Abstract
Regular exercise promotes brain function via a wide range of adaptive responses, including the increased expression of antioxidant and oxidative DNA damage-repairing systems. Accumulation of oxidized DNA base lesions and strand breaks is etiologically linked to for example aging processes and age-associated diseases. Here we tested whether exercise training has an impact on brain function, extent of neurogenesis, and expression of 8-oxoguanine DNA glycosylase-1 (Ogg1) and SIRT1 (silent mating type information regulation 2 homolog). To do so, we utilized strains of rats with low- and high- running capacity (LCR and HCR) and examined learning and memory, DNA synthesis, expression, and posttranslational modification of Ogg1 hippocampal cells. Our results showed that rats with higher aerobic/running capacity had better spatial memory, and expressed less Ogg1, when compared to LCR rats. Furthermore, exercise increased SIRT1 expression and decreased acetylated Ogg1 (AcOgg1) levels, a post-translational modification important for efficient repair of 8-oxoG. Our data on cell cultures revealed that nicotinamide, a SIRT1-specific inhibitor, caused the greatest increase in the acetylation of Ogg1, a finding further supported by our other observations that silencing SIRT1 also markedly increased the levels of AcOgg1. These findings imply that high-running capacity is associated with increased hippocampal function, and SIRT1 level/activity and inversely correlates with AcOgg1 levels and thereby the repair of genomic 8-oxoG.
Keywords: DNA repair, OGG1, exercise, VO2max, hippocampus
1. Introduction
Regular exercise has been shown to promote brain function via a wide range of adaptive responses, including the induction of brain-derived neurotrophic factor (Gomez-Pinilla and Vaynman, 2005), neuropeptide, glutamic acid decarboxylase (GAD)65 and GAD67 (Buck et al., 2007, Murray et al., 2010, Groves-Chapman et al., 2011), vascular endothelial growth (Fabel et al., 2003), enhanced metabolism and neurogenesis (van Praag et al., 1999, Raichlen and Gordon, 2011), and up-regulation of antioxidant and oxidative damage-repair systems (Radak et al., 2007b). The latter could be particularly important, since it has been shown that an elevated level of oxidative damage led to impairment of spatial memory, as assessed by the maze test (Radak et al., 2001).
It has been reported that regular exercise is a powerful tool to attenuate age-associated increases in the levels of protein carbonyls (Radak et al., 2001). Moreover, an increase in damage to proteins and accumulation of 8-oxo-7,8-dihydroguanine (8-oxoG) in DNA in neurons has been associated with a wide range of neurodegeneration (Aguirre et al., 2005, Wang et al., 2006, Lovell and Markesbery, 2007). Indeed, it was recently reported that aging results in increased levels of 8-oxoG in the hippocampus, which was associated with decreased levels of acetylation of the 8-oxoguanine DNA glycosylase (OGG1), that excises 8-oxoG during the DNA base excision repair (BER) pathway (Radicella et al., 1997). Acetylation of OGG1 on Lys338/Lys341 by p300/CBP increases its activity in the presence of apurinic apyrimidinic endonuclease1 (APE-1) by reducing its affinity for the abasic site product (Bhakat et al., 2006). OGG1 also interacts with class I histone deacetylases, which may be responsible for its deacetylation (Bhakat et al., 2006). The importance of OGG1’s acetylation is underlined by data showing that exercise increases the acetylated (Ac) OGG1 levels in the muscles of young individuals (Bori et al., 2012). Efficient DNA repair has been shown to protect against neurodegeneration and thus underline the significance of oxidative DNA damage repair in the brain (Yang et al., 2010, Liu et al., 2011).
SIRT1 is a redox-sensitive deacetylase, targets acetyl groups on DNA repair proteins, such as APE1, and a number of regulatory proteins, including forkhead homeobox type O protein, nuclear factor kappa B (NF-κB), p53, peroxisome proliferator-activated receptor gamma coactivator-1 alpha, hypoxia inducible factor 1 alpha (Herranz and Serrano, 2010, Kelly, 2010). Due to silent mating type information regulation 2 homolog 1’s (or NAD+-dependent deacetylase sirtuin-1, SIRT1) multiple roles in cellular physiology, the activation of SIRT1 has been shown to retard the aging process (Agarwal and Baur, 2011), as well as to increase the resistance against oxidative stress (Csiszar et al., 2009), and attenuate neurodegeneration (Chao et al., 2008).
To examine the incidence and development of life-style-related diseases, Koch and Britton (Koch and Britton, 2001) used artificial selection for intrinsic aerobic endurance running capacity to develop a heterogeneous N:NIH strain of rats [low running capacity (LCR) and high running capacity (HCR)]. These rat models allowed the study of the effects of exercise on a variety of factors, including the incidence and development of life-style related diseases (Wisloff et al., 2005, Schwarzer et al., 2010). Wisloff and co-workers have found that LCR rats develop mitochondrial dysfunction in the heart and metabolic syndromes earlier than do the HCR ones (Wisloff et al., 2005). Moreover, it has also been shown that LCR rats have increased insulin resistance, visceral obesity, dyslipidemia, and decreased life-span compared to HCR rats (Bowman et al., 2010, Koch et al., 2011).
By utilizing LCR and HCR rat models, we showed that HCR rats had higher cognitive abilities and running capacity and expressed significantly less Ogg1. Further, exercise via SIRT1-mediated deacetylation transiently decreased the levels of AcOgg1 levels when compared to those of the LCR. These unexpected results imply that the Ogg1-initiated base excision repair of 8-oxoG during exercise may have a complex effect on the brain’s function in terms of endurance and raises the possibility that a delay in repair of oxidized guanine lesions could be advantageous for hippocampal function during exercise.
2. Materials and Methods
2.1. Animals
Artificial selective breeding, starting with a founder population of 186 genetically heterogeneous rats (N: NIH stock), was used to obtain rat strains differing in inherent aerobic capacity. The procedure was described in detail previously (Koch and Britton, 2001). Briefly, endurance running capacity was assessed on a treadmill, and the total distance run during the test was used as a measure for maximal aerobic exercise capacity. Rats with the highest running capacity from each generation were bred to produce the HCR strain, and rats with the lowest capacity were bred with each other to produce the LCR strain. A subgroup of male rats (24 HCR and 24 LCR) from generation 22 was used in the present investigation, carried out according to the requirements of The Guiding Principles for Care and Use of Animals, EU, and approved by the local ethics committee.
2.2. Exercise protocol
Twenty-four LCR and HCR male, 13-month-old rats were assigned to groups as follows: control LCR, trained LCR (TrLCR), control HCR, and trained HCR (TrHCR). Exercised rats (6 animals per group) were introduced to treadmill running for three days, and, then, for the next two weeks, the running speed was set to 10 m/min on a 5% incline for 30 min. Next, the maximal oxygen uptake (VO2max) was measured on the treadmill (Columbus Inst. Columbus, Ohio) with a gradually increasing intensity. VO2max was measured for each animal by using three criteria: (i) no change in VO2 when speed is increased, (ii) rats could no longer keep their position on the treadmill, and (iii) respiratory quotient (RQ = VCO2/VO2) > 1. Then, based on the level of VO2max, the speed corresponding to the 60% VO2max was determined and used for daily training for 1 hr five times a week. The VO2max was measured every second week, and the running speed was adjusted. The training period lasted for 12 weeks. In order to monitor new cell formation, 5-bromo-2'-deoxyuridine (BrdU) was injected into each animal for the last four weeks of the program. The animals were sacrificed two days after the last exercise session to avoid the metabolic effects of the final run, part of the hippocampus was excised and frozen, and the other portion used for histochemistry.
2.3. Passive avoidance test
The test was performed according to the step-through method described by Jarvik and Kopp (Jarvik and Kopp, 1967). The apparatus consists of a two-compartment acrylic box with a lighted chamber connected to a darkened one by a guillotine door. As soon as the rats entered the dark chamber, they received an electrical shock (0.5 mA, 1 sec). The latency times for entering the dark chamber were measured in the training test, after 24h and 10 days in the retention test.
2.4. Cell culture
Rat medullary pheochromocytoma (PC12) cells (obtained from the American Type Culture Collection) were maintained in DMEM/F12. PC12 cells were terminally differentiated into neuronal cells by nerve growth factor (NGF: 50 ng/mL) and characterized as we described previously (Bacsi et al., 2005). HCT116 cells (ATCC no.: CCL-247, human colorectal carcinoma cells) were maintained in Ham’s F12 (GIBCO-BRL). All media were supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), penicillin (100 units/ml; GIBCO-BRL), and streptomycin (100 µg/ml; GIBCO-BRL). Cells were routinely tested for mycoplasma contamination.
2.5. Immunohistochemistry
The brain samples were sectioned into 5-µm sections and, after de-paraffination with xylene and dehydration with 96% ethanol. For antigen retrieval, sections in citrate buffer (pH 6.0) were heated to 95°C for 15 min. After blocking in normal goat serum (Vector, S-1000) primary mouse anti-BrdU antibody (BD Pharmingen) was added to the sections (dilution of 1:200) and incubated overnight at 4°C. After washing with 0.2% Triton X-PBS, the sections were incubated with primary antibody and then Alexa Fluor 546 goat anti-mouse secondary antibody (30 min, room temperature, 1:200, Molecular Probes) was added. Neurons in sections were co-stained with anti-Neuronal Nuclei (NeuN, MAB377; Millipore, Temecula, CA) and visualized via Alexa Fluor 488 conjugated monoclonal antibody. Hoechst 33342 (Molecular Probes #H3570) was applied to visualize nuclei. Confocal microscopy was performed on a Zeiss LSM510 META system (Carl Zeiss Microimaging, Inc) by using 488-nm (argon laser) for excitation of Alexa Fluor 488, while Alexa Fluor 546’s excitation wavelength was 543 nm (helium–neon). Appropriate dichroic mirrors and emission band filters were combined to allow us to discriminate between green and red fluorescence. Images were captured at a magnification of 123× magnification. Colocalization was visualized by superimposition of green and red images by using MetaMorph software version 9.0r (Universal Imaging Corp, Sunnyvale, CA).
Ogg1 and AcOGG1 was detected by use of a Mouse and Rabbit Specific horseradish peroxidase (HRP) -3,3-Diaminobenzidine (DAB) detection immunohistochemistry (IHC) kit (ab64264), according to the kit’s protocol. The primary antibodies were anti-AcOgg1 (acetyl K338 + K341) antibody (Abcam, ab93670) and an affinity purified mouse anti-OGG1 antibody (human OGG1 reactive) generated against a synthetic peptide, CDLRQSRHAQEPPAK, representing the C-terminus of OGG1. These were acquired from Antibodies-Online GmbH (Atlanta, GA, USA). Microscopy was performed on a NIKON Eclipse Ti System. Magnification: x125. All morphological measurements were used with coded slides, and the experimenter was blind to the animal groupings. The level of neurogenesis was quantified as we reported earlier (Koltai et al., 2011).
2.6. Western blot analysis
Cells were lysed (25 mM Tris-HCl /pH 7.5/, 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DTT, 5% glycerol, 20 mM NaF, 1 mM sodium orthovanadate, 1 µg/ml leupeptin, and 1 µg/ml aprotinin). Proteins were separated by 5–20% SDS-PAGE, and then transferred to a Hybond-ECL nitrocellulose (Amersham Biosciences UK Ltd, Uppsala, Sweden) membrane by electroblotting. The membranes were blocked with 3% BSA in TBS containing 0.1% Tween (TBS-T) for 3 h and incubated overnight at 4°C with the primary antibodies (Ogg1 Abcam ab204, 1:500; Nampt Abcam ab37299 1:500; Sirt1 Abcam ab53517 1:500; β-Actin Santa Cruz Biotechnology sc-81178 1:2000). The blots were then washed four times with TBS-T and incubated for 1 h with HRP-conjugated secondary antibody (anti-mouse IgG, GE Healthcare UK Ltd, Pittsburgh, PA), and immunoreactive bands on membranes were visualized by chemiluminescence by using an ECL substrate (Amersham Biosciences).
The levels of oxidized proteins were determined by using an Oxyblot kit (Chemicon/Millipore, Temecula, CA, USA). Proteins were derivatized with 4-dinitrophenylhydrazine (DNPH) for 15 min followed by incubation at room temperature with a neutralization buffer. Derivatized proteins were electrophoresed and transferred, and DNPH-labeled proteins were detected by an anti-DNP primary antibody (1:150, Chemicon/Millipore) and HRP-secondary antibodies (1:300, Chemicon/Millipore). The bands were quantified by ImageJ software and standardized to β-actin (1:2000, #sc-47778 Santa Cruz).
2.7. siRNA ablation of gene expression
The N-TER Nanoparticle siRNA Transfection System was utilized to introduce siRNAs as described by the manufacturer (Sigma-Aldrich). Briefly, siRNAs (25 nM final concentrations, determined in preliminary studies) were mixed with N-TER Nanoparticle and added to cells. SIRT1, SIRT3 and control siRNAs (siGENOME SMARTpool) were obtained from Dharmacon (Thermo Fisher Scientific Inc.). Downregulation of the target genes’ mRNA levels was determined by quantitative real-time PCR (qRT-PCR).
2.8. RNA isolation and cDNA synthesis
Total RNA isolation was executed with an Ambion RNAqueous Kit (Cat. No: #1912), according to the manufacturer’s recommendation, and as we previously described (Szaniszlo et al., 2009). Briefly, the washed cell pellets were lysed in 350 µl lysis-binding solution. Next, 350 µl of 70% ethanol was added to the lysates, and then they were centrifuged (1 min at 13,000 rpm at 4°C) through a filter cartridge. The filters were washed (3 times) with 700 µl wash solution. The RNA was eluted from the filter with 50-µl elution solution (preheated to 75 °C). RNA concentration was determined by using a DU530 Beckman spectrophotometer. The A260/A280 ratios for all samples were found to be within the range of 1.9–2.2. The RNA samples were stored at −80°C. cDNA synthesis was carried out by adding 1 µg of total RNA to SuperScript™ III First-Strand Synthesis SuperMix for real time PCR (qRT-PCR; Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The thermal profile used was: 25 °C for 10 min, 50 °C for 30 min, and 85 °C for 5 min, and then samples were used immediately or stored at −80 °C.
2.9. Quantitative real-time PCR (qRT-PCR)
Total RNAs from brain samples were isolated by using a NucleoSpin® RNA/Protein (Macherey-Nagel, Düren, Germany) extraction kit, according to the manufacturer’s protocol. cDNAs were synthesized by using SuperScript™ III First-Strand Synthesis SuperMix (as in 2.8). Analyses of the real-time quantitative PCR data were performed by using the comparative threshold cycle [Ct] method as suggested by Applied Biosystems (User Bulletin #2). The following primers were used:
| Reference genes | |
| β-actin | F: 5’ GCT CGT CGT CGA CAA CGG CTC 3’ |
| R: 5’ CAA ACA TGA TCT GGG TCA TCT TCT 3’ | |
| Target genes | |
| OGG1 | F: 5’ AAC ATT GCT CGC ATC ACT GGC 3’ |
| R: 5’ GAT GTC CAC AGG CAC AGC CTG 3’ | |
| SIRT1 | F: 5’ TGC GGG AAT CCA AAG GAT AAT TCA GTG TC 3’ |
| R: 5’ CTT CAT CTT TGT CAT ACT TCA TGG CTC TAT G 3’ |
2.10. Statistical analyses
The results were compared with a Kruskal-Wallis ANOVA and using Mann-Whitney post-hoc analysis. Statistical significance was set to p <0.05. Means and standard errors of means (SEM) were presented to demonstrate the results. All statistical analyses were done with the use of the Statistica 8.8 program. The asterix indicates significant differences among the variables at a (*)p<0.05. Therefore it represents the results of the non-parametric post hoc analysis.
3. Results
3.1. Exercise endurance increases memory and reinitiates DNA synthesis
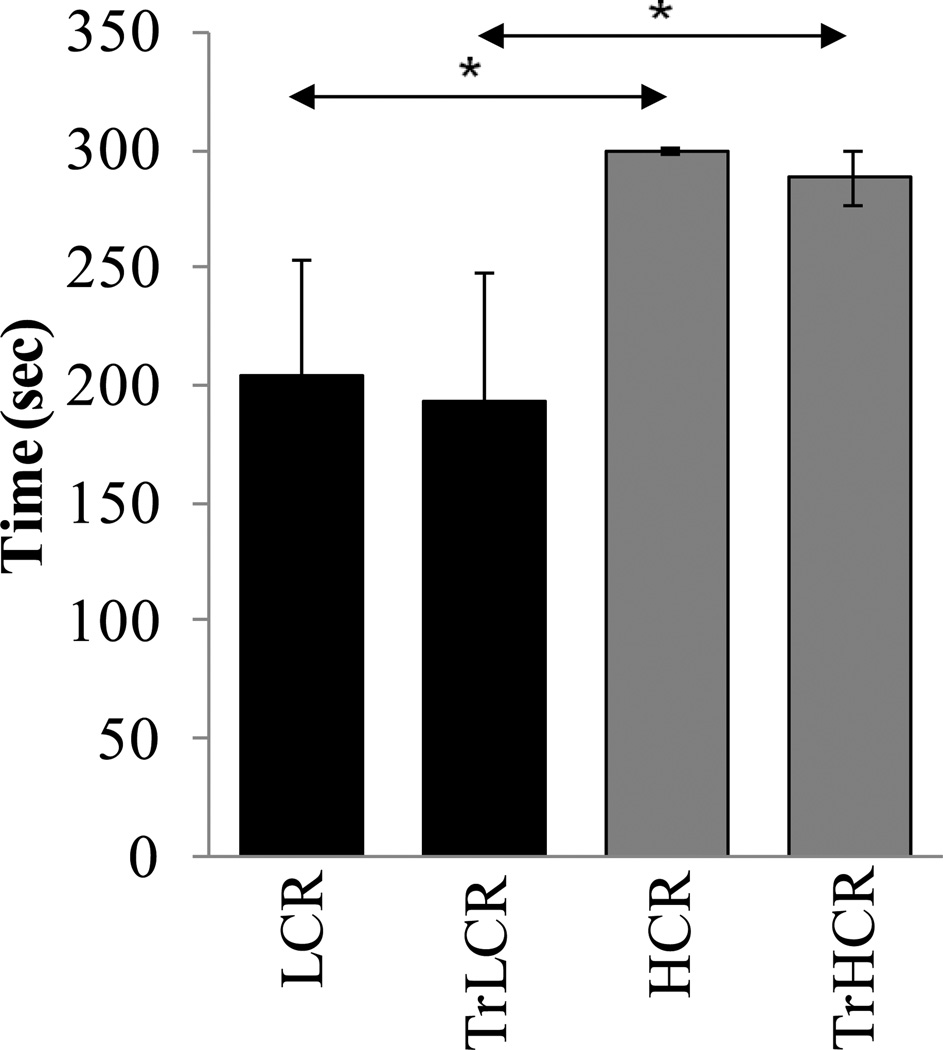
We used a passive avoidance test to assess the learning and memory of low- and highrunning capacity animals. As expected, HCR stayed longer in the bright chamber then in LCR rats, which showed that the HCR animals’ spatial memory was better (p<0.05), since they avoided entering the dark chamber where they were electrically shocked (Fig. 1).
Figure 1. Spatial memory of low- and high-running capacity rats.
The training period lasted for 12 weeks, and passive avoidance tests were carried out as in Materials and Methods. Spatial memory of animals was expressed by the time (min) they remained in the bright chamber where they received electric shock (Materials and Methods). LCR, low-running capacity animals, TrLCR, trained LCR; HCR, high-running capacity; TrHCR, trained HCR animals. Values were means ± SD for six subjects per group. * p<0.05
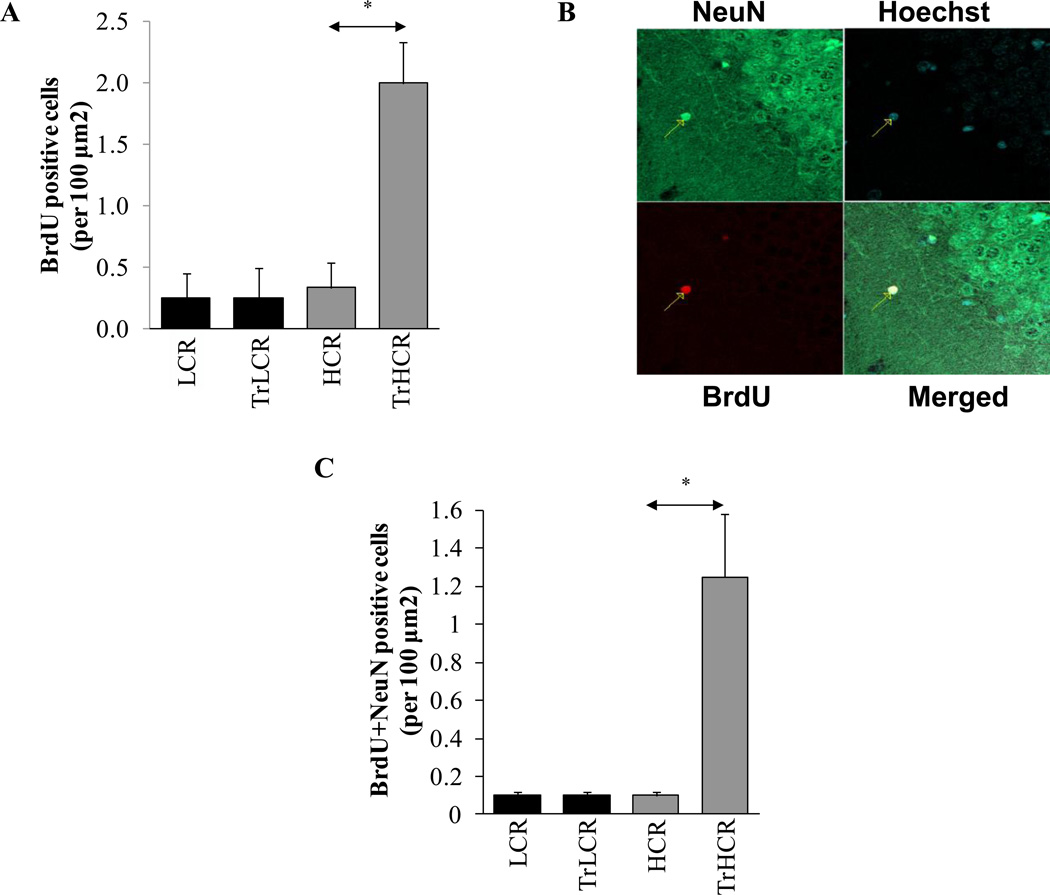
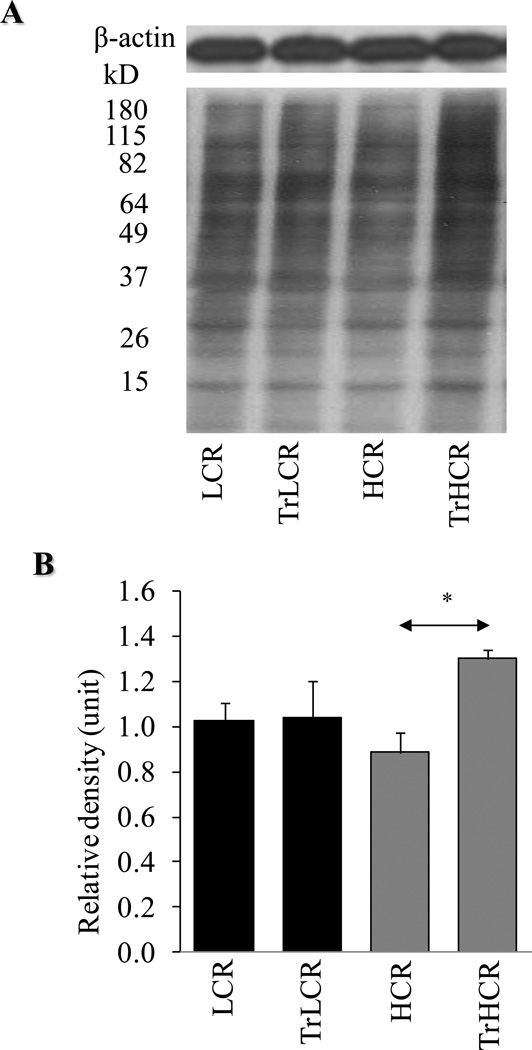
Terminally differentiated neurons in the hippocampus have been reported to have the capacity to reinitiate DNA replication in response to G1 regulatory activities induced by stimuli (Kuan et al., 2004). Indeed, our results showed increased BrdU incorporation in the DNA of the hippocampal cells of the trained HCR rats, while in LCR animals there was no change compared to that in the untrained controls (Fig. 2A). To confirm that DNA synthesis occurred in the neurons of the hippocampus, we showed that neuronal nuclear antigen (NeuN) and BrdU were co-localized primarily in hippocampal cells (Fig. 2B). Frequency of NeuN-stained cells were similar to the number of cells that incorporated BrdU, as shown in Fig. 2C lower panel. BrdU incorporation could be due to repair and/or replicative DNA synthesis, which presently is being further investigated. To provide evidence that hippocampal neurons are oxidatively stressed, we show that protein carbonyl levels were significantly increased. Prior to excersise, basal levels of oxidatively modified proteins were higher in LCR compared to HCR animals; however, in response to training, these modifications were unexpectedly elevated in HCR rats (Fig. 3A and B). Oxidative stress also inflicted damage to DNA, resulting in various base and strand lesions. Thus incorporation of BrdU may represent DNA synthesis associated with DNA repair.
Figure 2. Exercise endurance re-initiates DNA synthesis in cells of the hippocampus.
Twelve weeks of treadmill training and treatment with BrdU were undertaken as in Materials and Methods. (A), Incorporation of BrdU into hippocapal neurons per 100-µm2 area (B), Superimposition of BrdU (red) and NeuN (green) images. (C), Numerical illustration of cells showing BrdU and NeuN staining. HCR, TrLCR and TrHCR as in legend to Fig. 1. Values were means ± SD for six subjects per group. * p<0.05
Figure 3. Changes in oxidative protein modification in LCR and HCR animals in response to exercise.
After 12 weeks of training, protein carbonyl levels were determined in the hippocampus by using an immunoblot method. (A), Levels of oxidized proteins were determined by using an Oxyblot kit. Upper panel, β-actin was used as internal control. (B), Graphical depiction of lane intensities. Intensities of lanes were determined by ImageJ. HCR, TrLCR and TrHCR as in legend to Fig. 1. Values were means ± SD for six subjects per group. * p<0.05
3.2. Decreased expression of Ogg1 in high-performing animals
8-Oxoguanione (8-oxoG) is one of the most abundant DNA base lesions and is considered a marker of oxidative stress. 8-oxoG is repaired by Ogg1 during DNA BER (Radak et al., 2012). Efficient repair of this and other DNA base and strand lesions appeared to have a major role in the function of hippocampal cells. Ogg1 is a housekeeping gene whose basal expression is constitutive and unchanged during the cell cycle. This conclusion is based largely on the absence of TATA or CAAT boxes (Dhenaut, 2000, Boiteux et al. 2000). Its promoter contains binding sites for transcription factors, including NF-YA, AP4, NrF2, Sp1 and p53. The promoter’s composition implies that it may also be redox regulated.
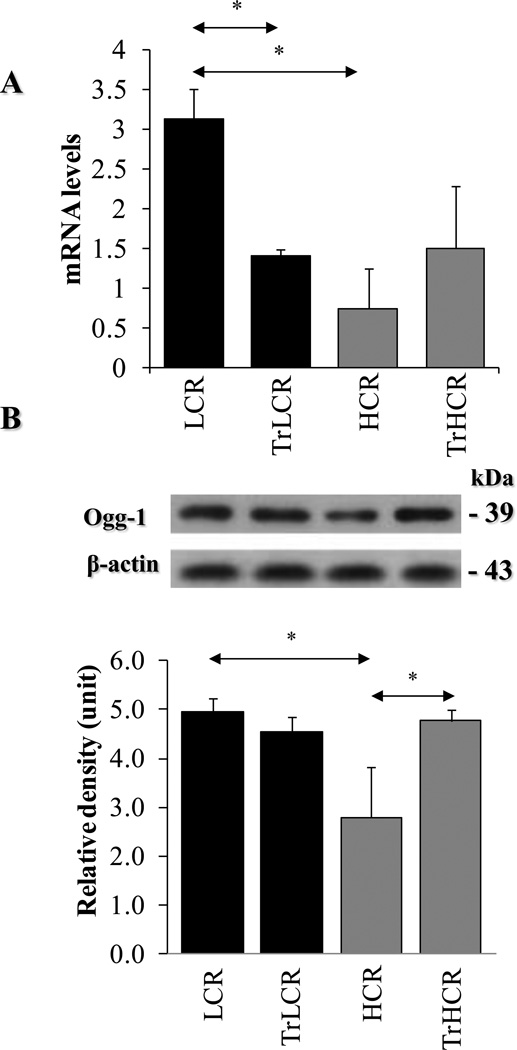
To examine if Ogg1 expression is altered in response to exercise, we determined RNA and protein levels. Our data show that the RNA levels of Ogg1 in hippocampal cells were unexpectedly lower in HCR compared to those in LCR animals (Fig. 4A). In line with RNA levels, Ogg1 protein levels were also significantly lower in the high-performing HCR rats (Fig. 4B, upper and lower panels). Interestingly, despite increased oxidative stress on proteins (Fig. 3A), training decreased Ogg1 mRNA levels in LCR, while protein levels were not altered (Fig. 4A, B). In high-performing animals, exercise increased Ogg1 expression both at the RNA and protein levels (Fig. 4A, B). These results indicate an inherent difference between low- and high-performing animals. Moreover, there was an inverse correlation between Ogg1 mRNA and protein levels in low running capacity animals. Importantly, these data underline a highly complex regulation of Ogg1 RNA and protein expression in hypocampal cells.
Figure 4. Expression of OGG1 in LCR and HCR animals in response to exercise.
After aerobic endurance treadmill training, the hippocampus was excised and qRT-PRC and Western blot analysis was undertaken as Materials and Methods. (A), Effect of training on Ogg1 mRNA levels in hippocampus of LCR and HCR animals. (B), Ogg1 in hippocampus of LCR and HCR animals. Upper panel, representative autoradiograms from Ogg1 and β-actin immunoblots. Lower panel, graphical depiction of Ogg1 levels as calculated by band intensities (ImageJ software). β-actin was used as an internal control. LCR, HCR, TrLCR and TrHCR as in legend to Fig. 1. Values were means ± SD for six subjects per group. * p<0.05
3.3. Decreased levels of AcOgg1 in neurons of hippocampus in HCR animals
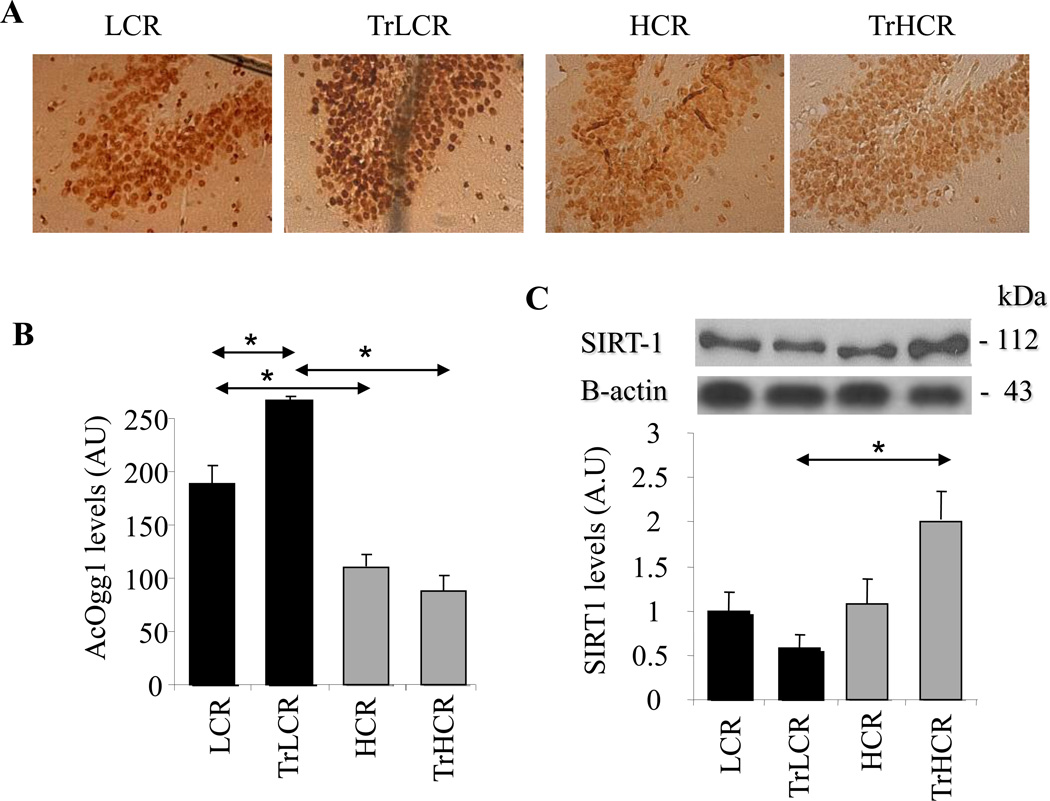
Activity of Ogg1 is modulated by phosphorylation(s) and acetylation and also by the redox state of some of the cysteine residues (Bravard et al., 2006). Reversible acetylation at Lys338/Lys341 is one of the most essential for Ogg1’s activity, as it decreases its affinity for the AP site product and allows recruitement of AP-endonuclease-1 (Bhakat et al., 2006). Therefore, the abundance of AcOgg1’ was determined in the hippocampus by using immunohistochemistry. Unexpectedly, the level of AcOgg1 was lower in the high-performing groups compared to those in LCR rats (Fig. 5A, upper and lower panels). Graphical depiction of staining densities showed statistically significant differences between LCR and HCR animals (Fig. 5B, left and right panels).
Figure 5. AcOgg1 and SIRT1 level is lower in HCR groups of animals.
LCR and HCR groups of animals were subjected to 12 weeks of training, and AcOgg1 as well as SIRT1 levels were determined by immune staining. (A) Sections of the hippocampus of exercise-trained animals were stained with AcOgg1 antibody and by the streptavidin-biotin immunoenzymatic antigen detection system. (B) Graphical representation of staining intensities is shown as determined by ImageJ software. (C) SIRT1 expression at protein level. Lower panel shows band intensities as determined by ImageJ software. HCR, TrLCR and TrHCR as in legend to Fig. 1. Values were means ± SD for six subjects per group. * p<0.05.
SIRT1 is a potent deacetylase, and therefore we examined whether lower AcOgg1 levels in hypocampus of HCR rats could be explained by increased levels of SIRT1. Western-blot analysis shows that SIRT1 levels were nearly identical in untrained HCR and LCR groups of animals (Fig. 5C, upper panel). Quantification of immune-blot images support this observation (Fig. 5C, lower panel). In response to training, SIRT1 levels were decreased in low-performing (LCR) rats in contrast to HCR animals in which SIRT1 levels were significantly increased (Fig. 5C, upper and lower panels). These results show an inverse correlation between AcOgg1 (Fig. 5A, B) and SIRT1 levels and suggested to us that SIRT1 could be a modulator of Ogg1-initiated DNA BER in hippocampal cells.
3.4. Increased levels of AcOgg1 by SIRT1 inhibition
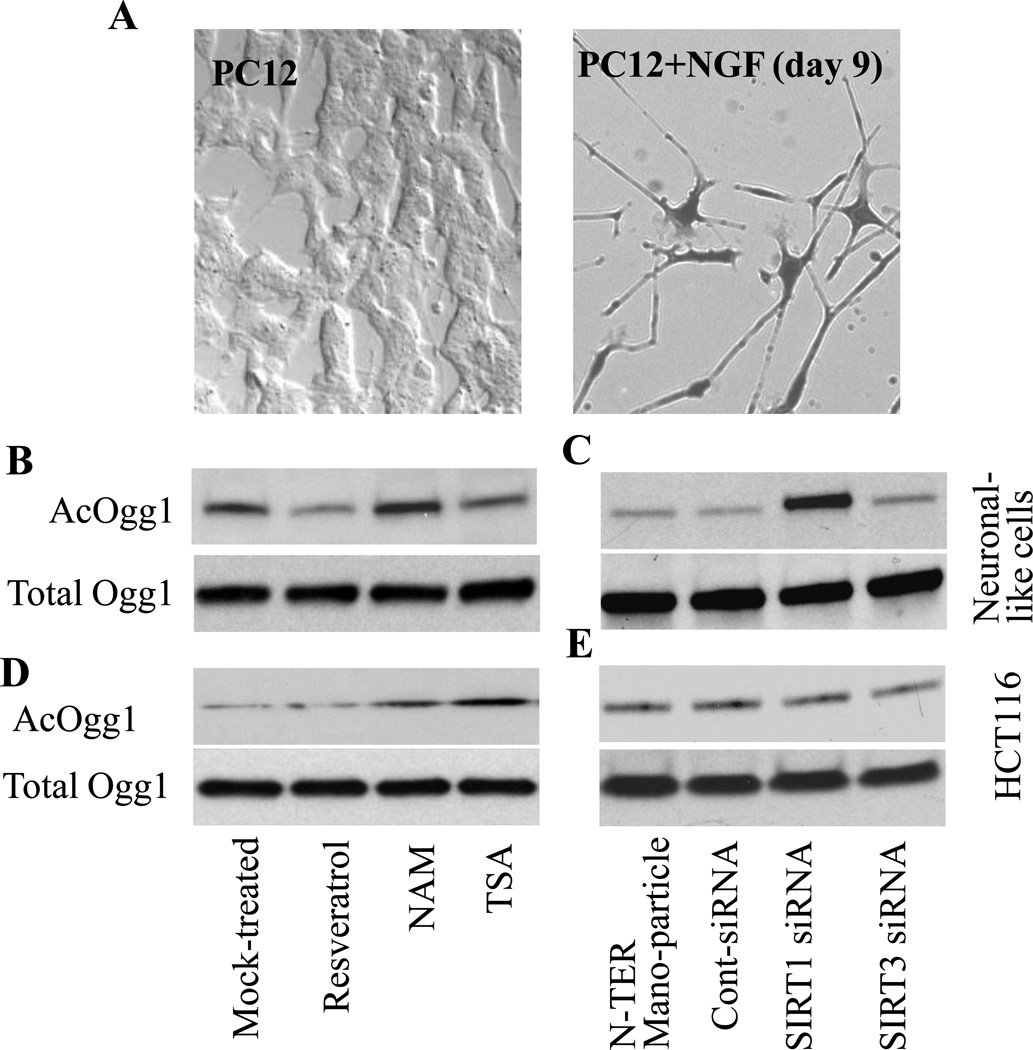
Next, we examined the validity of our observation on the inverse association between SIRT1 and AcOgg1 levels in the neurons of the hippocampus. To do so, we utilized terminally differentiated neuronal cell cultures developed from rat pheochromocytoma cells (Fig. 6A; Materials and Methods). Parallel cultures of neuronal cells were treated with resveratrol (100 µg/mL); nicotinamide (NAM, 10 mM), or trichostatin A (TSA, 10 ng per mL; histone deacetylase (HDAC) inhibitor). Then AcOgg1 levels were determined. As shown in Fig. 6B, resveratrol, an activator of SIRT1, decreased the level of AcOgg1, while AcOgg1 levels were significantly increased by NAM, a SIRT1 inhibitor. TSA had only an insignificant effect on AcOgg1 levels in neuronal cell cultures (Fig. 6B, upper panel). These agents did not alter total Ogg1 levels after 12h treatment (Fig. 6B, lower panel).
Figure 6. SIRT1 expression inversely correlates with levels of AcOgg1.
Neuronal cells were developed from pheochromocytoma (PC12) cells as described in Materials and Methods. (A) Phase contrast image of PC12 (left panel) and morphology of PC12 cells after nine days of nerve growth factor (NGF) treatment (neuronal-like cells, right panel). Parallel cultures neuronal-like (B,C) and HCT116 (D,E) cells were treated with NAM, resveratrol or TSA for 12h (B,D), or transfected with siRNA to SIRT1, SIRT3 or control siRNA by using N-TER transfection reagents (C,E; Materials and Methods). AcOgg1 levels were determined by Western blotting utilizing nuclear extracts. Upper panels show levels of AcOgg1 in 20 µg (per lane) nuclear extracts. NAM, nicotine amide, TSA, Trichostatin A. Lower panels: total Ogg1 levels in 5 µg nuclear extracts per lane. Representative images are shown from 3–4 experiments.
To confirm these results, we silenced SIRT1 expression via siRNA and found an increase in AcOgg1 level in neuronal-like cells (Fig. 6C). In controls, depletion of SIRT3 by siRNA had no effect on AcOgg1 levels (Fig.6C). SIRT1 and SIRT3 siRNA did not alter Ogg1 at protein levels, and control siRNA or transfection reagent (N-TER Nanoparticle) had no effect (Fig.6C, lower panel). These data suggested to us that SIRT1 could be responsible for the deacetylation of Ogg1 in neuronal cells, including those in the hippocampus of our experimental rat model. To investigate if SIRT1-mediated deacetylation of Ogg1 is neuronal cell specific only, we utilized HCT116 cells. Interestingly, resveratrol had no effect, while TSA caused an increase in AcOGG1 levels (Fig. 7D). NAM also increased levels of AcOGG1 in HCT116 cells (Fig. 7D). These data indicate that TSA-sensitive HDACs and not SIRT1s are involved in the deacetylation of AcOGG1 in HCT116 cells. SiRNA depletion of SIRT1 and SIRT3 had no effect, further supporting the idea that in neuronal cells SIRT1 is the primary deacetylase of Ogg1. These data strongly imply that acetylation levels of Ogg1 may be regulated by SIRT1 in the brain, specifically in hippocampal cells.
4. Discussion
Regular physical exercise increases the endurance of tissues to oxidative stress, increases vascularization, energy metabolism, and neurotrophin synthesis, all important in neurogenesis, memory improvement, and brain plasticity (Radak et al., 2010, Koltai et al., 2011, Radak et al., 2012). Here we show that animals with a higher aerobic/running capacity and better spatial memory have a lower expression and acetylated levels of Ogg1 compared to the levels in low-running capacity rats. Moreover, expression of SIRT1 showed an inverse correlation with AcOgg1 levels in hipocampal neurons. Similar observations were made in terminally differentiated neural cultures of cells. These findings are in accordance with the view that endurance running could play a beneficial role in brain function (Mattson, 2012), while lower expression of Ogg1 and SIRT1-mediated decrease in AcOgg1 levels was unexpected.
Accumulation of oxidatively modified molecules, DNA, and proteins (carbonyl groups in amino acid residues) has been linked to decreases in memory in aging gerbils (Carney et al., 1991). In the present study, we found that exercise training resulted in increased levels of protein carbonyls in HCR rats, but these animals showed better performance in passive avoidance tests compared to LCR. Indeed, it was suggested that certain types of carbonyl groups could be important to stimulate protein turnover (Radak et al., 2011). In support of the latter, there was an insignificant change in protein carbonyl levels in HCR rats, which could at least partly explain the better performance of these groups. Dispensable changes in protein carbonyl levels could also due to iron metabolism, as the interaction of H2O2 with iron led to its reduction and the formation of hydroxyl radicals, which is the most common inducer of carbonylation (Stadtman, 2006).
We had previously observed an increase in AMP-kinase activity in the skeletal muscle of these rats in response to exercise (Hart et al. 2013 and unpublished observation). Exercise mediates metabolic challenges, and, since SIRT1 is heavily involved in metabolic processes, finding elevated SIRT1 levels during exercise is not so surprising (Koltai et al., 2010). Without question, exercise is beneficial to the brain, but the level of metabolic stress is much smaller in the brain than in skeletal muscle. This could explain at least a part of the different organ response of SIRT1 to exercise stimuli.
Reactive species produce multiple oxidative DNA damage, such as oxidized DNA bases, oxidized sugar fragments, abasic (AP) sites, and single-strand breaks (ssbs). Furthermore, closely spaced ssbs or oxidized bases/AP sites (during repair) in the genome could form DNA double-strand breaks (Hegde et al., 2012). Training increased BrdU incorporation into hippocampal cells in high-performing animals. On the other hand, we did not observe any indication of mitotic cells and, thus, we considered that BrdU incorporation may represent DNA synthesis due to repair processes of the oxidative base and strand lesions. Among multiple types of DNA base damage generated by ROS, 8-oxoG accumulation in the genome has been associated with an increased frequency of mutations and various agingassociated diseases, including muscle wasting, cardiovascular diseases, malignancies, diabetes, and Alzheimers Disease (McMurray et al., 1998, Twisk et al., 2000, Radak et al., 2010, Radak et al., 2012, Park et al., 2013). 8-OxoG is repaired via the base excision repair pathway that is initiated by the Ogg1 (Hollenbach et al., 1999). Unexpectedly, in HCR rats there was a significantly lower Ogg1 expression in the hippocampus of HCR rats compared to those in the LCR at both protein and RNA levels. These results were unexpected, since efficient Ogg1-mediated genome repair is generally believed to increase life span and physical endurance and also has anti-apoptotic potential. These observations raise an unexpected issue about 8-oxoG repair. Intriguingly the activity-related, post-translational modification of Ogg1, namely acetylation, was lower in high-performing rats, when compared to LCR rats. These results appear to contradict previously published observations showing the imperative role of efficient oxidative DNA damage repair in the functioning of hippocampal cells.
SIRT1 is shown to deacetylate APE1, which is a rate-limiting enzyme in DNA base excision repair (Yamamori et al., 2010); therefore it cannot be excluded that this enzyme also deacetylates Ogg1. We used a neural cell culture model to test whether SIRT1 is a potential deacetylating enzyme of Ogg1. Our data revealed that NAM, a SIRT1-specific inhibitor, caused the greatest increase in the acetylation of Ogg1, a finding further supported by our other observations that silencing SIRT1 also markedly increased the level of AcOgg1. Resveratrol an activator of SIRT1 decreased AcOgg1 levels in neuronal cells. TSA (histone deacetylase inhibitor) had no significant effect on Ac-Ogg1 levels in neural cells. In contrast TSA increased AcOGG1 levels in HCT116 cells. These data strongly suggest to us that AcOgg1 levels are SIRT1- modulated in neural cells, while histone deacetylases are the primary modulator in non-neural cells. As acetylation modulates Ogg1’s activity on AP sites, it is reasonable to propose that SIRT1 modulates the repair of 8-oxoG. Exercise increases the activity of SIRT1, leading to a decreased acetylation of Ogg1, which implies a decreased enzymatic Ogg1 activity and lower efficiency of 8-oxoG repair in the brain. We have reported that exercise increases DNA repair activity of OGG1 in human skeletal muscle from young individuals (Radak et al., 2002, Radak et al., 2003, Radak et al., 2007a). Taking these observations into consideration, it is seems possible that Ogg1’s activity is differentially regulated in response to exercise, and that specifically its activity is transiently downregulated in the brain, while up regulated in muscle. These observations raise the possibility that a delay in the repair of 8-oxoG lesions could be beneficial for brain function. Despite an accumulation of 8-oxoG, Ogg1−/− mice appeared to have a normal phenotype and showed an increased resistance to inflammation. Moreover, no organ defects were observed, and these Ogg1−/− mice showed an increased tolerance to chronic oxidative stress (Arai et al., 2006). Although, the response of the Ogg1−/− mice to exercise is yet to be investigated, these observations imply that the 8-oxoG base released from the genome of the brain cells (and not the transient 8-oxoG accumulation in DNA) could have a higher physiological/pathophysiological relevance compared to that from skeletal muscle. Indeed, we have previously shown that OGG1 binds its excision product, the 8-oxoG base, but not 8-oxoguanine deoxynucleoside (8-oxodG) or FapyG (or other intact or oxidized nucleotides and nucleosides), at sites independent from its catalytic active site. In complex with the 8-oxoG base, OGG1 interacts with the canonical Ras family members and induces guanine nucleotide exchange. Activated Ras then initiates signal transduction via Raf1 kinase, dual-specificity mitogen-activated protein kinases (MEK1,2) and extracellular signal-regulated kinases (ERK1,2), leading to the transcriptional activation of genes (Boldogh et al., 2012).
Activation of Ras protein and the mitogen-activated kinase (MAPK) pathway has been shown to cause apoptosis in neurons (Sheng et al., 2012). Therefore de-activation of Ogg1 by SIRT1-mediated deacetylation could favor its control of the Ogg1-initiated repair of DNA, but also imply an anti-apoptotic role of SIRT1. Although the molecular mechanism of the inverse correlation between the lower expression of Ogg1 and high-running capacity of rats is yet to be determined, our data link the decreased repair of 8-oxoG with aerobic endurance capacity and spatial learning. A recent study showed that SIRT1 inhibition decreased phosphorylation of insulin receptor substrate 2 and also activation of the Ras/ERK1/2 pathway associated with resistance to oxidative damage (Li et al., 2008). In our system an important element of exercise in inducing neuroprotection could be the SIRT1-dependent deacetylation of Ogg1, which can transiently decrease excision of 8-oxoG, thereby activating the Ras/ERK1/2 pathway. We speculate that a higher Ogg1 expression and failure in the control of Ogg1 activity in LCR rats may lead to an excessive release of 8-oxoG from DNA, resulting in unscheduled activation of Ras family GTPases that could result in pathophysiological cellular responses, contributing to lower physical endurance and spatial learning.
Highlights.
-
►
Exercise training has an impact on brain function and increases neurogenesis
-
►
High running-capacity animals have better spatial memory and express less Ogg1
-
►
Exercise increased SIRT1 expression and decreased levels of acetylated Ogg1
-
►
Exercise increased hippocampal function and transiently decreased repair of genomic 8-oxoG
Acknowledgements
The present work was supported by Hungarian grants from TAMOP-4.2.2/B-10/1-2010-0013 awarded to Z. Radák, NIEHS RO1 ES018948 (IB), NIAID/AI062885 (IB) and NIA/AG 021830 (IB). We thank Mardelle Susman (Department of Microbiology and Immunology, University of Texas Medical Branch at Galveston) for editing our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal B, Baur JA. Resveratrol and life extension. Annals of the New York Academy of Sciences. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- Aguirre N, Beal MF, Matson WR, Bogdanov MB. Increased oxidative damage to DNA in an animal model of amyotrophic lateral sclerosis. Free radical research. 2005;39:383–388. doi: 10.1080/10715760400027979. [DOI] [PubMed] [Google Scholar]
- Arai T, Kelly VP, Minowa O, Noda T, Nishimura S. The study using wild-type and Ogg1 knockout mice exposed to potassium bromate shows no tumor induction despite an extensive accumulation of 8-hydroxyguanine in kidney DNA. Toxicology. 2006;221:179–186. doi: 10.1016/j.tox.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bacsi A, Stanton GJ, Hughes TK, Kruze M, Boldogh I. Colostrinin-driven neurite outgrowth requires p53 activation in PC12 cells. Cellular and molecular neurobiology. 2005;25:1123–1139. doi: 10.1007/s10571-005-8222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK, Mitra S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Molecular and cellular biology. 2006;26:1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK, Mitra S. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. The Journal of biological chemistry. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bori Z, Zhao Z, Koltai E, Fatouros IG, Jamurtas AZ, Douroudos II, Terzis G, Chatzinikolaou A, Sovatzidis A, Draganidis D, Boldogh I, Radak Z. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Experimental gerontology. 2012;47:417–424. doi: 10.1016/j.exger.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman TA, Ramakrishnan SK, Kaw M, Lee SJ, Patel PR, Golla VK, Bourey RE, Haram PM, Koch LG, Britton SL, Wisloff U, Lee AD, Najjar SM. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology. 2010;151:5157–5164. doi: 10.1210/en.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravard A, Vacher M, Gouget B, Coutant A, de Boisferon FH, Marsin S, Chevillard S, Radicella JP. Redox regulation of human OGG1 activity in response to cellular oxidative stress. Molecular and cellular biology. 2006;26:7430–7436. doi: 10.1128/MCB.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck BJ, Kerman IA, Burghardt PR, Koch LG, Britton SL, Akil H, Watson SJ. Upregulation of GAD65 mRNA in the medulla of the rat model of metabolic syndrome. Neuroscience letters. 2007;419:178–183. doi: 10.1016/j.neulet.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free radical biology & medicine. 2008;45:1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mechanisms of ageing and development. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. The European journal of neuroscience. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S. A "deficient environment" in prenatal life may compromise systems important for cognitive function by affecting BDNF in the hippocampus. Experimental neurology. 2005;192:235–243. doi: 10.1016/j.expneurol.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Groves-Chapman JL, Murray PS, Stevens KL, Monroe DC, Koch LG, Britton SL, Holmes PV, Dishman RK. Changes in mRNA levels for brain-derived neurotrophic factor after wheel running in rats selectively bred for high- and low-aerobic capacity. Brain research. 2011;1425:90–97. doi: 10.1016/j.brainres.2011.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N, Sarga L, Csende Z, Koltai E, Koch LG, Britton SL, Davies KJ, Kouretas D, Wessner B, Radak Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013 doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde ML, Izumi T, Mitra S. Oxidized base damage and single-strand break repair in mammalian genomes: role of disordered regions and posttranslational modifications in early enzymes. Progress in molecular biology and translational science. 2012;110:123–153. doi: 10.1016/B978-0-12-387665-2.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nature reviews Cancer. 2010;10:819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbach S, Dhenaut A, Eckert I, Radicella JP, Epe B. Overexpression of Ogg1 in mammalian cells: effects on induced and spontaneous oxidative DNA damage and mutagenesis. Carcinogenesis. 1999;20:1863–1868. doi: 10.1093/carcin/20.9.1863. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Kopp R. An improved one-trial passive avoidance learning situation. Psychological reports. 1967;21:221–224. doi: 10.2466/pr0.1967.21.1.221. [DOI] [PubMed] [Google Scholar]
- Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Alternative medicine review : a journal of clinical therapeutic. 2010;15:245–263. [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiological genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation research. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mechanisms of ageing and development. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai E, Zhao Z, Lacza Z, Cselenyak A, Vacz G, Nyakas C, Boldogh I, Ichinoseki-Sekine N, Radak Z. Combined exercise and insulin-like growth factor-1 supplementation induces neurogenesis in old rats, but do not attenuate age-associated DNA damage. Rejuvenation research. 2011;14:585–596. doi: 10.1089/rej.2011.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell metabolism. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, Jiang H, Mustafa K, Keijzers G, Bohr VA, Mattson MP. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic acids research. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Evolutionary aspects of human exercise--born to run purposefully. Ageing research reviews. 2012;11:347–352. doi: 10.1016/j.arr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RG, Ainsworth BE, Harrell JS, Griggs TR, Williams OD. Is physical activity or aerobic power more influential on reducing cardiovascular disease risk factors? Medicine and science in sports and exercise. 1998;30:1521–1529. doi: 10.1097/00005768-199810000-00009. [DOI] [PubMed] [Google Scholar]
- Murray PS, Groves JL, Pettett BJ, Britton SL, Koch LG, Dishman RK, Holmes PV. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides. 2010;31:2264–2268. doi: 10.1016/j.peptides.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Ko Y, Song SH, Kim S, Yoon HJ. Association of low aerobic fitness with hyperfiltration and albuminuria in men. Medicine and science in sports and exercise. 2013;45:217–223. doi: 10.1249/MSS.0b013e318271b39f. [DOI] [PubMed] [Google Scholar]
- Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G, Nakamoto H, Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life sciences. 2003;72:1627–1633. doi: 10.1016/s0024-3205(02)02476-1. [DOI] [PubMed] [Google Scholar]
- Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, Boldogh I. Exercise plays a preventive role against Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochemistry international. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. Journal of applied physiology (Bethesda, Md : 1985) 2007a;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007b;32:942–946. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Archiv : European journal of physiology. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Goto S, Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Molecular aspects of medicine. 2011;32:305–315. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen Consumption and Usage During Physical Exercise: The Balance Between Oxidative Stress and ROS-Dependent Adaptive Signaling. Antioxidants & redox signaling. 2012 doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicella JP, Dherin C, Desmaze C, Fox MS, Boiteux S. Cloning and characterization of hOGG1, a human homolog of the OGG1 gene of Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8010–8015. doi: 10.1073/pnas.94.15.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Gordon AD. Relationship between exercise capacity and brain size in mammals. PloS one. 2011;6:e20601. doi: 10.1371/journal.pone.0020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer M, Britton SL, Koch LG, Wisloff U, Doenst T. Low intrinsic aerobic exercise capacity and systemic insulin resistance are not associated with changes in myocardial substrate oxidation or insulin sensitivity. Basic research in cardiology. 2010;105:357–364. doi: 10.1007/s00395-010-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z, Oka S, Tsuchimoto D, Abolhassani N, Nomaru H, Sakumi K, Yamada H, Nakabeppu Y. 8-Oxoguanine causes neurodegeneration during MUTYH-mediated DNA base excision repair. The Journal of clinical investigation. 2012;122:4344–4361. doi: 10.1172/JCI65053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free radical research. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Szaniszlo P, German P, Hajas G, Saenz DN, Woodberry MW, Kruzel ML, Boldogh I. Effects of Colostrinin on gene expression-transcriptomal network analysis. International immunopharmacology. 2009;9:181–193. doi: 10.1016/j.intimp.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Twisk JW, Kemper HC, van Mechelen W. Tracking of activity and fitness and the relationship with cardiovascular disease risk factors. Medicine and science in sports and exercise. 2000;32:1455–1461. doi: 10.1097/00005768-200008000-00014. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science (New York, NY) 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung SB, Kim CS, Irani K. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic acids research. 2010;38:832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. The Journal of biological chemistry. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]