Abstract
OBJECTIVE
A rat model of diet-induced obesity (DIO) was used to determine dopamine transporter (DAT) function, impulsivity and motivation as neurobehavioral outcomes and predictors of obesity.
DESIGN
To evaluate neurobehavioral alterations following the development of DIO induced by an 8-week high-fat diet (HF) exposure, striatal D2-receptor density, DAT function and expression, extracellular dopamine concentrations, impulsivity, and motivation for high- and low-fat reinforcers were determined. To determine predictors of DIO, neurobehavioral antecedents including impulsivity, motivation for high-fat reinforcers, DAT function and extracellular dopamine were evaluated before the 8-week HF exposure.
METHODS
Striatal D2-receptor density was determined by in vitro kinetic analysis of [3H]raclopride binding. DAT function was determined using in vitro kinetic analysis of [3H]dopamine uptake, methamphetamine-evoked [3H]dopamine overflow and no-net flux in vivo microdialysis. DAT cell-surface expression was determined using biotinylation and western blotting. Impulsivity and food-motivated behavior were determined using a delay discounting task and progressive ratio schedule, respectively.
RESULTS
Relative to obesity-resistant (OR) rats, obesity-prone (OP) rats exhibited 18% greater body weight following an 8-week HF-diet exposure, 42% lower striatal D2-receptor density, 30% lower total DAT expression, 40% lower in vitro and in vivo DAT function, 45% greater extracellular dopamine and twofold greater methamphetamine-evoked [3H]dopamine overflow. OP rats exhibited higher motivation for food, and surprisingly, were less impulsive relative to OR rats. Impulsivity, in vivo DAT function and extracellular dopamine concentration did not predict DIO. Importantly, motivation for high-fat reinforcers predicted the development of DIO.
CONCLUSION
Human studies are limited by their ability to determine if impulsivity, motivation and DAT function are causes or consequences of DIO. The current animal model shows that motivation for high-fat food, but not impulsive behavior, predicts the development of obesity, whereas decreases in striatal DAT function are exhibited only after the development of obesity.
Keywords: diet-induced obesity, dopamine transporter, impulsivity, motivation, striatum
INTRODUCTION
Obesity is associated with excessive overeating and preference for palatable, high-fat foods.1,2 Increased impulsivity, a multifaceted behavioral construct involving urgent actions, lack of premeditation and perseverance, and increased sensation-seeking behaviors, plays a role in obesity.3–6 In the context of obesity, lack of perseverance refers to an inability to control thoughts about food and body shape.5 High impulsivity may underlie the inability to resist excessive eating. Increased sensitivity to palatable-food reward drives overeating only when accompanied by insufficient inhibitory control.6 Thus, cognitive and motivational facets of impulsivity are linked to obesity.
Ingestion of palatable foods activates brain reward circuits, leading to dopamine release in nucleus accumbens (NAc) and striatum.7,8 NAc dopamine mediates primary reward and incentive motivation for food reinforcers.9 A shift in control from NAc to striatal dopamine pathways occurs coincident with development of habitual behaviors.10,11 Inhibition of dopamine synthesis by tyrosine hydroxylase gene inactivation results in reduced preference for palatable foods.12 Gene rescue in NAc and/or striatum restores preference, whereas only striatal rescue restores consumption.12 Striatal involvement in food motivation is supported by findings that rats over-expressing delta Fos B exhibit high progressive ratio (PR) breakpoints.13 Following extended access to high-fat food, obese rats exhibit increased brain stimulation reward thresholds, increased resistance to aversive stimuli-induced disruption of food consumption and decreased D2 receptors.14 In obese humans, both striatal D2-receptor density and neuronal activity are decreased compared with non-obese individuals.15,16 Thus, dysregulated striatal function may underlie excessive food intake in obesity.
Extracellular dopamine is regulated by dopamine transporters (DAT) and vesicular monoamine transporters-2 (VMAT2), translocating dopamine across plasmalemma and synaptic vesicular membrane, respectively.17 DAT-deficient mice exhibit increased extracellular dopamine and greater food intake compared with wild-type mice.18 Genetic-linkage analysis reveals human DAT gene polymorphisms with a greater frequency of short alleles associated with decreased DAT expression and binge eating.19 Thus, DAT plays a prominent role in regulating binge eating.
Little information is available to indicate if alterations in dopamine, impulsivity and motivation precede or are a consequence of diet-induced obesity (DIO). Decreased striatal D2 receptors in obesity15 may be compensatory to decreased DAT function and increased extracellular dopamine. Evaluation of predictors of DIO is difficult in humans, but controlled animal models can provide insight.20 In the current study, striatal dopaminergic function, impulsivity and food-motivated behavior were evaluated as neurobehavioral outcomes and predictors of DIO.
MATERIALS AND METHODS
Animals
Outbred male Sprague Dawley rats (350–400 g; Charles River Laboratories, Wilmington, MA, USA) were housed individually in solid-bottom cages with bedding and received standard rat chow and water ad libitum. Rats were maintained on standard chow during acclimatization and neurobehavioral predictor assessment. Experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
DIO model
A naturalistic DIO model employing outbred rats, characterized as exhibiting hyperphagia, increased visceral, epididymal and retroperitoneal adiposity, increased plasma angiotensin peptides levels, cholesterol and triglycerides, and defective leptin- and insulin-signaling, was used to mimic human obesity.20–25 For evaluation of DIO outcomes, 30–32 rats were used to generate the DIO model (obesity-prone (OP), obesity-resistant (OR) and low-fat diet (LF) groups) for each assay. Rats were fed for 8 weeks with either a moderately high-fat diet (HF; n =24; D12266B, 31.8% kcal fat; total density =4.41 kcal g −1) or a LF diet (n =6–8; D12489B, 10.6% kcal fat; total density =3.9 kcal g −1; Research Diets, New Brunswick, NJ, USA). Food intake was determined daily; body weight three times weekly. Cage bedding was scanned for food spillage. Following an 8-week HF-diet exposure, rats were segregated into OP and OR groups based on bodyweight gain (top and bottom third, respectively; n = 6–8 per group).20,22 Energy intake was calculated by multiplying daily food intake (g) by total kcal per g for each respective diet.
For evaluation of predictors of DIO, assays were conducted in rats fed standard chow (n =22), then HF-diet for 8 weeks, and segregated into OP and OR groups (n = 6 rats per group) as described; correlations between neurobehavioral measures and body weight were determined. For evaluation of outcomes of DIO, LF groups served as control to determine if alterations were due to diet or obesity. An LF group was not included in the predictor study, because consumption of LF-diet does not result in DIO.
Experimental design, DIO outcomes
Study-1
Striatal D2-receptor density (Bmax) and affinity (Kd) were evaluated in vitro using [3H]raclopride saturation analysis. Between-subject differences in kinetic parameters were determined in OP, OR and LF (n = 6 per group). Treatment was a between-subject factor and [3H]raclopride concentration a within-subject factor. The brains were from rats previously used in Study -6 and Study-7.
Study-2
Maximal velocity (Vmax) and affinity (Km) of [3H]dopamine uptake by striatal VMAT2 into vesicular preparations were evaluated in vitro using saturation analysis. Between-subject differences in kinetic parameters were determined in OP, OR and LF (n = 6 per group). Treatment was a between-subject factor and [3H]dopamine concentration a within-subject factor. Rats had no prior experimental manipulations.
Study-3
Vmax and Km of [3H]dopamine uptake by striatal DAT into synaptosomal preparations were evaluated in vitro using saturation analysis. Between-subject differences in kinetic parameters were determined in OP, OR and LF (n =6–8 rats per group). Treatment was a between-subject factor and [3H]dopamine concentration a within-subject factor. Rats had no prior experimental manipulations.
Study-4
Methamphetamine-induced reverse transport of DAT in super-fused striatal slices was determined in vitro in OP, OR and LF (n = 6–8 rats per group). Treatment was a between-subject factor, and methampheta-mine concentration and time within-subject factors. Rats had no prior experimental manipulations.
Study-5
Striatal DAT protein in total, intracellular and cell-surface fractions was determined in vitro using biotinylation and western blot assay in OP, OR and LF (n =8 rats per group). Treatment was a between-subject factor, and individual fractions were within-subject factors. Rats had no prior experimental manipulations.
Study-6
Striatal dopamine uptake (extraction fraction) and extracellular dopamine concentration were evaluated in vivo using no-net flux microdialysis in OP, OR and LF (n = 8 rats per group). Treatment was a between-subject factor and dopamine concentration a within-subject factor. Rats were used subsequently in Study-1 and Study-7.
Study-7
Impulsivity was evaluated using delay discounting in OP, OR and LF (n = 6–8 per group). Treatment was a between-subject factor and session a within-subject factor. Rats were used previously in Study-6.
Study-8
Motivation for food reinforcement was evaluated using PR reinforcement in OP, OR and LF (n = 8 rats per group). Treatment was a between-subject factor and session a within-subject factor. Rats had no prior experimental manipulations.
Experimental design, DIO predictors
Study-9
Impulsivity was determined using delay discounting. Session was a within-subject factor. Experimentally naive rats (n = 22) were employed. Subsequently, this group was used in Study-10.
Study-10
Motivation for food reinforcement was determined using a PR schedule. Session was a within-subject factor. Subsequently, this group was used in Study-11.
Study-11
Striatal DAT function and extracellular dopamine concentration in vivo were determined using no-net flux microdialysis. Dopamine concentration was a within-subject factor. Rats were used previously in Study-9 and Study-10.
Assays
Detailed methods are provided in Supplementary Materials.
Striatal D2-receptor density was determined using saturation analysis of [3H]raclopride binding.26 In vitro striatal VMAT2 and DAT function were assessed using saturation analysis of [3H]dopamine uptake into vesicles27 and synaptosomes,28 respectively. To evaluate methamphetamine-induced DAT reverse transport, methamphetamine-evoked [3H]dopamine overflow was determined using superfused striatal slices.17,29 DAT cell-surface expression was determined using biotinylation and western blotting.30 β-Actin and PP2A, control proteins monitoring protein loading within each fraction (total, non-biotinylated and biotinylated), determined biotinylation efficiency.30
In vivo striatal DAT function and extracellular dopamine concentrations were evaluated using no-net flux microdialysis.31 Microdialysis probes were implanted stereotaxically in striatum (stereotaxic coordinates; AP =1.2 mm anterior to bregma, ML = 2.8 mm lateral to midline and DV =4 mm ventral to dura). Probes were perfused with artificial cerebrospinal fluid containing 5–20 nM dopamine (dopaminein). Microdialysates were analyzed by high performance liquid chromatography with electrochemical detection.
Behavioral procedures determining impulsivity and food motivation were carried out during the light cycle in operant chambers (MED Associates, St Albans, VT, USA). To facilitate lever responding during evaluation of behavioral outcomes of DIO, rats received their daily food allowance (50 g) in the home cage during a 3-h period after each operant session; OP and OR groups received HF-diet; LF group received LF-diet. Impulsivity was evaluated using delay discounting.32 Rats were trained for 28 consecutive sessions during which responses on one lever delivered one sucrose pellet immediately, whereas responses on the other delivered three pellets after an adjusted delay.32 Responses on the lever delivering three pellets increased the delay(s) for the subsequent large reinforcer.
Food motivation was determined in OP and OR rats receiving HF-reinforcers, and in LF rats receiving LF-reinforcers using a fixed ratio (FR) schedule, followed by three PR sessions (3-h duration). Subsequently, three PR sessions employed the alternative reinforcer. Both levers were extended; however, only active lever responses were reinforced. Following delivery of each reinforcer, response ratio for subsequent reinforcer delivery was increased according to [5e(response number ×0.2)] − 5, and specific response requirements were: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62.33 Last ratio completed defined PR breakpoint.
To evaluate impulsivity and motivation as predictors of DIO, rats were trained on delay discounting for sucrose reinforcers for 28 consecutive sessions, followed by 6 daily PR sessions (3-h) for HF-reinforcers. Immediately following each session, rats received their home-cage food allowance (15 g standard chow, provided overnight). Following behavioral experiments, striatal DAT function and extracellular dopamine were evaluated as predictors of DIO using no-net flux microdialysis.
Data analysis
Details of the data analysis are provided in Supplementary Materials.
RESULTS
Development of DIO
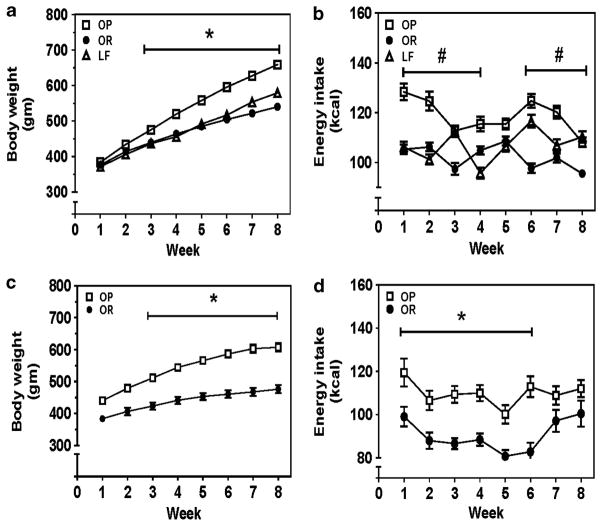
For DIO-outcomes studies, mean body weight and mean energy intake for OP, OR and LF groups were not different between assays. Body weight and energy intake data for each group were collapsed across all assays. Analysis of body-weight gain during 8-week HF- or LF-diet revealed a group × time interaction (F14,1043 = 32.66, P<0.0001; Figure 1a). At 8 weeks, OP group exhibited 18% and 11% greater body weight than OR and LF groups, respectively. Mean daily energy intake across 8 weeks was 14% greater in OP compared with OR groups (119±2 and 102±2 kcal per day, respectively). For the predictor study, analysis of body-weight data revealed a group × time interaction (F 7,70 = 16.83, P<0.0001; Figure 1c). At 8 weeks, the OP group exhibited 20% greater body weight than the OR group. OP group exhibited 17% greater mean daily energy intake across the 8-week period compared with the OR group (Figure 1d).
Figure 1.
Development of DIO. Outcome studies: (a) The OP group exhibited greater body weight compared with OR and LF groups; *P<0.001, OP vs OR and LF. (b) The OP group exhibited greater mean daily energy intake compared with the OR group; #P<0.001, OP vs OR. Food intake was determined daily, and the amount of food intake was multiplied by caloric density of the respective diet to obtain the daily energy intake. The daily energy intake was averaged across the 7 days of the week. Thus, each symbol in panels b and d represents the mean daily energy intake across the 7 days of the respective week. (a, b) Data were pooled from groups employed in studies 1–8 and expressed as mean±s.e.m. (s.e.m. smaller than symbol size); n = 48–52 rats per group. The number of rats employed for different assays was VMAT2 function, n = 6 per group; DAT uptake, n = 12–14 per group (two separate series of DAT uptake assays); METH-induced DAT reverse transport, n = 6–8 per group; DAT cellular localization, n = 8 per group; striatal DAT function and extracellular dopamine concentration, n = 8 per group; motivation for food reinforcement, n = 8 per group. Predictor study: (c) The OP group exhibited greater body weight and (d) greater energy intake compared with the OR group. Data are expressed as mean±s.e.m.; n = 6 rats per group; *P<0.05, OP vs OR.
Effects of DIO
Striatal D2-receptor density
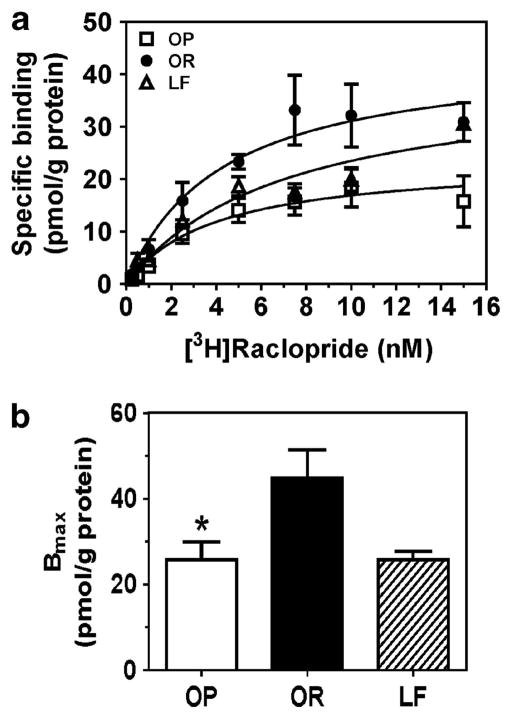
OP group exhibited 42% lower Bmax compared with OR group (F2,13 = 6.08, P<0.01; Figure 2). No between-group differences in Kd were found (Supplementary Figure 1).
Figure 2.
[3H]Raclopride binding to striatal membranes from OP, OR and LF groups. Saturation curves for specific [3H]raclopride binding to striatal D2 receptors (a). Bmax for the OP group was lower than for the OR group (b). Specific [3H]raclopride binding and Bmax are expressed as mean±s.e.m. pmol g −1 protein; n = 4–5 rats per group; *P<0.05, OP vs OR.
In vitro striatal DAT function and cellular expression
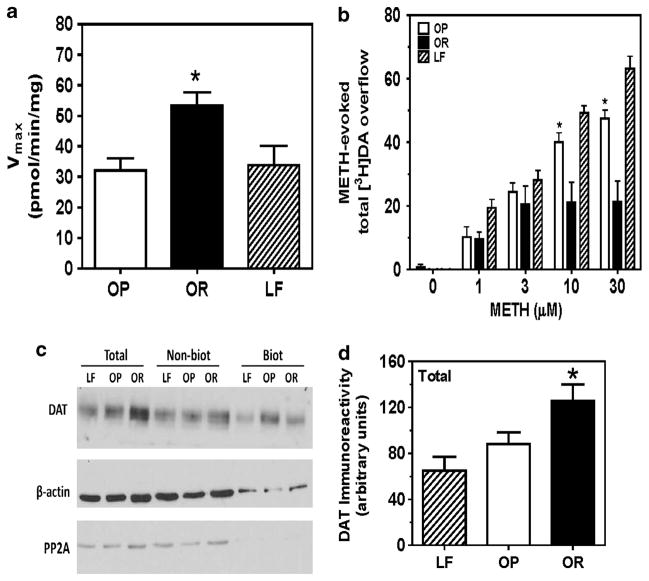
Striatal VMAT2 function was not different between groups (Supplementary Table 1). Vmax for [3H]dopamine uptake at DAT was 40% lower in OP compared with OR groups (F2,35 = 6.285, P<0.01; Figure 3a), but not different from LF. No between-group differences in Km were found (Supplementary Figure 2b).
Figure 3.
Effect of DIO on striatal DAT function and expression in vitro. Vmax of striatal [3H]dopamine (DA) uptake at DAT was lower in the OP group compared with the OR group (a). Data are expressed as mean±s.e.m. pmol per min per mg protein; n = 10–13 rats per group; *P<0.05, OR vs OP and LF. DIO increased methamphetamine-evoked striatal [3H]DA overflow (expressed as mean±s.e.m.; b); n = 5–7 rats per group; *P<0.01, OP vs OR and LF. Representative immunoblot showing DIO associated decrease in total striatal DAT expression (c). Mean total DAT immunoreactivity expressed as mean±s.e.m. from LF, OP and OR (d); n = 6 rats per group; *P<0.05, OR vs OP and LF.
No group differences in basal [3H]dopamine outflow before methamphetamine were detected. Across time, superfusate [3H]dopamine increased following methamphetamine addition, peaked 10–15 min after methamphetamine addition, and over time declined towards basal, despite continued methamphetamine presence (Supplementary Figure 3). Analysis of methamphetamine-evoked total [3H]dopamine overflow revealed a group × concentration interaction (F8,75 = 6.45, P<0.0001; Figure 3b). At 10 and 30 μM methamphetamine, total [3H]dopamine overflow was greater in OP compared with OR groups, and greater in LF compared with the OP group.
Western blot analysis revealed DAT and β-actin immunoreactive bands located at ~75 and 42 kDa, respectively (Figure 3c). β-Actin levels were not different between groups, within fraction. PP2A immunoreactive bands located at ~34 kDa were not detected in biotinylated fraction, indicating efficient surface biotinylation. Total striatal DAT levels were 30% and 48% higher in OR compared with OP and LF groups, respectively (F2,17 = 6.143, P<0.05; Figure 3d). No between-group differences in non-biotinylated and biotinylated fractions (F2,17 = 0.586 and F2,17 = 3.035 and, ps>0.05, respectively) were found (Supplementary Figure 4).
In vivo striatal DAT function and extracellular dopamine
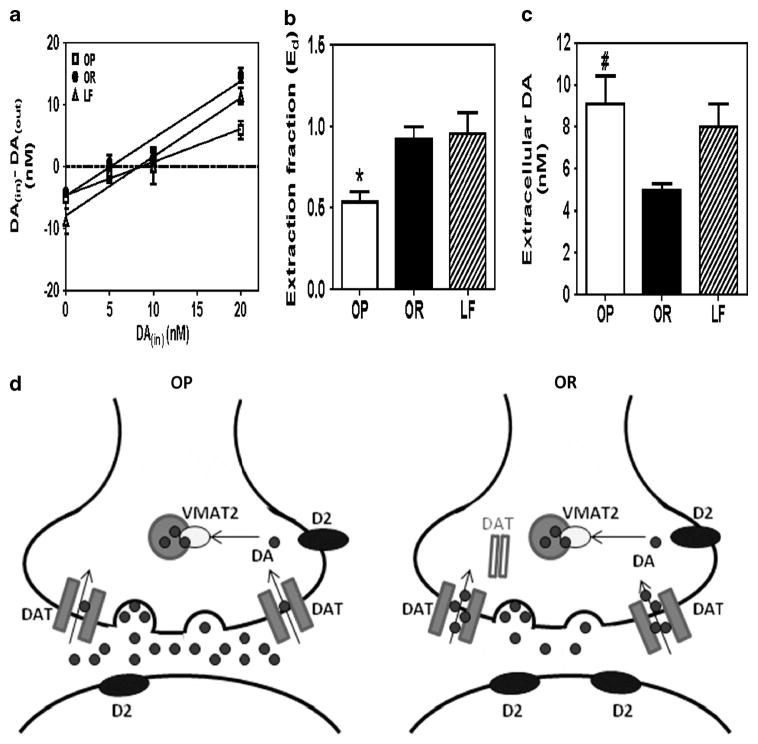
Striatal extraction fraction for the OP group was 40% lower than for OR and LF groups (F2,16 = 5.313, P<0.05; Figure 4b). Striatal extracellular dopamine in OP was 45% greater than in OR group (F2,16 = 4.767, P<0.05; Figure 4c), but not different from LF group.
Figure 4.
Effect of DIO on striatal DAT function and extracellular dopamine in vivo. DIO results in decreased slope of the linear regression line from the no-net flux microdialysis plot (a), decreased extraction fraction (b) and increased extracellular DA (c). Data are expressed as nM (mean± s.e.m.) for DAin – DAout and extracellular DA; n = 5–6 rats per group; *P<0.05, OP vs OR and LF; #P<0.05, OP vs OR. Schematic of DIO effects observed on striatal DA function (d). Striatal D2-receptor density and DAT function were decreased and extracellular DA concentration increased in the OP group compared with the OR group. Cell-surface DAT expression and VMAT2 function were not different between OP and OR groups. Filled dots represent DA.
Impulsivity and motivation for food reinforcement
Level of caloric restriction (58 and 54%) imposed by the 3-h access to daily food allowance was not different between OP and OR groups, respectively (data not depicted).
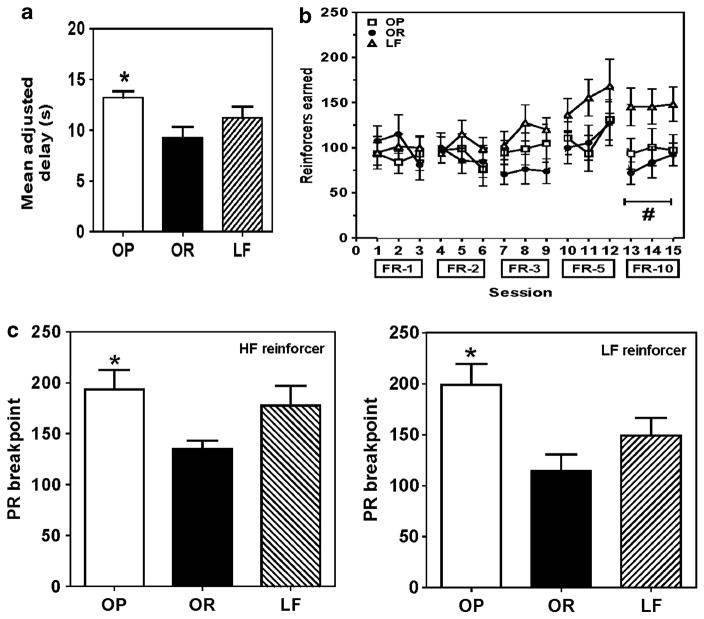
Mean adjusted delay for each group varied by <5 s across the last five sessions (median = 10.5 s). OP group exhibited 30% greater delay compared with OR group (F2,14 = 4.219, P<0.05; Figure 5a).
Figure 5.
Effect of DIO on impulsivity and motivation for food reinforcement. OP rats exhibited a greater mean adjusted delay (decreased impulsivity) compared with OR rats (a). During the FR-10 schedule, number of reinforcers earned was lower in OP and OR rats compared with LF rats (b). OP rats exhibited a higher PR breakpoint compared with OR rats for both HF- and LF-reinforcers (c). Data are expressed as mean±s.e.m.; n = 5–8 rats per group; *P<0.05, OP vs OR; #P<0.001, OP and OR vs LF.
During FR training, number of reinforcers earned was not different between groups across incremental FR increases (Figure 5b), and number of active lever responses, but not inactive responses, increased correspondingly (Supplementary Figure 5). Analysis of the number of reinforcers earned revealed a group × FR interaction (F8,84 = 2.751, P<0.05). At FR-10, number of reinforcers earned by OP and OR groups was lower than that for LF group (P<0.05). PR breakpoint for HF- and LF-reinforcement was greater for OP than for OR groups (F2,22 = 3.655 and F2,18 = 4.639, ps<0.05, respectively; Figure 5c).
Neurobehavioral predictors of DIO
All rats received identical treatment, standard chow ad libitum for 1 week during acclimatization, 15 g standard chow after each daily operant session, and HF-diet ad libitum for 8 weeks. Level of caloric restriction during behavioral assays was not different in rats subsequently designated as OP and OR groups.
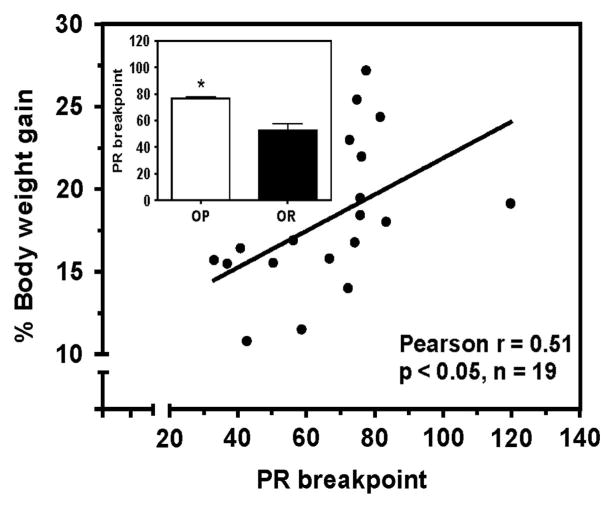
No correlation (Pearson r = 0.227, P = 0.35) was found between mean adjusted delay and %body-weight gain after the 8-week HF-diet exposure (Supplementary Figure 6a). There were no differences (P>0.05) between groups subsequently designated as OP and OR for mean adjusted delay (10.9±2.75 and 5.9±1.64 s, respectively). A positive correlation between PR breakpoint for HF reinforcers and %body-weight gain was found (Pearson r = 0.51, P<0.05; Figure 6). Mean PR breakpoint for HF reinforcers across the six sessions was greater in OP compared with OR groups (P<0.01; Figure 6, inset). Extraction fraction and extracellular dopamine did not correlate with %body-weight gain (Pearson r = 0.275 and −0.291; P-values>0.05; Supplementary Figures 6b and c, respectively). No differences (P>0.05) were found between groups subsequently designated as OP and OR, for either dopamine extraction fraction (0.94±0.14 and 0.86±0.22, respectively) or extracellular dopamine concentration (7.7±1.64 and 11.3±1.92 nM, respectively).
Figure 6.
Neurobehavioral predictors of DIO. A positive correlation between motivation for HF-reinforcers and %body-weight gain was found. The OP group exhibited greater PR breakpoint compared with the OR group (inset). Data points represent individual rats; for inset, data are mean±s.e.m.; n = 6 rats per group; *P<0.01, OP vs OR.
DISCUSSION
The current study provides novel findings that motivation to obtain HF-food predicts the development of obesity in an outbred animal model of DIO, whereas impulsivity does not predict DIO. Increased motivation for HF-food persisted following the development of DIO, consistent with observations that orosensory properties and post-ingestive effects of HF-diets engender overeating.34,35 Pre-existing dopamine reward deficiency has been suggested to contribute to compensatory overeating of carbohydrates leading to human obesity.36 In the current model, striatal D2-receptor density was decreased, similar to human obesity.15 Striatal DAT function and extracellular dopamine did not predict DIO, whereas DAT function and expression decreased and extracellular dopamine increased once DIO was established (Figure 4d). Thus, a deficiency in striatal DAT function is an outcome of DIO, but does not underlie obesity resulting from consumption of an HF diet.
Primary food reward is mediated by NAc dopamine.9 Following ingestion of an HF diet for 12 weeks, NAc dopamine turnover in rats with free-access (obese) or restricted-access (non-obese) was decreased compared with rats with free-access to standard chow,37 supporting alterations in dopamine release, uptake and/or metabolism. In contrast, extracellular NAc dopamine was decreased in inbred adult obese rats compared with OR rats fed standard chow;38 however, contributions of functionally distinct NAc core and shell were not evaluated. In another study, dopamine release from NAc shell, striatum and medial prefrontal cortex slices was decreased in obese inbred rats compared with OR inbred rats.38 Decreased expression of mRNA for VMAT2, tyrosine hydroxylase, DAT and D2 was found in cultured VTA neurons from OP inbred compared with OR inbred rats.38 Collectively, deficient NAc dopamine function contributes to decreased primary food reward and obesity. While decreased mesolimbic dopamine function is suggested to promote compensatory hyperphagia leading to obesity, the current research focused on striatal dopaminergic mechanisms.
A shift in underlying dopaminergic control from NAc to dorsal striatum coincides with development of habitual behaviors.11,39,40 A decrease in dorsal-striatal D2 receptors was found herein using an outbred-DIO model, consistent with the results from obese humans.15 Decreased D2-receptor density may precede the development of obesity or result from pre-existing decreases in DAT function and/or increased extracellular dopamine associated with repeated food reward. Herein, decreased DAT function and increased extracellular dopamine was found only after DIO was established. Since clearance of extracellular dopamine is primarily regulated by DAT localized at the cell surface,41 one explanation for the decreased DAT function is decreased cell-surface DAT expression. Thus, the effect of methamphetamine was anticipated to be decreased as a result of diminished cell-surface DAT expression. Surprisingly, methamphetamine-induced DAT reverse transport was increased in OP relative to OR groups. In contrast, dopamine uptake was decreased in OP compared with OR groups. Thus, obesity may differentially alter the bidirectional DAT function, dopamine uptake and reverse transport, by modulating distinct signaling mechanisms.
Decreased DAT function in OP relative to the OR group could be explained by decreased D2 autoreceptor function, since D2 autoreceptors regulate DAT function.42 Alternatively, decreased DAT function in the OP group may be explained by activation of protein kinase C (PKC), shown previously to decrease dopamine uptake at hDAT expressed in Xenopus oocytes.43 With respect to DAT reverse transport, PKC activation increases amphetamine-induced dopamine reverse transport in rat striatal slices and DAT-transfected HEK-293 cells.44–46 Thus, PKC activation may underlie the increased methamphetamine-induced reverse transport of DAT herein. Furthermore, PKC phosphorylation of DAT occurs to a greater extent in lipid-raft membrane compartments, compared with non-raft.47 Thus, DAT partitioning between lipid-raft and non-raft compartments may underlie decreases in dopamine uptake and increases in methamphetamine-induced reverse transport in obesity.
Another potential underlying mechanism for altered DAT function in OP and OR rats was altered DAT cellular localization. Previous studies showed reduced striatal DAT density in rats fed the HF-diet for 20 days compared with chow-fed controls.48 Current work extends previous findings by showing decreased total striatal DAT protein in OP compared with the OR group, consistent with decreased striatal dopamine uptake in OP compared with OR group. However, no differences in cell-surface or intracellular DAT expression were observed, suggesting that regulation of DAT function in DIO is trafficking-independent. Alternative mechanisms underlying decreased striatal DAT function/expression in the OP group may include differential partitioning of DAT into lipid-raft and non-raft membrane domains and differential regulation of DAT turnover.
Obesity and drug addiction are hypothesized to share common underlying neurobehavioral mechanisms.49 Personality traits including high sensation seeking and impulsivity predispose and are affected by drug addiction.50 Herein, impulsivity measured using delay discounting was decreased following development of DIO in male rats. Current findings contrast with increased impulsivity observed in obese women,4 possibly due to different species, sex or reinforcers employed. Delay discounting, which measures only one facet of impulsivity, was employed in both studies. Reward-based motivational processes may have overcome impulsive choice in the animal model, but not in the human study. Although OP and OR rats did not differ in acquisition of FR responding for HF-reinforcers, PR breakpoint was greater in OP than the OR group, indicating greater food motivation in OP. Further, PR breakpoint was higher in the OP group regardless of employment of HF or LF reinforcer. Thus, caloric density was not a factor in food motivation. A high level of motivation for either reinforcer in the OP group suggests development of compulsivity.51
Controversy exists regarding effects of obesity on food motivation across studies using different animal models. In contrast to the current results, PR breakpoint for HF-reinforcers did not differ between DIO-prone Osborne-Mendel and DIO-resistant S5B/PI inbred rats fed standard chow in the home-cage.52 Similarly, no differences in PR breakpoint for sucrose pellets were found between outbred Sprague Dawley rats with 12-week free-access (obese) or restricted-access (non-obese) to HF-diet in the home-cage.37 However, both groups receiving HF-diet in the home-cage had decreased PR breakpoint for sucrose pellets compared with another group with free-access to standard-chow in the home-cage, suggesting that the value of the sucrose reward was decreased following long-term access to HF-diet. Decreased PR breakpoint for sucrose reinforcement was found in Sprague Dawley rats selectively inbred for obesity compared with obesity resistance; all rats being fed standard chow in the home-cage.37 Discrepancies between previous results and the current study showing an increase in PR breakpoint for HF-reinforcers in the OP group may be due to differences in animal models of obesity, reinforcer-type (sucrose vs HF, different satiety mechanisms), and/or home-cage diet. However, it is unlikely that the HF-diet is responsible for increased PR breakpoint in OP rats, because PR breakpoint for HF- or LF-reinforcers was not different between OR and LF rats, fed HF- and LF-diets, respectively, in the home-cage. Thus, differences in food motivation may be due to obesity rather than diet per se.
Consistent with the current results, CCK-1 receptor-deficient OLETF rats and leptin-receptor deficient Zucker rats fed standard chow in the home-cage exhibited increased PR breakpoints for sucrose reinforcers compared with lean controls.53,54 Similarly, outbred Wistar rats fed a HF/high-sugar home-cage diet demonstrated increased PR breakpoint for sucrose reinforcers compared with chow-fed rats.55 The current study extends these findings showing increased motivation for HF-reinforcers in the OP group, and, that pre-existing increased motivation for HF-reinforcers predicts obesity. While preference for high fat was not determined in the current study, previous studies show increased choice for fat compared with carbohydrate and protein in inbred OP (Osborne-Mendel) compared with OR (S5B/PI) rats.56 Similar increases in fat preference may underlie increased motivation for HF-reinforcers in the OP group, herein.
An important contribution of the current research is the evaluation of impulsivity, motivation and DAT function as predictors of DIO. Using delay discounting, impulsivity has been shown previously to predict psychostimulant intake.32 In contrast, impulsivity, measured using delay discounting, did not predict DIO, suggesting that distinct mechanisms underlie predisposition for obesity and drug addiction. Importantly, increased motivation for HF-reinforcers predicted DIO.
In the current study, striatal DAT function and extracellular dopamine concentration did not predict obesity that developed following an 8-week HF-diet, leading to large body-weight differences (150 g). Decreased extracellular dopamine in NAc-shell has been reported to predict obesity,57 and excessive food intake was interpreted as compensatory for low basal dopamine in NAc-shell. However, these results were obtained only after 5 days of HF-diet, between-group body-weight differences were only 20 g, and lower dopamine levels may have resulted from transient exposure to HF-diet per se. In contrast, in the current study, striatal dopamine did not predict obesity evaluated before HF-diet exposure. That is, individual differences in dopamine before HF-diet exposure did not correlate with long-term body-weight gain. Further studies evaluating NAc dopamine as a predictor of obesity are needed.
In both current in vitro and in vivo studies, outcomes of DIO included decreased striatal DAT function and increased extra-cellular dopamine. Obesity, not diet per se, decreased DAT function, since OP and OR groups received the same HF diet. Also, between-group differences in caloric intake may have contributed to observed differences in DAT function. Despite ad libitum access to HF-diet, resistance of OR phenotype to develop obesity may be due to compensatory increases in DAT function and decreased extracellular dopamine. Thus, decreases in striatal DAT function as an obesity outcome, rather than as a predictor, may be important for maintenance of obesity.
In the outcome study, comparisons of results from OP and OR groups to the LF group varied depending on outcome measure. Caloric intake was not different between OP and LF groups and between OR and LF groups. Further, there were no differences between OP and LF groups on in vitro dopamine uptake, DAT expression and extracellular dopamine concentration, and no differences between OR and LF groups on impulsivity and food motivation. Thus, caloric intake may have contributed to comparisons between groups fed HF and LF diets. Also, despite exposure to the same HF diet, neither OP nor OR groups were different from the LF group (fed LF diet), suggesting that these outcomes are not altered by diet per se, but by obesity. A different pattern emerged upon comparison of OP and OR groups to the LF group for methamphetamine-induced DAT reverse transport, with decreased effect in OP vs LF and OR vs OP group. Thus, an outcome of prolonged exposure to HF-diet per se may be downregulation of signaling mechanisms modulating methamphetamine–DAT interactions. Since the OP group exhibited greater effects of methamphetamine compared with the OR group, obesity may have mitigated effects of the HF-diet on this outcome. Thus, both development of obesity and effects of diet per se contribute to differential between-group effects of methamphetamine.
In contrast to the pattern of effect for methamphetamine, the OP group exhibited lower DAT function in vivo compared with the LF group; however, the OR group was not different from the LF group, indicating that diet per se does not affect this outcome measure. The OP group exhibited decreased DAT function in vivo compared with the OR group, which was not dependent on diet, since both OP and OR groups had the HF-diet. Thus, obesity alone appears to contribute to differences in in vivo DAT function. Importantly, the LF group is comprised of an unknown distribution of both OP and OR phenotypes. Based on sampling distribution, the pattern of response might vary across experiments. Thus, cross-experiment direct statistical comparisons to the LF group are inappropriate and were not conducted.
Given the interaction between homeostatic and reward systems, sustained stimulation of dopamine reward circuits by adiposity hormones may underlie increased food-motivated behavior following DIO. Leptin inhibits dopamine neuronal firing rate and decreases extracellular NAc dopamine,58,59 whereas insulin increases DAT mRNA.60 Furthermore, leptin and insulin (icv) decrease sucrose self-administration and reverse HF-food-conditioned place preference.61,62 Also, knockdown of midbrain leptin receptors increases PR responding for sucrose reinforcers.63 Importantly, OP rats exhibit defective central leptin- and insulin-signaling relative to OR rats.23,25 Taken together, DIO and increased food intake may lead to increased adiposity hormones stimulation of the reward circuitry, including decreased striatal DAT function and increased extracellular dopamine.
Overall, current results demonstrate that as a predictor, motivation for HF-reinforcers was greater in rats which subsequently became obese following 8-week HF-diet exposure, compared with those not developing obesity when exposed to the same diet for the same period. Once DIO developed, greater food motivation persisted in the OP compared with the OR group, indicating that food motivation is linked to both initiation and maintenance of obesity. In contrast, impulsivity, DAT function and extracellular striatal dopamine did not predict DIO upon HF-diet exposure. Once DIO was established, impulsivity decreased, DAT function decreased and extracellular striatal dopamine increased in the OP relative to OR group. Although the impact of obesity on dopamine function was expected, decreased impulsivity was an unanticipated outcome. Importantly, obesity results in decreased striatal DAT function, which may underlie maintenance of compulsive food intake associated with obesity.
Supplementary Material
Acknowledgments
We acknowledge Agripina Deaciuc, Andrew Smith, Andrew Chip Meyer, David Lee, Deann Hopkins, Emily Denehy, Gurpreet Dhawan, Kate Fischer, Kiran Babu Siripurapu, Travis McCuddy and Victoria English for help with the assays. We thank Dr Robert Lorch for help with the statistical analyses. This research was supported by NIH P50 DA05312 (Linda P Dwoskin), NIH HL73085 and P20RR021954 (Lisa A Cassis) and a Pre-doctoral Fellowship from the American Heart Association, AHA 715489B (Vidya Narayanaswami).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Sclafani A. Psychobiology of food preferences. Int J Obesity Related Metabolic Disorders. 2001;25:S13–S16. doi: 10.1038/sj.ijo.0801905. [DOI] [PubMed] [Google Scholar]
- 2.Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci. 2008;1141:163–175. doi: 10.1196/annals.1441.028. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Indiv Differ. 2001;30:669–689. [Google Scholar]
- 4.Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Mobbs O, Crépin C, Thiéry C, Golay A, Van der Linden M. Obesity and the four facets of impulsivity. Patient Educ Couns. 2010;79:372–377. doi: 10.1016/j.pec.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) 2011;19:2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 8.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 9.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 13.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. Delta FosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher ‘wanting’ but not ‘liking’ for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, et al. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004;29:134–137. [PMC free article] [PubMed] [Google Scholar]
- 20.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 21.Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- 22.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 23.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 24.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol. 2002;283:R941–R948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: differential findings with [3H]raclopride versus [3H]spiperone. Mol Pharmacol. 2003;63:456–462. doi: 10.1124/mol.63.2.456. [DOI] [PubMed] [Google Scholar]
- 27.Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, et al. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2010;332:612–621. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Crooks PA, Ayers JT, Sumithran SP, Dwoskin LP. N-n-Alkylnicotinium and N-n-alkylpyridinium analogs inhibit of dopamine transporter function: selectivity as nicotinic receptor antagonists. Drug Dev Res. 2003;60:270–284. [Google Scholar]
- 29.Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, et al. Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther. 2001;296:1023–1034. [PubMed] [Google Scholar]
- 30.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neuroschem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- 31.Acri JB, Thompson AC, Shippenberg T. Modulation of pre- and postsynaptic dopamine D2 receptor function by the selective kappa-opioid receptor agonist U69593. Synapse. 2001;39:343–350. doi: 10.1002/1098-2396(20010315)39:4<343::AID-SYN1018>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 34.Kern DL, McPhee L, Fisher J, Johnson S, Birch LL. The postingestive consequences of fat condition preferences for flavors associated with high dietary fat. Physiol Behav. 1993;54:71–76. doi: 10.1016/0031-9384(93)90045-h. [DOI] [PubMed] [Google Scholar]
- 35.Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–R37. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- 36.Blum K, Chen TJ, Meshkin B, Downs BW, Gordon CA, Blum S, et al. Reward deficiency syndrome in obesity: a preliminary cross-sectional trial with a genotrim variant. Adv Ther. 2006;23:1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- 37.Davis JF, Tracy AL, Schurdak JD, Tschöp MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;112:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavioral under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 41.Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–263. doi: 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]
- 44.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:594–598. [PubMed] [Google Scholar]
- 45.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:387–393. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39:73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- 49.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 50.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thanos PK, Cho J, Kim R, Michaelides M, Primeaux S, Bray G, et al. Bromocriptine increased operant responding for high fat food but decreased chow intake in both obesity-prone and resistant rats. Behav Brain Res. 2011;217:165–170. doi: 10.1016/j.bbr.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glass MJ, O’Hare E, Cleary JP, Billington CJ, Levine AS. The effect of naloxone on food-motivated behavior in the obese Zucker rat. Psychopharmacology (Berl) 1999;141:378–384. doi: 10.1007/s002130050847. [DOI] [PubMed] [Google Scholar]
- 54.Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC, Obese OLETF. rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol. 2007;2293:R1846–R1854. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- 55.Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes. 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 56.Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C. Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol. 1992;262(6 Part 2):R1111–R1116. doi: 10.1152/ajpregu.1992.262.6.R1111. [DOI] [PubMed] [Google Scholar]
- 57.Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krügel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 59.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 60.Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994;644:331–334. doi: 10.1016/0006-8993(94)91698-5. [DOI] [PubMed] [Google Scholar]
- 61.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- 62.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 63.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.