Abstract
Exposure to opioids results in the activation of opioid receptors; this is follow ed by receptor endocytosis. Previously, we showed that delta opioid receptors undergo rapid agonist-mediated internalization and that mutations in the C-tail result in a substantial loss of agonist-mediated internalization. In this study, we investigated the fate of receptors following rapid internalization. We found that the majority of the wild type receptors recycled back to the surface after acute agonist treatment. The kinetics of internalization and recycling of the receptor were virtually identical to the kinetics of internalization and recycling of the radiolabeled agonist. In contrast, the kinetics of internalization and recycling of a C-tail mutant receptor were substantially altered, suggesting an involvement of the C-tail in the recycling process. It is possible that in addition to agonist-mediated internalization, opioid receptors undergo constitutive, agonist-independent internalization. We directly examined this possibility using an antibody-prebinding assay. The wild type delta opioid receptors exhibited agonist-independent internalization via the clathrin-coated pit pathway. We also examined the role of receptor internalization and recycling in the modulation of its function by quantitating the level of opioid-stimulated phosphorylation of MAP kinase (MAPK) under conditions of receptor internalization and recycling. We found that agonist treatment caused a rapid increase in the level of phosphorylated MAPK that was rapidly desensitized. The removal of the agonist, which results in receptor recycling, led to the resensitization of the receptor, as evidenced by the agonist’s ability to reinduce MAPK phosphorylation. Mutant receptors that underwent rapid recycling exhibited enhanced resensitization, suggesting a role for receptor recycling in the re-sensitization process. Taken together, these results indicate that agonist-mediated internalization and recycling modulate opioid receptor function and that the receptor C-tail plays an important role in both processes.
INTRODUCTION
The binding of an opioid agonist to opioid receptors initiates the activation of the associated G proteins, followed by the induction of a number of second-messenger systems (Herz, 1993; Zaki et al., 1996; Smart and Lambert, 1996). This action is accompanied by rapid agonist-induced internalization of the receptor. By regulating the number of receptors on the cell surface, receptor endocytosis plays an important role in modulating the biologic actions of opioids.
The agonist-induced internalization of the opioid receptor is a rapid and selective event (Jordan and Devi, 1998). Mu and delta opioid receptors are internalized with a t1/2 of 5 to 10 min, and only high-efficacy selective agonists induce receptor internalization (Keith et al., 1996, 1998; Koch et al., 1998; Trapaidze et al., 1996). Morphine, a low-efficacy agonist, does not induce internalization of mu or delta opioid receptors in transfected cells or in neurons endogenously expressing the receptor (Arden et al., 1995; Keith et al., 1996, 1998; Sternini et al., 1996). Interestingly, etorphine, a high-efficacy universal opioid agonist, is able to preferentially internalize mu and delta but not kappa opioid receptors, suggesting that internalization of opioid receptors also exhibits receptor type specificity (Chu et al., 1997; Jordan et al., in press). Opioid receptor internalization is blocked by agents that disrupt clathrin-coated pit formation (Keith et al., 1996; Trapaidze et al., 1996). This finding, as well as studies with colocalization of opioid receptors within the transferrin-containing compartment (Keith et al., 1996), indicates that the clathrin-coated pit-mediated endocytic pathway is used for agonist-mediated internalization of opioid receptors.
Internalization of G protein-coupled receptors (GPCRs) is initiated by the agonist-induced phosphorylation of the receptor by G-protein receptor kinases (GRKs). Adapter proteins such as beta-arrestin recruit GPCRs to the clathrin-coated pit pathway by binding to the phosphorylated receptor as well as clathrin with high affinity (Gurevich et al., 1995; Goodman et al., 1996). Both GRK-mediated phosphorylation and beta-arrestin binding have been shown to be crucial for internalization of certain GPCRs but not others (Tsuga et al., 1994; Ferguson et al., 1996; Zhang et al., 1996). For instance, internalization of m2-muscarinic cholinergic receptor is enhanced by GRK overexpression and decreased by a dominant-negative mutant (Tsuga et al., 1994). Similarly, internalization of a sequestration-defective beta-adrenergic receptor mutant is rescued by overexpression of GRK (Ferguson et al., 1996), whereas internalization of angiotensin II 1A receptor is not substantially affected by overexpression of GRK, beta-arrestin, or dynamin (Zhang et al., 1996). In the case of opioid receptors, the agonist-mediated internalization appears to be a GRK- and beta-arrestin-mediated phenomenon (Zhang et al., 1998; Schulz et al., 1999; Whistler and Von Zastrow, 1998).
Although a number of studies have examined the mechanisms of agonist-mediated endocytosis of opioid receptors, only a few have explored agonist-independent endocytosis or receptor recycling. A study examining the trafficking of muopioid receptor mutants has found that the truncated receptors are constitutively internalized and recycled at a rapid rate (Segredo et al., 1997). In the present study, we have examined opioid receptor recycling and correlated it with the recycling of the ligand. We found that delta receptors recycle following rapid endocytosis. Receptor mutants that did not undergo rapid agonist-mediated internalization underwent robust agonist-independent internalization and recycling. Receptor recycling plays a role in receptor resensitization, as examined using the opioid-mediated phosphorylation of MAP kinase (MAPK).
MATERIALS AND METHODS
Generation of cell lines expressing wild type and mutant delta opioid receptor
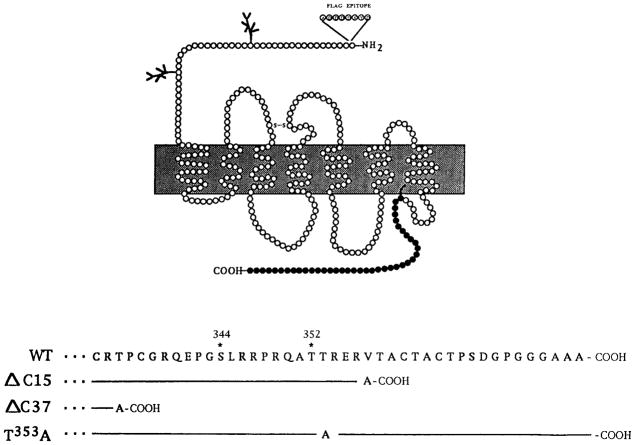
Chinese hamster ovary (CHO) cells stably expressing N-terminally Flag-epitope (ADDDDKYD)-tagged wild type delta opioid receptor or mutant receptors Δ C15, Δ C37, and T353A (see Fig. 1 for the schematic of the C-tail) were generated and characterized for their binding affinities, coupling to adenylyl cyclase, internalization, and downregulation as described previously (Cvejic et al., 1996; Trapaidze et al., 1996). For the generation of N18-DOR cells, the N18TG2 cells maintained in Dulbecco Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) were stably transfected with epitope-tagged wild type delta receptors and characterized. The binding affinities and coupling properties of these cells are similar to those of CHO cells stably expressing Flag-tagged delta receptors. The NG108-15 cells were maintained in DMEM containing hypoxanthine, aminopterin, and thymidine (HAT) and 10% FBS.
FIG. 1.
Schematic representation of the C-terminal tail of wild type and mutant delta opioid receptors. The C-terminal tail residues 333–372 of the wild type receptor are in single-letter amino acid code. The numbering is according to Evans et al. (1992); the amino acid sequence of the mutants identical to the wild type is represented by a line, and changes are as indicated.
Detection of agonist-mediated receptor internalization by confocal microscopy
The N18TG2 cells stably transfected with delta receptors were grown on coverslips and were treated without or with 100 nM agonist for 30 min or 24 h. Following incubation, cells were washed with ice-cold 50 mM Tris Cl, pH 7.5, containing 150 mM NaCl and 1 mM CaCl2 (TBS) and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). Fixed cells were washed with TBS, permeabilized, and blocked with 0.1% Triton X-100 in Blotto (3% nonfat dry milk in 50 mM Tris Cl, pH 7.5). Cells were incubated for 1 h at room temperature with primary antibody (anti-Flag M1; Sigma) 10 μg/ml diluted in Blotto, then washed with TBS, incubated for 30 min with fluoresceine isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Vector Laboratories) 2 μg/ml diluted in Blotto, washed with TBS, and mounted on glass slides using Permount. Cells were examined using an oil-immersion objective and standard fluorescein epifluorescence optics, and confocal fluorescence microscopy was performed using a laser-scanning microscope.
Receptor internalization and recycling
Receptor internalization was measured by flow cytometry as described previously (Trapaidze et al., 1996; Cvejic and Devi, 1997). For quantifying receptor recycling, the CHO or N18 cells expressing wild type delta opioid receptors were pretreated with 10 μ M cycloheximide for 1 h to block protein synthesis and then exposed to a single dose of 100 nM DADLE for 30 min. The agonist was removed by extensive washing and incubated for various periods of time with buffer without the agonist in the absence or presence of 100 μ M monensin; treatment of cells with 100 μ M monensin blocks > 50% of receptor recycling. At the end of the incubation, cells were chilled to 4°C, washed three times with 0.5 ml of PBS, and incubated for 1 h at 4°C with primary antiserum 10 μ g/ml in PBS containing 50% FBS. Cells were washed with 1% FBS in PBS and incubated with FITC-conjugated goat anti-mouse IgG 5 μ g/ml for 1 h. Cells were washed with 1% FBS in PBS followed by a PBS wash, collected from the wells with 1 mM EDTA in PBS, and analyzed on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Inc). Live cells were gated by light scatter or exclusion of propidium iodide, and 5000 to 10,000 cells were acquired for each time point. The mean fluorescence of all live cells, minus the mean fluorescence of cells stained only with FITC-conjugated second antibody, was used for the calculation (Trapaidze et al., 1996).
Internalization and recycling of the ligand-[3H] DPDPE
CHO cells expressing the Flag-tagged wild-type delta receptors or T353A mutant delta receptors and NG108-15 cells expressing endogenous delta receptors were plated (1–2 × 105 cells/well) in 24-well plates. After 24 h, the medium was removed, and cells were incubated with 2 nM [3H]-DPDPE in Krebs Ringer–HEPES buffer, pH 7.4 (buffer A) in a final volume of 300 μ l. Incubation was carried out for different time periods, after which cells were chilled at 4°C, washed in 50 mM Tris Cl, pH 7.5, and collected to measure the total binding. The amount of ligand internalized was determined by washing a parallel set of wells with ice-cold 0.2 M sodium acetate, pH 4.8, containing 500 mM sodium chloride (acid buffer); a wash with this buffer has been previously shown to remove cell-surface binding (Sorokin et al., 1989). The acid-washed cells were collected to determine the amount of internalized radiolabeled agonist. To quantify ligand recycling, the acid buffer-washed cells were washed with 50 mM Tris Cl, pH 7.5 and incubated at 37°C for various time periods. The spent medium was collected, and the cells were washed again in acid buffer (“acid wash”). The spent medium + the acid wash samples were combined, and the radioactivity was determined using BioSafe scintillation fluid. The radioactivity remaining in the cells was determined by dissolving them in 1 N NaOH, neutralizing with 1 N HCl, and measuring in scintillation fluid.
Detection of constitutive internalization by confocal microscopy
Cells expressing Flag-tagged wild type receptors were grown on coverslips. Cells were incubated for 1 h at 4°C to label cell-surface receptors or for 30 min and 60 min at 37°C to enable antibody uptake with primary antibody 5 μ g/ml diluted in Blotto containing TBS. To examine the effect of sucrose on receptor internalization, cells were labeled with primary antiserum 5 μ g/ml at 4°C, washed, and warmed for 60 min in the absence or presence of 0.65 M sucrose. To examine the effect of sucrose on antibody uptake, cells were incubated for 30 min at 37°C with 0.65 M sucrose followed by 60 min at 37°C with M1 antibody in 0.65 M sucrose. After treatment, cells were washed with ice-cold TBS. In order to visualize only the internalized receptor, the antibody bound to the cell surface receptors was removed by washing three times with ice-cold PBS containing 5 mM EDTA (stripped). Cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 in Blotto. The internalized receptors were visualized by incubation for 60 min with FITC-conjugated goat anti-mouse IgG 2 μ g/ml (Vector Laboratories) diluted in Blotto.
MAPK phosphorylation
To determine the level of opioid-mediated increase in the phosphorylation of MAPK, CHO cells expressing either wild-type or mutant receptors were plated in a 24-well plate and grown to 80% confluency. Cells were incubated in serum-free medium for 2 h before the addition of ligands and treated with 100 nM DPDPE for 30 min (conditions of internalization). This was followed by the removal of the agonist. A second 5-min treatment with 100 nM DPDPE was used to determine the extent of opioid-mediated phosphorylation following receptor internalization. Cells not pretreated with agonists but exposed to a 5-min pulse of 100 nM DPDPE were used as the control. To examine opioid-mediated phosphorylation during recycling, cells were treated with 100 nM DPDPE for 30 min (to induce internalization). The cells were then washed and incubated in the medium without the agonist for various periods of time (recycling). The cells were extracted by lysing in 100 μ l/well of 2% SDS in 50 mM Tris Cl, pH 6.8, and ~10 μ g of protein was subjected to SDS-PAGE on an 8% gel. The protein concentration of the lysates was determined using BCA protein assay reagent (Pierce). To detect the phosphorylated MAPKs, proteins were transferred to a nitrocellulose membrane (MSI Inc.) and incubated overnight with 5% milk in 50 mM Tris Cl, pH 7.4, and 150 mM NaCl, 0.1% Tween 20 (TBST). Membranes were incubated with a 1:2000 dilution of p44/42 phospho-ERK antibody E10 (New England Biolabs) in 5% milk/TBST for 1 h and washed with TBST four times for 15 min each. This was followed by incubation with a 1:3000 dilution of horseradish peroxidase-conjugated anti-mouse IgG (Vector Laboratories) in 5% milk in TBST for 1 h. The membranes were washed with TBST four times for 15 min each, and the signal was detected with enhanced chemiluminescence SuperSignal® West Pico Chemiluminescent Substrate as described by the manufacturer (Pierce). To confirm equal loading and quantitation, membranes were stripped by incubation in 0.1 M glycine, pH 2.6, for 20 min at room temperature. The membranes were incubated with a 1:2000 dilution of monoclonal tubulin antibody (Sigma), and the signal was detected as described above. For densitization of the blots, a LaCie Silverscanner attached to a Macintosh Quadra 950 running NIH Image software was used. Typically, two or three exposures of each membrane were scanned, and only the values in the linear range of the film were used.
RESULTS
We have previously shown that opioid receptors expressed in fibroblast cell lines are internalized rapidly upon acute exposure to agonists (Trapaidze et al., 1996). Chronic (prolonged) exposure to agonists results in the degradation of the receptor (Cvejic et al., 1996; Trapaidze et al., 2000a). In this study, we examined the fate of the receptor following internalization by acute (single) exposure to agonists. Because neuroblastoma cell lines are a more appropriate model than the previously used fibroblast cell line, we first examined the trafficking of delta receptors in the N18TG2 neuroblastoma cell line. We found that in cells not treated with the agonist, delta receptors were localized primarily on the plasma membrane (Fig. 2A). Acute treatment with 100 nM DADLE (30 min) resulted in the redistribution of the receptor to an intracellular location (Fig. 2B), whereas chronic treatment (24 h) resulted in a substantial loss of the receptor fluorescence from these cells (Fig. 2C). These results are consistent with the rapid internalization of the receptor followed by the degradation of the receptor on chronic treatment; these properties are similar to the reported properties of delta receptors in neuronal cells expressing endogenous or exogenous receptors (Law et al., 1984; Trapaidze et al., 1996; Cvejic et al., 1996; Afify et al., 1998).
FIG. 2.
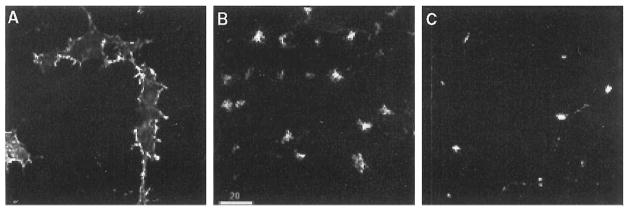
Neuroblastoma cells expressing delta opioid receptors. The N18TG2 cells expressing wild type mouse delta opioid receptors were incubated in the absence (A) or presence of 100 nM DADLE for 30 min (B) or 24 h (C). Fixation, permeabilization, staining, and confocal microscopy of receptors with the anti-Flag antibody were carried out as described in the text. Bright staining of the plasma membrane is seen in (A), while prominent intracellular staining is seen inside the cells in (B) and (C).
Next, we quantified the extent of receptor internalization by flow cytometry. Approximately 50% of the receptors had been internalized by about 10 min of agonist treatment, and more than 60% of the receptors were internalized by about 30 min both in neuroblastoma cells and in CHO cells expressing delta receptors (Fig. 3). Receptor internalization exhibited agonist selectivity in that treatment with delta-selective peptide agonists (100 nM DADLE or DPDPE) or a universal opioid agonist (30 nM etorphine) caused substantial internalization, whereas treatment with the muselective agonist 100 nM DAMGO did not (Table 1).
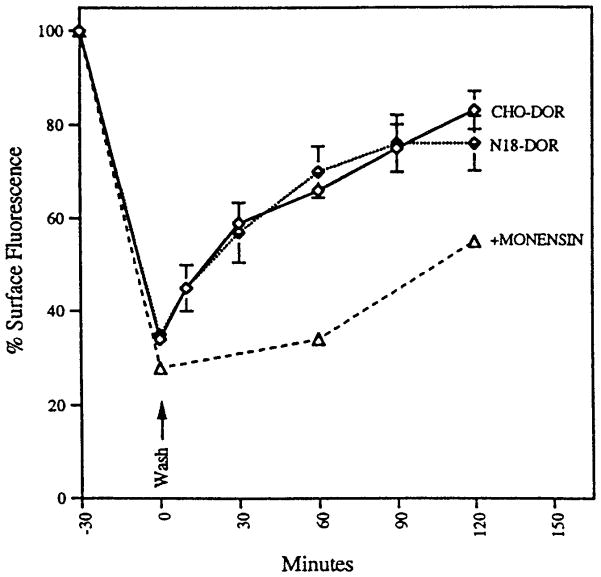
FIG. 3.
Time course of recycling of epitope-tagged mouse delta opioid receptors. CHO cells or N18G2 cells expressing epitope-tagged delta opioid receptor (CHO-DOR and N18-DOR) were treated with 10 μ M cycloheximide for 1 h, followed by incubation with 100 nM DADLE at 37°C for 30 min. The cells were washed and incubated for various periods of time in buffer without the agonist in the absence or presence of 100 μ M monensin. Cycloheximide was included in all the incubations. The cells were stained with M1 antibody followed by FITC-conjugated second antibody. Cell-surface fluorescence was measured by flow cytometry as described (Trapaidze et al., 1996). The mean fluorescence, after subtracting autofluorescence of cells (stained with second antibody alone) without DADLE treatment, was taken as 100%. The data represent mean ± SEM from three independent experiments.
Table 1.
Effect of Various Ligands on Delta Opioid Receptor Internalization and Recycling
| Ligand | % Surface receptors
|
|
|---|---|---|
| Internalization | Recycling | |
| DADLE | 34 ± 1.5 | 66 ± 0.9 |
| DPDPE | 43 ± 2.1 | 83 ± 5.6 |
| DSLET | 49 ± 3.1 | 74 ± 4.2 |
| Etorphine | 50 ± 1.4 | 59 ± 5.6 |
| DAMGO | 102 ± 4.5 | 93 ± 5.3 |
The cells expressing wild type receptors were treated with 10 μ M cycloheximide for 1 h and with the various agents (at 100 nM except etorphine at 30 nM) for 30 min. The cells were washed and incubated in medium without the ligand (but with cycloheximide) for 60 min. The cells were stained and analyzed by flow cytometry as described (Trapaidze et al., 1996). The data represent the mean and SEM of triplicate determinations.
We next examined receptor recycling. For this, the cells were treated with cycloheximide (to block protein synthesis) and exposed to a single dose of 100 nM DADLE for 30 min (to cause internalization of ~60% of the surface receptors), and the level of cell-surface receptors at various times after agonist removal was examined. We found a rapid increase in the cell-surface fluorescence intensity on removal of agonist; within 30 min of agonist removal, approximately 50% of the internalized receptors had been recycled, and by about 90 min, the majority of the receptors were recycled back to the surface (Fig. 3). The receptor recycling was substantially reduced by monensin. We found that the receptors internalized in response to selective as well as nonselective agonists recycled to about the same extent (Table 1). Because the treatment of cells with 100 to 1000 μ M morphine (low-efficacy alkaloid agonist) did not induce internalization of the receptor (not shown), we did not examine receptor recycling in cells treated with morphine. Blocking protein synthesis by treatment of the cells with cycloheximide did not affect the extent of receptor recycling, suggesting that new receptor synthesis does not contribute to the level of surface receptors seen during recycling. Taken together, these results suggest that the rapid internalization of the delta opioid receptor is not a cell-line-specific phenomenon and that N18 neuroblastoma as well as the CHO fibroblast cell lines are well suited for studies of delta receptor trafficking.
We next compared the time course of receptor internalization/recycling with the time course of ligand internalization/recycling. We took advantage of the availability of NG108-15 cells that express endogenous mouse delta receptors to examine the time course of ligand internalization/recycling. The findings were compared with the time course of ligand internalization and recycling in CHO cells transfected with delta receptors. For this, confluent cells in 24-well plates were incubated with 2 nM [3H]-DPDPE for different periods. At the end of the incubation, the cells were washed with mild acid buffer to remove surface-bound ligand. The amount of ligand released into the acid wash and that remaining in the cells were quantified independently. A substantial increase in internalization as well as recycling of the agonist was detected both in NG108-15 cells and in CHO cells expressing wild type delta receptors (Fig. 4). We have previously shown that the delta receptor mutants with mutations in the C-tail exhibit deficient agonist-induced internalization (Trapaidze et al., 1996). The extent of ligand internalization in these cells is lower than in wild type cells (Fig. 4A). Interestingly, the internalized ligand was rapidly recycled, and the extent of ligand recycling was significantly higher in cells expressing mutant receptor (Fig. 4B), suggesting that both endocytosis and recycling of these receptors are affected. It is possible that the apparent lack of agonist-mediated internalization of the mutant receptors observed previously (Trapaidze et al., 1996) is attributable to an increase in the rate of constitutive internalization, as well as an increase in the kinetics of recycling of these receptors.
FIG. 4.
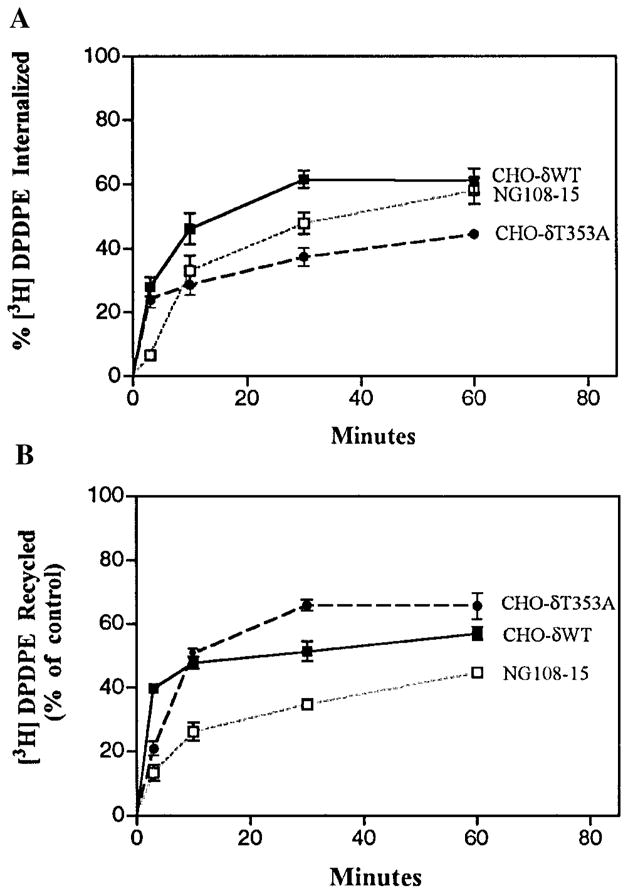
Internalization and recycling of opioid ligand. (A) Internalization. CHO cells expressing wild type or T353A receptors or NG108-15 cells expressing endogenous receptors were incubated at 37°C with 2 nM [3H]-DPDPE for different time intervals. Cells were acid washed to remove surface-bound ligand, and the radiolabel associated with the cells was determined as described in Materials and Methods. (B) Recycling. Cells were incubated for 30 min with 2 nM [3H]-DPDPE, acid washed to remove surface-bound ligand, and incubated in buffer without radioactivity for various periods of time. The radioactivity in acid-washed cells after 30 min of incubation with radiolabeled agonist is taken as 100%. The data represent mean ± SEM of three to five experiments.
To examine agonist-independent internalization directly, we used an antibody-prebinding assay and confocal microscopy to visualize the receptors and flow cytometry to quantitate the extent of receptor internalization. At 4°C, the receptor fluorescence was primarily on the cell surface (Fig. 5A), whereas warming the cells to 37°C for 30 or 60 min, resulted in a cytoplasmic localization of these receptors (Fig. 5B, C). These results suggest that delta receptors undergo agonist-independent internalization. Pretreatment of cells with hypertonic medium is thought to disrupt the clathrin-mediate dendocytic pathway (Heuser and Anderson, 1989), and under these conditions, the receptor fluorescence was primarily on the cell surface even on incubation for 60 min at 37°C, suggesting that the constitutive internalization was significantly reduced by treatment with sucrose (Fig. 5D). To further explore this possibility, we carried out antibody uptake studies in the absence or presence of hypertonic medium. We found that in the absence of the hypertonic medium, the receptor fluorescence was seen mainly as punctate staining within the cytoplasm (Fig. 5G) and on treatment was mainly on the cell surface (Fig. 5E, H). These results suggest involvement of clathrin-coated pits in the agonist-independent internalization process. Treatment of cells with 1 μ M naloxone alone did not affect agonist-independent internalization (not shown). Taken together, these results suggest that the delta receptors undergo slow constitutive endocytosis via the classic clathrin-coated-pit endocytic pathway.
FIG. 5.
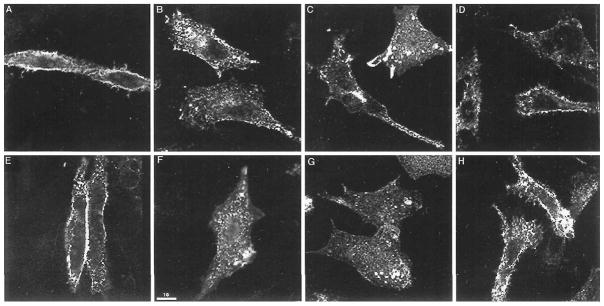
Confocal immunofluorescence microscopy of epitope-tagged wild type delta opioid receptors expressed in CHO cells after constitutive internalization. Cells expressing wild type delta opioid receptors were incubated for 1 h with M1 antibody at 4°C, washed, and warmed at 37°C for 0 (A), 30 min (B), or 60 min either in the absence of sucrose (C) or in the presence of 0.65 M sucrose (D). Cells were incubated with antibody at 37°C in the absence of sucrose for 30 min (F) or 60 min (G) or in the presence of 0.65 M sucrose for 60 min (H). Cells incubated with 0.65 M sucrose prior to staining with M1 antibody are shown in (E). To visualize both the cell-surface and internal receptors, cells were subjected to acid wash to remove primary antibody from the cell-surface receptors in the experiments shown in panels B, C, F, and G, then fixed and stained with FITC-conjugated second antibody following permeabilization. Immunofluorescence staining of the receptors with the monoclonal antibody against the epitope tag was as described in Materials and Methods. Cells were imaged by confocal fluorescence microscopy using a plane of focus adjusted 3 to 6 mm above the surface of the coverslip. This produces a cross-section through the center of the cell. Bright staining of the plasma membrane is apparent in panels A, D, E, and H. Prominent intracellular staining within the cytoplasm is seen in B, C and F, G.
Internalization and recycling have been implicated in the re-sensitization and thus modulation of function of a number of GPCRs (Lefkowitz, 1998). In order to examine the role of internalization and recycling in the modulation of opioid receptor function, we used the opioid activation of the MAPK pathway as a measure of receptor activity and examined the relative level of phosphorylated MAPK under the conditions of internalization and recycling. Several reports have shown that many GPCRs, including opioid receptors, activate the MAPK pathway (Polakiewicz et al., 1998; Li and Chang, 1996; Daaka et al., 1998). We found that both the p44 and p42 forms of MAPKs were phosphorylated within 5 min of agonist treatment of wild-type receptor-expressing cells (Fig. 6). After 30 min of agonist treatment, the receptors were desensitized, as a second dose of agonist was not able to induce phosphorylation of these MAPKs. Under the conditions of recycling (incubation in the absence of the agonist following internalization), the receptors were resensitized, as they were able to respond to a second dose of the agonist, leading to phosphorylation of the MAPK. Treatment of cells with monensin (a known blocker of recycling) that caused substantial reduction in receptor recycling (see Fig. 3) also caused a substantial reduction in the extent of resensitization of the receptor (Fig. 6). When the T353A mutant receptor was examined for the kinetics of MAPK phosphorylation under conditions of recycling, we found that both the rate and the extent of phosphorylation were significantly enhanced in these receptors compared with the wild type receptors (Fig. 6). These results are consistent with the notion that receptor recycling plays a role in the resensitization of the opioid receptors. Taken together, these results suggest that the rapid internalization and recycling of the delta opioid receptor play an important role in the desensitization/resensitization of the receptor.
FIG. 6.
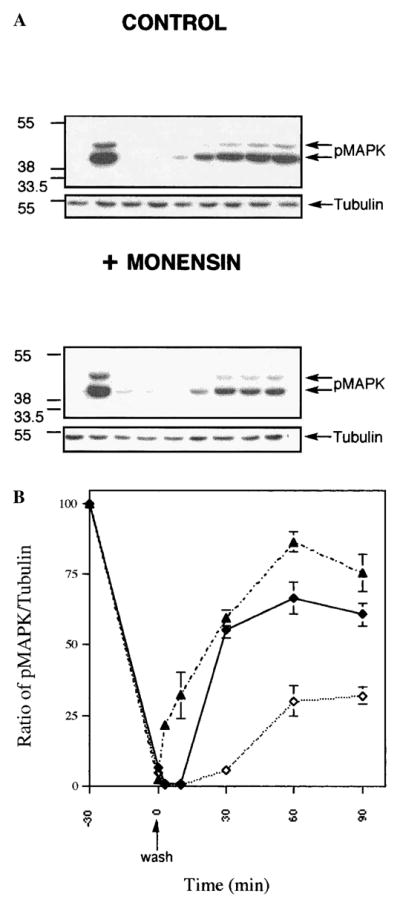
Phosphorylation of MAPK under conditions of internalization and recycling. (A) CHO cells expressing wild type or T353A mutant receptors were exposed to ligand for various periods of time (for internalization studies) or for 30 min. (B) The cells were washed and incubated without the agonist for various periods of time in the absence or presence of 100 μ M monensin (for recycling studies). The level of phosphorylated MAPK was determined by immunoblotting using phospho-MAPK (pMAPK) antibodies as described in the text. The blots were reprobed with tubulin antibody for standardization. A representative figure from three independent experiments is presented.
DISCUSSION
In this study, we characterized the internalization and recycling of the delta opioid receptors, as well as the receptor-selective ligand DPDPE, in fibroblast or neuronal cells expressing exogenous or endogenous receptors. We found that the kinetics of internalization and recycling of the receptor and ligand were similar in these cells and consistent with the kinetics reported by others (Afify et al., 1998; Law et al., 1984). These results, taken together with those of other studies that have used these cells to examine GPCR endocytosis, indicate that CHO cells contain the machinery for endocytosis of this and other GPCRs and thus are suitable for studies on GPCR trafficking.
Using an antibody-prebinding assay to examine receptor internalization, we found that opioid receptors undergo slow constitutive internalization via the classic clathrin-coated pit pathway. It is possible that the constitutive internalization and recycling of opioid receptors maintains a steady-state level of active receptors on the cell surface. Previous studies have shown that a number of cell-surface receptors undergo constitutive internalization via the classic clathrin-coated pit-mediated pathway (Watts, 1985; Moore et al., 1995; Brown and Greene, 1991; Tan et al., 1993). Constitutive internalization is thought to play an active role in the process of mating-type switching in the case of alpha-mating factor receptor (Tan et al., 1993) by constantly clearing the surface receptors so that after a mating-type switch, the older receptors can be replaced by newly synthesized ones. Constitutive internalization is also thought to lead to polarized distribution of other GPCRs (Jackson et al., 1991). Thus, it is possible that agonist-independent constitutive internalization is an additional mechanism that is involved in the modulation of opioid receptor function.
Internalized GPCRs colocalize with the transferrin receptor in an endosomal pathway characterized by the presence of rab5 (Moore et al., 1995; Koenig and Edwardson, 1997). The internalized receptor is processed in the endosomes, which is thought to be necessary for the resensitization of the receptor. This process could include dissociation of the ligand–receptor complex in the acidified pH of the endosomes, dephosphorylation of the receptor, dissociation of arrestins, and recycling of the receptor to the plasma membrane (Lefkowitz, 1998). It appears that structurally related receptors such as the D1 and D2 dopamine receptors can be selectively endocytosed to distinct endocytic compartments by dynamin-dependent and -independent mechanisms (Vickery and von Zastrow, 1999). After this initial segregation, both these receptors recycle back to the plasma membrane. Thus, it appears that the differential endocytic processes, rather than targeting the receptors to lysosomes, may in fact physically segregate structurally homologous receptors. The fate of the endocytosed peptide ligand is less clear because of the complications of peptide degradation by proteases. Significant recycling of a stable enkephalin analog or somatostatin analog in a neuroblastoma cell line has been demonstrated and shown to be the factor limiting the amount of radiolabeled agonist retained inside the cells (this study; Law et al., 1984; Koenig and Edwardson, 1997).
We found that the endocytosed receptor recycles back to the cell surface and that the receptors lacking the C-tail are able to recycle better than wild type receptors. The previously observed lack of agonist-mediated internalization of these receptors could be attributable to rapid agonist-independent internalization and recycling that would mask the agonist-induced internalization. The results from the ligand internalization and recycling studies support such a view. Segredo et al. (1997) have shown that a muopioid receptor C-tail deletion mutant exhibits rapid constitutive internalization and recycling. The fact that mutations in the C-tail affect both constitutive and agonist-mediated internalization suggests that multiple regulatory elements within the C-tail govern receptor trafficking; the removal of negative regulatory elements would allow increased constitutive endocytosis, and the receptor would rapidly be recycled back to the cell surface. In naive cells, the majority of the wild type receptors exist as dimers and monomers (Cvejic and Devi, 1997) that are transiently retained on the cell surface, presumably via interaction of the C-tail with intracellular proteins. In the absence of the agonist, there would be a slow dissociation of these factors, leading to a low level of receptor internalization. Agonist binding could lead to changes in the conformation of the receptor, resulting in the monomerization of the delta dimers (Cvejic and Devi, 1997). This change would be accompanied by an increased rate of dissociation of these proteins that could allow the phosphorylation of the receptor or binding of adapter proteins such as β-arrestin, thus leading to rapid endocytosis.
One of the signal transduction pathways that is activated by opioids appears to be the MAPK pathway. Although many studies have used the activation/inhibition of adenylate cyclases or phospholipase C as a measure of GPCR activity, a relatively small number of studies have used the activation of MAPK to examine receptor activity. Furthermore, opioid-mediated desensitization/resensitization of the MAPK pathway has not been well explored. We have found that activation of all three opioid receptor types (mu, delta, and kappa) by agonists results in the rapid phosphorylation of MAPKs (this study; Trapaidze et al., 2000b; Jordan et al., 2000). Furthermore, on receptor internalization, the level of MAPK phosphorylation is significantly reduced in the case of mu and delta receptors (Trapaidze et al., 2000b). These results suggest a role for receptor internalization in the desensitization of MAPK pathways. Recent findings with rat kappa receptors further support such a model: these receptors do not exhibit substantial agonist-mediated internalization, and they do not exhibit a significant reduction in the level of MAPK phosphorylation even on prolonged (120–240-min) exposure to agonists (Jordan et al., in press). Taken together, these results support a role for opioid receptor internalization/recycling in the desensitization/resensitization of receptor function.
In summary, we have demonstrated that the wild type delta opioid receptor undergoes agonist-independent internalization and recycling. The receptor C-tail is involved in this process, as mutations of the C-tail result in increased recycling. Receptor internalization/recycling are important for the recovery of receptor activity.
Acknowledgments
Flow cytometric analysis was performered in the Kaplan Comprehensive Cancer Center at NYU School of Medicine. This work is supported in part by grants DA08863 and NS1788 (to L.A.D.).
References
- AFIFY EA, LAW PY, RIEDL M, ELDE R, LOH HH. Role of carboxyterminus of mu and delta receptor in agonist-induced down-regulation. Brain Res Mol Brain Res. 1998;54:24–34. doi: 10.1016/s0169-328x(97)00315-x. [DOI] [PubMed] [Google Scholar]
- ARDEN JR, SEGREDO V, WANG Z, LAMEH J, SADEE W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged muopioid receptor expressed in HEK-293 cells. J Neurochem. 1995;65:1636– 1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- BROWN VI, GREENE MI. Molecular and cellular mechanisms of receptor-mediated endocytosis. DNA Cell Biol. 1991;10:399–409. doi: 10.1089/dna.1991.10.399. [DOI] [PubMed] [Google Scholar]
- CHU P, MURRAY S, LISSIN D, VON ZASTROW M. Delta and kappa opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J Biol Chem. 1997;272:27124– 27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- CVEJIC S, DEVI LA. Dimerization of the delta opioid receptor: Implications for a role in receptor internalization. J Biol Chem. 1997;272:26959– 26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- CVEJIC S, TRAPAIDZE N, CYR C, DEVI LA. Thr353, located within the COOH-terminal tail of the delta opiate receptor, is involved in receptor down-regulation. J Biol Chem. 1996;271:4073– 4076. doi: 10.1074/jbc.271.8.4073. [DOI] [PubMed] [Google Scholar]
- DAAKA Y, LUTTRELL LM, AHN S, DELLA ROCCA GJ, FERGUSON SS, CARON MG, LEFKOWITZ RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- FERGUSON SS, DOWNEY WE, COLAPIETRO AM, BARAK LS, MEN ARD L, CARON MG. Role of beta-arrestin in mediating agonist promoted G-protein coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- EVANS CJ, KEITH DE, MORRISON JR, MAGENDZO HK, EDWARDS RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- GOODMAN OB, KRUPNICK JG, SANTINI F, GUREVICH VV, PENN RB, GAGNON AW, KEEN JH, BENOVIC JL. β-arrestin acts as a clathrin adaptor in endocytosis of the b2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- GUREVICH VV, DION SB, ONORATO JJ, PTASIENSKI J, KIM CM, STERNE-MARR R, HOSEY MM, BENOVIC JL. Arrestin interactions with G protein coupled receptors: Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- HERZ A. Opioids. Vol. 1. Springer-Verlag; Berlin: 1993. [Google Scholar]
- HEUSER JE, ANDERSON RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON CL, KONOPKA JB, HARTWELL LH. S. cerevisiae alpha pheromone receptors activate a novel signal transduction pathway for mating partner discrimination. Cell. 1991;67:389–402. doi: 10.1016/0092-8674(91)90190-a. [DOI] [PubMed] [Google Scholar]
- JORDAN BA, DEVI LA. Molecular mechanisms of opiate receptor signal transduction. Br J Anaesth. 1998;81:12–19. doi: 10.1093/bja/81.1.12. [DOI] [PubMed] [Google Scholar]
- JORDAN BA, CVEJIC S, DEVI LA. Kappa opioid receptor endocytosis by dynorphin peptides. DNA Cell Biol. 2000;19:19–27. doi: 10.1089/104454900314672. [DOI] [PubMed] [Google Scholar]
- KEITH DE, MURRAY SR, ZAKI PA, CHU PC, LISSIN DV, KANG L, EVANS CJ, VON ZASTROW M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021– 19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- KEITH DE, ANTON B, MURRAY SR, ZAKI PA, CHU PC, LISSIN DV, MONTEILLET-AGIUS G, STEWART PL, EVANS CJ, VON ZASTROW M. Muopioid receptor internalization: Opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- KOCH T, SCHULZ S, SCHRODER H, WOLF R, RAULF E, HOLT V. Carboxy-terminal splicing of the rat muopioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem. 1998;273:13652– 13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- KOENIG JA, EDWARDSON JM. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- LAW PY, HOM DS, LOH HH. Down-regulation of opiate receptor in neuroblastoma × glioma NG108-15 hybrid cells. J Biol Chem. 1984;259:4096– 4104. [PubMed] [Google Scholar]
- LEFKOWITZ RJ. G protein-coupled receptors. J Biol Chem. 1998;273:18677– 18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- LI LY, CHANG KJ. The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express muopioid receptors. Mol Pharmacol. 1996;50:599–602. [PubMed] [Google Scholar]
- MOORE RH, SANDOVNIKOFF N, HOFFENBERG S, LIU S, WOODFORD P, ANGELIDES K, TRIAL J, CARSURD V, DICKEY BF, KNOLL BJ. Ligand-stimulated β2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J Cell Sci. 1995;108:2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- POLAKIEWICZ RD, SCHIEFERL MS, DORNER LF, KAN-SRA V, COMB MJ. A mitogen-activated protein kinase pathway is required for mopioid receptor desensitization. J Biol Chem. 1998;273:12402– 12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- SCHULZ R, WEHMEYER A, MURPHY J, SCHULZ K. Phosducin, beta-arrestin and opioid receptor migration. Eur J Pharmacol. 1999;375:349–357. doi: 10.1016/s0014-2999(99)00223-x. [DOI] [PubMed] [Google Scholar]
- SEGREDO V, BURFORD NT, LAMEH J, SADEE W. A constitutively internalizing and recycling mutant of the mu opioid receptor. J Neurochem. 1997;68:2395–2404. doi: 10.1046/j.1471-4159.1997.68062395.x. [DOI] [PubMed] [Google Scholar]
- SMART D, LAMBERT DG. The stimulatory effects of opioids and their possible role in the development of tolerance. Trends Pharmacol Sci. 1996;17:264–269. doi: 10.1016/0165-6147(96)10023-7. [DOI] [PubMed] [Google Scholar]
- SROKIN A, KORNILOVA E, TESLENKO L, SOROKIN A, NIKOLSKY N. Recycling of epidermal growth factor-receptor complexes in A432 cells. Biochem Biophys Acta. 1989;1101:88–96. doi: 10.1016/0167-4889(89)90083-9. [DOI] [PubMed] [Google Scholar]
- STERNINI C, SPANN M, ANTON B, KEITH DE, BUNNETT NW, VON ZASTROW M, EVANS CJ, BRECHA NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci USA. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN PK, DAVIS NG, SPRAGUE GF, PAYNE GS. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707– 1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAPAIDZE N, CVEJIC S, NIVARTHI NR, ABOOD M, DEVI LA. The role of C-tail residues in delta opioid receptor down-regulation. DNA Cell Biol. 2000a;19:93–101. doi: 10.1089/104454900314609. [DOI] [PubMed] [Google Scholar]
- TRAPAIDZE N, GOMES I, CVEJIC, DEVI LA. Opioid receptor internalization is not required for the activation of MAP kinase pathway. Mol Brain Res. 2000b doi: 10.1016/s0169-328x(00)00002-4. (in press) [DOI] [PubMed] [Google Scholar]
- TRAPAIDZE N, KEITH DE, CVEJIC S, EVANS CJ, DEVI LA. Sequestration of the delta opioid receptor: Role of the C terminus in agonist-mediated internalization. J Biol Chem. 1996;271:29279– 29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUGA H, KAMEYAMA K, HAGA T, KUROSE H, NA-GAO T. Sequestration of muscarinic acetylcholine receptor m2 subtypes. J Biol Chem. 1994;269:32522– 32527. [PubMed] [Google Scholar]
- VICKERY RG, VON ZASTROW M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHISTLER JL, VON ZASTROW M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985;100:633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKI PA, BILSKY EJ, VANDERAH TW, LAI J, EVANS CJ, PORECCA F. Opioid receptor types and subtypes: The delta receptor as a model. Annu Rev Pharmacol Toxicol. 1996;36:379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- ZHANG J, FERGUSON SSG, BARAK LS, MENARD L, CARON MG. Dynamin and beta-arrestin reveal distinct mechanisms for G-protein coupled receptor internalization. J Biol Chem. 1996;271:18302– 18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- ZHANG J, FERGUSON SSG, BARAK LS, BODDULURI SR, LAPORTE SA, LAW PY, CARON MG. Role of G protein-coupled receptor kinase in agonist-specific regulation of muopioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157– 7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]