Abstract
Background
The issue of osteoporosis-induced fractures has attracted the world’s attention. Postmenopausal women are particularly at risk for this type of fracture. The nonmedicinal intervention for postmenopausal women is mainly exercise. Whole body vibration (WBV) is a simple and convenient exercise. There have been some studies investigating the effect of WBV on osteoporosis; however, the intervention models and results are different. This study mainly investigated the effect of high-frequency and high-magnitude WBV on the bone mineral density (BMD) of the lumbar spine in postmenopausal women.
Methods
This study randomized 28 postmenopausal women into either the WBV group or the control group for a 6-month trial. The WBV group received an intervention of high-frequency (30 Hz) and high-magnitude (3.2 g) WBV in a natural full-standing posture for 5 minutes, three times per week, at a sports center. Dual-energy X-ray absorptiometry was used to measure the lumbar BMD of the two groups before and after the intervention.
Results
Six months later, the BMD of the WBV group had significantly increased by 2.032% (P=0.047), while that of the control group had decreased by 0.046% (P=0.188). The comparison between the two groups showed that the BMD of the WBV group had increased significantly (P=0.016).
Conclusion
This study found that 6 months of high-frequency and high-magnitude WBV yielded significant benefits to the BMD of the lumbar spine in postmenopausal women, and could therefore be provided as an alternative exercise.
Keywords: whole body vibration, osteoporosis, postmenopausal women
Introduction
Owing to the aging of the global population, the prevention and treatment of chronic diseases in the elderly have become important health issues. The increase in the number of cases of osteoporosis-induced fractures in the elderly is noteworthy.1,2 The World Health Organization has defined osteoporosis as a disease characterized by “low bone density and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture.”3 More than 200 million postmenopausal women around the world suffer from osteoporosis.4 In the United States, osteoporosis affects 2% of men and 10% of women aged 50 years and above. In addition, 49% of older women and 30% of older men have osteopenia.5 In Taiwan, 1.63% of men aged 50 years and above suffer from osteoporosis, and 11.35% of women suffer from it.6 Osteoporosis and falls are related to fractures, which can lead to increased morbidity and mortality, as well as decreased functional ability. The mortality of patients with hip fractures within 1 year is 20%, and only one-third of the patients have recovered their original functions.7 A large-scale, multinational study on vertebral compression fractures in Asia found that the incidence of fractures in women aged 65–74 years ranges from 9.2% to 18.8%, and that in women aged 75 years and above ranges from 18% to 28.7%.8
Most strategies for treating bone loss have focused on dietary and pharmacologic interventions;9 however, drug treatments can have adverse effects and poor long-term adherence, despite their effectiveness.10 Weight-bearing and resistance exercises can be an alternative therapy. Some studies have shown that these exercises can increase bone mineral density (BMD).11,12 In comparison with pharmacological interventions, the compliance with exercise for treating osteoporosis is better.13 Furthermore, fall incidence is multifactorial; it may strengthen the case for exercise interventions, and exercise itself is effective in reducing fall incidence, whereas pharmacological and other interventions are not.14
Whole body vibration (WBV) is a popular exercise where individuals stand on an oscillating plate, and the motor transmits vertical acceleration to muscle and bone.15 Wolff stated the bone will increase where the load is placed, which leads to the remodeling of bone; it was also found that the morphology (density, size, and width) of a bone will be changed by the external forces acting on it – hence, he proposed the famous Wolff’s law.16 WBV can produce osteogenic effects by changing the flow of bone fluid through direct bone stimulation and mechanotransduction, or it can generate indirect bone stimulation through skeletal muscle activation by means of tone stretch reflex.17–19
The results of animal trials had shown that vibration stimulation can increase the anabolic activity of bone tissue, as well as increase the bone volume and area.20,21 In addition, the study by Wenger et al22 found that mice exhibited a shift toward higher bone density in the femur and an increase in mineralizing surface in the radius after vibration. Studies and systemic reviews on postmenopausal women have found that WBV has a significant effect on femoral neck BMD; however, it does not have a significant effect on lumbar spine BMD.3,22–33 These studies have also found that the frequency and magnitude of the applied WBV used in these studies differ greatly. The aim of this study was to determine whether 6 months of high-frequency and high-magnitude WBV training at a neutral full standing position would be effective for the BMD of the lumbar spine in postmenopausal women.
Methods
Subjects
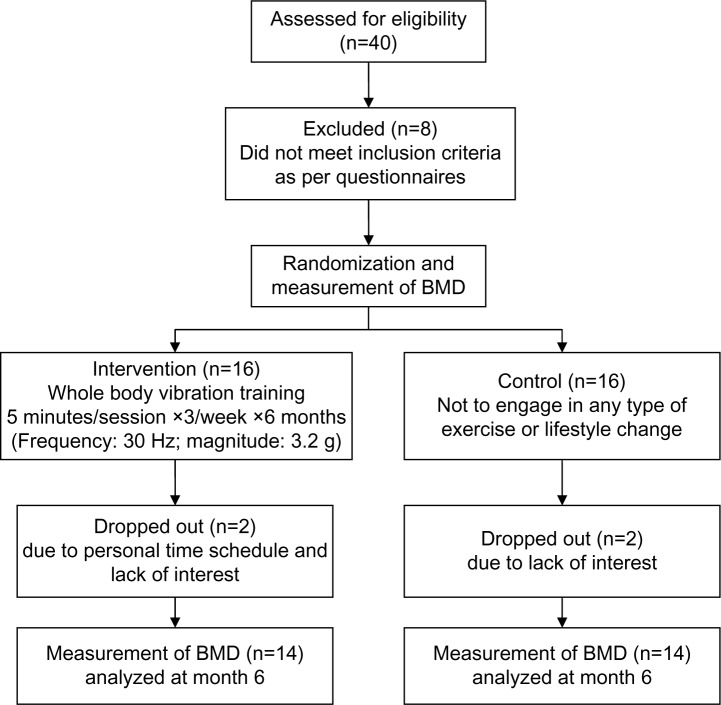
This study was a randomized clinical trial, in which subjects that met the inclusion criteria in community volunteer groups were recruited through advertisements from January 2010 to October 2011. As shown in Figure 1, a total of 40 postmenopausal women were recruited, and 32 of them met the inclusion criteria. In the end, a total of 28 subjects completed this study. The inclusion criteria were: postmenopausal; nonsmokers; adequate nutritional status (body mass index [BMI] ≥18.5), a lack of regular exercise at least three times per week, and the ability to follow the protocol. The exclusion criteria were: acute hernias or thrombosis; kidney or bladder stones; epilepsy or seizures; pregnancy; arrhythmia; use of a pacemaker; serious cardiovascular or pulmonary disease; dizziness; undergoing surgery or being hospitalized for treatment within the last 6 months; and receiving any osteoporosis drugs within the last year. The subjects were fully informed of the research purpose, possible adverse events, and expected health benefits, and all subjects signed the approved informed consent form for this study after being verbally informed of the relevant information. This study was approved by Institutional Review Board at the Taichung Hospital (Taichung, Taiwan) (IRB-05-06).
Figure 1.
Flowchart of this trial.
Abbreviations: n, number; BMD, bone mineral density.
Study design
A total of 32 subjects who met the inclusion criteria were randomized into two groups using computer-generated numbers: the WBV group and the control group (CON group). During the study, the two groups were asked to maintain their daily life habits and not to use any osteoporosis drugs, including calcium and vitamin D. The WBV group received vibration training three times per week at a sports center in a hospital. The subjects stood on the platform in a natural full standing posture with their bare feet. The stimulation source of the WBV device (LV-1000; X-trend Fitness Equipment, Luntai Enterprise Co., Ltd, Taichung, Taiwan) was a horizontal vibration with a frequency of 30 Hz (1 Hz =1 oscillation/second) and a magnitude (acceleration) of 3.2 g (gravity; 1 g =9.81 m/second2) for 5 minutes each time. A well-trained physical therapist was responsible for executing the vibration training and for monitoring the safety of the subjects (Figure 2). The subjects all underwent BMD (g/cm2) tests of the lumbar spine before and after the 6-month intervention. The first to fourth lumbar spine BMD was assessed using dual-energy X-ray absorptiometry (DEXA) (QDR4500; Hologic® Inc, Bedford, MA, USA). A physician who was certified by the International Society for Clinical Densitometry interpreted the test data to ensure the consistency of the DEXA quality. The day-to-day precision coefficient of variation percentage of this DEXA machine was about 1% at the lumbar spine.
Figure 2.

The subject stood on the platform in a natural full standing posture and was monitored by a well-trained physical therapist during whole body vibration.
Statistical analysis
This study used the Statistical Package for the Social Sciences, Windows version 14 (SPSS Inc., Chicago, IL, USA) to analyze the research data. Descriptive statistics included the mean and standard deviation and the chi-square test for the baseline characteristics. This study used a paired samples t-test to compare the change in BMD of the two groups before and after the intervention. The effects between the two groups were tested using analysis of covariance, and were adjusted by body weight, age, and baseline data to compare the change in BMD of the two groups. This study adopted P<0.05 as the level of statistical significance in the two-tailed analysis.
Results
Thirty-two postmenopausal women met the inclusion criteria. During the trial, four participants dropped out due to schedule problems or a lack of interest. Therefore, 28 (88%) of the subjects completed the program. None of the subjects experienced adverse effects, such as dizziness or pain, during the entire program.
As shown in Table 1, the basic information of the 28 participants was as follows: the average age was 60.1±7.1 years (46–69 years); the average BMI was 22.7±1.9 kg/m2 (19.8–26.1 kg/m2); the average BMD of the lumbar spine was 0.818±0.088 g/cm2 (0.684–0.984 g/cm2); and the average years after menopause was 9.8±8.7 years (1–30 years) in the WBV group. The average age was 62.4±7.1 years (53–75 years); the average BMI was 23.1±4.4 kg/m2 (18.5–28.6 kg/m2); the average BMD of the lumbar spine was 0.819±0.078 g/cm2 (0.674–1.015 g/cm2); and the average number of postmenopausal years was 10.6±6.9 years (0–25 years) in the CON group. There were no significant differences in the age, BMI, BMD of lumbar spine, and number of postmenopausal years between the two groups, suggesting that the subjects were properly randomized. According to the criteria of osteoporosis recommended by the World Health Organization, which compares youths aged 20–29, a calculated T-score >−1.0 is normal, while a T-score =−1.0 to −2.5 indicates osteopenia (low bone mass or low bone density). A T-score ≤−2.5 is used to diagnose osteoporosis.34 The incidence of osteopenia and osteoporosis of the two groups in the trial was 100% for the WBV group and 85% for the CON group.
Table 1.
Baseline characteristics of the sample (mean ± SD)
| Assessment | WBV group | CON group | P-value |
|---|---|---|---|
| Number (N=28) | 14 | 14 | |
| Age (years) | 60.1±7.1 | 62.4±7.1 | 0.386 |
| Years after menopause | 9.8±8.7 | 10.6±6.9 | 0.776 |
| BMI (kg/m2) | 22.7± 1.9 | 23.1 ±4.4 | 0.749 |
| BMD of lumbar spine (g/cm2) | 0.818±0.088 | 0.819±0.078 | 0.992 |
| Proportion of osteopenia and osteoporosis (%) | 100 | 85 | 0.481 |
Abbreviations: SD, standard deviation; WBV, whole body vibration; CON, control; N, number; BMI, body mass index; BMD, bone mineral density.
The BMD of the WBV group and the CON group after 6 months was 0.835±0.098 g/cm2 (compared to the pretest, P=0.047) and 0.815±0.076 (compared to the pre-test, P=0.188), respectively. There was a significant increase in the BMD of the lumbar spine of the WBV group, while there was a decrease in that of the CON group (Table 2). The variables (age, BMI, and number of postmenopausal years) that might affect the BMD were further adjusted using analysis of covariance. The comparison of the change in the BMD between the two groups before and after the 6-month intervention indicated that the BMD of the lumbar spine in the WBV group increased by 2.032%±3.332%, while that of the CON group decreased by 0.046%±1.245%. The difference between the two groups reached statistical significance (P=0.016), as shown in Table 3.
Table 2.
Comparative effects of the WBV and CON groups after 6 months (mean ± SD)
| Month 0 BMD (g/cm2) | Month 6 BMD (g/cm2) | P-value | Change in BMD (%) | P-value | |
|---|---|---|---|---|---|
| WBV group | 0.818±0.088 | 0.835±0.098 | 0.047* | 2.032%±3.332% | |
| CON group | 0.819±0.078 | 0.815±0.076 | 0.188 | −0.046%± 1.245% | 0.016* |
Note:
P<0.05.
Abbreviations: WBV, whole body vibration; CON, control; SD, standard deviation; BMD, bone mineral density.
Table 3.
Comparative effects of the WBV and CON groups after six months (mean ± SD)
| WBV group | CON group | P-value | |
|---|---|---|---|
| Difference | 0.017±0.029 | −0.004±0.011 | 0.018* |
| Change in BMD | 2.032%±3.332% | −0.046%± 1.245% | 0.016* |
Notes: Analysis of covariance was used to compare the differences between the two groups and adjust factors such as age, BMI, and number of post-menopausal years.
P<0.05.
Abbreviations: WBV, whole body vibration; CON, control; SD, standard deviation; BMD, bone mineral density; BMI, body mass index.
Discussion
Osteoporosis has become one of the most important health issues for postmenopausal women, and it has been found that multicomponent exercise programs based on strength, aerobic, high impact, and/or weight-bearing training are beneficial to postmenopausal women.35 However, some weight-bearing exercises are not suitable for patients with muscle weakness or joint and nerve diseases; therefore, WBV training can be provided as an alternative exercise. WBV had negative effects if the exposure was of large intensity or long duration, which could damage the peripheral nerves and blood vessels. On the other hand, the side effects, including dizziness, headache, and fall, could be minor when exposure includes low intensity and is of short duration.3 Thus, the choice of the vibration model and the duration of the intervention are important.
The oscillating plate of a WBV machine can be adjusted to alter the exercise stimulus. According to the frequency/magnitude of the applied vibration, the oscillating plate can be divided into high-frequency (Hz >20) or low-frequency plates (Hz ≤20), and they can be categorized as high magnitude (≥1 g) or low magnitude (<1 g), according to the strength of the exercise.15,17 This study used high-frequency (30 Hz) and high-magnitude (3.2 g) horizontal WBV to conduct a trial on postmenopausal women. After the 6-month intervention, the WBV group showed significantly improved lumbar spine BMD (P=0.016).
Several randomized controlled studies have compared WBV training groups with CON groups and found that there is no significant effect on lumbar spine BMD or volumetric bone density in postmenopausal women.24,25,27,28 The study by Rubin et al24 used a quiet standing posture to receive high-frequency and low-magnitude WBV. In the study by von Stengel et al,30 the patients received a multifunctional training program at the high-frequency and low-magnitude WBV platform. The subjects in this study received high-frequency and high-magnitude WBV, which was different from the two studies mentioned above. The study by Verschueren et al25 used the same high-frequency and high-magnitude WBV as that used in this study; however, the subjects engaged in static and dynamic knee-extensor exercises and osteoporosis cases were excluded. In the study by Gusi et al,27 the subjects stood on a WBV platform and maintained a 60° angle of knee flexion. The posture used on the WBV platform will affect the transmissibility of WBV. An erective posture can enhance the transmissibility of vibration through the hip and spine.36 The neutral full-standing position used in this study could enhance the effect of WBV on the bones in the lumbar spine.
In recent large-scale studies, Ruan et al28 enrolled 91 postmenopausal women with osteoporosis in a study and provided a 6-month intervention of high-frequency (30 Hz, five times/week) WBV, and found that the WBV group’s lumbar spine BMD increased by 4.3% (P=0.000); conversely, the CON group’s lumbar spine BMD decreased by 1.9% (P<0.05). In the study by Beck and Norling,37 following an 8-month intervention of high-frequency (30 Hz) and low-magnitude (0.3 g) WBV twice per week, the CON group experienced bone loss at the lumbar spine (−6.6%; P=0.02), while the WBV group did not. However, there was no between-group difference. The results of the aforementioned studies were similar to those of this study; however, the study by Ruan et al28 required a series of five, 10-minute sessions/week, and only three, 5-minute sessions/week were performed in this study, which is more reasonable for the participants to adhere to. This would be strengthened by highlighting the dropout rate in the Ruan et al study,28 which was 23%, as compared to the 88% retention rate in this study. In contrast, Slatkovska et al38 conducted a study on 202 postmenopausal women who were taking calcium and vitamin D supplements, in which high-frequency (30 Hz and 90 Hz) and low-magnitude (0.3 g) WBV was used. Comparisons to the CON group showed that there was no significant change in the lumbar spine BMD in the WBV training group. However, the author noted some limitations, including inconsistent medical adherence (65%–79%) and the fact that participants self-administered the WBV at home. In this study, the participants who received high-frequency and high-magnitude WBV did not take calcium or vitamin D supplements, and they exhibited good adherence. The entire WBV training program was performed at the sports center of a hospital and was supervised by a well-trained physical therapist.
Although this study observed a significant increase in the lumbar spine BMD from baseline, there were some limitations. First, the overall results may not be applicable to the general population, because the samples were low and only consisted of postmenopausal women, not from a random sampling of the general population. Second, blank WBV was not provided to the CON group and a double-blind design could not be implemented in this study. Moreover, not all the participants had osteopenia or osteoporosis. Therefore, it was impossible to identify the effect of WBV on preventing or improving osteoporosis. The rate of osteoporosis and osteopenia might affect the trial results. It has been generally speculated that the increased rate of osteoporosis and osteopenia could result in a greater increase of BMD due to a low baseline BMD.39,40 However, the mechanism of WBV on lumbar spine BMD remains unclear.
WBV training is a very convenient exercise. This study found that high-frequency (30 Hz) and high-magnitude (3.2 g) WBV training could be used by postmenopausal women to improve bone loss at the lumbar spine. In order to determine guidelines for the use of WBV, including the posture used on the platform, and the oscillation type (amplitude, frequency, and duration), a large-scale study should be conducted on elderly participants, or on patients with disabilities who are unable to engage in resistant exercise or other weight-bearing exercises.
Conclusion
This study concluded that 6 months of high-frequency, high-magnitude WBV using a neutral full standing posture is a feasible exercise for reducing bone loss at the lumbar spine for postmenopausal women.
Acknowledgments
This research was supported by the foundation of the Taichung Hospital, Ministry of Health and Welfare, Taichung, Taiwan.
Author contributions
Study concept: Chung-Liang Lai, Meng-Chih Lee, and Pi-Shan Hsu. Planning and designing: Chung-Liang Lai, Shiuan-Yu Tseng, and Pi-Shan Hsu. Acquisition of data: Chung-Nan Chen, Shiuan-Yu Tseng, and Wan-Chun Liao. Analysis of the data: Chun-Hou Wang, Wan-Chun Liao, and Meng-Chih Lee. Interpretation of the data: Chung-Liang Lai, Chung-Nan Chen, and Chun-Hou Wang. Writing/revising the manuscript: Chung-Liang Lai, Shiuan-Yu Tseng, Chung-Nan Chen, Wan-Chun Liao, Chun-Hou Wang, Meng-Chih Lee, Pi-Shan Hsu.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health. 2003;57(9):740–744. doi: 10.1136/jech.57.9.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Totosy de Zepetnek JO, Giangregorio LM, Craven BC. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: a review. J Rehabil Res Dev. 2009;46(4):529–542. doi: 10.1682/jrrd.2008.09.0136. [DOI] [PubMed] [Google Scholar]
- 4.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(Suppl 2):S3–S11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Melton LJ, Harris TB, Borrud LG, Shepherd JA. Prevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES III. J Bone Miner Res. 2010;25(1):64–71. doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang NP, Deng CY, Chou YJ, et al. Estimated prevalence of osteoporosis from a Nationwide Health Insurance database in Taiwan. Health Policy. 2006;75(3):329–337. doi: 10.1016/j.healthpol.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 8.Kwok AW, Leung JC, Chan AY, et al. Prevalence of vertebral fracture in Asian men and women: comparison between Hong Kong, Thailand, Indonesia and Japan. Public Health. 2012;126(6):523–531. doi: 10.1016/j.puhe.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Hamdy RC, Baim S, Broy SB, et al. Algorithm for the management of osteoporosis. South Med J. 2010;103(10):1009–1015. doi: 10.1097/SMJ.0b013e3181f0e8d6. quiz 1016. [DOI] [PubMed] [Google Scholar]
- 10.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H, Kim KJ, Komatsu T, Park SK, Mutoh Y. Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J Bone Miner Metab. 2008;26(3):254–259. doi: 10.1007/s00774-007-0819-z. [DOI] [PubMed] [Google Scholar]
- 12.Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46(2):294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GA, Kelley KS. Dropouts and compliance in exercise interventions targeting bone mineral density in adults: a meta-analysis of randomized controlled trials. J Osteoporos. 2013;2013:250423. doi: 10.1155/2013/250423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch F, Sievanen H, Boonen S, et al. International Society of Musculoskeletal and Neuronal Interactions Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 16.Wolff (1836–1902) Julius. Morphogenesis of bone. JAMA. 1970;213(13):2260. [PubMed] [Google Scholar]
- 17.Judex S, Rubin CT. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact. 2010;10(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6(1):50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2(2):73–85. [PubMed] [Google Scholar]
- 20.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol (1985) 2008;104(4):1056–1062. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 22.Wenger KH, Freeman JD, Fulzele S, et al. Effect of whole-body vibration on bone properties in aging mice. Bone. 2010;47(4):746–755. doi: 10.1016/j.bone.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Russo CR, Lauretani F, Bandinelli S, et al. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil. 2003;84(12):1854–1857. doi: 10.1016/s0003-9993(03)00357-5. [DOI] [PubMed] [Google Scholar]
- 24.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 25.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 26.Iwamoto J, Takeda T, Sato Y, Uzawa M. Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res. 2005;17(2):157–163. doi: 10.1007/BF03324589. [DOI] [PubMed] [Google Scholar]
- 27.Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan XY, Jin FY, Liu YL, Peng ZL, Sun YG. Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin Med J (Engl) 2008;121(13):1155–1158. [PubMed] [Google Scholar]
- 29.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25(11):975–988. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 30.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22(1):317–325. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 31.Wysocki A, Butler M, Shamliyan T, Kane RL. Whole-body vibration therapy for osteoporosis: state of the science. Ann Intern Med. 2011;155(10):680–686. doi: 10.7326/0003-4819-155-10-201111150-00006. W206. [DOI] [PubMed] [Google Scholar]
- 32.Sitjà-Rabert M, Rigau D, Fort Vanmeerghaeghe A, Romero-Rodríguez D, Bonastre Subirana M, Bonfill X. Efficacy of whole body vibration exercise in older people: a systematic review. Disabil Rehabil. 2012;34(11):883–893. doi: 10.3109/09638288.2011.626486. [DOI] [PubMed] [Google Scholar]
- 33.Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010;21(12):1969–1980. doi: 10.1007/s00198-010-1228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 35.Gómez-Cabello A, Ara I, González-Agüero A, Casajús JA, Vicente-Rodríguez G. Effects of training on bone mass in older adults: a systematic review. Sports Med. 2012;42(4):301–325. doi: 10.2165/11597670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine (Phila Pa 1976) 2003;28(23):2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 37.Beck BR, Norling TL. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil. 2010;89(12):997–1009. doi: 10.1097/PHM.0b013e3181f71063. [DOI] [PubMed] [Google Scholar]
- 38.Slatkovska L, Alibhai SM, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med. 2011;155(10):668–679. doi: 10.7326/0003-4819-155-10-201111150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15(12):2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 40.Torvinen S, Kannus P, Sievänen H, et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res. 2003;18(5):876–884. doi: 10.1359/jbmr.2003.18.5.876. [DOI] [PubMed] [Google Scholar]