Abstract
Previous results from our lab suggest that hypofunctioning of the serotonergic (5-HT) dorsal raphe nucleus (DRN) is involved in stress-induced opiate reinstatement. To further investigate the effects of morphine dependence and withdrawal on the 5-HT DRN system, we measured gene expression at the level of mRNA in the DRN during a model of morphine dependence, withdrawal and post withdrawal stress exposure in rats. Morphine pellets were implanted for 72h and then either removed or animals were injected with naloxone to produce spontaneous or precipitated withdrawal, respectively. Animals exposed to these conditions exhibited withdrawal symptoms including weight loss, wet dog shakes and jumping behavior. Gene expression for brain-derived neurotrophic factor (BDNF), TrkB, corticotrophin releasing-factor (CRF)-R1, CRF-R2, GABAA-α1, μ-opioid receptor (MOR), 5-HT1A, tryptophan hydroxylase2 and the 5-HT transporter was then measured using quantitative real-time PCR at multiple time-points across the model of morphine exposure, withdrawal and post withdrawal stress. Expression levels of BDNF, TrkB and CRF-R1 mRNA were decreased during both morphine exposure and following seven days of withdrawal. CRF-R2 mRNA expression was elevated after seven days of withdrawal. 5-HT1A receptor mRNA expression was decreased following 3 hours of morphine exposure, while TPH2 mRNA expression was decreased after seven days of withdrawal with swim stress. There were no changes in the expression of GABAA-α1, MOR or 5-HT transporter mRNA. Collectively these results suggest that alterations in neurotrophin support, CRF-dependent stress signaling, 5-HT synthesis and release may underlie 5-HT DRN hypofunction that can potentially lead to stress-induced opiate relapse.
1.0 Introduction
The serotonin (5-hydroxytryptamine, 5-HT) system plays an important role in stress-related psychiatric disorders and substance abuse (Charney et al., 1990; Meltzer, 1990; Valentino et al., 2010; Kirby et al., 2011; Waselus et al., 2011). Previous studies from our laboratory and others have shown that the serotonergic dorsal raphe nucleus (DRN) expresses receptors for the stress neuropeptide corticotrophin-releasing factor (CRF) (Swanson et al., 1983; Sakanaka et al., 1987; Chalmers et al., 1995) and is densely innervated by CRF terminals (Kirby et al., 2000; Valentino et al., 2001; Waselus et al., 2003). Both CRF and forced swim stress (FS) have the capacity to inhibit 5-HT output to specific forebrain regions (Price et al., 1998; Kirby et al., 2000; Kirby et al., 2008; Waselus et al., 2009; Valentino et al., 2010). Furthermore, CRF release within the DRN mediates the inhibitory effects of FS on 5-HT release (Price et al., 2002). Electrophysiology studies have demonstrated that intra-DRN CRF inhibits 5-HT neuronal activity (Price et al., 1998; Kirby et al., 2000), potentially via stimulation of GABAergic synaptic activity at 5-HT DRN neurons (Kirby et al., 2008).
In addition to an abundance of CRF receptors, the DRN expresses receptors for a number of other potential regulatory neuropeptides associated with drug abuse including opiates (Mansour et al., 1994; Neal, Jr. et al., 1999) and brain-derived neurotrophic factor (BDNF) (Merlio et al., 1992; Madhav et al., 2001). Opiate exposure has been shown to increase 5-HT output from the DRN through disinhibition of inhibitory GABA afferents caused by the activation of the μ-opioid receptor (MOR) (Jolas and Aghajanian, 1997; Tao and Auerbach, 2002a; Tao and Auerbach, 2002b). Interestingly, during opiate withdrawal, 5-HT levels decrease below baseline (Tao et al., 1998), an effect caused by MOR-mediated stimulation of GABA synaptic activity at 5-HT DRN neurons (Jolas et al., 2000).
There is also a high degree of comorbidity of affective disorders including major depression with opioid dependence (Woody et al., 1975; Rounsaville et al., 1982; Brooner et al., 1997; Pani et al., 1997; Mason et al., 1998). Additionally, stress has been shown to be a potential trigger of opioid relapse in both the clinic and in animal models of drug abuse (de Wit H. and Stewart, 1981; de Wit H. and Stewart, 1983; Self and Nestler, 1998; De Vries and Shippenberg, 2002; Shaham et al., 2003; Brown and Lawrence, 2009). Thus the 5-HT system may contribute to negative affective states which underlie vulnerability to both mood disorders as well as drug abuse, potentially via overlapping neural mechanisms.
Recent work from our laboratory shows that CRF increases GABA synaptic activity in 5-HT cells within the DRN (Kirby et al., 2008). FS has been shown to inhibit 5-HT DRN activity by increasing CRF within the DRN (Price and Lucki, 2001). Furthermore FS can trigger reinstatement of previously extinguished morphine conditioned place-preference (Staub et al., 2012). Additionally 5-HT DRN neurons from animals exposed to both morphine and forced swim showed an increase in GABAergic synaptic activity (Staub et al., 2012). A more recent study from our lab shows that intra-DRN injections of GABA agonists can produce reinstatement of CPP without swim stress, while GABA antagonists can block swim stress-induced reinstatement of CPP (Li et al., 2013). These results underscore the importance of FS, CRF, and GABA in behavioral models of opiate relapse.
In the current study we hypothesize that the dynamic regulation of the 5-HT system during opiate exposure, withdrawal and stress-induced relapse is mediated by adaptations in neuropeptides and neurotransmitters within the DRN and that opiate history induces long term changes to the plasticity of the 5-HT DRN system. To test this hypothesis we measured the expression of genes that potentially regulate 5-HT DRN transmission at multiple time-points in a model for opiate dependence, withdrawal and post withdrawal stress exposure. These time points included baseline, acute and chronic opiate exposure, precipitated withdrawal, spontaneous withdrawal, seven days post withdrawal and seven days post withdrawal with forced swim. While post withdrawal swim stress is not a model of stress-induced relapse, inclusion of this group in our model allows us to test the hypothesis that stress and opiate history interact, producing neuroadaptations within the DRN that could contribute to stress induced relapse.
Nine genes were chosen to study under the conditions of our model: BDNF, TrkB, CRF-R1, CRF-R2, GABAA-α1, MOR, 5-HT1A, tryptophan hydroxylase2 (TPH2) and 5-HT transporter (SERT). BDNF (Boyarskikh et al., 2013) and its receptor TrkB (Merlio et al., 1992; Madhav et al., 2001) are known to be expressed within the DRN. Additionally intracerebroventricular BDNF administration has been shown to increase 5-HT activity (Siuciak et al., 1996). The receptors CRF-R1, CRF-R2, GABAA, and MOR are known to affect 5-HT DRN activity under our experimental conditions (Jolas and Aghajanian, 1997; Kirby et al., 2008). The α1 subunit of the GABAA receptor was specifically chosen because it is expressed at high levels in the DRN (Sieghart and Sperk, 2002) and is the most abundantly expressed subunit in 5-HT DRN neurons (Lemos et al., 2011). Finally, 5-HT1A, TPH2 and SERT are widely known to have more global effects on 5-HT regulation (Jacobs and Azmitia, 1992; Shishkina et al., 2007; Donner et al., 2012).
2.0 Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) between 8–9 weeks of age were housed 2 per cage under standard temperature (20 °C) and humidity (40%). Rats were kept under a 12 h light/dark cycle (lights on at 7:00 AM). Food and water were provided ad libitum. Animal protocols were approved by the Temple University Institutional Animal Care and Use Committee and were conducted in accordance to the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2 Surgery and post-operative care
Prior to the start of experiments, rats were handled for 5 min over 2 days after which one rat per cage was assigned to either the placebo or morphine group. Before surgery two 2 cellulose placebo pellets or 2 morphine pellets (75 mg each pellet) (The National Institute on Drug Abuse (NIDA), Rockville, MD) were encased in a 1 × 1-cm square nylon mesh bag for each animal undergoing surgery. Rats were anesthetized with isoflurane after which nylon mesh bags containing either the placebo or morphine pellets were implanted subcutaneously in the scruff of the neck. In some groups, pellets were surgically removed 72 after implantation under isoflurane anesthesia. Rats were weighed and monitored daily for the duration of the experiment.
2.3 Animal behavior and experimental groups
One baseline and six experimental groups were generated by euthanasia at different time-points during a model of opiate exposure, withdrawal and post-withdrawal stress exposure (Figure 1) followed by tissue collection for PCR analysis. In addition to the baseline (untreated) group, the experimental group was implanted with placebo or morphine pellets and then sacrificed 3 hr post-pellet implantation (group 1, acute morphine), 72 hr post-pellet implantation (group 2, chronic morphine), 72 hr post-pellet implantation with naloxone injection (1mg/kg, subcutaneous injection) (group 3, precipitated withdrawal), 18 hr post-pellet removal (group 4, spontaneous withdrawal), 7 days following pellet removal (group 5, abstinence) or 7 days following pellet removal with subsequent exposure to swim stress (animals placed in a swim tank that is 20 cm in diameter and filled with 21–22°C water to depth of 30 cm for 6-min) (group 6, abstinence + stress) to examine the effects of opiate history x stress interactions.
Figure 1. Experimental groups.
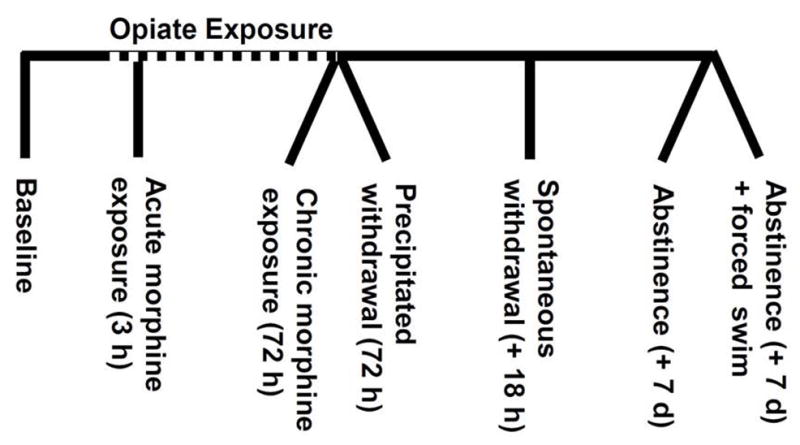
Gene expression at the mRNA level was assessed at the following seven time points: baseline, 3 hours of morphine exposure, 72 hours of morphine exposure, precipitated withdrawal (naloxone injection following 72 hours of morphine exposure), spontaneous withdrawal (18 hours following surgical removal of morphine pellets), abstinence (seven days following surgical removal of morphine pellets), and abstinence with forced swim (6 minutes of swim stress following seven days of abstinence). Placebo pellet controls were included for each experimental group.
Animals undergoing withdrawal were recorded on video camera in a behavioral chamber and assessed for quantified behavioral signs of withdrawal including wet dog shakes, jumping, teeth chattering and writhing as well as the presence or absence of chromodacryorrhea, eye-twitching, ptosis, rhinorrhea, salivation and diarrhea over a 15 min period, then euthanized 30 minutes later (Cerletti et al., 1975).
2.4 RNA processing
Rats were sacrificed by decapitation and their brains were rapidly frozen on dry ice and stored at −80 °C for RNA extraction and real time PCR processing. Rat brains were sliced (200 μm) in a cryostat at −20 °C and mounted on slides while RNA Later Ice (Invitrogen, Grand Island, NY) was added to maintain RNA stability. Following at least 24 hours of incubation with RNA Later Ice, 15 punches were collected from 5 sections spanning the DR using a 0.5 mm circular punch (Ted Pella, Inc., Redding, CA) (see Figure 2), pooled together and placed in TRIzol (Invitrogen) utilizing the small tissue extraction method supplied by Invitrogen. Briefly, tissues were added to 800 μl of TRIzol, and homogenized using an electric tissue homogenizer. Samples were spun down to remove cellular debris and genomic DNA was sheared by passing TRIzol solution through a 26 gauge needle before adding chloroform. RNA was then precipitated by addition of isopropanol and glycogen (GlycoBlue, Invitrogen). Samples were further cleaned through ethanol precipitation. Following RNA purification integrity of the samples was measured by spectrophotometry. All samples had A260/280 ratios between 1.8–2.0. Samples were run on a formaldehyde gel to confirm RNA integrity.
Figure 2. DRN tissue collection.
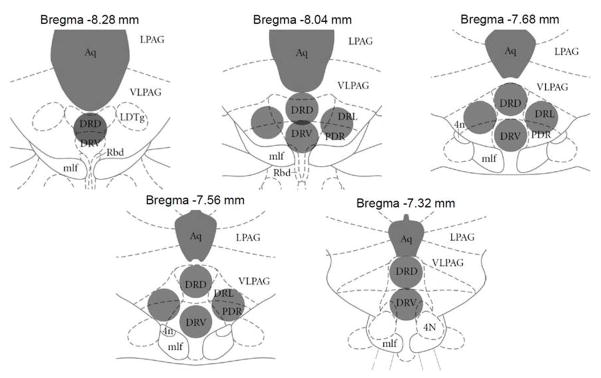
Brains were sliced into 200 μm sections on a cryostat and incubated for 24 hours in RNA Later ICE solution to preserve RNA integrity. Fifteen DRN tissue punches (500 μm diameter) from five brain slices (7.32–8.26 mm posterior to Bregma) were collected as indicated by grey circles (Paxinos and Watson, 2007). Tissue punches were pooled together placed immediately into TRIzol solution for RNA extraction. Aq = aqueduct; LPAG = lateral periaqueductal gray; VLPAG = ventrolateral periaqueductal gray; LDTg = lateral dorsal tegmental nucleus; DRD = dorsal raphe nucleus, dorsal part; DRV = dorsal raphe nucleus, ventral part; DRL = dorsal raphe nucleus, lateral part; PDR = posterodorsal dorsal raphe nucleus; Rbd = rhabdoid nucleus; mlf = medial longitudinal fasciculus; 4N = trochlear nucleus.
Following resuspension, 1 μg of total RNA went through a DNase I digestion protocol (Invitrogen) and was reverse transcribed using Affinity Script QPCR cDNA Synthesis Kit (Agilent Technologies, Santa Clara, CA). The cDNA samples were then diluted to a concentration of 2 ng/μl and stored for real time PCR.
2.5 Housekeeping gene selection
Three potential housekeeping genes: β actin (actb, Rn00667869_m1), GAPDH (Gapdh, Rn01775763_g1), HPRT (Hprt1, Rn01527840_m1) were tested at sample timepoints including 3 hours, 72 hours, precipitated withdrawal, and 7 days abstinence to assess consistency under the experimental conditions. Both Data Assist Software (Applied Biosystems, Foster City, CA) and REST Software (Qiagen, Germantown, MD) indicated that β actin showed the least differences between the treatment groups.
2.6 Real time quantitative PCR
Real time PCR was performed using TaqMan gene expression assays with TaqMan universal PCR master mix (both Applied Biosystems). Assays utilized were: BDNF (Bdnf, Rn02531967_s1), TrkB (Ntrk2, Rn01441749_m1), CRF-R1 (Crhr1, Rn00578611_m1), CRF-R2 (Crhr2, Rn00575617_m1), 5-HT1A (Htr1a, Rn00561409_s1), TPH2 (Tph2, Rn00598017_m1), GABAA-α1 (Gabra1, Rn00788315_m1), MOR (Oprm1, Rn01430371_m1), SERT, (Slc6a4, Rn00564737_m1) and β actin (Actb, Rn00667869_m1). Each well consisted of 20 μl reaction mixture containing 10 μl master mix, 1 μl enzyme, 5 μl cDNA and 4 μl double autoclaved deionized water. Reactions were performed in triplicate using an ABI 7500 Real-Time PCR System (Applied Biosystems) under manufacturers recommended settings.
2.7 Data analysis
Gene expression analysis was performed using the comparative CT (cycle threshold) method as described by Schmittgen and Livak (Schmittgen and Livak, 2008). A one tailed Student’s T test was used for morphine to placebo comparisons as well as baseline to morphine comparisons, as all comparisons were a priori planned. Outliers were excluded based on the result of the Grubbs’ test for outliers (Grubbs, 1969; Stefansky, 1972).
3.0 Results
The hypothesis of the study is that the regulation of the 5-HT system across the opiate addiction cycle is mediated by adaptations in regulatory neurotransmitter and neuropeptide systems within the DRN. To test the hypothesis we quantified mRNA for genes that could potentially regulate 5-HT DRN neurotransmission within a model for opiate dependence, withdrawal, and stress exposure. All mRNA levels were normalized to a naïve baseline group or to placebo controls (Figs. 5–9; Table 1). The genes examined were BDNF, TrkB, CRF-R1, CRF-R2, 5-HT1A, TPH2, GABAA-α1, MOR, and SERT.
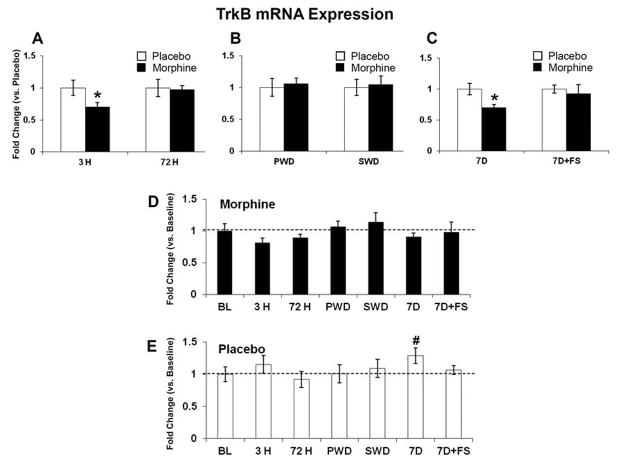
Figure 5. TrkB mRNA expression.
TrkB mRNA was quantified relative to placebo (AC), and baseline (D and E). TrkB mRNA expression was decreased after 3 hours of morphine exposure; no differences were seen at 72 hours of morphine exposure (A). No differences were seen in either withdrawal group (B). TrkB mRNA expression was also decreased following seven days of abstinence (C). There were no differences in the morphine groups compared to baseline (D), but there was a significant increase in placebo at 7 days (E). Data indicate mean ± SEM. * P < 0.05 vs. placebo; # P < 0.05 vs. baseline by Student’s t-test. Baseline data: BL (n=8); placebo: 3 H (n=6), 72 H (n=6), PWD (n=6), SWD (n=5), 7D (n=6), 7D+FS (n=6); morphine: 3 H (n=6), 72 H (n=7), PWD (n=7), SWD (n=5), 7D (n=5), 7D+FS (n=6).
Figure 9. TPH2 mRNA expression.
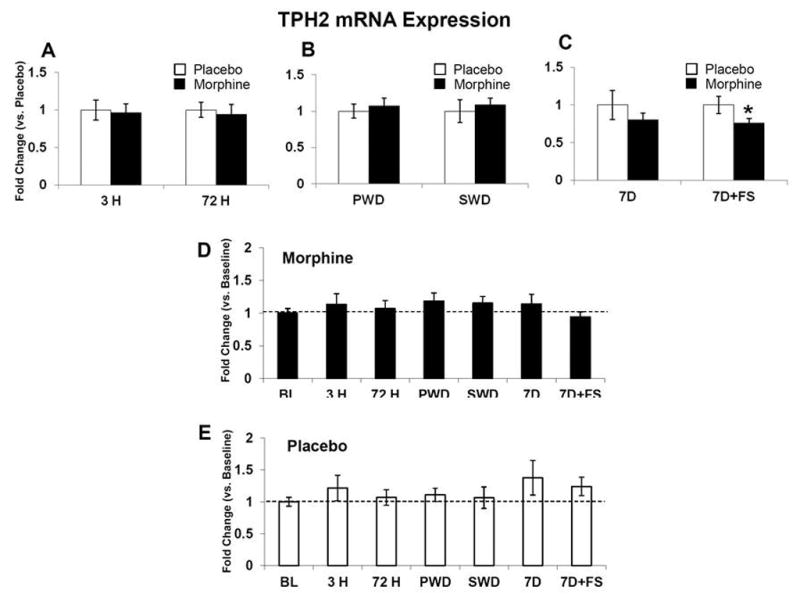
TPH2 mRNA was quantified relative to placebo (A–C), and baseline (D and E). TPH2 mRNA expression was not changed after 3 hours or 72 hours of morphine exposure (A). No differences were seen in either withdrawal group (B). TPH2 mRNA expression was decreased following seven days of abstinence with swim stress (C). There were no differences in the morphine or placebo groups compared to baseline (D and E). Data indicate mean ± SEM. * P < 0.05 vs. placebo by Student’s t-test. Baseline data: BL (n=8); placebo: 3 H (n=5–6), 72 H (n=5–6), PWD (n=6), SWD (n=5), 7D (n=6), 7D+FS (n=6); morphine: 3 H (n=5–6), 72 H (n=6–7), PWD (n=7), SWD (n=5), 7D (n=5–6), 7D+FS (n=6).
Table 1.
Summary of mRNA expression changes
| A. Direction of mRNA expression changes with respect to placebo
| |||
|---|---|---|---|
| Treatment Group | |||
| Morphine: 3 D | 5-HT1A ↓ | TrkB ↓ | CRF-R1 ↓ |
| Morphine: PWD | BDNF ↑ | ||
| Morphine: 7D Abstinence | BDNF ↓ | TrkB ↓ | CRF-R1 ↓ |
| Morphine: 7D + Swim | TPH2 ↓ | ||
| B. Direction of mRNA expression changes with respect to baseline
| |
|---|---|
| Treatment Group | |
| Morphine: 3 H | 5-HT1A ↓ |
| Morphine: 72 H | BDNF ↓ |
| Morphine: 7D Abstinence | CRF-R2 ↑ |
Data represent the direction of mRNA changes compared to placebo (panel A) or baseline (panel B). Treatment groups include 3 hours of morphine exposure, 72 hours of morphine exposure, morphine precipitated withdrawal, seven days abstinence from morphine and seven days abstinence from morphine with forced swim.
3.1 Withdrawal syndrome
For precipitated withdrawal, following 72 hours of placebo or morphine exposure the animals were weighed, given a single injection of naloxone (1 mg/kg), and observed for 15 minutes for withdrawal symptoms. All other animals had the placebo or morphine pellets surgically removed and 18 hours later were observed for withdrawal symptoms for 15 minutes. Animal experiencing both precipitated and spontaneous withdrawal experienced significant weight loss (precipitated: p < 0.01; spontaneous: p < 0.01) (Figure 3A). Both groups of animals were monitored for withdrawal behavior as well. In the precipitated and spontaneous withdrawal group, morphine-treated subjects showed a significant elevation of wet dog shakes compared to placebo controls (precipitated: p < 0.01; spontaneous: p < 0.01) (Figure 3B). In contrast, jumping behavior was seen in morphine treated animals undergoing precipitated but not spontaneous withdrawal (p < 0.05 vs. placebo controls) (Figure 3C). Additionally, we observed the presence of diarrhea in both withdrawal groups as well as writhing within the precipitated withdrawal group. These data indicate somatic and behavioral signs of withdrawal in both withdrawal groups, though in precipitated withdrawal the behavioral signs are most prominent whereas in spontaneous withdrawal, somatic elements (i.e. weight loss) predominate.
Figure 3. Withdrawal syndrome.
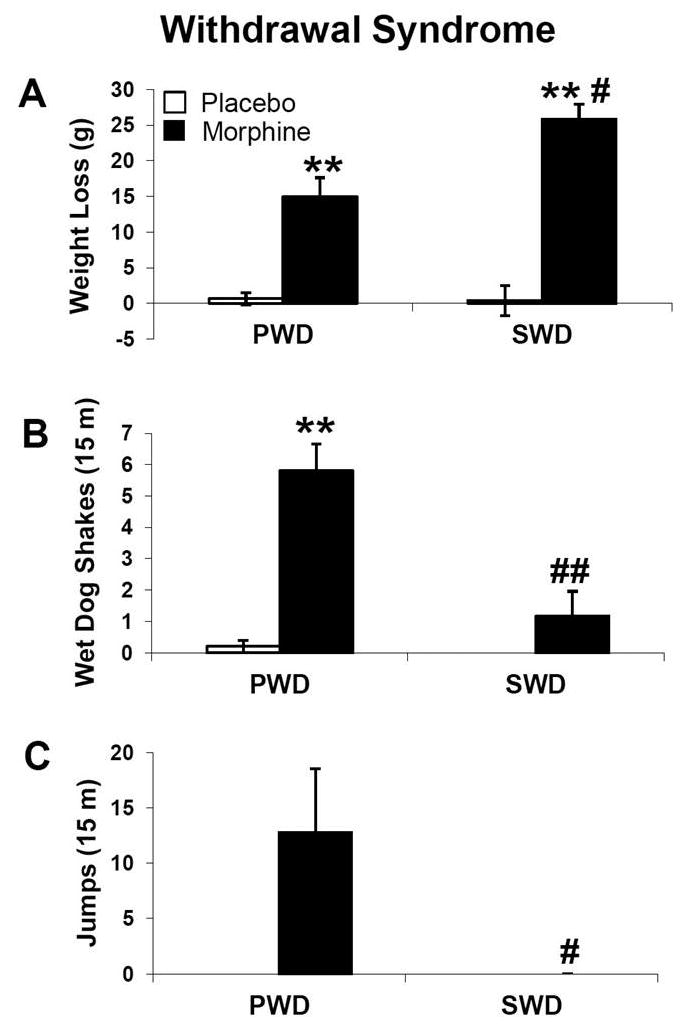
Physiological and behavioral withdrawal symptoms were measured in animals undergoing both precipitated withdrawal (PWD) and spontaneous withdrawal (SWD). For PWD, weights were recorded before and 30 minutes after subcutaneous naloxone injection and behavior was scored over 15 min following naloxone injection (n=4). For SWD animals, weights were recorded immediately and 18 hours after surgical pellet removal (n=23–24) and behavior was scored over 15 min at the 18-hour time-point (n=5). Data indicate mean ± SEM. ** P < 0.01 vs. placebo; # P < 0.05 vs. PWD, ## P < 0.01 vs. PWD by Student’s t-test.
3.2 mRNA expression
Utilizing all baseline groups, the mRNA of each gene was compared to CRF-R1, the lowest expressed gene. Generally we found that CRF-R1, CRF-R2, MOR and BDNF mRNA had low levels of expression. GABAA-α1, 5-HT1A and TrkB were expressed at middle levels and SERT and TPH2 mRNA were expressed most abundantly.
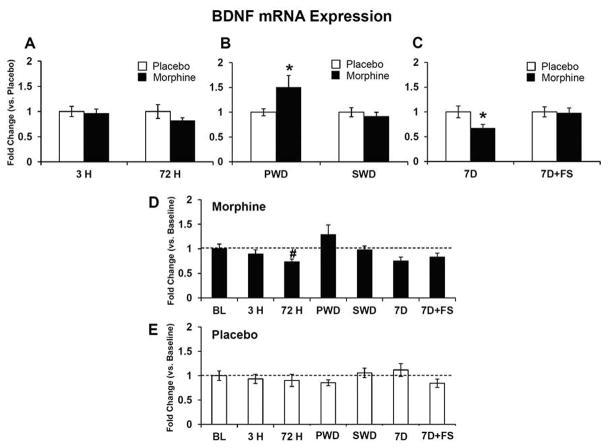
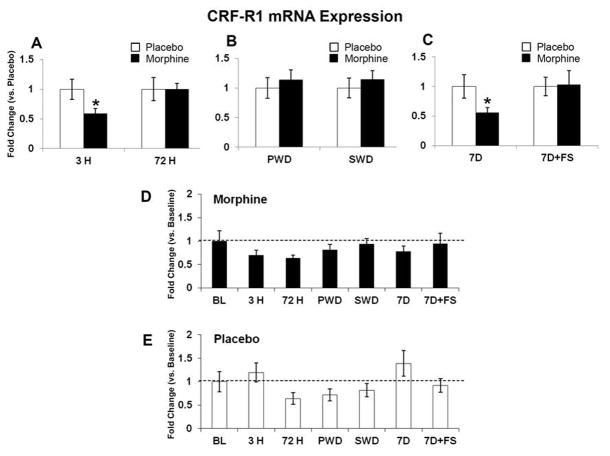
mRNA levels were measured at baseline, 3 hours and 72 hours after pellet implantation, following precipitated and spontaneous withdrawal, and 7 days following pellet removal with and without forced swim. Expression of BDNF mRNA was significantly decreased after 72 hours of morphine exposure compared to baseline (Figure 4D, p<0.05). In contrast precipitated withdrawal increased BDNF mRNA compared to placebo (Figure 4B, p<0.05) and then by 7 days following pellet removal, BDNF mRNA was downregulated in the morphine group compared to placebo (Figure 4C, p< 0.05). The BDNF receptor TrkB was significantly decreased compared to placebo after 3 hours of morphine exposure (Figure 5A, p<0.05) and again 7 days following morphine pellet removal compared to placebo (Figure 5C, p<0.05). TrkB mRNA was also elevated in the placebo group at 7 days post-pellet removal compared to baseline (Figure 5E, p< 0.05). CRF-R1 mRNA was decreased after 3 hours of morphine exposure (Figure 6A, p<0.05) and again 7 days following pellet removal compared to placebo (Figure 6C, p<0.05). Seven days after morphine pellet removal, CRF-R2 mRNA was significantly increased compared to baseline (Figure 7D, p<0.01). The 5-HT1A receptor mRNA was significantly decreased after 3 hours of morphine exposure compared to placebo (Figure 8A, p<0.01) and baseline (Figure 8D, p<0.01). 5-HT1A receptor mRNA was also elevated in the placebo group exposed to forced swim stress at 7 days post-pellet removal compared to baseline (Figure 8E, p<0.05). The TPH2 mRNA was significantly decreased compared to placebo 7 days following morphine pellet removal in the forced swim group (Figure 9C, p<0.05). These changes in mRNA expression are summarized in Table 1. All other mRNA expression data (GABAA-1, MOR, and SERT) that were unaffected by the experimental conditions can be found in Table 2.
Figure 4. BDNF mRNA expression.
BDNF mRNA was quantified relative to placebo (A–C), and baseline (D and E). No differences were seen at 3 hours and 72 hours of morphine exposure (A). Precipitated withdrawal elevated BDNF mRNA expression (B), while seven days of abstinence reduced BDNF mRNA expression (C). BDNF mRNA expression was also decreased following 72 hours of morphine exposure compared to baseline (D). There were no differences in the placebo groups compared to baseline (E). Data indicate mean ± SEM. * P < 0.05 vs. placebo; # P < 0.05 vs. baseline by Student’s t-test. Baseline data: BL (n=8); placebo: 3 H (n=6), 72 H (n=6), PWD (n=5–6), SWD (n=5–6), 7D (n=6), 7D+FS (n=6); 3 H (n=6); morphine: 3 H (n=6), 72 H (n=7), PWD (n=7), SWD (n=5), 7D (n=5), 7D+FS (n=6).
Figure 6. CRF-R1 mRNA expression.
CRF-R1 mRNA was quantified relative to placebo (A–C), and baseline (D and E). CRF-R1 mRNA expression was decreased after 3 hours of morphine exposure; no differences were seen at 72 hours of morphine exposure (A). No differences were seen in either withdrawal group (B). CRF-R1 mRNA expression was also decreased following seven days of abstinence (C). There were no differences in the morphine or placebo groups compared to baseline (D and E). Data indicate mean ± SEM. * P < 0.05 vs. placebo by Student’s t-test. Baseline data: BL (n=8); placebo: 3 H (n=5), 72 H (n=5), PWD (n=6), SWD (n=5), 7D (n=6), 7D+FS (n=6); morphine: 3 H (n=5), 72 H (n=6), PWD (n=7), SWD (n=5), 7D (n=5), 7D+FS (n=6).
Figure 7. CRF-R2 mRNA expression.
CRF-R2 mRNA was quantified relative to placebo (A–C), and baseline (D and E). No differences were seen in any group with respect to placebo (A–C). CRF-R2 mRNA expression was significantly higher than baseline following seven days of abstinence (D). There were no differences in the placebo groups compared to baseline (E). Data indicate mean ± SEM. ## P < 0.01 vs. baseline by Student’s t-test. Baseline data: BL (n=8); placebo: 3 H (n=6), 72 H (n=6), PWD (n=6), SWD (n=5), 7D (n=6), 7D+FS (n=6); morphine: 3 H (n=6), 72 H (n=6–7), PWD (n=7), SWD (n=5), 7D (n=6), 7D+FS (n=5–6).
Figure 8. 5-HT1A mRNA expression.
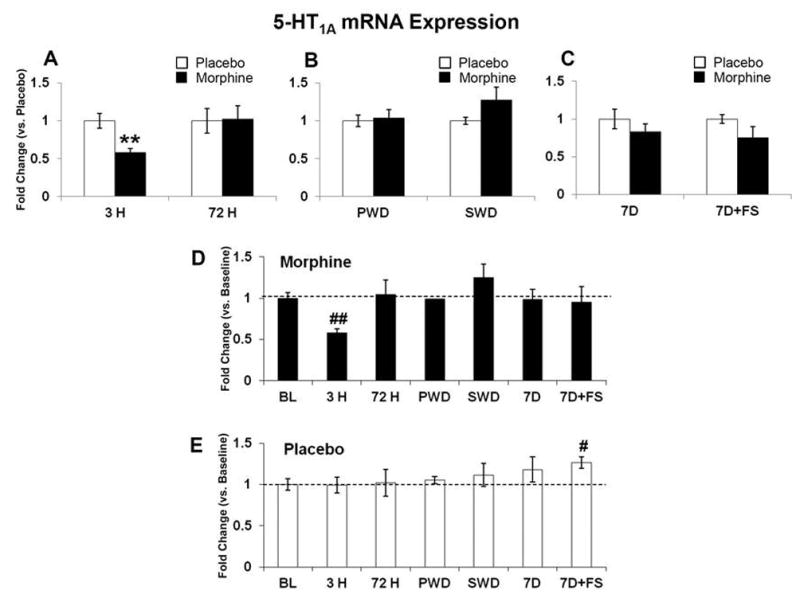
5-HT1A mRNA was quantified relative to placebo (A–C), and baseline (D and E). 5-HT1A mRNA expression was decreased after 3 hours of morphine exposure; no differences were seen at 72 hours of morphine exposure (A). No differences were seen in either withdrawal group or in abstinence with or without stress (B and C). 5-HT1A mRNA expression was also decreased following 3 hours of morphine exposure compared to baseline (D) and there was a significant increase in placebo at 7 days + FS compared to baseline (E). Data indicate mean ± SEM. ** P < 0.01 vs. placebo; # P < 0.05 and ## P < 0.01 vs. baseline by Student’s t-test. BL (n=8); placebo: 3 H (n=6), 72 H (n=6), PWD (n=6), SWD (n=4), 7D (n=6), 7D+FS (n=6); morphine: 3 H (n=5), 72 H (n=7), PWD (n=7), SWD (n=5), 7D (n=6), 7D+FS (n=6).
Table 2.
GABAA-α1, MOR and SERT mRNA Expression
| Gene and treatment group | Fold change (vs. Placebo) | Fold change (vs. Baseline) | ||
|---|---|---|---|---|
| GABA-α1 | Placebo | Morphine | Placebo | Morphine |
| BL (N = 7) | 1.00 ± 0.13 | 1.00 ± 0.13 | ||
| 3 H (N = 6) | 1.00 ± 0.09 | 0.91 ± 0.07 | 0.95 ± 0.09 | 0.86 ± 0.06 |
| 72 H (N = 6–7) | 1.00 ± 0.13 | 0.97 ± 0.09 | 1.02 ± 0.13 | 0.99 ± 0.10 |
| PWD (N = 6–7) | 1.00 ± 0.13 | 1.31 ± 0.12 | 0.98 ± 0.13 | 1.28 ± 0.12 |
| SWD (N = 5) | 1.00 ± 0.11 | 1.08 ± 0.09 | 1.22 ± 0.13 | 1.31 ± 0.11 |
| 7D (N = 6) | 1.00 ± 0.12 | 0.98 ± 0.21 | 1.24 ± 0.15 | 1.21 ± 0.26 |
| 7D + FS (N = 6) | 1.00 ± 0.12 | 1.05 ± 0.16 | 1.12 ± 0.13 | 1.18 ± 0.18 |
| MOR | Placebo | Morphine | Placebo | Morphine |
| BL (N = 7) | 1.00 ± 0.27 | 1.00 ± 0.27 | ||
| 3 H (N = 6) | 1.00 ± 0.34 | 0.64 ± 0.18 | 1.11 ± 0.38 | 0.72 ± 0.20 |
| 72 H (N = 6–7) | 1.00 ± 0.18 | 1.14 ± 0.17 | 0.92 ± 0.16 | 1.05 ± 0.15 |
| PWD (N = 6–7) | 1.00 ± 0.14 | 1.25 ± 0.17 | 0.90 ± 0.13 | 1.13 ± 0.10 |
| SWD (N = 5) | 1.00 ± 0.16 | 0.95 ± 0.15 | 1.20 ± 0.19 | 1.14 ± 0.18 |
| 7D (N = 6) | 1.00 ± 0.30 | 0.81 ± 0.29 | 1.33 ± 0.39 | 1.08 ± 0.38 |
| 7D + FS (N = 6) | 1.00 ± 0.11 | 1.13 ± 0.23 | 0.99 ± 0.10 | 1.12 ± 0.23 |
| SERT | Placebo | Morphine | Placebo | Morphine |
| BL (N = 8) | 1.00 ± 0.13 | 1.00 ± 0.13 | ||
| 3 H (N = 6) | 1.00 ± 0.20 | 0.92 ± 0.17 | 1.13 ± 0.22 | 1.03 ± 0.19 |
| 72 H (N = 6–7) | 1.00 ± 0.14 | 1.08 ± 0.20 | 0.87 ± 0.12 | 0.95 ± 0.18 |
| PWD (N = 6–7) | 1.00 ± 0.12 | 1.14 ± 0.12 | 1.05 ± 0.12 | 1.20 ± 0.12 |
| SWD (N = 5) | 1.00 ± 0.25 | 1.37 ± 0.22 | 0.91 ± 0.23 | 1.25 ± 0.20 |
| 7D (N = 5–6) | 1.00 ± 0.20 | 0.83 ± 0.16 | 1.52 ± 0.31 | 1.26 ± 0.24 |
| 7D + FS (N = 6) | 1.00 ± 0.09 | 0.91 ± 0.18 | 1.07 ± 0.09 | 0.97 ± 0.19 |
Data represent mean ± SEM. BL = baseline, FS = forced swim, MOR = μ-opioid receptor, PWD = precipitated withdrawal, SERT = 5-HT transporter, SWD = spontaneous withdrawal.
4.0 Discussion
The main results of this study show that morphine dependence, withdrawal, and post withdrawal stress bring about differential regulation of mRNA expression for BDNF, TrkB, CRF receptors, and other genes regulating 5-HT in the rat DRN.
4.1 Baseline DRN mRNA expression
We ranked mRNA levels in three distinct groups based on relative expression. Our results are consistent with published literature in many cases. CRF-R2 mRNA has previously been shown to be expressed at higher levels than CRF-R1 mRNA within the DRN (Van Pett et al., 2000; Day et al., 2004). BDNF mRNA has only recently been shown to be expressed in the DRN by quantitative PCR (Boyarskikh et al., 2013) as early in situ hybridization studies failed to measure expression in the raphe (Hofer et al., 1990; Wetmore et al., 1990; Gall et al., 1992), indicating that the high sensitivity of quantitative PCR is necessary to detect BDNF mRNA in the DRN. Genes for the 5-HT synthetic enzyme TPH2, the 5-HT transporter and the 5-HT1A autoreceptor were, as expected, abundant in the serotonergic DRN.
4.2 Opiate regulation of BDNF and TrkB mRNA expression
The neurotrophin BDNF and its receptor TrkB have been implicated in addiction-related neural plasticity in several monoaminergic nuclei (Koo et al., 2012; Mashayekhi et al., 2012) and reward-related brain regions (Berglind et al., 2007; Graham et al., 2007). More generally, BDNF activation of TrkB has been proposed to play a role in blocking the default pro-apoptotic pathway that is theoretically present in all neurons (Ichim et al., 2012). Additionally BDNF signaling is involved in neural differentiation and maturation (Waterhouse et al., 2012), regulating dendrite structure, modulation of synapse strength and circuit formation (English et al., 2012). Decreases in BDNF and TrkB activity have been associated with neuronal atrophy in both the hippocampus and frontal cortex as well as depression (for a review see (Gray et al., 2013) and (Duman, 2009) respectively). Our data show that following 3 hours of morphine exposure TrkB mRNA was decreased in the DRN relative to placebo, while a decrease in BDNF mRNA relative to baseline was seen after 72 hours. Interestingly a transient upregulation of DRN BDNF mRNA following precipitated opiate withdrawal was seen as well. While withdrawal-induced upregulation of BDNF and TrkB have been observed in other brain regions (Numan et al., 1998; Grimm et al., 2003; McGinty et al., 2010; Lu et al., 2010), the effect that we see in the DRN is less persistent. More experiments are needed to determine the functional implications of such a transient BDNF mRNA response.
Both chronic and repeated morphine exposure has been shown to reduce the number and complexity of dendritic spines in several reward-related brain regions including nucleus accumbens medium spiny neurons, medial prefrontal cortex and hippocampus pyramidal neurons (for a review see (Russo et al., 2009)). Opioid exposure has also been shown to impact morphology of neurons within the mesocorticolimbic dopamine system. Ventral tegmental area (VTA) dopamine neurons in rats treated with chronic morphine were shown to have a decreased area (≈20–25%) as well as reduced process length (≈30%), results that were prevented by BDNF pretreatment followed by administration of a mixture of BDNF and morphine (Sklair-Tavron et al., 1996). Both chronic morphine and heroin self-administration in rats led to significant decreases in the surface area of VTA dopamine neurons (Russo et al., 2007). These morphological effects in VTA dopamine neurons persist when opioids are removed, lasting beyond the withdrawal phase. Fourteen days following opiate withdrawal, VTA neurons are reduced in size (Chu et al., 2007; Russo et al., 2007), an effect that is accompanied by a significant reduction in BDNF positive cells and VTA BDNF content (Chu et al., 2007). Collectively these other studies show that opiate exposure leads to long term changes in plasticity of limbic brain regions that are in part BDNF dependent. In a similar fashion, our data indicate that both BDNF mRNA and TrkB mRNA were downregulated compared to placebo seven days after the removal of morphine pellets. It is interesting to speculate that the decrease in neurotrophic support in the DRN following opiate exposure could potentially lead to a decrease in cellular surface area, process length or overall synaptic activity within DRN neurons. If so, these effects could potentially contribute to hypofunction of the 5-HT system that has been observed during protracted abstinence from chronic morphine (Goeldner et al., 2011) as well as susceptibility to stress induced reinstatement that we have observed in our previous studies (Staub et al., 2012; Li et al., 2013).
4.3 Opiate regulation of CRF receptor mRNA expression
Anatomical data indicate that CRF axonal projections are present throughout the DRN and make contact with both GABA and 5-HT neurons (Kirby et al., 2000; Roche et al., 2003; Waselus et al., 2005). Electrophysiology studies further show that CRF stimulates inhibitory GABA synaptic activity at 5-HT DRN neurons at the pre- and postsynaptic levels with contributions from both CRF-R1 and –R2 receptor subtypes (Kirby et al., 2008). In the DRN of naïve animals CRF-R2 is expressed at higher levels than CRF-R1 (Van Pett et al., 2000; Day et al., 2004) and most of the CRF-R2 protein is intracellular leaving a higher functional ratio of CRF-R1/CRF-R2 on the cell surface (Waselus et al., 2009). Stress has been shown to induce a cellular redistribution of CRF receptor subtypes in DRN neurons, the majority of CRF-R1 receptors become internalized while CRF-R2 receptors are recruited to the membrane, effectively reversing the functional ratio of CRF-R1/CRF-R2 receptors on 5-HT DRN neurons (Waselus et al., 2009). Waselus et al (2009) further hypothesize that this functional CRF receptor redistribution underlies the switch from active to passive behavioral strategies to cope with the initiating stressor. Our results show that following 7 days of opiate abstinence CRF-R1 mRNA expression is decreased compared to placebo, while CRF-R2 mRNA expression is increased compared to baseline. These results indicate that this functional reversal of CRF receptors in the DRN may occur more ubiquitously, in this case produced by opiate history rather than by stress, and may occur at the level of the genes that encode CRF receptors as well as at the level of cell surface expression of the receptor protein (Waselus et al., 2009). It is also possible that this shift in CRF receptor mRNA expression in the DRN has similar behavioral consequences, predisposing subjects to passive, depression-like behaviors that might contribute to opiate relapse vulnerability. Previous studies from our lab (Staub et al., 2012) show that animals with an opiate history show sensitization of CRF-mediated inward current in 5-HT DRN neurons (a CRF-R2-mediated response; (Kirby et al., 2008) but CRF-R1-mediated stimulation of presynaptic GABA release that is normally seen in naïve subjects (Kirby et al., 2008) is absent in these animals. This opiate-induced shift in CRF receptor sensitivity of 5-HT DRN neurons is consistent with, and may be the result of the upregulation of the CRF-R2 mRNA and downregulation of the CRF-R1 mRNA that we observe in animals with opiate history in the current study. These alterations within the CRF stress system further support the hypothesis that opiate history produces an enduring change in plasticity of the 5-HT DRN system, potentially contributing to relapse susceptibility.
4.4 Opiate regulation of serotonin-related genes
In the DRN the 5-HT1A receptor is located on the soma and dendrites of 5-HT neurons and effectively functions as an autoreceptor to maintain 5-HT homeostasis by regulating DRN electrical activity as well as 5-HT synthesis and release (Sprouse and Aghajanian, 1987; Hamon et al., 1988; Sharp et al., 1989). Expression of 5-HT1A receptor mRNA was significantly decreased following 3 hours of morphine exposure compared to both placebo and baseline, yet remained at baseline for the duration of the experiment. Given that acute opiate exposure increases 5-HT output (Jolas and Aghajanian, 1997), 5-HT1A mRNA downregulation may be an acute compensatory adaptation to elevated 5-HT levels. The net effect of this downregulation would be to further increase 5-HT levels. Therefore this mechanism may contribute to the overall stimulation of 5-HT in response to acute morphine exposure. The fact that the expression returns to baseline at 72 hours and remains steady for the rest of the experiment indicates that this 5-HT1A mRNA response is transient and not be recruited beyond the acute response phase of the 5-HT system to morphine exposure.
Expression of TPH2 mRNA in contrast, was significantly decreased at seven days of abstinence with swim stress, but remained constant across all other time points. We have previously demonstrated that in animals with a morphine history, there is an interaction with swim stress leading to 5-HT hypofunction, an adaptation that may contribute to stress-induced opiate relapse in the CPP model (Staub et al., 2012). This adaptation was seen as an increased sensitivity of 5-HT DRN neurons (but not non 5-HT neurons) to GABAergic inhibition (Staub et al., 2012). Furthermore we have shown that intra-DRN injections of GABA agonists can produce reinstatement of CPP without swim stress, while GABA antagonists can block reinstatement of CPP following swim stress (Li et al., 2013). TPH2 is the main enzyme that drives 5-HT synthesis, and TPH2 mRNA is expressed almost 150 fold higher in the rodent brain stem compared to TPH1 (Walther and Bader, 2003). It is remarkable that TPH2 is steady across all non-stress time points examined suggesting that 5-HT synthesis within the DRN could be steady as well. Exposure to stress in subjects with an opioid history, in contrast, may decrease 5-HT synthesis. Furthermore the decrease in TPH2 mRNA is the opposite response seen in animals without exposure to opiates administered forced swim, as it has been demonstrated that forced swim increases TPH2 mRNA levels (Shishkina et al., 2008). These studies are consistent with our model that opiate history and stress interactions drive relapse through a decrease in 5-HT DRN activity. Results from the current study show that the interaction is not limited to GABAergic activity (Staub et al., 2012), as 5-HT synthesis may be altered as well. Finally this is a further example of the enduring plasticity in the 5-HT DRN system that results from an opiate history.
4.5 Gene expression changes in the placebo control group
It is interesting to note that at two time points the placebo group significantly differed from baseline values (see elevated TrkB mRNA in 7-day group, Fig. 5 and elevated 5-HT1A mRNA in the 7-day group exposed to FS, Fig. 8). The baseline group was included for comparison of all experimental groups to a naïve control group that had not experienced the stress of the surgery, anesthesia, etc. It is possible that in the placebo group, gene expression in these cases was upregulated in response to stress (related to surgery, anesthesia, and/or FS) but this effect was mitigated in the corresponding morphine group because the analgesic effects of the chronic morphine treatment may have reduced the stressfulness of these procedures (Buckingham and Cooper, 1984; Houshyar et al., 2001).
4.6 Conclusions
In this study we have shown that morphine exposure, withdrawal and post withdrawal stress regulate mRNA expression for BDNF, TrkB, CRF receptors, and other genes regulating 5-HT in the rat DRN. Following seven days of abstinence, we found decreases in BDNF, TrkB and CRF-R1, with an increase in CRF-R2. Additionally swim stress following abstinence decreased TPH2 mRNA. Our previous results indicate possible hypofunction of the 5-HT system in a model of stress-induced opiate relapse (Staub et al., 2012; Li et al., 2013). In this study we have identified differential mRNA expression patterns in neurotrophins, the CRF system and other aspects of 5-HT regulation that may contribute to this hypofunction and confer susceptibility to relapse.
Highlights.
Morphine decreased BDNF, TrkB and 5-HT1A mRNA expression in the serotonergic DRN
Morphine decreased CRF-R1 and increased CRF-R2 mRNA expression in the DRN
Opiate history and swim stress interact to reduce DRN TPH2 mRNA expression
Opiate modulation of DRN circuits may promote 5-HT hypofunction
5-HT hypofunction may contribute to vulnerability to stress-induced opiate relapse
Acknowledgments
This research was supported by NIH R01 DA 20126 and NIH P30 DA 13429. We would also like to thank Dr. Fabiola Del Carpio-Cano and Chrissy Mundy for assistance with real time PCR, Alessandra Cathel, Jonathan Palma and Dr. Daniel Staub for their assistance with surgical procedures as well as Dr. Alexandra Monroy for use of the ABI 7500 instrument.
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- 5-HT1A
serotonin 1A receptor
- BDNF
brain-derived neurotrophic factor
- CPP
conditioned place preference
- CRF
corticotropin releasing factor
- CRF-R1
corticotropin releasing factor receptor 1
- CRF-R2
corticotropin releasing factor receptor 2
- DRN
dorsal raphe nucleus
- FS
forced swim
- GABA
gamma aminobutyric acid
- GABAA
GABA receptor A
- GABAA α1
alpha 1 subunit of the GABAA receptor
- MOR
μ opiate receptor
- NAc
nucleus accumbens
- SERT
serotonin transporter
- TrkB
tyrosine kinase receptor B
- TPH2
tryptophan hydroxylase enzyme 2
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Boyarskikh UA, Bondar NP, Filipenko ML, Kudryavtseva NN. Downregulation of serotonergic gene expression in the raphe nuclei of the midbrain under chronic social defeat stress in male mice. Mol Neurobiol. 2013;48:13–21. doi: 10.1007/s12035-013-8413-y. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Brown RM, Lawrence AJ. Neurochemistry underlying relapse to opiate seeking behaviour. Neurochem Res. 2009;34:1876–1887. doi: 10.1007/s11064-009-9967-y. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinol. 1984;38:411–417. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Cerletti C, Keinath SH, Geller EB, Reidenberg MM, Adler MW. Comparison of Morphine Withdrawal Syndrome in Injected and Pellet-Implanted Rats. Pharmacologist. 1975;17:237. [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Krystal JH, Delgado PL, Heninger GR. Serotonin-specific drugs for anxiety and depressive disorders. Annu Rev Med. 1990;41:437–446. doi: 10.1146/annurev.me.41.020190.002253. [DOI] [PubMed] [Google Scholar]
- Chu NN, Zuo YF, Meng L, Lee DY, Han JS, Cui CL. Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res. 2007;1182:90–98. doi: 10.1016/j.brainres.2007.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shippenberg TS. Neural systems underlying opiate addiction. J Neurosci. 2002;22:3321–3325. doi: 10.1523/JNEUROSCI.22-09-03321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety. 2012;29:307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialogues Clin Neurosci. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CN, Vigers AJ, Jones KR. Genetic evidence that brain-derived neurotrophic factor mediates competitive interactions between individual cortical neurons. Proc Natl Acad Sci U S A. 2012;109:19456–19461. doi: 10.1073/pnas.1206492109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall CM, Gold SJ, Isackson PJ, Seroogy KB. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs are expressed in ventral midbrain regions containing dopaminergic neurons. Mol Cell Neurosci. 1992;3:56–63. doi: 10.1016/1044-7431(92)90009-q. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neurosci. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs F. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H. Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther. 1988;246:745–752. [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshyar H, Cooper ZD, Woods JH. Paradoxical effects of chronic morphine treatment on the temperature and pituitary-adrenal responses to acute restraint stress: a chronic stress paradigm. J Neuroendocrinol. 2001;13:862–874. doi: 10.1046/j.1365-2826.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- Ichim G, Tauszig-Delamasure S, Mehlen P. Neurotrophins and cell death. Exp Cell Res. 2012;318:1221–1228. doi: 10.1016/j.yexcr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Jolas T, Nestler EJ, Aghajanian GK. Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neurosci. 2000;95:433–443. doi: 10.1016/s0306-4522(99)00436-4. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacol. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacol. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, LaPlant Q, Ferguson D, Feng J, Sun H, Scobie KN, mez-Werno D, Crumiller M, Ohnishi YN, Ohnishi YH, Mouzon E, Dietz DM, Lobo MK, Neve RL, Russo SJ, Han MH, Nestler EJ. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Zhang G, Walsh T, Kirby LG, Akanwa A, Brooks-Kayal A, Beck SG. Stress hyperresponsive WKY rats demonstrate depressed dorsal raphe neuronal excitability and dysregulated CRF mediated responses. Neuropsychopharmacology. 2011;36:721–734. doi: 10.1038/npp.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Staub DR, Kirby LG. Role of GABAA receptors in dorsal raphe nucleus in stress-induced reinstatement of morphine conditioned place-preference in rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3182-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav TR, Pei Q, Zetterstrom TS. Serotonergic cells of the rat raphe nuclei express mRNA of tyrosine kinase B (trkB), the high-affinity receptor for brain derived neurotrophic factor (BDNF) Mol Brain Res. 2001;93:56–63. doi: 10.1016/s0169-328x(01)00183-8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mashayekhi FJ, Rasti M, Rahvar M, Mokarram P, Namavar MR, Owji AA. Expression levels of the BDNF gene and histone modifications around its promoters in the ventral tegmental area and locus ceruleus of rats during forced abstinence from morphine. Neurochem Res. 2012;37:1517–1523. doi: 10.1007/s11064-012-0746-9. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Kocsis JH, Melia D, Khuri ET, Sweeney J, Wells A, Borg L, Millman RB, Kreek MJ. Psychiatric comorbidity in methadone maintained patients. J Addict Dis. 1998;17:75–89. doi: 10.1300/J069v17n03_07. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. Role of serotonin in depression. Ann N Y Acad Sci. 1990;600:486–499. doi: 10.1111/j.1749-6632.1990.tb16904.x. [DOI] [PubMed] [Google Scholar]
- Merlio JP, Ernfors P, Jaber M, Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neurosci. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605. [PubMed] [Google Scholar]
- Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Contu P, Agus A, Gessa GL. Psychiatric severity and treatment response in a comprehensive methadone maintenance treatment program. Drug Alcohol Depend. 1997;48:119–126. doi: 10.1016/s0376-8716(97)00115-4. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacol. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Weissman MM, Kleber H, Wilber C. Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry. 1982;39:161–168. doi: 10.1001/archpsyc.1982.04290020027006. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacol. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Grahame-Smith DG. 5-HT1 agonists reduce 5-hydroxytryptamine release in rat hippocampus in vivo as determined by brain microdialysis. Br J Pharmacol. 1989;96:283–290. doi: 10.1111/j.1476-5381.1989.tb11815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neurosci. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Serotonergic changes produced by repeated exposure to forced swimming: correlation with behavior. Ann N Y Acad Sci. 2008;1148:148–153. doi: 10.1196/annals.1410.074. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Staub DR, Lunden JW, Cathel AM, Dolben EL, Kirby LG. Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Psychoneuroendocrinology. 2012;37:859–870. doi: 10.1016/j.psyneuen.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansky W. Rejecting outliers in factorial designs. Technometrics. 1972;14:469–479. [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinol. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002a;303:704–710. doi: 10.1124/jpet.102.038133. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002b;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286:481–488. [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van BE. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Axon terminals containing corticotropin-releasing factor (CRF) preferentially contact GABA-containing somatodendritic processes in the rat dorsal raphe nuceus (DRN) lateral wing region. Soc Neurosci Abs. 2003:712.4. [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu B. BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci. 2012;32:14318–14330. doi: 10.1523/JNEUROSCI.0709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Woody GE, O’Brien CP, Rickels K. Depression and anxiety in heroin addicts: a placebo-controlled study of doxepin in combination with methadone. Am J Psychiatry. 1975;132:447–450. doi: 10.1176/ajp.132.4.447. [DOI] [PubMed] [Google Scholar]