Abstract
Objective
The binding protein FKBP5 is an important modulator of the function of the glucocorticoid receptor, the main receptor of the stress horm one system. This turns the FKBP5 gene into a key candidate for gene-environment interactions, which are considered critical for pathogenesis of stress-related disorders. The authors explored gene-environment interactions between FKBP5 gene variants and adverse life events in predicting the first occurrence of a major depressive episode.
Method
The analyses were based on 884 Caucasians in a 10-year prospective community study. At baseline, they were 14–24 years old and did not fulfill criteria for a major depressive episode. The DSM-IV-based Munich Composite International Diagnostic Interview was used to assess adverse life events preceding baseline and major depressive episodes during follow-up. On the basis of previous findings, five single-nucleotide polymorphisms (SNPs) within the FKBP5 gene were selected for genotyping.
Results
While the authors did not observe genetic main effects, they found interactions between the five SNPs and traumatic (but not separation) events, with the strongest effect for severe trauma. The effect of trauma on incident major depressive episodes was evident among subjects homozygous for the minor alleles but not subjects with other genotypes. The findings were replicated in the U.K. Environmental Risk Longitudinal Twin Study.
Conclusions
These hypothesis-driven results suggest that an interaction between FKBP5 genotype and trauma is involved in the onset of depression. Subjects homozygous for the minor alleles of the investigated FKBP5 SNPs seem to be particularly sensitive to effects of trauma exposure in terms of triggering depression onset.
The gene-environment interaction approach (1) suggests that genes can control an individual's sensitivity for stressful life events. After the report by Caspi et al. (2) of an interaction between the serotonin transporter gene 5-HTTLPR and life events that predicts depression, most studies in this field focused on 5-HTTLPR. The role of other relevant genetic variants is still insufficiently explored.
Neurobiological mechanisms involved in causality of disease are considered to be appropriate sources for candidate genes. In this context, the hypothalamic-pituitary-adrenal (HPA) axis is important, as impaired functioning of this neuroendocrine system has been implicated in the pathogenesis and course of depression (3). Since adverse life events might contribute to the development of depression through their effects on HPA axis functioning (4, 5), genes involved in the regulation of this system are promising candidates for studies of gene-environment interactions.
The major regulatory element of the HPA axis is the glucocorticoid receptor, whose functioning is moderated by chaperone proteins forming a molecular heterocomplex. Proper folding of the heterocomplex is required for ligand binding and translocation to the cell nucleus, where the activated glucocorticoid receptor exhibits its effects. In this respect, the FK506 binding protein 51 (FKBP5), coded by the FKBP5 gene, is relevant as it is a co-chaperone to the heat shock protein 90 (hsp90) and modulates glucocorticoid receptor functioning (6, 7). FKBP5 acts as an inhibitor of the glucocorticoid-receptor-mediated glucocorticoid action. In addition, glucocorticoids induce FKBP5 expression as part of an intracellular ultrashort negative feedback loop for glucocorticoid receptor activity (8).
Our group (9) and others (10) have demonstrated that single-nucleotide polymorphisms (SNPs) in the FKBP5 gene, including rs1360780, rs3800373, rs9296158, and rs9470080, are associated with an altered induction of FKBP5 expression by cortisol, leading to differences in glucocorticoid receptor sensitivity. These differences in receptor sensitivity correspond to other findings from our group, showing that three variants in the FKBP5 gene (rs4713916, rs1360780, and rs3800373) are associated with an incomplete normalization of stress-elicited cortisol secretion in healthy subjects after psychosocial stress (11). This suggests a genotype-dependent risk of chronically elevated plasma cortisol levels in the context of chronic or recurrent stress, which also may increase the risk of stress-related disorders, such as depression. This is further supported by the association of the same gene variants with unipolar depression (12–14), bipolar disorder (15), and suicidality (16). However, other investigators have not found a genetic main effect of FKBP5 SNPs on depression (9, 17). These inconsistencies could result from a failure to consider moderating influences, such as interactions with environmental factors (18).
There are four studies published to date (10, 12, 19, 20) exploring gene-environment interactions with regard to FKBP5. Two of these indicated that FKBP5 SNPs interact with child abuse to influence risk of posttraumatic stress disorder (PTSD) (10, 20). Another study showed that the same FKBP5 SNPs moderate the influence of childhood trauma on suicide risk (19). Only one of these studies examined depression as an outcome, and it indicated a genetic main effect of one FKBP5 SNP on depression in males only but no interaction with retrospectively assessed childhood problems (12).
The aim of the present investigation was to examine the interaction between adverse life events and variants in the FKBP5 gene in relation to predicting the first occurrence of a major depressive episode.
Method
Design and Sample
The subjects were 996 respondents who participated in the molecular-genetic part of the Early Developmental Stages of Psychopathology study. This is a prospective longitudinal community study comprising a baseline and three follow-up waves covering an overall period of about 10 years. The representative baseline sample was drawn randomly from the 1994 government registries of all residents in Munich and surrounding counties between the ages of 14 and 24 years. The first follow-up, approximately 20 months after baseline, was conducted for subjects who were age 14–17 years at baseline; the second and third follow-ups included all subjects and occurred at approximately 42 and 101 months after baseline, respectively. The rate of retention from baseline (N=3,021) to the third follow-up (N=2,210) was 73.2%, and there was no selective attrition due to age, gender, geographic distribution, major depressive episodes, or the investigated adverse event categories (21, 22).
The molecular-genetic part of the study was initiated at the third follow-up. There were 996 respondents who were European Caucasians of German nationality, according to interviewer ratings and respondents’ self-reports on the country of origin for themselves, their natural parents, and their grandparents. Five individuals were of ethnicities other than European Caucasian and were excluded. Sociodemographic characteristics are presented in section SA1a of Appendix SA1 of the data supplement accompanying the online version of this article.
Of the 996 participants, 112 reported a lifetime history of a major depressive episode before or at baseline and were excluded from the investigation, leaving 884 individuals in the analysis. This prospective approach was done to prevent confounding of adverse event reports by depression status and to ensure that the adverse events occurred before the first major depressive episode, which is a prerequisite for causal interpretation.
The Early Developmental Stages of Psychopathology study was approved by the Ethics Committee of the Bayerische Landesaerztekammer Munich and the Medical Faculty of the Technische Universitaet Dresden. After complete description of the study to the subjects, written and oral informed consent was obtained.
Diagnostic Assessment
At each wave participants were interviewed with the computer-assisted Munich version of the Composite International Diagnostic Interview (M-CIDI) (23), which allows for the standardized assessment of symptoms, syndromes, and diagnoses of DSM-IV disorders and information about onset, duration, and severity. It is supplemented by a respondent's booklet including disorder-specific questionnaires and symptom lists. Test-retest reliability and validity have been reported elsewhere (24–26). Trained clinical interviewers carried out the interviews face to face, mostly in the respondents’ homes. At baseline, lifetime and 12-month disorders were assessed. At each follow-up interview, the M-CIDI covered the time interval since the previous interview.
Major depressive episodes and other mental disorders used as covariates (anxiety disorders, substance use disorders) were assessed by using the M-CIDI algorithms according to DSM-IV (see section SA1b of Appendix SA1 in the online data supplement). The main outcome measure, an incident major depressive episode, was defined as meeting the DSM-IV criteria for a major depressive episode for the first time during follow-up.
Adverse Life Events
In the evaluation of lifetime separation events, “death of a parent” (identified in 40 of 884 respondents, 4.5%) and “divorce of parents” (N=194, 21.9%) were assessed at baseline by using items from the family history section of the M-CIDI. The category “any separation event” covers “death” and “divorce” in any parent (N=223, 25.2%).
Lifetime trauma exposure before baseline was assessed at the beginning of the M-CIDI section for PTSD. Respondents were presented with a list of seven categories of traumatic events (see section SA1c of online Appendix SA1). “Any trauma” comprises all traumatic events (N=118, 13.3%). “Any severe trauma” includes only qualified traumas, which were reported to have caused intense fear, a feeling of helplessness, or horror (DSM-IV criterion A2 for PTSD) (N=90, 10.2%).
The category “any adverse event” (N=298, 33.7%) indicated exposure to any separation or traumatic event. Lifetime events preceding baseline occurred predominantly during childhood and adolescence (75% of severe traumas happened by age 16).
DNA Extraction and SNP Genotyping
The participants were asked to provide blood samples (40 ml) either at the Max Planck Institute of Psychiatry or at the practice of their general practitioner. Participants who did not comply were additionally asked for saliva samples, which were collected in four 10-ml vials containing an alcohol-based commercial mouthwash. DNA was extracted from fresh blood (for 885 of the 996 total respondents in the molecular-genetic study) or saliva (N=111).
Referring to our previous FKBP5 studies (9, 11), we selected five polymorphisms for genotyping: the three potentially functional SNPs (rs3800373, rs1360780, rs4713916) and two further SNPs (rs9296158, rs9470080) previously identified as relevant in a gene-environment analysis of PTSD (10). These SNPs represent the FKBP5 gene region at an average pairwise r2 value (representing linkage disequilibrium) of 0.85 (see supplemental Figure SF1 in the data supplement accompanying the online version of this article). Genotyping was performed on a Sequenom platform employing the iPlex technology (Sequenom, San Diego). Hardy-Weinberg equilibrium was tested with Stata software (27). Online supplemental Table ST1 presents information on the genotyped SNPs, Hardy-Weinberg equilibrium, minor allele frequency, and call rates (proportions of unambiguous genotypes). The linkage disequilibrium structure of the SNPs was determined by Haploview 4.0 (28) with the r2 statistic. It showed that four of the SNPs belong to the same linkage disequilibrium block and one (rs4713916) is highly associated with this block (see supplemental Figure SF1). The linkage disequilibrium structure in our sample is a good match with the linkage disequilibrium structures reported by the HapMap Project and in other samples (9, 11). Power calculations are provided in section SA1d of the online supplemental Appendix SA1.
Statistical Analyses
The analyses followed the genotypic model, comparing the three possible genotypes with each other, and produced odds ratios based on logistic regressions adjusted for age and gender.
To examine interactions between FKBP5 polymorphisms and adversity on an incident major depressive episode, an additive statistical interaction model was used. This model determines interaction effects as a deviation from additivity (instead of multiplicativity), which has been recommended for testing biologic interaction (29–32). A superadditive risk difference interaction means that the combined effect of two factors is larger (or smaller) than the sum of their individual effects (30, 33). The risk difference has been proposed as an appropriate measure in terms of the standard models of causality (counterfactual causality and the sufficient component cause model) (29–31). We used the LOGISTIC procedure in Stata (27) to fit logistic regressions and the MFX procedure to compute risk differences from the results. In the latter procedure, a method proposed by Joffe and Greenland was applied (33, 34).
To illustrate the effect over the lifetime, age-specific cumulative lifetime incidences for major depressive episodes were calculated by means of the Kaplan-Meier method (35, p. 7). Differences were tested with hazard ratios from stratified Cox regression (35, p. 39).
We corrected for two independent event categories (separation and traumatic events) and for the five SNPs. Using the conservative Bonferroni correction for multiple testing, we set the level of significance to 0.005 (0.05/10).
To assure that interaction findings were not confounded by other preexisting disorders, we additionally controlled the logistic regressions for baseline anxiety and substance use disorders. Control variables were chosen a priori on the basis of previous research (21, 36). All analyses were carried out with Stata 9.0 (27).
Replication Studies
Samples
Participants of the Dunedin Multidisciplinary Health and Development Study and the mothers of the participants in the Environmental Risk (E-Risk) Longitudinal Twin Study served as replication samples (37). Briefly, the Dunedin sample was recruited from infants born in Dunedin, New Zealand, between April 1972 and March 1973 (N=1,037, 91% of eligible births; 52% male) and represents the full range of socioeconomic status in the general population of New Zealand's South Island. More than 90% of the participants are Caucasian. Assessments were conducted between the ages of 3 and 32, when 96% of the 1,015 study members still alive in 2004–2005 were interviewed. Evidence of childhood maltreatment was assessed between ages 3 and 11 years from five sources (shown in section SA1e of online supplemental Appendix SA1).
The base sample of the E-Risk study was formed in 1999–2000, when 1,116 mothers of 5-year-old twins (93% of eligible families) participated in home visit assessments. The participants represent the full range of socioeconomic status in the general population of England, and more than 90% are white. Four assessments were performed when the women were on average between 33 and 40 years (range=26–55 years, SD=5.8). The mothers completed the Childhood Trauma Questionnaire (38) at an average age of 40 years (see section SA1e of online supplemental Appendix SA1).
In both replication samples, a major depressive episode during adulthood was the measured outcome and was evaluated with the Diagnostic Interview Schedule (39) (see section SA1e of supplemental Appendix SA1).
DNA extraction and SNP genotyping
In both replication samples, genotyping was restricted to the rs1360780 SNP because of technical and financial restrictions. We used the Applied Bio-systems 7900HT TaqMan genotyping platform (Applied Biosystems, Foster City, Calif.). The genotypes were in Hardy-Weinberg equilibrium in both samples (Dunedin: p=0.94; E-Risk: p=0.88).
Statistical analyses
For replicating the findings on gene-environment interactions, we performed a general log-linear model, which is a general procedure for model fitting and hypothesis testing in multidimensional contingency tables. We generated a three-dimensional contingency table that included the following dimensions: 1) lifetime major depressive episode: yes, no; 2) childhood maltreatment or trauma: “no, possible” or “some, severe”; and 3) genotype for rs1360780: CC, CT, TT. Hierarchical and nonstandard log-linear models were conducted by using the GENLOG procedure in SPSS 17.01 (IBM, Armonk, N.Y.). For the nonstandard log-linear analysis, the main effects of a lifetime major depressive episode, maltreatment, and genotype and the two-way interactions were defined as the base model. In the prediction model, the gene-environment interaction vector was additionally included to define the concurrent effects of the risk genotype (TT) and severe maltreatment on lifetime occurrence of a major depressive episode. Improvement in model fit was tested by using an incremental likelihood ratio test, and the significance of the gene-environment interaction vector was analyzed by using the z statistic. Because of the confirmatory nature of this analysis, one-sided p values are reported.
Results
Relation of FKBP5 Genotypes and Adverse Life Events to Incident Major Depressive Episodes
Although the incidence of a major depressive episode slightly varied among genotypes, no significant genetic main effect could be observed (p=0.22–1.00) (Table 1).
TABLE 1.
Associations of Selected FKBP5 Single-Nucleotide Polymorphisms (SNPs) With the First Occurrence of a Major Depressive Episode in 884 Young Community Subjects in a 10-Year Prospective Study
| Incident Major Depressive Episode During Follow-Upa |
Heterozygous Versus Homozygous for Minor Allele |
Homozygous for Major Allele Versus Homozygous for Minor Allele |
Heterozygous Versus Homozygous for Major Allele |
|||||
|---|---|---|---|---|---|---|---|---|
| FKBP5 SNP and Genotype | N | % of Genotype Group | Odds Ratiob | 95% CI | Odds Ratiob | 95% CI | Odds Ratiob | 95% CI |
| rs3800373c | 0.8 | 0.4 to 1.6 | 0.7 | 0.4 to 1.4 | 0.9 | 0.6 to 1.4 | ||
| Homozygous for minor allele (CC) | 15 | 20.8 | ||||||
| Heterozygous (AC) | 56 | 18.4 | ||||||
| Homozygous for major allele (AA) | 87 | 17.3 | ||||||
| rs9296158 | 1.0 | 0.5 to 1.8 | 0.9 | 0.5 to 1.8 | 1.0 | 0.6 to 1.5 | ||
| Homozygous for minor allele (AA) | 16 | 18.0 | ||||||
| Heterozygous (AG) | 59 | 18.2 | ||||||
| Homozygous for major allele (GG) | 84 | 17.8 | ||||||
| rs1360780 | 0.9 | 0.5 to 1.8 | 1.0 | 0.5 to 1.9 | 1.1 | 0.7 to 1.6 | ||
| Homozygous for minor allele (TT) | 16 | 17.8 | ||||||
| Heterozygous (CT) | 57 | 17.4 | ||||||
| Homozygous for major allele (CC) | 86 | 18.4 | ||||||
| rs9470080 | 1.1 | 0.6 to 2.1 | 1.2 | 0.6 to 2.2 | 1.0 | 0.7 to 1.6 | ||
| Homozygous for minor allele (TT) | 16 | 15.5 | ||||||
| Heterozygous (CT) | 64 | 18.9 | ||||||
| Homozygous for major allele (CC) | 79 | 18.6 | ||||||
| rs4713916 | 1.5 | 0.7 to 3.1 | 1.5 | 0.7 to 3.1 | 1.0 | 0.6 to 1.5 | ||
| Homozygous for minor allele (AA) | 11 | 12.6 | ||||||
| Heterozygous (AG) | 64 | 18.7 | ||||||
| Homozygous for major allele (GG) | 84 | 18.5 | ||||||
A total of 159 individuals first met the DSM-IV criteria for a major depressive episode during follow-up. The reference group comprised the subjects without an incident major depressive episode during follow-up.
Based on multiple logistic regression including age and gender as covariates.
Data were missing for one individual who first met the criteria for a major depressive episode during follow-up.
A major depressive episode occurred in 26.7% of the respondents with prebaseline exposure to severe trauma, compared to 17.0% of those without severe trauma prior to baseline. The association between severe trauma and onset of a major depressive episode was significant (odds ratio=1.9, 95% CI=1.16–3.22, p=0.02), even after correction for multiple comparisons (corrected p=0.03). The associations with onset of a major depressive episode were not significant for trauma or separation events (see Appendix SA2 in the data supplement accompanying the online version of this article).
Effect of Interaction Between FKBP5 Genotypes and Adverse Life Events on Prediction of Incident Major Depressive Episode
Table 2 shows the effects of the interaction between homozygosity for the minor allele and adverse life events on the subsequent incidence of a major depressive episode. As shown by the risk difference in percentage points (pp), significant interactions with the FKBP5 genotype that withstood corrections for multiple testing and adjustment for anxiety and substance use disorders were observed for trauma (range of risk differences: ppunadjusted=13.1–16.6 [followed by “f” in Table 2], ppadjusted=12.3–16.3 [not shown in table]) and particularly, severe trauma (range of risk differences: ppunadjusted=13.3–16.7 [followed by “f” in Table 2], ppadjusted=12.9–15.9 [not shown in table]) in all five SNPs. This means that the sum of the risk differences for the independent effects of trauma (in the absence of the risk genotype) and the risk genotype (homozygosity for the minor allele, in the absence of trauma) was exceeded by the joint effect of these factors (trauma in the presence of the risk genotype), indicating a gene-environment interaction effect on the first occurrence of a major depressive episode. For rs3800373 (minor allele homozygosity versus heterozygosity) and severe trauma, for example, the sum of the risk differences of the single factors was significantly exceeded by the joint effect of both factors by 16.7%. Note that the strongest interaction effects were revealed for rs3800373 and rs4713916 (range of risk differences: ppunadjusted=15.2–16.7), showing significant interactions with any trauma after adjustment for anxiety and substance use disorders and Bonferroni correction.
TABLE 2.
Interactions Among Selected FKBP5 Single-Nucleotide Polymorphisms (SNPs), Prebaseline Adverse Life Events, and the Risk of the First Occurrence of a Major Depressive Episode in 884 Young Community Subjects in a 10-Year Prospective Study
| Additive Interaction Effect Between Prebaseline Adverse Life Event and Homozygosity for SNP Minor Allele on Incident Major Depressive Episode During Follow-Upb |
||||||
|---|---|---|---|---|---|---|
| Homozygous for Minor Allele Versus Heterozygous |
Homozygous for Minor Allele Versus Homozygous for Major Allele |
|||||
| SNPa and Presence of Lifetime Adverse Event Preceding Baseline | Risk Difference in Percentage Pointsc | 95% CI | p | Risk Difference in Percentage Pointsc | 95% CI | p |
| rs3800373 (CC/AC/AA) | ||||||
| Any adverse life event | 13.0d | 3.2 to 22.7 | 0.009 | 7.0 | –7.6 to 21.6 | 0.35 |
| Any separation event | 11.0d | 0.0 to 22.0 | 0.05 | 3.7 | –13.0 to 20.4 | 0.67 |
| Any trauma | 15.3d,e,f | 9.0 to 21.6 | <0.001 | 15.2d,e,f | 8.0 to 22.3 | <0.001 |
| Any severe trauma | 16.7d,e,f | 12.0 to 21.4 | <0.001 | 16.1d,e,f | 10.1 to 22.1 | <0.001 |
| rs9296158 (AA/AG/GG) | ||||||
| Any adverse life event | 13.0d | 3.7 to 22.4 | 0.006 | 6.4 | –7.9 to 20.6 | 0.39 |
| Any separation event | 12.2d | 2.9 to 21.6 | 0.01 | 2.2 | –14.4 to 18.7 | 0.80 |
| Any trauma | 12.3d | 3.5 to 21.2 | 0.006 | 14.0d,e,f | 6.4 to 21.7 | <0.001 |
| Any severe trauma | 13.3d,e | 5.6 to 21.0 | <0.001 | 14.4d,e,f | 7.3 to 21.4 | <0.001 |
| rs1360780 (TT/CT/CC) | ||||||
| Any adverse life event | 12.7d | 3.1 to 22.4 | 0.009 | 6.1 | –8.2 to 20.5 | 0.40 |
| Any separation event | 11.6d | 1.6 to 21.6 | 0.03 | 2.0 | –14.6 to 18.6 | 0.82 |
| Any trauma | 12.7d,e | 4.1 to 21.2 | 0.003 | 14.0d,e,f | 6.4 to 21.6 | <0.001 |
| Any severe trauma | 13.5d,e,f | 5.9 to 21.0 | <0.001 | 14.4d,e,f | 7.5 to 21.3 | <0.001 |
| rs9470080 (TT/CT/CC) | ||||||
| Any adverse life event | 11.6d | 1.3 to 21.8 | 0.03 | 2.4 | –13.6 to 18.3 | 0.78 |
| Any separation event | 9.4 | –2.3 to 21.2 | 0.12 | –3.4 | –23.3 to 16.5 | 0.74 |
| Any trauma | 13.1d,e,f | 5.2 to 21.0 | 0.001 | 13.2d,e,f | 5.3 to 21.1 | 0.001 |
| Any severe trauma | 13.6d,e,f | 6.7 to 20.8 | <0.001 | 13.3d,e,f | 5.7 to 20.9 | <0.001 |
| rs4713916 (AA/AG/GG) | ||||||
| Any adverse life event | 18.0d,e,f | 10.2 to 25.7 | <0.001 | 15.2d | 3.3 to 27.1 | 0.02 |
| Any separation event | 12.8d | 3.0 to 22.6 | 0.01 | 5.6 | –10.4 to 21.6 | 0.50 |
| Any trauma | 15.9d,e,f | 10.6 to 21.2 | <0.001 | 16.6d,e,f | 11.3 to 21.9 | <0.001 |
| Any severe trauma | 16.0d,e,f | 11.5 to 20.6 | <0.001 | 16.3d,e,f | 11.5 to 21.2 | <0.001 |
Minor allele is listed first, i.e., for rs3800373 the minor allele is C; for rs9296158 the minor allele is A; for rs1360780 the minor allele is T; for rs9470080 the minor allele is T; and for rs4713916 the minor allele is A.
A total of 159 individuals first met the DSM-IV criteria for a major depressive episode during follow-up. The reference group comprised the subjects without an incident major depressive episode during follow-up.
The amount by which the sum of the independent effects of an adverse life event (in the absence of the risk genotype, i.e., homozygosity for the minor allele) and the risk genotype (in the absence of adverse life events) is exceeded by the joint effect of both factors, i.e., an adverse life event in the presence of the risk genotype.
Significant risk difference (p<0.05) when controlled for age and gender.
Significant risk difference (p<0.05) when controlled for age and gender and after Bonferroni correction for multiple testing (two independent event conditions, five SNPs).
Significant risk difference (p<0.05) when controlled for age, gender, and baseline anxiety and substance use disorders and after Bonferroni correction for multiple testing (two independent event conditions, five SNPs).
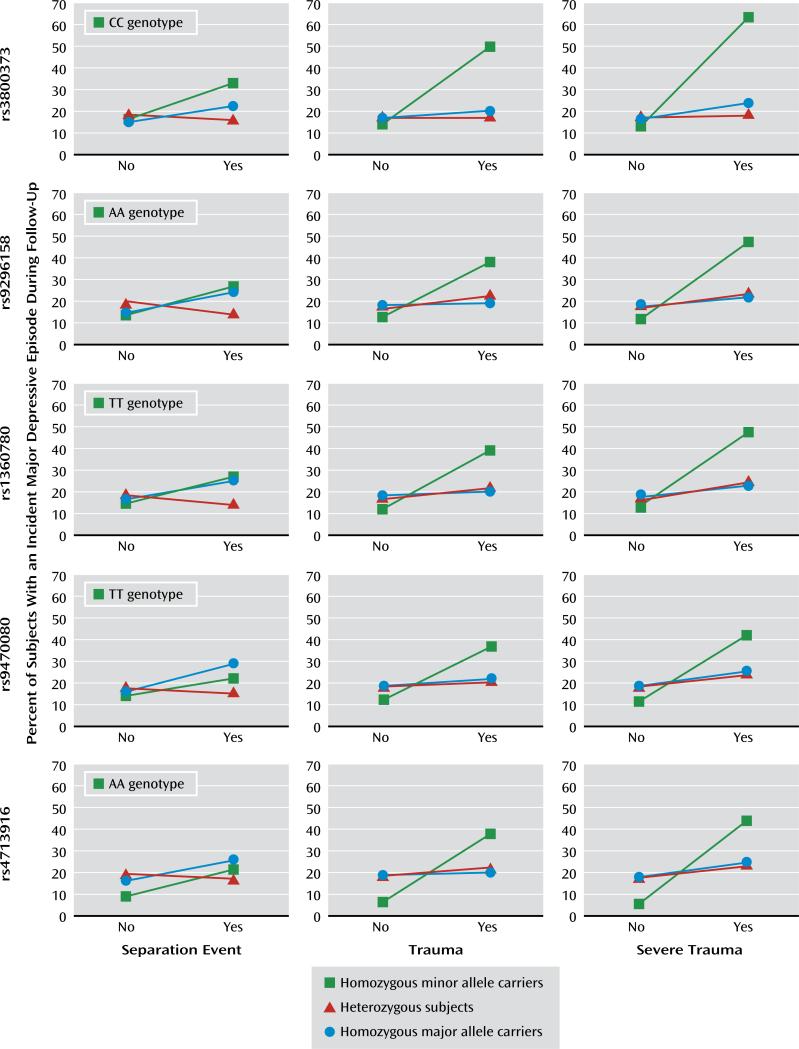
The incidence rates for major depressive episodes markedly differed between participants with and without trauma among homozygous minor allele carriers (ranges for those with and without trauma or severe trauma were 35.0%–63.6% and 5.6%–13.8%, respectively) but not among those who were heterozygous or who were homozygous for the major allele (Figure 1). Individuals homozygous for the minor allele had the lowest risk for an incident major depressive episode if they did not have baseline trauma exposure, but those exposed to trauma had the highest risk, compared with the other two genotype groups.
FIGURE 1.
Relation of Prebaseline Adverse Life Events to the First Occurrence of a Major Depressive Episode for 884 Young Community Subjects in a 10-Year Prospective Study, by Genotype for Selected FKBP5 Single-Nucleotide Polymorphisms
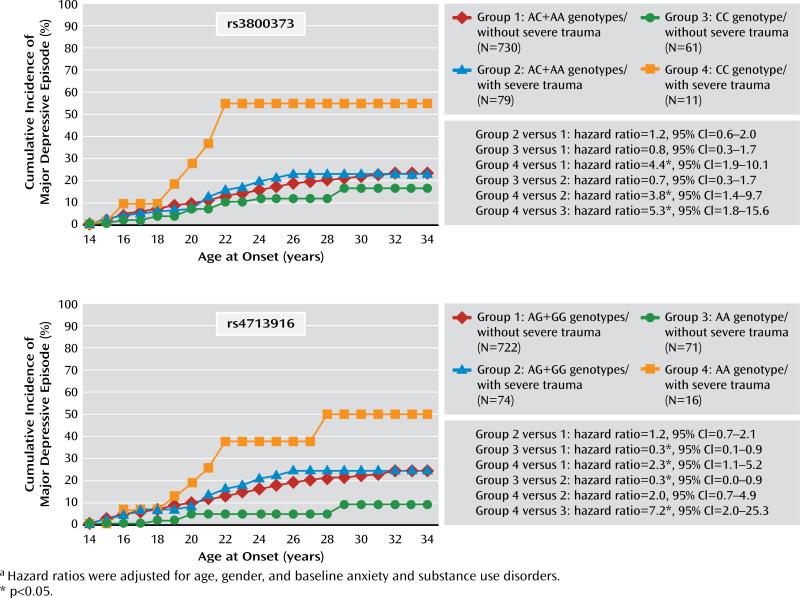
Figure 2 illustrates the results for the two SNPs with the strongest interaction effects across the age groups in terms of age-dependent cumulative lifetime incidences. Concerning rs3800373, respondents homozygous for the minor allele (C) with severe trauma prior to baseline (group 4) had the highest cumulative incidence of major depressive episodes (up to 55% by age 34). Also for rs4713916, individuals who were homozygous for the minor allele (A) and had severe trauma (group 4) had a higher incidence rate than all other groups; they differed the most from group 3, who had the same genotype (AA) but no exposure to severe trauma.
FIGURE 2.
Cumulative Incidence of the First Occurrence of a Major Depressive Episode in 884 Young Community Subjects in a 10-Year Prospective Study, by Genotype for two FKBP5 Single-Nucleotide Polymorphisms and Prebaseline Severe traumaa
Since gene-environment correlation effects are important for the interpretation of gene-environment interactions, associations between genotype and adverse life events were tested. No effect withstood correction for multiple testing (see Table ST2 in the online data supplement).
Replication Analysis
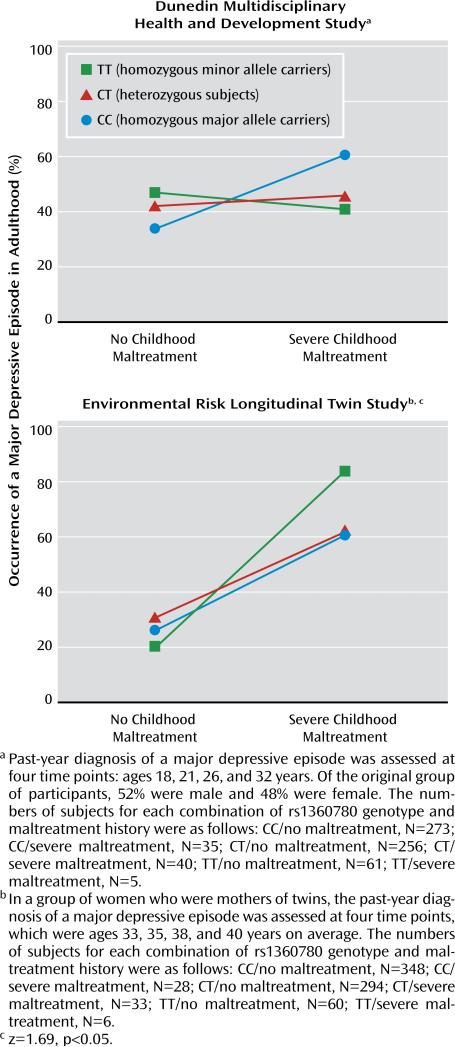
The Dunedin sample failed to yield a significant gene-environment interaction predicting an adult major depressive episode (p=1.0, one-sided), and the inclusion of the prediction vector in the log-linear model did not improve model fit (Δlikelihood ratio=0.63, df=1, p=0.22, one-sided). The E-Risk sample, however, yielded a significant effect (p<0.05, one-sided) of the gene-environment interaction prediction vector in the expected direction (Figure 3), and model fit improved significantly after the prediction vector was included (Δlikelihood ratio=2.80, df=1, p<0.05, one-sided). Also, the hierarchical log-linear model suggested an additive effect of the three-way interaction of major depressive episode, maltreatment, and genotype (likelihood-ratio=11.34, df=4, p=0.03).
FIGURE 3.
Effect of Severe Childhood Maltreatment and Genotype of the FKBP5 Single-Nucleotide Polymorphism rs1360780 on the Occurrence of Adult Major Depressive Episodes in two Replication Samples
Discussion
Stimulated by a series of investigations from our group (7, 9, 11) and others (10, 13, 15, 40) suggesting a prominent role of FKBP5 in stress vulnerability and depression, we investigated FKBP5 polymorphisms as promising candidates for gene-environment interactions affecting depression onset. This was done in a prospective community study with a 10-year follow-up covering the first three decades of life. In agreement with previous studies (9–11), we identified homozygosity for the minor allele of selected FKBP5 polymorphisms as a risk genotype for the development of a major depressive episode in individuals with prior trauma exposure.
Several findings are noteworthy. First, whereas no genetic main effects for FKBP5 polymorphisms were found, severe trauma was a risk factor for depression onset. Second, consistent with a recessive model, significant interactions between the five FKBP5 SNPs and trauma indicate that subjects homozygous for the minor alleles are particularly sensitive to the pathogenic effects of trauma. The consistent effects across all five investigated polymorphisms are plausible since all SNPs are in high linkage disequilibrium. Third, while no stable interaction for separation events was revealed, there was an increasing interaction effect from trauma to severe trauma on subsequent depression onset. This dose-response relationship, together with the temporal priority, suggests a causal role of trauma exposure in this interaction process. Fourth, as variants in the five FKBP5 SNPs were not robustly associated with any of the adverse life event categories, a bias of interaction effects by gene-environment correlations is unlikely. Fifth, the interactions largely remained stable after we adjusted for controls (such as PTSD) and multiple testing. This stability indicates that the results are not confounded by these factors and further supports a causal relationship.
The interaction finding is supported by data from the E-Risk study: Subjects homozygous for the minor allele of the intronic FKBP5 SNP rs1360780 with exposure to severe childhood trauma showed the highest prevalence of adult major depressive episodes. No effects were found in the Dunedin study. In this study, however, exposure to adverse life events was defined in a broader fashion; in addition to self-reports, information about parent discipline style, caregiver changes, and mother-child interactions was included.
The following methodological points should be considered. First, although the analyses were based on a large community sample, the number of individuals with depressive episodes was still small for detecting interactions. However, the a priori power analysis suggested sufficient power for detecting gene-environment interactions within the limits of a small or medium effect. Second, the prospective approach required exclusion of individuals with a lifetime major depressive episode at baseline. This could have led to omission of people with depression onset shortly after trauma exposure, and thus the effect size of the association might be underestimated. Third, since adverse life events occurring after baseline were not considered in our analyses, the interpretation of our findings is restricted to traumas before age 24 and cannot be applied to traumas that occur beyond early adulthood. Fourth, because of the young age of our sample, some of the subjects without depression onset during follow-up could develop depression later in life. Such potentially false negatives may have led to more conservative results. Fifth, the SNPs of the respondents without a major depressive episode revealed a nominal deviation from the Hardy-Weinberg equilibrium. The high call rates (>99%) and further quality checks support the validity of the genotyping results (see Table ST1 in the online data supplement).
We did not find main effects of the five FKBP5 SNPs on depression. This is in accordance with findings in some studies (9, 17, 41) but not in others (13–15). The inconsistencies could be explained by the failure of these studies to consider trauma as an environmental factor. Moreover, the studies indicating a genetic main effect were based on clinical samples (13, 14) and a sample with a high familial load for mood disorders (15), who were probably suffering from more severe forms of depression than our community subjects. As negative life events and mood disorders are linked (36, 42), trauma rates might tend to be higher in clinical samples, and consequently there might be a higher chance of determining genetic main effects. Furthermore, our findings are in contrast to results in a study by Lavebratt et al. (12), who did not observe elevated depression rates among adult carriers of the TT genotype for rs1360780 who had a history of childhood problems. This discrepancy could be due to important methodological differences between our study and theirs (e.g., retrospective and different measures of childhood problems and depression, different age groups).
Regarding the underlying biological mechanisms, our results conform to those of functional studies showing a greater induction of FKBP5 expression by cortisol and, accordingly, an impaired glucocorticoid receptor sensitivity after stress in individuals who are homozygous for the minor alleles of these FKBP5 SNPs (9, 10). In a study with healthy subjects, we demonstrated an insufficient recovery of the cortisol secretory response to psychosocial stress in homozygous minor allele carriers of the same FKBP5 SNPs (11). This can be interpreted as a predisposition to chronically elevated cortisol levels in response to stress and thus may lead to long-lasting detrimental effects of chronically elevated cortisol levels. Since hypercortisolism due to an impaired regulation of the HPA axis can be regarded as a prominent feature of depression (3, 43, 44), our result (elevated risk of major depressive episodes in trauma-exposed homozygous minor allele carriers) can be explained by direct effects of the investigated genotypes on FKBP5 expression. The increased expression might reduce glucocorticoid receptor sensitivity, subsequently impair the regulation of the HPA axis in response to stress, and therefore increase the risk for the development of a major depressive episode in trauma-exposed individuals. On the other hand, in nonexposed individuals it appears that carrying the risk genotype of the promoter SNP rs4713916 protects against the development of depression (see Figure 2). Such opposing effects of the genotype depending on stress exposure level, which translate into a disordinal gene-environment interaction, have also been reported by Xie et al. (20). In their participants who were homozygous for the minor allele of FKBP5 SNP rs9470080, PTSD risk was highest for those exposed to childhood adversity and lowest for those not exposed. While functional data are still not available, it is conceivable that a hyper-responsive HPA axis might exhibit beneficial effects in the case of mild nontraumatic stress by mobilizing individual resources and enabling a sustained coping with low-level stress. In the case of traumatic stress, the negative effects of a hyperresponsive HPA axis might predominate through the induction of a chronically elevated cortisol level. It is notable, moreover, that the same risk alleles that apparently predispose a person to a major depressive episode also predict severity of PTSD symptoms when they interact with trauma (10, 20), suggesting a shared role of FKBP5 in the pathophysiology of these disorders. However, further prospective studies with large samples are required to provide sufficient power for a combined gene-environment analysis of both disease phenotypes.
Overall, the essential finding of our study is that trauma exposure during childhood and adolescence was a strong risk factor for developing subsequent depression in genetically vulnerable individuals, even within the extended time period of 10 years. Such medium- or long-term effects of adverse life events are explained by the model of Heim et al. (4) proposing that a genetic predisposition together with early adverse experiences during sensitive developmental phases induce a persistent vulnerability to stress later in life. The present results suggest that this effect could be moderated by variants of the FKBP5 gene. As it has been shown that early abuse is related to altered glucocorticoid receptor activity in adults (45), FKBP5 could be an important factor in such developmental processes.
Supplementary Material
Acknowledgments
Dr. Zimmermann has received grant support from the German Federal Ministry of Education and Research. In the past, Dr. Binder has received grant support from Pfizer, Glaxo-Smith-Kline, and NARSAD; currently she receives grant support from NIMH, the Doris Duke Charitable Foundation, the Behrens-Weise Foundation, the NeuroNova AG Foundation, and PharmaNeuroboost; she is also co-inventor on the following patent applications: “FKBP5: a novel target for antidepressant therapy” (international publication number WO 2005/054500) and “Polymorphisms in ABCB1 associated with a lack of clinical response to medicaments” (international application number PCT/EP2005/005194). Dr. Uhr is co-inventor on the following patent applications: “FKBP5: a novel target for antidepressant therapy” (international publication number WO 2005/054500) and “Polymorphisms in ABCB1 associated with a lack of clinical response to medicaments” (international application number: PCT/EP2005/005194). Dr. Lieb has received grant support from the German Federal Ministry of Education and Research and has received speakers honoraria from Wyeth. Dr. Holsboer receives grant support from the German Federal Ministry of Education and Research and is founder/shareholder of Affectis Pharmaceuticals; he is also owner of several patents related to depression. Dr. Ising has received grant support from the German Federal Ministry of Education and Research.
Supported by the German Federal Ministry of Education and Research through funding for the Early Developmental Stages of Psychopathology (EDSP) investigation (projects 01EB9405/6, 01EB9901/6, EB01016200, 01EB0140, and 01EB0440) and for the genetic part of the study (project 01GS0481); by Deutsche Forschungsgemeinschaft funding of part of the fieldwork and analyses (grants LA1148/1-1, WI2246/1-1, WI709/7-1, and WI709/8-1); by U.K. Medical Research Council funding of the Dunedin and E-Risk Studies (G9806489, G0100527, G0601483, G1002190); by NIMH (MH-077874); by the National Institute on Aging (AG-032282); and by the New Zealand Health Research Council.
The authors thank H.-U. Wittchen, initiator and founder of the EDSP study, for his major contributions to the project; the staff members and interviewers; the study members and their families; Richie Poulton for providing the Dunedin data; Gisela Gajewsky, Maik Koedel, Susann Sauer, and Susanne Zie glgaensberger for technical support; Benno Puetz, Bertram Mueller-Myhsok, and Darina Roeske for data processing and statistical advice; and Thomas Bettecken for genotyping support.
Footnotes
Dr. Brückl, Dr. Nocon, Ms. Pfister, Dr. Moffitt, and Dr. Caspi report no financial relationships with commercial interests.
References
- 1.Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977;84:309–322. [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 3.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- 5.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 6.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 7.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 8.Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003;88:277–284. doi: 10.1210/jc.2002-020354. [DOI] [PubMed] [Google Scholar]
- 9.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Künzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Brückl T, Müller N, Pfister H, Lieb R, Müller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Müller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 10.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Müller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavebratt C, Aberg E, Sjoholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- 13.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zobel A, Schuhmacher A, Jessen F, Hofels S, von Widdern O, Metten M, Pfeiffer U, Hanses C, Becker T, Rietschel M, Scheef L, Block W, Schild HH, Maier W, Schwab SG. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int J Neuropsychopharmacol. 2010;13:649–660. doi: 10.1017/S1461145709991155. [DOI] [PubMed] [Google Scholar]
- 15.Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, Goes FS, Zandi PP, Lee RS, Mackinnon DF, Mondimore FM, Schweizer B, DePaulo JR, Jr, Gershon ES, McMahon FJ, Potash JB. Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitiello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, DeBar L, Keller M. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am J Psychiatry. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 19.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traum atic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieb R, Isensee B, von Sydow K, Wittchen HU. The Early Developm ental Stages of Psychopathology Study (EDSP): a methodological update. Eur Addict Res. 2000;6:170–182. doi: 10.1159/000052043. [DOI] [PubMed] [Google Scholar]
- 22.Wittchen HU, Perkonigg A, Lachner G, Nelson CB. Early Developm ental Stages of Psychopathology Study (EDSP): objectives and design. Eur Addict Res. 1998;4:18–27. doi: 10.1159/000018921. [DOI] [PubMed] [Google Scholar]
- 23.Wittchen H-U, Pfister H. DIA-X-Interviews: Manual für Screening-Verfahren und Interview; Interviewheft Längsschnittuntersuchung (DIA-X-Lifetim e); Ergänzungsheft (DIA-X-Lifetim e); Inter view heft Querschnittuntersuchung (DIA-X-12 Monate); Ergän z ung sheft (DIA-X-12Monate); PC-Programm zur Durchführung des Interviews (Längs und Querschnittuntersuchung); Auswertungsprogramm. Swets & Zeitlinger; Frankfurt, Germany: 1997. [Google Scholar]
- 24.Lachner G, Wittchen HU, Perkonigg A, Holly A, Schuster P, Wunderlich U, Turk D, Garczynski E, Pfister H. Structure, content and reliability of the Munich Composite International Diagnostic Interview (M-CIDI) substance use sections. Eur Addict Res. 1998;4:28–41. doi: 10.1159/000018922. [DOI] [PubMed] [Google Scholar]
- 25.Reed V, Gander F, Pfister H, Steiger A, Sonntag H, Trenkwalder C, Hundt W, Wittchen HU. To what degree does the Composite International Diagnostic Interview (CIDI) correctly identify DSM-IV disorders? testing validity issues in a clinical sample. Int J Methods Psychiatr Res. 1998;7:142–155. [Google Scholar]
- 26.Wittchen HU. Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI)—a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 27.Stata Statistical Software: Release 9.0. Stata Corp; College Station, Tex: 2005. [Google Scholar]
- 28.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 29.Darroch J. Biologic synergism and parallelism. Am J Epidemiol. 1997;145:661–668. doi: 10.1093/oxfordjournals.aje.a009164. [DOI] [PubMed] [Google Scholar]
- 30.Höfler M. Causal inference based on counterfactuals. BMC Med Res Methodol. 2005;5:28. doi: 10.1186/1471-2288-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ. Epidemiology: An Introduction. Oxford University Press; New York: 2002. p. 168. [Google Scholar]
- 32.Rutter M, Thapar A, Pickles A. Gene-environm ent interactions—biologically valid pathway or artifact? Arch Gen Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S. Basic problems in interaction assessment. Environ Health Perspect. 1993;101:59–66. doi: 10.1289/ehp.93101s459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe MM, Greenland S. Standardized estimates from categorical regression models. Stat Med. 1995;14:2131–2141. doi: 10.1002/sim.4780141907. [DOI] [PubMed] [Google Scholar]
- 35.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- 36.Zimmermann P, Brückl T, Lieb R, Nocon A, Ising M, Beesdo K, Wittchen HU. The interplay of familial depression liability and adverse events in predicting the first onset of depression during a 10-year follow-up. Biol Psychiatry. 2008;63:406–414. doi: 10.1016/j.biopsych.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Polanczyk G, Caspi A, William s B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein D, Fink L. Childhood Trauma Questionnaire Manual. Psychological Corp; San Antonio, Tex: 1998. [Google Scholar]
- 39.Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM-IV. University School of Medicine; St Louis, Washington: 1995. [Google Scholar]
- 40.Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, Brockm oller J. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. doi: 10.2217/14622416.9.7.841. [DOI] [PubMed] [Google Scholar]
- 41.Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, Valle D, Pulver AE. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Tijssen JG, Wiersinga WM, Schene AH. Glucocorticoids and relapse of major depression (dexam ethasone/corticotropin-releasing horm one test in relation to relapse of major depression). Biol Psychiatry. 2006;59:696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Aubry JM, Gervasoni N, Osiek C, Perret G, Rossier MF, Bertschy G, Bondolfi G. The DEX/CRH neuroendocrine test and the prediction of depressive relapse in remitted depressed outpatients. J Psychiatr Res. 2007;41:290–294. doi: 10.1016/j.jpsychires.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 45.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in hum an brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.