Abstract
Objectives
Relationship between low-level air pollution and stroke is conflicting. This study was conducted to document the relationship between outdoor air pollution and ischaemic stroke occurrence.
Design
Time-stratified case-crossover analysis.
Setting
University Hospital of Nice, France.
Participants
All consecutive patients with ischaemic stroke living in Nice admitted in the University Hospital of Nice (France) between January 2007 and December 2011.
Main outcome measure
Association (adjusted OR) between daily levels of outdoor pollutants (ozone (O3), nitrogen dioxide (NO2), particulate matter (PM10) and sulfur dioxide (SO2)) and ischaemic stroke occurrence.
Results
1729 patients with ischaemic stroke (mean age: 76.1±14.0 years; men: 46.7%) were enrolled. No significant association was found between stroke occurrence and short-term effects of all pollutants tested. In stratified analysis, we observed significant associations only between recurrent (n=280) and large artery ischaemic stroke (n=578) onset and short-term effect of O3 exposure. For an increase of 10 µg/m3 of O3 level, recurrent stroke risk (mean D-1, D-2 and D-3 lag) was increased by 12.1% (95% CI 1.5% to 23.9%) and large artery stroke risk (mean D-3 and D-4 lag) was increased by 8% (95% CI 2.0% to 16.6%). Linear dose–response relationship for both subgroups was found.
Conclusions
Our results confirm the relationship between low-level O3 exposure and ischaemic stroke in high vascular risk subgroup with linear exposure–response relation, independently of other pollutants and meteorological parameters. The physiopathological processes underlying this association between ischaemic stroke and O3 exposure remain to be investigated.
Keywords: Public Health
Strengths and limitations of this study.
The relationship between low-level air pollution and stroke is conflicting. This article confirms the relationship between low-level ozone exposure and ischaemic stroke in high vascular risk subgroup with linear exposure–response relationship, independently of other pollutants and meteorological parameters. PM2.5 was not studied because it was not monitored in Nice.
Introduction
Outdoor air pollution is considered as a major environmental health issue, responsible for an excess of death in the world. It is defined as any undesirable modification of air by substances either toxic or likely to have adverse effects on health. Outdoor air pollutants are known to increase morbidity and mortality of respiratory diseases.1 However, in the 1950s and 1960s, epidemiological studies of acute severe pollution episodes have also shown an increasing cardiovascular and cerebrovascular mortality risk.2 A link between acute air pollution and stroke mortality has been reported for the first time in the London fog incident study in December 1952.2 In the last decades, the consequences of low-level air pollution on cardiovascular mortality and morbidity have been clearly described.3–6 By analogy, a few studies have examined the role of short-term air pollution on ischaemic stroke but actually no conclusion could be generalised.3 5–24 The purpose of the present study was to document the relationship between the characteristics of outdoor air pollution and the occurrence of ischaemic stroke.
Materials and methods
Population studied
We performed a 5-year (2007–2011) case-crossover analysis in Nice, France. We retrospectively enrolled consecutive patients with stroke admitted at the University Hospital of Nice between January 2007 and December 2011. Querying French DRG-based database (PMSI: Programme de Médicalisation des Systèmes d'Information) with I60–I69 codes from the International Classification of Diseases (10th revision), we screened all patients hospitalised for stroke. We filtered the sample to patients living in Nice (geographical area defined by zip codes: 06000, 06100, 06200 and 06300). The diagnosis of ischaemic stroke was reviewed and confirmed by a panel of neurologists using clinical and radiological data of medical records. Patients with another diagnosis than stroke were excluded. Demographic data, vascular risk factors (WHO definitions), clinical and radiological characteristics of stroke were also collected from medical records.
Outdoor air pollution and meteorological data
Nice is an urban city situated in the south-eastern part of France on the Mediterranean coast. According to the latest census, Nice has a population of 340 735 in 2009. Its climate is temperate and qualified as Mediterranean type. Surrounded by hills and mountains (south Alps), the city of Nice is sheltered from continuous violent winds. Outdoor air pollution comes mainly from traffic due to high density of roads and an international airport (first one in France after Paris airports).
Air pollution data were obtained from the regional agency for air quality monitoring (AirPACA). Exposure measurements during the study period were carried out in 2 of 13 permanent monitoring stations in the study area. Measures (µg/m3) were performed in an urban station (Cagnes Ladoumègue) for following atmospheric pollutants: particulate matter (PM10; tapered element oscillating microbalance), nitrogen dioxide (NO2) (chemiluminescence), sulfur dioxide (SO2; ultraviolet photometry) and ozone (O3; ultraviolet photometry). Missing values were replaced by measures performed by the observational monitoring station located at Nice Airport. We computed for each pollutant during 24 h average and specifically for O3 during 8 h daytime periods.
Daily meteorological data were obtained from the National Meteorological Office of Nice, including temperature (°C) and humidity (%). Moreover, data on influenza epidemics (weekly count) in the region of Provence-Alpes-Cote-d'Azur were obtained from the Sentiweb network.
Statistical analysis
Continuous variables were expressed as mean (SD) or median (IQR), and categorical variables as percentages. Spearman correlation coefficients (r) between air pollutants and atmospheric parameters were calculated. The time-stratified case-crossover design was used to examine the relationship between short-term effects of outdoor air pollutants and stroke. In this design, each participant enrolled was his own control. Case days were defined as the day of stroke. Control days were defined as the same day of the same stratum as the case day. Study time was stratified by months. Therefore, explicative variable levels at the case day were compared with levels of the same variables at control days. This method has the main advantage to control individual factors, the day of the week, season and time trend.24 Conditional logistic regression was performed to estimate the association between short-term effects of each air pollutant measured and stroke onset. OR and 95% CI for a 10 µg/m3 increase of pollutant level were adjusted for temperature and humidity with a 1-day lag, influenza epidemics and holidays without day lag. The pollutant exposure was tested in models for 1-day, 2-day or 3-day lag. Stratified analyses by subgroups were performed according to age, gender, risk vascular factors (tobacco use, diabetes mellitus, hypercholesterolaemia and hypertension) and stroke aetiological subtypes according to the Trial of ORG 10 172 in acute stroke treatment (TOAST). We evaluated dose–response relationships across four exposure levels of pollutants studied, and the first quartile was used as the reference group. A p value less than 0.05 was considered as significant. The data were analysed using Stata V.10.0 SE software.
Results
During the study period (January 2007 to December 2011), there were 2067 patients living in Nice and were admitted to the University Hospital Center for ischaemic stroke based on the DRG database. After neurologists review of medical records, 1729 patients with ischaemic stroke were enrolled for final analysis. Six hundred and twenty (35.9%) of these patients were hospitalised in the stroke unit. According to the last population census of 2009, annual ischaemic stroke incidence rates (by 100 000) in the studied area were, respectively, from 2007 to 2011: 100, 100, 98, 96 and 112. The mean age was 76.1±14.0 years, and 46.7% were men (table 1).
Table 1.
Baseline characteristics of patients with ischaemic stroke (incident and recurrent) hospitalised in Nice University Hospital from 2007 to 2011
| All patients | Incident | Recurrent | p Value | |
|---|---|---|---|---|
| (n=1729) | n=1449 (83.81%) | n=280 (16.19%) | ||
| Demographic data | ||||
| Men | 808 (46.73) | 683 (47.14) | 125 (44.64) | 0.0044 |
| Age (years) | 76.06±14.04 | 75.48±14.29 | 79.01±12.33 | <0.0001 |
| <55 | 141 (8.16) | 132 (9.11) | 9 (3.21) | 0.0011 |
| 55–64 | 186 (10.76) | 155 (10.70) | 31 (11.07) | 0.8532 |
| 65–74 | 324 (18.74) | 279 (19.25) | 45 (16.07) | 0.2114 |
| 75–84 | 524 (30.31) | 438 (30.23) | 86 (30.71) | 0.8712 |
| ≥85 | 554 (32.04) | 445 (30.71) | 109 (38.93) | 0.0071 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 311 (17.99) | 249 (17.18) | 62 (22.14) | 0.0481 |
| Hypertension | 998 (57.72) | 803 (55.42) | 195 (69.64) | <0.0001 |
| Dyslipidaemia | 441 (25.51) | 348 (24.02) | 93 (33.21) | 0.0012 |
| Current smoker | 410 (23.71) | 357 (24.64) | 53 (18.93) | 0.0398 |
| Overweight | 226 (13.07) | 204 (14.08) | 22 (7.86) | 0.0047 |
| Coronary artery disease | 263 (15.21) | 209 (14.42) | 54 (19.29) | 0.0381 |
| Atrial fibrillation | 527 (30.48) | 433 (29.88) | 94 (33.57) | 0.2198 |
| Classification of stroke aetiological subtypes (TOAST) | ||||
| Large artery | 578 (33.43) | 479 (33.06) | 99 (35.36) | 0.4552 |
| Cardioembolic | 563 (32.56) | 469 (32.37) | 94 (33.57) | 0.6938 |
| Lacunar stroke | 153 (8.85) | 129 (8.90) | 24 (8.57) | 0.8582 |
| Other determined aetiology | 43 (2.49) | 40 (2.76) | 3 (1.07) | 0.0966 |
| Undetermined aetiology | 392 (22.67) | 332 (22.91) | 60 (21.43) | 0.5872 |
| Hospitalisation in stroke unit | 620 (35.86) | 546 (37.68) | 74 (26.43) | 0.0003 |
TOAST, Trial of ORG 10 172 in acute stroke treatment.
The distribution of air pollutants and meteorological variables is shown in table 2. Spearman correlation coefficients (r) were ranged from 0.01 to 0.25 between each studied pollutants, except between O3 and NO2 (r=−0.54). Correlation coefficient between minimal temperature and O3 was r=0.67 (see online supplementary table I).
Table 2.
Distribution of air pollution concentrations and meteorological parameters in Nice (France) between 2007 and 2012
| Mean | SD | Minimum | Quartile 1 | Median | Quartile 3 | Maximum | |
|---|---|---|---|---|---|---|---|
| Ozone daily 8 h average (μg/m3) | 80.74 | 31.78 | 4.63 | 54.75 | 84.38 | 105.38 | 157.27 |
| Ozone daily 1 h maximum (μg/m3) | 92.37 | 31.32 | 7.00 | 69.00 | 94.00 | 115.00 | 197.00 |
| Ozone 24 h average (μg/m3) | 52.20 | 22.89 | 4.00 | 32.58 | 53.29 | 69.02 | 111.13 |
| PM10 (μg/m3) | 28.48 | 9.81 | 1.00 | 22.00 | 28.00 | 34.00 | 74.00 |
| NO2 (μg/m3) | 26.22 | 8.69 | 3.00 | 20.00 | 25.00 | 32.00 | 59.00 |
| SO2 (μg/m3) | 1.23 | 1.18 | 0.00 | 0.00 | 1.00 | 2.00 | 10.00 |
| Minimum temperature (°C) | 13.02 | 5.98 | −1.60 | 7.80 | 12.90 | 18.20 | 25.90 |
| Maximum humidity (%) | 81.40 | 9.07 | 40.00 | 76.00 | 83.00 | 88.00 | 97.00 |
NO2, nitrogen dioxide; PM10, particulate matter; SO2, sulfur dioxide.
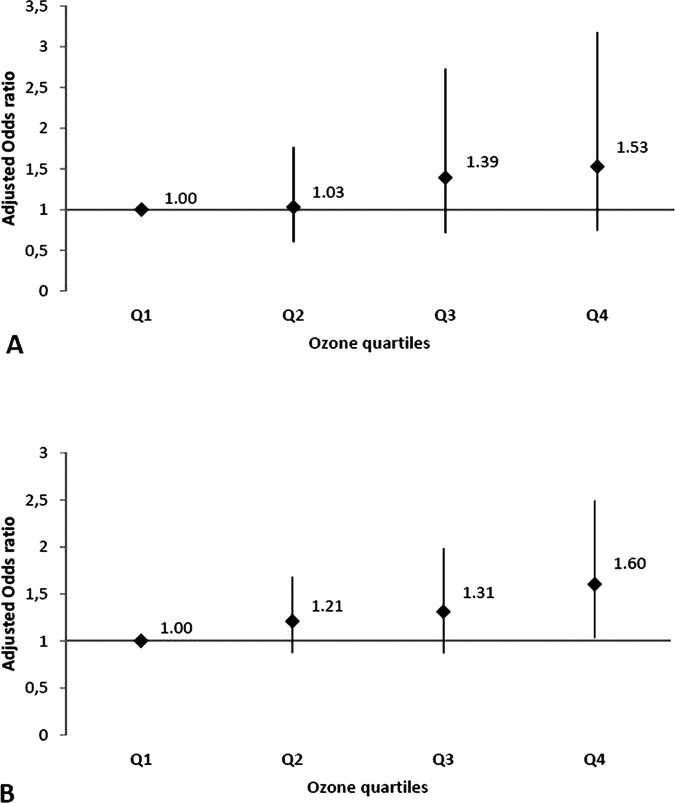
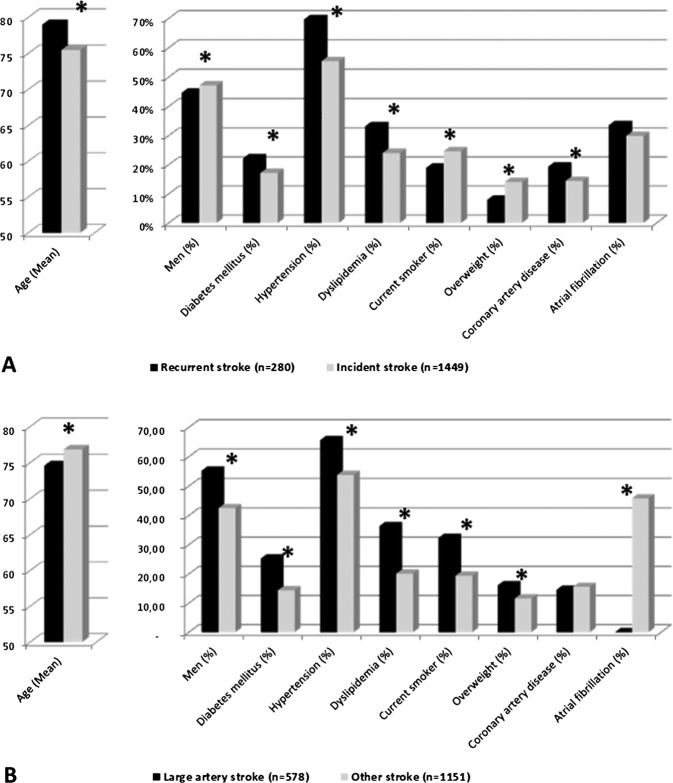
No significant association was found between stroke occurrence and short-term effects of all pollutants tested. In addition, we performed stratified subgroup analysis according to gender, age by decade, incident/recurrent stroke status, vascular risk factors, presence of atrial fibrillation and stroke aetiological subgroups. We measured only significant associations between stroke and short-term effect of O3 in following both groups: recurrent (n=280) and large artery stroke (n=578) (table 3). In recurrent stroke subgroup, for an increase of 10 µg/m3 of O3 level (mean D-1, D-2 and D-3 lag), stroke risk was significantly increased by 12.1% (95% CI 1.5% to 23.9%). Adjusted OR between O3 exposure (mean D-3 and D-4) and large artery stroke subgroup was 1.080 (95% CI 1.002 to 1.166). No significant association was observed with other pollutants than O3. Adjusted in two-pollutant models, OR was not affected. Using O3 quartiles (1st quartile as the reference group), linear dose–response relationship for both subgroups was observed (figure 1). Baseline characteristics in recurrent stroke and large artery stroke subgroups are shown in figure 2.
Table 3.
Adjusted ORs between ischaemic stroke and outdoor pollutants exposure for an increase of 10 µg/m3 in Nice (France) between 2007 and 2011
| All ischaemic stroke (n=1729) |
Recurrent stroke (n=280) |
Large artery stroke (n=578) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI |
p Value | aOR | 95% CI |
p Value | aOR | 95% CI |
p Value | |||||||
| Ozone | |||||||||||||||
| D-1 | |||||||||||||||
| 8 h average | 0.9917 | 0.9584 | to | 1.0261 | 0.633 | 1.0899 | 1.0009 | to | 1.1867 | 0.047 | 0.9697 | 0.9119 | to | 1.0310 | 0.326 |
| 1 h maximum | 0.9957 | 0.9644 | to | 1.0281 | 0.795 | 1.0641 | 0.9824 | to | 1.1527 | 0.127 | 0.9881 | 0.9334 | to | 1.0460 | 0.682 |
| 24 h average | 1.0036 | 0.9578 | to | 1.0517 | 0.877 | 1.0793 | 0.9616 | to | 1.2115 | 0.195 | 0.9983 | 0.9190 | to | 1.0843 | 0.968 |
| D-2 | |||||||||||||||
| 8 h average | 0.9976 | 0.9657 | to | 1.0306 | 0.888 | 1.0957 | 1.0086 | to | 1.1903 | 0.030 | 0.9903 | 0.9347 | to | 1.0491 | 0.741 |
| 1 h maximum | 1.0040 | 0.9738 | to | 1.0351 | 0.795 | 1.0955 | 1.0144 | to | 1.1831 | 0.020 | 0.9979 | 0.9457 | to | 1.0529 | 0.940 |
| 24 h average | 1.0015 | 0.9598 | to | 1.0451 | 0.942 | 1.0638 | 0.9587 | to | 1.1804 | 0.244 | 1.0097 | 0.9365 | to | 1.0885 | 0.801 |
| D-3 | |||||||||||||||
| 8 h average | 0.9987 | 0.9670 | to | 1.0314 | 0.939 | 1.0601 | 0.9784 | to | 1.1487 | 0.154 | 1.0261 | 0.9703 | to | 1.0852 | 0.366 |
| 1 h maximum | 0.9968 | 0.9671 | to | 1.0273 | 0.836 | 1.0380 | 0.9635 | to | 1.1184 | 0.326 | 1.0254 | 0.9728 | to | 1.0808 | 0.349 |
| 24 h average | 1.0046 | 0.9644 | to | 1.0466 | 0.822 | 1.0838 | 0.9788 | to | 1.2000 | 0.122 | 1.0519 | 0.9802 | to | 1.1289 | 0.160 |
| D-4 | |||||||||||||||
| 8 h average | 1.0067 | 0.9751 | to | 1.0393 | 0.681 | 1.0169 | 0.9395 | to | 1.1006 | 0.678 | 1.0359 | 0.9808 | to | 1.0941 | 0.205 |
| 1 h maximum | 0.9978 | 0.9684 | to | 1.0280 | 0.887 | 1.0038 | 0.9321 | to | 1.0811 | 0.918 | 1.0290 | 0.9777 | to | 1.0829 | 0.272 |
| 24 h average | 1.0114 | 0.9711 | to | 1.0534 | 0.583 | 1.0248 | 0.9260 | to | 1.1342 | 0.635 | 1.0787 | 1.0065 | to | 1.1561 | 0.032 |
| PM10 | |||||||||||||||
| D-1 | 1.0143 | 0.9518 | to | 1.0806 | 0.659 | 1.0041 | 0.5586 | to | 1.7995 | 0.989 | 1.0347 | 0.9282 | to | 1.1527 | 0.536 |
| D-2 | 0.9861 | 0.9238 | to | 1.0523 | 0.674 | 0.9518 | 0.8106 | to | 1.1167 | 0.545 | 0.9350 | 0.8349 | to | 1.0464 | 0.242 |
| D-3 | 0.9788 | 0.9203 | to | 1.0405 | 0.493 | 1.0047 | 0.8532 | to | 1.182 | 0.955 | 0.9436 | 0.8475 | to | 1.0501 | 0.288 |
| D-4 | 0.9780 | 0.9202 | to | 1.0391 | 0.473 | 0.9911 | 0.8520 | to | 1.152 | 0.908 | 0.9544 | 0.8572 | to | 1.0620 | 0.392 |
| NO2 | |||||||||||||||
| D-1 | 1.0307 | 0.9367 | to | 1.1336 | 0.533 | 0.8960 | 0.7689 | to | 1.0434 | 0.158 | 1.0293 | 0.8699 | to | 1.2169 | 0.735 |
| D-2 | 0.9931 | 0.9029 | to | 1.0918 | 0.887 | 0.9427 | 0.7403 | to | 1.1991 | 0.631 | 1.0494 | 0.8894 | to | 1.2372 | 0.565 |
| D-3 | 0.9462 | 0.8607 | to | 1.0396 | 0.250 | 1.1262 | 0.8767 | to | 1.4449 | 0.349 | 0.9147 | 0.7743 | to | 1.0796 | 0.292 |
| D-4 | 0.9462 | 0.8607 | to | 1.0396 | 0.250 | 0.8931 | 0.7047 | to | 1.1306 | 0.348 | 0.9147 | 0.7743 | to | 1.0796 | 0.292 |
| SO2 | |||||||||||||||
| D-1 | 1.0069 | 0.5986 | to | 1.6893 | 0.979 | 0.653 | 0.164 | to | 2.5822 | 0.544 | 1.0789 | 0.4507 | to | 2.5712 | 0.864 |
| D-2 | 0.8763 | 0.5138 | to | 1.4905 | 0.626 | 0.8916 | 0.2525 | to | 3.1284 | 0.858 | 1.1784 | 0.4790 | to | 2.8858 | 0.719 |
| D-3 | 1.2539 | 0.7405 | to | 2.1174 | 0.397 | 0.7231 | 0.1983 | to | 2.6188 | 0.622 | 0.9351 | 0.3630 | to | 2.3973 | 0.889 |
| D-4 | 1.4852 | 0.8956 | to | 2.4567 | 0.123 | 1.3587 | 0.3735 | to | 4.9101 | 0.640 | 1.6564 | 0.7140 | to | 3.8260 | 0.237 |
NO2, nitrogen dioxide; PM10, particulate matter; SO2, sulfur dioxide; aOR, adjusted odds ratio.
Figure 1.
Dose relationship between ozone and ischaemic stroke events ((A), recurrent ischaemic stroke subgroup and (B), large artery ischaemic stroke subgroup).
Figure 2.
Baseline characteristics according to recurrent stroke subgroup (A) and large artery stroke subgroup (B) (*p<0.05).
Discussion
Our study assessed the short-term effect of O3 exposure on a selected population of ischaemic stroke in a city especially polluted by O3. An elevation of 10 µg/m3 of O3 concentration increases stroke risk with few days lag in recurrent (≈12%) and large artery stroke (≈8%) subgroups only. Linear dose–response relationship was observed systematically in both groups. In these groups, the common feature of the patients was that they cumulate vascular risk factors. No significant association was found between all ischaemic stroke groups and atmospheric pollutants studied (O3, NO2, SO2 and PM10).
Several studies have investigated the association between outdoor air pollution and stroke.3 5–24 Results of these studies are conflicting and hamper generalisation of conclusions. Heterogeneous methodological considerations are the main explanation of this conflict. Methodological differences are observed in patient selection, study design, outcomes choice (incidence, hospital admission, mortality) and assessment of individual exposure to selected pollutants.20 Few published studies investigated especially the association between occurrence of ischaemic stroke and O3 exposure using the case-crossover design8 15 17 19 21 or the time series analysis method.3 12 16 22 Consistent with our results, the majority of these studies do not observe the relationship between O3 exposure and occurrence of ischaemic stroke.3 8 17 19 21 22 Whenever a relationship was revealed, the association was borderline significant16 or was not confirmed by a second study on the same area of investigation.15 19 Despite the fact that the link between ischaemic stroke and O3 exposure is not obvious, results in subgroup analyses seem to identify a population at risk for O3 exposure. In a recurrent ischaemic stroke subgroup, a significant increase of 12.1% (95% CI 1.5% to 23.9%) in stroke risk was observed for each increase of 10 µg/m3 of O3 concentration during previous days (mean D-1, D-2 and D-3 lag). Consistent with this result, a population-based study in Dijon (France) revealed the same association (OR 1.150; 95% CI 1.027 to 1.209) with 3 days lag.19 Similarly, a significant association was observed in a large artery stroke subgroup (mean D-3, D-4, OR 1.080; 95% CI 1.002 to 1.166). This link was observed in the previous study (Dijon) especially in this stroke aetiological subgroup.15 Associations in other ischaemic stroke subgroups are not systematically confirmed (age, gender, vascular risk factors and season).3 15 19 21 22 Our study confirms the short-term effects of O3 exposure on patients with stroke with high vascular risk.15 19
Our findings suggest that exposure to O3, the main photochemical pollutant, could increase the risk of ischaemic stroke in population subgroups (recurrent stroke, large arteries stroke) particularly exposed to vascular risk factors inducing atherosclerosis. Physiopathological pathways linking ischaemic stroke and O3 exposure still remain largely unclear and probably complex. Some studies support a delayed effect (1–3 days lag) between acute exposure of O3 pollution and stroke onset.15 19 O3 urban pollution effects on healthy participants are associated with systemic inflammatory responses, oxidative stress and blood coagulation.25 26 These acute phenomena induced by even low levels of O3 could be the trigger of ischaemic event consecutively to atherosclerotic plaque instability, alterations in endothelial function, and increased coagulation and thrombosis.27 As suggested by Henrotin et al,19 we hypothesised that short-term effect of O3 exposure could be involved especially among participants with high vascular risk.
In order to establish a causal relationship between O3 exposure and stroke onset, we studied the exposure–response relationship, the main criteria identified by Hill.28 Consistent with previous reports, we show a linear exposure–response relationship between O3 concentration and ischaemic stroke in subgroups identified in previous reports.15 19
Our study has several limitations. The question of completeness of patients with stroke living in Nice in this hospital-based study was discussed. In Nice, patients with suspicion of stroke are admitted in priority in the University Hospital Center. Likewise, incidence of ischaemic stroke was consistent with epidemiological data in France. The question of individual exposure measurement is generally discussed. The main limitation is that we used air pollution levels from air monitoring station to represent individual exposure. However, we limited our investigations to a small geographical area (72 km2), not considered as a polluted town except for O3 (median 53.3 (32.6–69.2) µg/m3). Moreover, in the stroke population studied, elderly patients are mostly retired and have daily activity in the study area. Since O3 concentration is correlated with meteorological parameters, temperature and humidity were incorporated into our models. Association between O3 pollution and stroke can be confounded by other pollutants studied, especially particles. Effects of O3 alone are not modified using adjusted models for each of the other pollutants (NO2, SO2 and PM10). PM2.5 was not studied because it was not monitored in Nice.
Summary
The consequences of O3 pollution on the respiratory system and mortality are well documented.1 Our results confirm the relationship between low-level O3 exposure and ischaemic stroke in high vascular risk subgroup with linear exposure–response relationship, independently of other pollutants and meteorological parameters. Reproducibility of previous results is one of the main Hill’s criterion to induce causality of O3 exposure. Even if the individual's risk is low, to identify an association between O3 and ischaemic stroke incidence is important from a public health point of view, since a large population is concerned. The physiopathological processes underlying this association between ischaemic stroke and O3 exposure remain to be investigated.
Supplementary Material
Acknowledgments
The authors would like to thank AirPACA association and Sentiweb network who provided, respectively, daily measures of outdoor air pollution and data on influenza epidemics in the region of Provence-Alpes-Cote-d'Azur.
Footnotes
Contributors: All authors have contributed to (1) substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; (2) drafting the manuscript or revising it critically for important intellectual content and (3) final approval of the version to be published.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–42 [DOI] [PubMed] [Google Scholar]
- 2.Logan WP. Mortality in the London fog incident, 1952. Lancet 1953;1:336–8 [DOI] [PubMed] [Google Scholar]
- 3.Ponka A, Virtanen M. Low-level air pollution and hospital admissions for cardiac and cerebrovascular diseases in Helsinki. Am J Public Health 1996;86:1273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samet JM, Dominici F, Curriero FC, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 2000;343:1742–9 [DOI] [PubMed] [Google Scholar]
- 5.Zeller M, Giroud M, Royer C, et al. Air pollution and cardiovascular and cerebrovascular disease: epidemiologic data. Presse Med 2006;35:1517–22 [DOI] [PubMed] [Google Scholar]
- 6.Larrieu S, Jusot JF, Blanchard M, et al. Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight French cities: the PSAS program. Sci Total Environ 2007;387:105–12 [DOI] [PubMed] [Google Scholar]
- 7.Hong YC, Lee JT, Kim H, et al. Air pollution: a new risk factor in ischemic stroke mortality. Stroke 2002;33:2165–9 [DOI] [PubMed] [Google Scholar]
- 8.Tsai SS, Goggins WB, Chiu HF, et al. Evidence for an association between air pollution and daily stroke admissions in Kaohsiung, Taiwan. Stroke 2003;34:2612–16 [DOI] [PubMed] [Google Scholar]
- 9.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke 2005;36:2549–53 [DOI] [PubMed] [Google Scholar]
- 10.Maheswaran R, Haining RP, Brindley P, et al. Outdoor air pollution and stroke in Sheffield, United Kingdom: a small-area level geographical study. Stroke 2005;36:239–43 [DOI] [PubMed] [Google Scholar]
- 11.Low RB, Bielory L, Qureshi AI, et al. The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, September 11, 2001, and day of the week. Stroke 2006;37:951–7 [DOI] [PubMed] [Google Scholar]
- 12.Chan CC, Chuang KJ, Chien LC, et al. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J 2006;27:1238–44 [DOI] [PubMed] [Google Scholar]
- 13.Ballester F, Rodriguez P, Iniguez C, et al. Air pollution and cardiovascular admissions association in Spain: results within the EMECAS project. J Epidemiol Community Health 2006;60:328–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettunen J, Lanki T, Tiittanen P, et al. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke 2007;38:918–22 [DOI] [PubMed] [Google Scholar]
- 15.Henrotin JB, Besancenot JP, Bejot Y, et al. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med 2007;64:439–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisabeth LD, Escobar JD, Dvonch JT, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol 2008;64:53–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudin A, Stromberg U, Jakobsson K, et al. Estimation of short-term effects of air pollution on stroke hospital admissions in southern Sweden. Neuroepidemiology 2010;34:131–42 [DOI] [PubMed] [Google Scholar]
- 18.Maheswaran R, Pearson T, Smeeton NC, et al. Impact of outdoor air pollution on survival after stroke: population-based cohort study. Stroke 2010;41:869–77 [DOI] [PubMed] [Google Scholar]
- 19.Henrotin JB, Zeller M, Lorgis L, et al. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon Vascular Project (DIVA). Heart 2010;96:1990–6 [DOI] [PubMed] [Google Scholar]
- 20.Hennerici MG. Report of the 20th European Stroke Conference, Hamburg, May 24–27, 2011. Cerebrovasc Dis 2011;32:589–613 [DOI] [PubMed] [Google Scholar]
- 21.Mechtouff L, Canoui-Poitrine F, Schott AM, et al. Lack of association between air pollutant exposure and short-term risk of ischaemic stroke in Lyon, France. Int J Stroke 2012;7:669–74 [DOI] [PubMed] [Google Scholar]
- 22.Maheswaran R, Pearson T, Smeeton NC, et al. Outdoor air pollution and incidence of ischemic and hemorrhagic stroke: a small-area level ecological study. Stroke 2012;43:22–7 [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Zhang Y, Yang C, et al. Acute effect of ambient air pollution on stroke mortality in the China air pollution and health effects study. Stroke 2013;44:954–60 [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Sun Y, Ha S, et al. Association between ozone exposure and onset of stroke in Allegheny County, Pennsylvania, USA, 1994–2000. Neuroepidemiology 2013;41:2–6 [DOI] [PubMed] [Google Scholar]
- 25.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 2007;176:370–6 [DOI] [PubMed] [Google Scholar]
- 26.Jang AS, Choi IS, Yang SY, et al. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration 2005;72:79–84 [DOI] [PubMed] [Google Scholar]
- 27.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation 2005;111:2913–20 [DOI] [PubMed] [Google Scholar]
- 28.Dab W, Segala C, Dor F, et al. Air pollution and health: correlation or causality? The case of the relationship between exposure to particles and cardiopulmonary mortality. J Air Waste Manag Assoc 2001;51:220–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.