ASEAN Food J. 2008;15(1):89–96. [Google Scholar]
- 37.Hutadilok-Towatana N, Wattanapiromsakul C, Thammarutwasik P. Acute and subchronic toxicity evaluation of the hydroethanolic extract of germinated brown rice.
- 38.Ismail M, Al-Naqeeb G, Mamat WA, Ahmad Z. Gamma-oryzanol rich fraction regulates the expression of antioxidant and oxidative stress related genes in stressed rat’s liver. Nutr Metab (Lond) 2010;7:23. doi: 10.1186/1743-7075-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res. 1999;67(1):165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 40.Nys Y, Baker K, Lawson DE. Estrogen and a calcium flux dependent factor modulate the calbindin gene expression in the uterus of laying hens. Gen Comp Endocrinol. 1992;87(1):87–94. doi: 10.1016/0016-6480(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 41.Perret C, L’Horset F, Thomasset M. DNase I-hypersensitive sites are associated, in a tissue-specific manner, with expression of the calbindin-D9k-encoding gene. Gene. 1991;108(2):227–235. doi: 10.1016/0378-1119(91)90438-h. [DOI] [PubMed] [Google Scholar]
- 42.Reiswig JD, Frazer GS, Inpanbutr N. Calbindin-D9k expression in the pregnant cow uterus and placenta. Histochem Cell Biol. 1995;104(2):169–174. doi: 10.1007/BF01451576. [DOI] [PubMed] [Google Scholar]
- 43.Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C, Lyttle CR. Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem. 1989;264(28):16941–16947. [PubMed] [Google Scholar]
- 44.Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7(6):560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 45.Koshiyama M, Konishi I, Nanbu K, et al. Immunohistochemical localization of heat shock proteins HSP70 and HSP90 in the human endometrium: correlation with sex steroid receptors and Ki-67 antigen expression. J Clin Endocrinol Metab. 1995;80(4):1106–1112. doi: 10.1210/jcem.80.4.7714077. [DOI] [PubMed] [Google Scholar]
- 46.Choi KC, Leung PC, Jeung EB. Biology and physiology of Calbindin-D9k in female reproductive tissues: involvement of steroids and endocrine disruptors. Reprod Biol Endocrinol. 2005;3:66. doi: 10.1186/1477-7827-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen TH, Lee GS, Ji YK, Choi KC, Lee CK, Jeung EB. A calcium binding protein, calbindin-D9k, is mainly regulated by estrogen in the pituitary gland of rats during estrous cycle. Brain Res Mol Brain Res. 2005;141(2):166–173. doi: 10.1016/j.molbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Brown EO, Sundstrom SA, Komm BS, Yi Z, Teuscher C, Lyttle CR. Progesterone regulation of estradiol-induced rat uterine secretory protein, complement C3. Biol Reprod. 1990;42(4):713–719. doi: 10.1095/biolreprod42.4.713. [DOI] [PubMed] [Google Scholar]
- 49.Papaconstantinou AD, Fisher BR, Umbreit TH, Goering PL, Lappas NT, Brown KM. Effects of beta-estradiol and bisphenol A on heat shock protein levels and localization in the mouse uterus are antagonized by the antiestrogen ICI 182,780. Toxicol Sci. 2001;63(2):173–180. doi: 10.1093/toxsci/63.2.173. [DOI] [PubMed] [Google Scholar]
- 50.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285(2):H687–H692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 51.Sgroi DC, Teng S, Robinson G, LeVangie R, Hudson JR, Elkahloun AG. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res. 1999;59(22):5656–5661. [PubMed] [Google Scholar]