Abstract
Methamphetamine (METH) is an addictive stimulant drug. In addition to drug craving and lethargy, METH withdrawal is associated with stress-triggered anxiety. However, the cellular basis for this stress-triggered anxiety is not understood. The present results suggest that during METH withdrawal (24 h) following chronic exposure (3 mg/kg, i.p. for 3-5 weeks) of adult, male mice, the effect of one neurosteroid released by stress, 3α,5α-THP (3α-OH-5α-pregnan-20-one), and its 3α,5β isomer reverse to trigger anxiety assessed by the acoustic startle response (ASR), in contrast to their usual anti-anxiety effects. This novel effect of 3α,5β-THP was due to increased (3-fold) hippocampal expression of α4βδ GABAA receptors (GABARs) during METH withdrawal (24 h – 4 wk) because anxiogenic effects of 3α,5β-THP were not seen in α4−/− mice. 3α,5β-THP reduces current at these receptors when it is hyperpolarizing, as observed during METH withdrawal. As a result, 3α,5β-THP (30 nM) increased neuronal excitability, assessed with current clamp and cell-attached recordings in CA1 hippocampus, one CNS site which regulates anxiety. α4βδ GABARs were first increased 1 h after METH exposure and recovered 6 wk after METH withdrawal. Similar increases in α4βδ GABARs and anxiogenic effects of 3α,5β-THP were noted in rats during METH withdrawal (24 h). In contrast, the ASR was increased by chronic METH treatment in the absence of 3α,5β-THP administration due to its stimulant effect. Although α4βδ GABARs were increased by chronic METH treatment, the GABAergic current recorded from hippocampal neurons at this time was a depolarizing, shunting inhibition, which was potentiated by 3α,5β-THP. This steroid reduced neuronal excitability and anxiety during chronic METH treatment, consistent with its typical effect. Flumazenil (10 mg/kg, i.p., 3x) reduced α4βδ expression and prevented the anxiogenic effect of 3α,5β-THP after METH withdrawal. Our findings suggest a novel mechanism underlying stress-triggered anxiety after METH withdrawal mediated by α4βδ GABARs.
Keywords: CA1 hippocampus, pregnanolone, allopregnanolone, stress, withdrawal, flumazenil, methamphetamine
1. Introduction
Methamphetamine (METH) is an addictive, stimulant drug. Dependence on this drug is difficult to treat (Cretzmeyer et al., 2003; Shoptaw et al., 2009) because of the severity of the symptoms of METH withdrawal. In contrast to chronic METH exposure which is well-correlated with increased anxiety and hyperactivity (Barr, 2009) due to the stimulant actions of the drug, METH withdrawal is characterized by sedation and depression. However, stress can trigger paradoxical anxiety during METH withdrawal even though the stimulant effect of the drug is no longer present (London et al., 2004; Mancino et al., 2011). A variety of systems associated with the stress response, including norepinephrine, corticosterone and corticotropin releasing factor (CRF), have been implicated in cocaine and ethanol withdrawal (Basso et al., 1999; Koob, 2009; Roberto et al., 2010; Sarnyai et al., 1995; Smith and Aston-Jones, 2008; Zorrilla et al., 2001). However, these systems have not been correlated with anxiety associated with withdrawal from amphetamines (Barr et al., 2010), suggesting alternative mechanisms. Although both METH and cocaine are stimulants, METH effects are distinct from cocaine due to its unique pharmacokinetics (Fowler et al., 2008). One CNS system not yet considered as a mediator of stress-triggered anxiety in METH withdrawal is the GABAA receptor (GABAR). This receptor is a likely candidate because it plays a pivotal role in generating anxiety and has a high degree of plasticity. Therefore the present study assessed the role of this system in METH dependence.
METH exerts stimulant actions in multiple brain regions, including the CA1 hippocampus (Hori et al., 2010), one CNS site which regulates anxiety (Bannerman et al., 2004; Bitran et al., 1999). METH increases glutamate release and depolarizes CA1 pyramidal cells when first administered (Yamamoto et al., 1999). These stimulant actions of the drug diminish during prolonged drug exposure (Yamamoto et al., 1999), suggesting that compensatory mechanisms occur to prevent neurotoxicity. Although compensatory mechanisms involving the glutamate and monoaminergic systems have been investigated after METH exposure (Yamamoto et al., 1999), compensatory regulation of the inhibitory GABAergic system in the CA1 hippocampus has not yet been studied in response to chronic METH exposure.
The GABAR is a pentameric membrane protein which gates a Cl− current and is the primary source of inhibition in the brain (Olsen and Sieghart, 2009). These receptors can either localize subsynaptically, where they generate a phasic current, or extrasynaptically (Wei et al., 2003), where they generate a tonic current (Bai et al., 2000; Stell and Mody, 2002) in response to ambient GABA. Of the many diverse subtypes, the extrasynaptic α4βδ GABAR normally has low expression in areas such as CA1 hippocampus (Pirker et al., 2000; Wei et al., 2003), an area in the limbic CNS important for generating mood (Bannerman et al., 2004). However, this receptor is highly regulatable, providing compensatory tonic inhibition across fluctuations in ovarian steroids as well as in response to increased neuronal excitability (Maguire et al., 2005; Mtchedlishvili et al., 2010; Shen et al., 2007), such as would occur with METH exposure. The α4βδ GABAR is also a sensitive target for 3α,5β-THP (3αOH-5β-pregnan-20-one or pregnanolone) and related neurosteroids, including 3α,5α-THP (3αOH-5α-pregnan-20-one or allopregnanolone) (Belelli et al., 2002; Bianchi and Macdonald, 2003; Brown et al., 2002). These THP isomers have nearly identical effects to increase GABA-gated current (Weir et al., 2004), and to reduce anxiety in adult rodents (Bitran et al., 1999; Rhodes and Frye, 2001; Toufexis et al., 2004).
3α,5α-THP is released during certain types of moderate stress in both rodents (Mukai et al., 2008; Purdy et al., 1991) and humans (Droogleever Fortuyn et al., 2004; Girdler et al., 2001). Studies conducted in humans have shown increases in circulating levels of this steroid during mental and/or social stress associated with performance (Droogleever Fortuyn et al., 2004; Girdler et al., 2001), which reflect stress states relevant for daily life. In contrast, stimulants such as cocaine do not have consistent effects in releasing 3α,5α-THP into the circulation (Grobin et al., 2005; Quinones-Jenab et al., 2008). There are also site-specific effects: recent studies show increased levels of 3α,5α-THP in striatum (Quinones-Jenab et al., 2008) but not in cortex (Grobin et al., 2005) or hippocampus (Quinones-Jenab et al., 2008) after cocaine administration to male rats at doses 5 to 10-fold greater than used in the present study. The source of stress-induced release of 3α,5α-THP includes the adrenal gland. However, the cellular machinery for 3α,5α-THP synthesis is also found in neurons such as CA1 pyramidal cells (Agis-Balboa et al., 2006), suggesting that this steroid (and its isomer, 3α,5β-THP) may be synthesized directly in the brain. This possibility is also suggested by studies showing stress-triggered 3α,5α-THP release in adrenalectomized animals (Purdy et al., 1991). Both THP isomers are metabolites of progesterone (Compagnone and Mellon, 2000), an ovarian hormone, and 3α,5α-THP is also increased on the proestrous stage of the estrous cycle in rodents (Palumbo et al., 1995) as well as before the onset of puberty (Fadalti M et al., 1999; Shen et al., 2010b) and during pregnancy (Concas et al., 1998; Luisi et al., 2000) in both rodents and humans. Circulating levels of 3α,5α-THP and 3α,5β-THP are similar in men and (Porcu et al., 2010) and women (Havlikova et al., 2006; Porcu et al., 2009), and they are also altered in parallel in response to anti-depressant treatment (Girdler et al., 2012) suggesting that both isomers likely play a role in the stress response. In addition, neurosteroids have been shown to be neuroprotective (Rhodes et al., 2004) and anti-nociceptive (Charlet et al., 2008), suggesting they may have diverse functions in the CNS in addition to stress and reproductive function.
3α,5α-THP, 3α,5β-THP and related neurosteroids such as THDOC (3α,21-dihydroxy-5 α -pregnan-20-one) are well known as potent positive modulators of the GABAR, where most definitive pharmacological studies have typically employed standard whole cell recording techniques to measure the effects on depolarizing, inward current (i.e., outward Cl- flux). However, recent evidence suggests that neurosteroids have unique effects at α4βδ GABARs which are dependent upon the direction of Cl− flux, such that they increase depolarizing current but decrease hyperpolarizing Cl− current (Shen et al., 2007), an effect initially demonstrated in recombinant receptors. In dentate gyrus granule cells, which normally have a high level of α4βδ expression (Wei et al., 2003), the neurosteroid THDOC increases the tonic inhibitory current produced by α4βδ GABARs and reduces neuronal excitability (Stell et al., 2003; Chiang et al., 2012). While GABAergic current recorded from these cells has been shown to be depolarizing, it results in a shunting inhibition (Staley and Mody, 1992). Thus, the net effect of neurosteroids here would be to enhance this inhibition and decrease excitability, as has been demonstrated (Stell et al., 2003). However, in mature CA1 hippocampal pyramidal cells, GABAergic inhibition is hyperpolarizing (Shen et al., 2007;(Lambert et al., 1991). Although, expression of α4βδ GABARs here is normally very low (Shen et al., 2010), under conditions where expression of these receptors increases significantly, such as puberty, 3α,5β-THP produces a paradoxical excitatory effect (Shen et al., 2007). At the onset of puberty, 3α,5β-THP reduces the tonic inhibitory (hyperpolarizing) current, thereby increasing neuronal excitability in vitro and increasing anxiety (Shen et al., 2007). These effects are not observed in the δ −/− mouse, consistent with the polarity-dependent effect of the steroid observed in recombinant receptors (Shen et al., 2007). Anxiogenic effects mediated by α4βδ GABARs at puberty are also seen after stress-related increases in endogenous 3α,5α-THP (Shen et al., 2007).
We tested the possibility that α4βδ GABARs would be increased compensatorily in CA1 hippocampus by the stimulant actions of METH (Yamamoto et al., 1999). The unique characteristics of this receptor may explain the seemingly paradoxical outcomes of METH withdrawal, which include lethargy and depression combined with stress-induced anxiety (London et al., 2004) because α4βδ receptors generate a shunting inhibition which can be reduced by both 3α,5α-THP and 3α,5β-THP leading to increased anxiety. In addition, increased expression of α4βδ GABARs is reported to be one compensatory mechanism for increased neuronal excitability which may serve a neuroprotective role (Santhakumar et al., 2010). Our findings in this study indeed suggest that α4βδ GABARs are increased by chronic METH treatment and withdrawal, and both 3α,5α-THP and 3α,5β-THP exert anxiety-producing effects during METH withdrawal.
2. Experimental Procedure
2.1. Subjects
Adult, male Long Evans rats (350-400 grams, Charles River) and adult, male C57/BL6 mice (10-12 weeks old) were used. Mice were initially bred from heterozygous GABAR α4 +/− pairs (supplied from G. Homanics, U. Pittsburgh), and genotyped from tail clips. Both +/+ and α4 −/− mice were used. (α4 −/− mice were used rather than δ−/− in order to maintain α1βδ on the interneurons (Glykys et al. 2007).) Animals were housed two to a cage in an animal facility controlled for light (12:12) and temperature (20-22°C), and tested in the light. Procedures were in accordance with the SUNY Downstate Institutional Animal Care and Use Committee and with the National Institute of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2.2. Drug treatment
Rats were injected with 3 mg/kg methamphetamine (METH, i.p., in sterile saline) 2x/d, 4d/week for 3-5 weeks to establish dependence (Kamei and Ohsawa, 1996; Kitanaka et al., 2010). Mice were administered the drug with a similar protocol, except that they were injected once a day (Kitanaka et al., 2010; Kamei and Ohsawa, 1996). (Because results (GABAR assessment in Western blots, gaboxadol responses) were similar for animals injected 3 weeks vs. 5 weeks, the results were pooled.) Animals were injected for 4 consecutive days with the drug and then allowed a 3 day break to simulate a modified binge schedule. Both the doses and frequency of METH administration we have used are below those shown to produce neurodegeneration (>10 mg/kg or multiple doses of lower concentrations within an 8-10 h period) (Cadet and Krasnova, 2009; Ladenheim et al., 2000; Zhu et al., 2006).
Animals were tested either 1 h after the last injection of the chronic injection protocol (chronic METH) or after a 1d or 2, 3, 4, 5 or 6 weeks following withdrawal from METH. The withdrawal time in all cases is measured after the final injection of the METH. In some cases, animals received flumazenil (Flu, 10 mg/kg, i.p., in oil) or vehicle injections 1, 8 and 24 h after the final METH injection following the chronic administration paradigm. In other cases, mice were tested 1 h after a single METH injection (acute METH). Animals received either 3α,5β-THP (pregnanolone or 3αOH-5β-pregnan-20-one, 10 mg/kg, i.p., in oil), 3α,5α-THP (allopregnanolone or 3αOH-5α-pregnan-20-one, 10 mg/kg, i.p., in oil) or vehicle 20 min before behavioral testing.
2.3. Western blot
Standard procedures were used to detect α1 (51 kDa, EMD Millipore), α2 (51 kDa, Novus), α4 (67 kDa, Santa Cruz), α5 (52 kDa, Aviva Systems Biology), and δ (54 kDa, Novus) immunoreactivity in crude membrane preparations from hippocampus using protein concentrations in the linear range (Shen et al., 2007). Following electrophoresis on a 10% NuPage Bis-Tris gel, proteins were transferred to nitrocellulose membranes and probed for immunoreactivity. Band density was visualized by enhanced chemiluminescence, and optical density determined using Adobe Photoshop software. Results were normalized to the GAPDH optical density from the same samples (36 kDa).
2.4. Electrophysiology
2.4.1. Whole cell patch clamp recording
Following standard preparation (Shen et al., 2007), hippocampal slices were used for recording CA1 pyramidal cells, visualized with a Leica infrared-differential interference contrast (IR-DIC) upright microscope. Current was recorded at –60 mV with whole cell patch clamp procedures (22-24° C), using an Axopatch 200B amplifier, at a 10 kHz sampling frequency (2 kHz 4-pole Bessel filter) and pClamp 9.2 software. Patch pipets were fabricated from borosilicate glass using a Flaming-Brown puller (Sutter Instruments) to yield open tip resistances of 2–4 MΩ. For whole cell recordings of pharmacologically isolated (2 mM kynurenic acid) inhibitory current, the pipet solution contained in mM: CsCl 140, HEPES 5, EGTA 5, CaCl2-H2O 0.5, QX-314 5, Mg-ATP 2, Li-GTP 0.5, pH 7.2, 290 mOsm. When investigating effects of the polarity of the GABAergic current, the pipet solution either contained 45 mM CsCl + 95 mM K-gluconate (depolarizing, ECl = −30 mV) or 140 mM K-gluconate (hyperpolarizing, ECL = −70 mV) instead of 140 mM CsCl, and currents were recorded at a −50 mV holding potential. Electrode capacitance and series resistance were monitored and compensated. Recordings were discarded if the series resistance changed more than 20% during the experiment. The bath contained (in mM): NaCl 124, KCl 5, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26, and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. The tonic GABAergic current was recorded as the difference current produced by the selective GABAA receptor antagonist SR95531 (120 μM, Stell and Mody, 2003), and the current generated by 100 nM gaboxadol (THIP or 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol), which at this concentration is selective for δ-containing GABARs (Brown et al., 2002; Meera et al., 2011), reflected the tonic current produced by α4βδ GABARs. In addition, the peak-to-peak noise produced by gaboxadol is an additional measure of α4βδ current, referred to herein as δ-noise.
Frequency, amplitude and half-width were determined for averaged sIPSCs or mIPSCs (1 μM TTX) using a threshold delimited event detection program in pClamp 10.1 (Hsu et al., 2003). Probability plots of sIPSC amplitude were constructed using Origin (Microcal). mIPSCs were recorded before and after bath application of 10 μM lorazepam to test for the presence of α4-containing GABARs because these receptors lack a benzodiazepine binding site (Weiland et al., 1992) and are thus benzodiazepine-insensitive (Wafford et al., 1996). Therefore, determining the effect of lorazepam on the half-width of mIPSCs can be used as an estimate of the relative presence of α4-containing GABARs subsynaptically, as we have reported before (Hsu et al., 2003).
2.4.2. Cell-attached recording
We used tight-seal cell-attached recording techniques in current clamp mode to assess whether GABA agonists yielded a hyperpolarizing or depolarizing potentials in hippocampal slices from different treatment groups (Mason et al., 2005; Perkins, 2006). With a >1 gigohm seal and passing 0 current, Rseal>>Rpatch+cell allows determination of the direction of potential change in response to a GABA agonist because a high resistance seal produces a detectable voltage change in response to the ligand-gated current as we have previously described (Shen et al., 2007). Therefore, for this study, tight-seal (>1 gigohm) cell-attached recording of membrane potentials were made from the soma of CA1 hippocampal pyramidal cells in the slice in current clamp mode. The pipet solution contained 150 mM NaCl. The response of the membrane potential to bath application of the GABA agonist gaboxadol (5 μM, THIP) was recorded (with no additional current injected). For this study and perforated patch recordings, bath-applied CGP 55845A (1 μM) was used to block GABAB receptors (Davies et al., 1993). TTX (0.5 μM) and GABA (1 μM) were added to isolate the post-synaptic component, TEA (5 mM) was used to block K+ channels and 200 nM SR95531 added to block synaptic GABAergic current (Stell and Mody, 2003).
2.4.3. Perforated patch recording
Because the effect of 3α,5β-THP is dependent upon the direction of Cl− flux through α4βδ GABAR, we recorded current using gramicidin perforated patch techniques to maintain the internal Cl− milieu, as we have previously described (Shen et al., 2007). Following standard slice preparation (see above), CA1 hippocampal pyramidal cells were patched with pipets containing 140 mM KCl, 25 μg ml–1 gramicidin (mixed by sonication), 0.1 mM MgCl2, .07 mM CaCl2, 10 mM HEPES and 0.1 mM EGTA, pH 7.3, 300 mOsm. The pipet was front-filled with gramicidin-free solution to permit successful tight-seal formation. After >1 gigohm seals were formed, access resistance was periodically checked as the steady-state response to a 10 mV hyperpolarizing step. Recording commenced after a stable access resistance <60 megohm had been achieved, indicating a stable level of perforation. The holding current was recorded before and after bath application of 30 nM 3α,5β-THP.
2.4.4. Paired pulse ratio
IPSCs were recorded from pyramidal cells in response to paired stimuli (0.05 Hz, 50 ms interpulse interval; 500 μs duration, 100-200 μA) delivered to the stratum radiatum with a bipolar tungsten electrode ~500 μm from the recording electrode in order to test potential presynaptic effects (Galante and Marty, 2003) of 3α,5β-THP. The paired pulse ratio was defined as IPSC2/iPSC1, and adjusted to ~2 before bath application of 30 nM 3α,5β-THP.
2.4.5. Current clamp recording
Whole cell current clamp recordings were used to assess effects of 3α,5β-THP on neuronal excitability (Shen et al., 2007) (–58 mV holding potential); (The pipet solution contained 140 mM K-gluconate, but without QX-314.) Current was injected in 20 pA-1 s steps, and the current necessary for triggering a spike determined before and after bath application of 30 nM 3α,5β-THP. The voltage threshold for spike generation, spike amplitude and half-width were also determined. Input resistance (Rm) was calculated using Ohm's Law. Only cells with a resting membrane potential < –58 mV, access resistance < 20 megohm, input resistance > 30 megohm, action potential amplitude > 70 mV and regularly timed spiking, as well as a stable baseline, were used for the current clamp recordings.
2.4.6. Cell-attached recordings of pyramidal cell spiking
In some cases, cell-attached recordings were made in voltage clamp mode to assess cell spiking before and after bath application of 30 nM 3α,5β-THP (150 mM NaCl pipet solution). Spiking (action potential currents) was assessed after achieving a >1 gigohm seal in cells where spontaneous activity was observed. The command potential was set to the potential at which the holding current was 0 pA to avoid direct cell stimulation by the electrode. This technique is advantageous in permitting evaluation of neuronal excitability without disturbing the internal Cl− milieu (Shen et al., 2007).
2.5. Animal model of anxiety
2.5.1. Acoustic startle response (ASR) paradigm
Rats were placed in an 8.8 cm diameter Plexiglas cylinder attached to a piezoelectric transducer platform to detect the motion of the rat (S-R Laboratory device, San Diego Instruments, San Diego, CA). Mice were tested with a 5.1 cm diameter cylinder in the same manner. Movement of the platform results in a voltage change in the transducer, which was digitized and analyzed by the S-R Laboratory program on an attached computer. After a 5 min. acclimatization period in the startle apparatus, animals were presented with a 120 dB sound pulse of 40 ms duration. Startle magnitude was defined as the integrated total body movement in response to the sound pulse (Curzon et al., 2009; Gulinello et al., 2003).
Because some studies have suggested that adult mice have hearing loss in the high frequencies (Willott et al., 2000), we verified that the volume of the white noise tone and signal duration were optimal for the acoustic startle response (ASR). To this end, mice were tested for their response to a range of decibels (dB): 85-120 and signal duration (10-90 ms), presented in random order to 10 untreated adult, male mice. Studies confirmed that a 40 ms, 120 dB tone was optimal (Fig 11A).
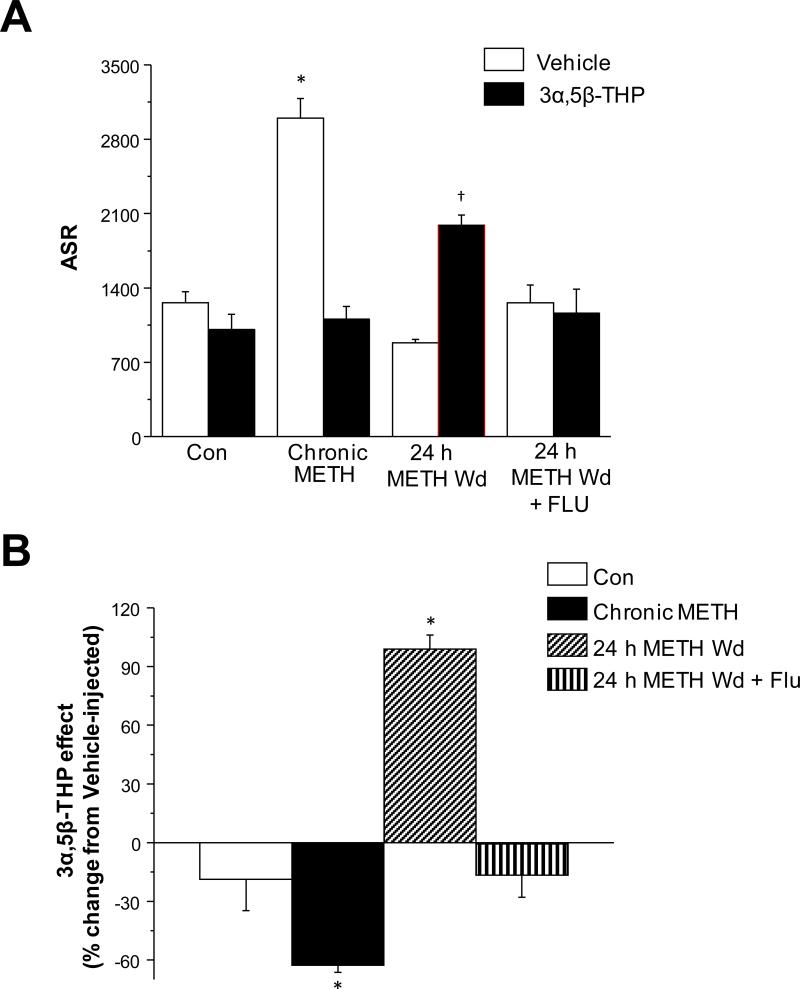
Fig. 11. 3α,5β-THP increases anxiety after METH withdrawal in rats: reversal by flumazenil.
Anxiety-like behavior was tested using the acoustic startle response (ASR) across METH treatments. All animals were injected with 3α,5β-THP (10 mg/kg, i.p.) or oil vehicle 20 min. before testing. A, ASR in vehicle or 3α,5β-THP-injected rats. Mean ± SEM, 2-factor ANOVA, F(7,61)=19.51, P<0.0001; *P<0.05 vs. all other vehicle-treatment groups; †P<0.05 vs. all other 3α,5β-THP -treatment groups; n=9 rats/group. B, 3α,5β-THP effects on the ASR across METH treatment groups. Mean ± SEM, 1-factor ANOVA, F(3,31)=47.7, P<0.0001; *P<0.05 vs. all other groups, n=9 rats/group. 3α,5β-THP increased the startle response only after a 24 h METH Wd (90% of animals), while Flu prevented this effect.
2.5.2. Analysis of the effect of 3α,5α-THP and 3α,5β-THP on anxiety behavior
Both 3α,5α-THP and 3α,5β-THP were tested for their effect on the acoustic startle response (ASR) compared to the effects of oil vehicle, where these neurosteroids could either increase or decrease the peak startle amplitude. Therefore, the data for ASR is presented both as the raw startle response as well as the 3α,5α-THP or 3α,5β-THP-induced change (as a percentage change from the response obtained from vehicle-injected animals for each METH or saline treatment group).
2.6. Drugs and chemicals
All Western blot supplies were from Invitrogen (Carlsbad, CA). All other chemicals used for solutions were from Sigma Chemical Co. (St. Louis, MO) except for QX-314 (Calbiochem, San Diego, CA). Drugs used for injection, methamphetamine and flumazenil, were also from Sigma Chemical Co. 3α,5α-THP and 3α,5β-THP were from Steraloids, Inc. (Newport, RI). The vehicle used for METH was sterile saline, while the vehicle used for flumazenil and THP was 100% sesame oil.
2.7. Statistics
All data are presented as the mean ± standard error of the mean (SEM). For most experiments with more than two groups, comparisons were made using a one-factor analysis of variance (indicated as ANOVA). For the raw behavioral data, a two-factor ANOVA was used, with METH/saline treatment and THP/oil injection used as the two factors, respectively. Analysis of the effects of 3α,5α-THP and 3α,5β-THP on the ASR was accomplished with a one-factor ANOVA. In all cases, a Tukey's post-hoc test was used to identify significant differences between individual groups. For experiments where two groups were compared, the student's t-test was used (unpaired). A paired t-test was used for experiments where effects of 3α,5β-THP were determined in the same cell. All data fit a normal distribution, and significance was determined with a P<0.05 (Origin, Statistica).
3. Results
3.1. METH and α4βδ GABAR expression in rat hippocampus
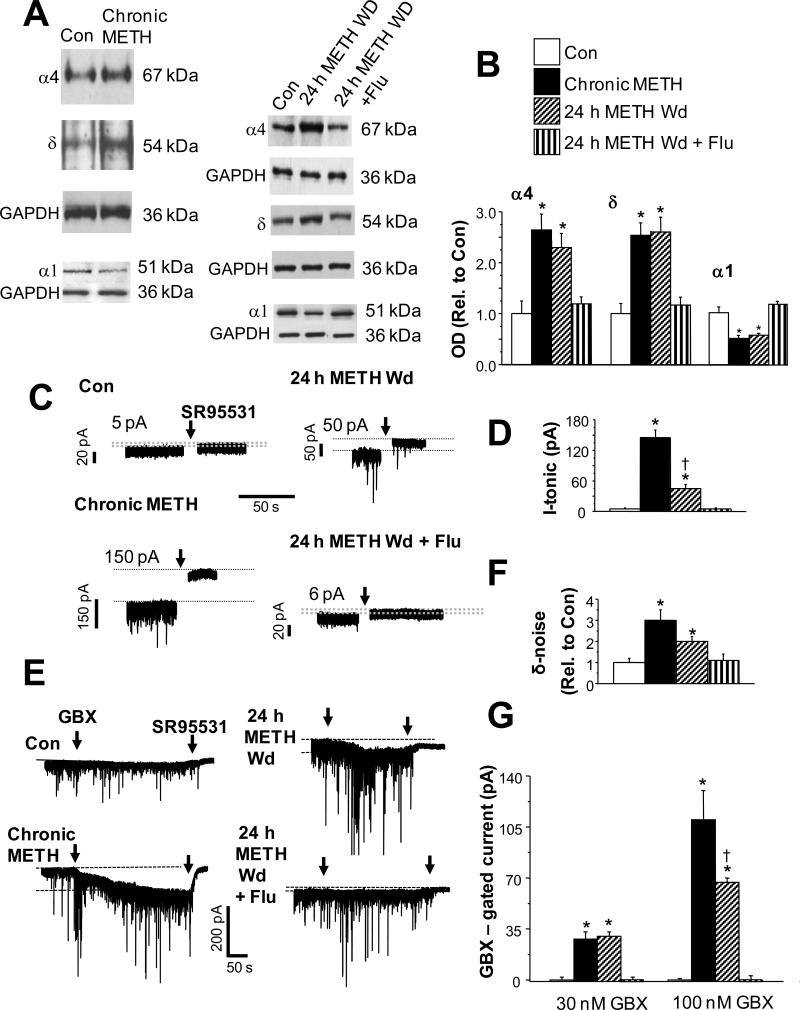
We initially used adult, male rats, in order to compare with the body of published work on stimulant drugs using this species (Kitanaka et al., 2008), to assess the effect of chronic METH exposure on expression of hippocampal α4βδ GABAR. Following the chronic METH administration protocol, hippocampal expression of both α4 and δ subunits was increased 2-3-fold compared to sham-injected controls, assessed by Western blot techniques (Fig. 1A,B). Increased levels of expression were maintained during the 24 h period of withdrawal (METH Withdrawal). We also tested the effect of flumazenil, a drug classically known as a benzodiazepine antagonist (Olsen and Sieghart, 2009), which can also bind to α4βδ receptors (Wallner et al., 2006) and regulate α4 expression (Biggio et al., 2007). When administered 3 times across the 24 h withdrawal period, flumazenil (10 mg/kg, i.p.) effectively reduced hippocampal expression of α4 and δ subunits to levels not significantly different from control (Fig. 1A,B). Expression of the α1 subunit was decreased ~50% by chronic METH treatment as well as after a 24 h METH withdrawal, an effect also reversed by flumazenil treatment (Fig. 1A,B) (n=5 rats/group, performed in duplicate).
Fig. 1. Chronic methamphetamine increases expression of α4 and δ GABAA receptor subunits in rat hippocampus: Western blot and pharmacological analysis.
A, Western blot analysis of GABAA receptor (GABAR) α4 (67 kDa), δ (54 kDa) and α1 (51 kDa) subunit expression in hippocampus following chronic methamphetamine (METH) or a 24 h METH Wd, with or without Flu (flumazenil). B, Averaged optical densities (OD), mean ± SEM. Results are normalized to the housekeeping protein GAPDH and are expressed as a ratio relative to the vehicle-treated control (OD drug treatment/OD Con). ANOVA, α4, F(3,16)=12.6, P=0.0002; δ, F(3,16)=20.1, P<0.0001; α1, F(3,16)=14.05, P<0.0001; *P<0.01 vs. Con, 24 h METH Wd + Flu; n=5 rats/group, performed in duplicate. C,D, Tonic current. C, Representative traces. Dashed lines indicate holding current before and after GABAR blockade. The difference current (pA) indicated for each trace. D, Mean ± SEM, the current shift in response to the GABA antagonist SR95531 (arrow, 120 μM) (ANOVA, F(3,24)=72.5, P<0.0001). *P<0.01 vs. Con, 24 h METH Wd + Flu; †P<0.05 METH Wd vs. Chronic METH; n=7 rats/group. In this and the following figures, arrows indicate continuous bath application of the indicated drug. E-G, Response of CA1 pyramidal cells to low concentrations of the GABA agonist gaboxadol (100 nM GBX, arrow) followed by SR95531 (120 μM, arrow). Chronic METH and 24 h METH Wd groups showed greater responsiveness to GBX, consistent with increased expression of α4βδ GABARs. E, Representative traces. Dashed lines indicate holding current before and after GBX. F, mean ± SEM, peak-to-peak noise generated by α4βδ (δ-noise, ANOVA, F(3,24)=6.0, P=0.0032). G, mean ± SEM, GBX-gated current: ANOVA, 30 nM, F(3,24)=26.37, P<0.0001; 100 nM, F(3,24)=22.6, P<0.0001; *P<0.0001 vs. Con, 24 h METH Wd + Flu, †P<0.05 vs. Chronic METH, n=7 rats/group.
3.2. METH and the inhibitory tonic current in rat hippocampus
α4βδ GABARs underlie a tonic current (Stell and Mody, 2002) which can be assessed by the shift in the holding current in response to the GABA antagonist SR95531 (120 μM, Fig. 1C,D) using whole cell patch clamp recordings from CA1 hippocampal pyramidal cells in the slice preparation. This parameter was significantly increased by chronic exposure to METH (by 30-fold) as well as by 24 h METH withdrawal (by 10-fold). Chronic METH treatment also reduced Rm by 40 ± 6% (t(8)=2.89; P=0.02). Flumazenil treatment during 24 h METH withdrawal reduced both measures of tonic current to control levels (n=7 rats/group).
In order to determine the functional expression of α4βδ receptors, we assessed the response of the current to the GABA agonist gaboxadol (100 nM, THIP), which at this concentration is selective for δ-containing GABAR (Brown et al., 2002; Meera et al., 2011). Responses to this drug were increased from negligible levels in control recordings to 26-30 pA (30 nM gaboxadol) and 65-108 pA (100 nM gaboxadol), and 100 nM gaboxadol also increased the peak-to-peak noise generated by α4βδ GABARs by 3-fold after METH exposure and withdrawal conditions, suggesting functional expression of α4βδ (Fig. 1E-G). Flumazenil treatment reduced the response to gaboxadol to control levels during METH withdrawal, as predicted by its effect to reduce α4 and δ expression (n=7 rats/group).
3.3. METH and the inhibitory synaptic current in rat hippocampus
sIPSC frequency was increased by ~16-fold (P<0.001) and sIPSC amplitude was increased by about 80% (P<0.05) by chronic METH treatment (Fig. 2A-C). Increases in both parameters were maintained following cessation of drug treatment in the METH withdrawal state (n=4-5 rats/group).
Fig. 2. Chronic METH increases the inhibitory synaptic current in rat CA1 hippocampal pyramidal cells.
A, Whole cell voltage clamp recordings from CA1 hippocampal pyramidal cells in the slice. Representative traces illustrating the increase in sIPSC amplitude and frequency following chronic treatment of rats with METH or after a 24 h withdrawal (24 h METH Wd). B, Probability plots demonstrating the range of amplitudes for sIPSCs. C, Mean ± SEM, sIPSC amplitudes (means ± SEM). ANOVA, F(2,391)=31.2, P<0.0001; *P<0.05 vs. Con. D, Mean ± SEM, sIPSC frequencies. ANOVA, F(2,11)=8.3, P=0.0063; *P<0.001 vs. Con; †P<0.05 vs. Chronic METH; n=4-5 rats/group. D, Mean ± SEM, mIPSC half-width before and after 10 μM lorazepam (LZM). E, Representative traces. LZM produced significant increases in mIPSC half-width of similar magnitude in recordings from Con and Chronic METH groups. (LZM effect, *Con, t(10)=4.47, P=0.0012; *Chronic METH, t(10)=3.0, P=0.0133; Con vs. Chronic METH, t(10)=1.32, P=0.22); n=5-6 rats/group.
We explored the possibility that α4-containing GABARs were synaptically localized following METH exposure. This was tested by examining the sensitivity of mIPSCs to the benzodiazepine lorazepam (Hsu et al., 2003). Lorazepam is a benzodiazepine agonist which potentiates GABA-gated current at γ2-containing GABARs that do not contain the α4 or α6 subunit (Wafford et al., 1996). Inclusion of the α4/6 subunit renders the receptor benzodiazepine-insensitive because the presence of an arginine at residue 99, instead of a histidine at homologous residues of benzodiazepine-sensitive receptors, such as α1, prevents binding of the benzodiazepine agonist (Wieland et al., 1992). Thus, lorazepam sensitivity can be a useful predictor of α4 expression sub-synaptically. (Hsu et al., 2003; Knoflach et al., 1996; Wafford et al., 1996). In fact, 10 μM lorazepam produced similar increases in the mIPSC half-width in pyramidal cell recordings from control and chronic METH rats suggesting that α4 is not expressed subsynaptically following METH exposure (Fig. 2D,E) (n=5-6 rats/group).
3.4. Polarity of GABAergic current in rat hippocampus
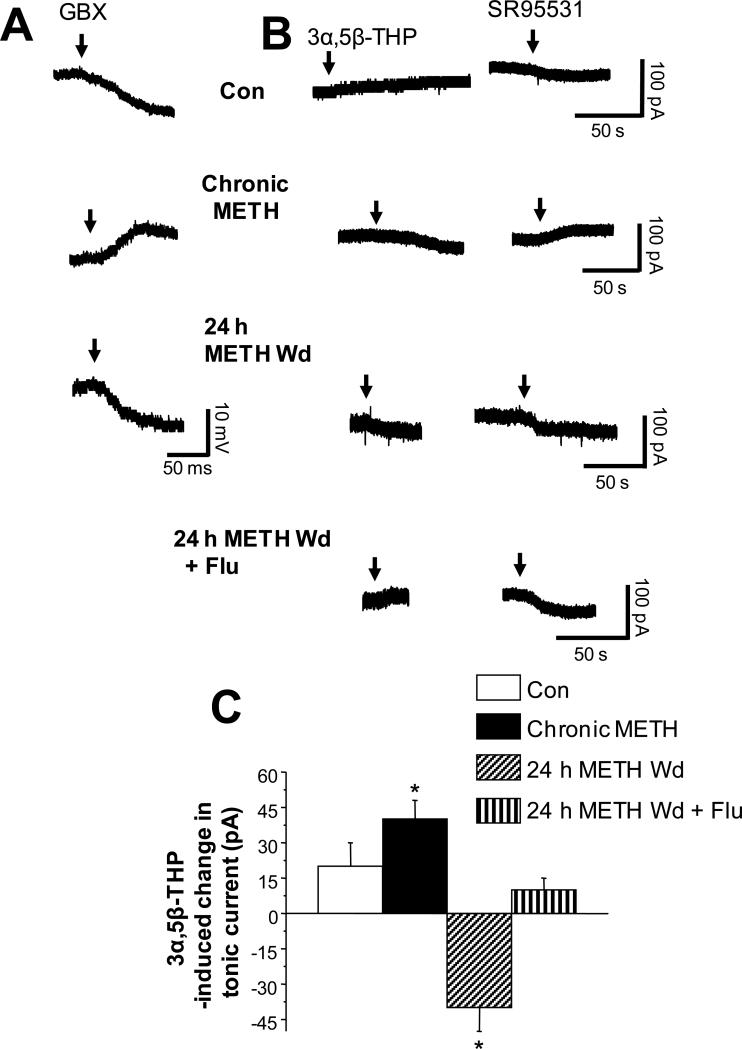
Because the effect of 3α,5β-THP at α4βδ GABAR is dependent upon the direction of Cl− flux (Shen et al., 2007), we assessed this parameter using tight seal (>1 gigohm), cell-attached recording techniques (Mason et al., 2005; Perkins, 2006) in current clamp mode in the presence of TTX (tetrodotoxin). Under these conditions, because the resistance of the pipet seal is much greater than that of the patch, changes in cell membrane potential can be recorded by the electrode. Thus, the polarity of the membrane potential change caused by a GABA agonist can be measured without disturbing the intracellular milieu, where a hyperpolarizing (downward) response to agonist indicates an outward current (inward Cl− flux).
As expected, the GABA agonist gaboxadol was hyperpolarizing in control neurons (Fig. 3A). In contrast, this agonist was depolarizing during chronic METH treatment, as indicated by the upward shift in the membrane potential (Fig. 3A, ANOVA, F(2,9)=20.5, P<0.0001, n=3 rats/group). However, 24 h after METH withdrawal, the direction of GABAergic current reverted back to outward.
Fig. 3. 3α,5β-THP reduces outward GABAergic tonic current after a 24 h METH withdrawal in rat hippocampus.
A, Representative traces of tight seal cell-attached recordings in current clamp mode (with no current injection) from CA1 hippocampal pyramidal cells testing polarity of GABAergic current by the direction of response to bath applied GBX (arrow): Con, hyperpolarization (downward deflection); chronic METH, depolarization; 24 h METH Wd, hyperpolarization. Rep. of results from 3 cells, each. B, Gramicidin perforated patch recordings of the holding current in voltage clamp mode (−65 mV holding potential) in response to 30 nM 3α,5β-THP (left, arrow) and 120 μM SR95531 (right, arrow). 3α,5β-THP decreased the tonic current after a 24 h METH Wd (produced a shift in the same direction as SR95531), but not in the other groups. C, Mean ± SEM, ANOVA, F(3,24)=62.6, P<0.0001; *P<0.05 vs. all other groups n=7 rats/group.
3.5. Effects of 3α,5β-THP in rat hippocampus
We predicted that the effect of 3α,5β-THP on tonic inhibition during chronic METH treatment and 24 h METH withdrawal would depend upon the direction of GABAergic current, because depolarizing current mediated by α4βδ GABARs is enhanced by 3α,5β-THP while hyperpolarizing current is reduced (Shen et al., 2007) (Summary Fig. 4). In this case, we recorded the GABAergic tonic current from CA1 hippocampal pyramidal cells using gramicidin perforated patch voltage clamp techniques to maintain the physiological Cl− gradient in the presence of TTX. Because the direction of GABAergic current varies across groups, the effect of 3α,5β-THP can best be determined by comparing its effect with that of a GABA antagonist, SR95531. This antagonist produced shifts in the holding current of opposite direction in recordings from pyramidal cells of chronic METH versus 24 h METH withdrawal (Fig. 3C,D), consistent with the cell-attached and perforated patch recordings in Fig. 3A,B showing that chronic METH produces depolarizing GABAergic current, while 24 h METH withdrawal leads to hyperpolarizing GABAergic current. As predicted by studies in recombinant receptors (Shen et al., 2007), the depolarizing GABAergic current recorded from the chronic METH group was enhanced by 3α,5β-THP (Fig. 3C), because it shifted the holding current downward, in a direction opposite to the GABA antagonist. In contrast, the hyperpolarizing GABAergic current recorded from the 24 h METH withdrawal group was attenuated by 3α,5β-THP, which also produced a downward shift in the holding current, but in this case, in the same direction as the GABA antagonist. Thus, 3α,5β-THP decreased tonic inhibition following a 24 h METH withdrawal (Table 1, summary; n=7 rats/group). This effect of the steroid was prevented by flumazenil such that 3α,5β-THP had minimal effects on the tonic current.
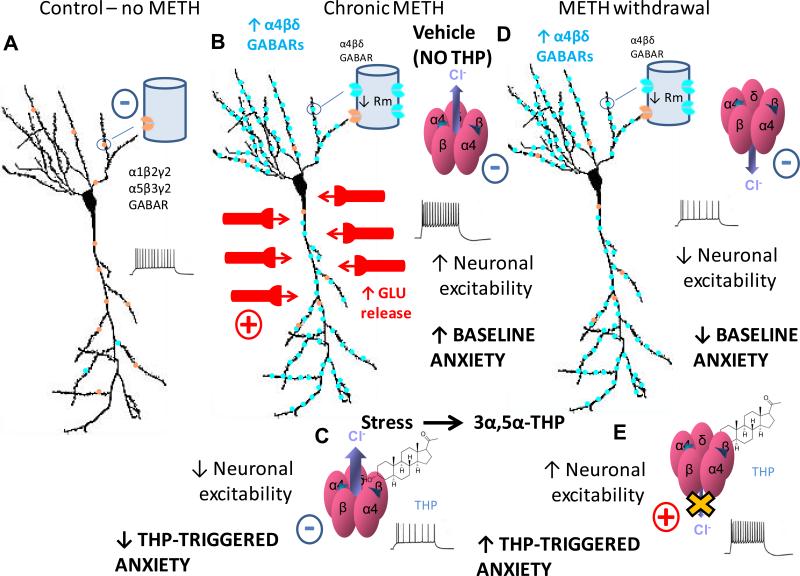
Fig. 4. Summary diagram of GABAA receptor changes and functional outcome due to METH exposure and withdrawal.
A, In the absence of METH treatment (control conditions), the predominant GABAA receptors (GABARs) on CA1 pyramidal cells are α1β2γ2 and α5β3γ2. B, During chronic METH treatment, glutamate (GLU) release increases significantly, which increases neuronal excitability despite compensatory increases in expression of α4βδ GABARs. This is because the excitatory input >> inhibitory compensation (α4βδ GABARs). Excitatory events indicated by +, inhibitory events indicated by -. Thus, during chronic METH, BASELINE ANXIETY is increased. C, Cl- flux through these GABARs is depolarizing (outward flux) probably as a result of the increase in neuronal activity, producing a shunting inhibition. (A shunting inhibition is produced (rather than excitation) when the Cl- reversal potential is close to the membrane potential, and the driving force for Cl- flux is small. Thus, the primary effect of GABAR activation is to reduce the membrane resistance which reduces the voltage produced by depolarizing current.) Now the neurosteroid 3α,5β-THP (“THP”) increases the GABA-gated current at these receptors, which increases the shunting inhibition, thereby decreasing neuronal excitability. As would be predicted, 3α,5β-THP decreases anxiety (“THP-TRIGGERED ANXIETY”). (Although not tested in this study, it is predicted that 3α,5α-THP would have a similar effect based on our findings after METH withdrawal.) D, After METH withdrawal (1d – 4 wk), the increase in α4βδ GABAR expression is maintained, but the stimulatory effects of METH are now gone, thus GLU release is no longer increased. As a result, neuronal excitability is decreased. BASELINE ANXIETY is also reduced compared to the chronic METH condition. E, However, after a 24 h METH withdrawal, the GABAergic Cl- current reverts to hyperpolarizing (inward Cl- flux) probably because neuronal excitability is decreased. Under these conditions, 3α,5β-THP (“THP”) decreases hyperpolarizing current at α4βδ GABARs, increasing neuronal excitability. Both 3α,5α-THP and 3α,5β-THP increase anxiety after METH withdrawal. Because 3α,5α-THP is released by stress, this suggests that one outcome of the stress response during METH withdrawal is to reduce GABAergic inhibition and increase anxiety (“THP-TRIGGERED ANXIETY”). This may represent a mechanism for stress-triggered anxiety, which is exacerbated after METH withdrawal (24 h – 4 weeks).
Table 1.
Summary of the effects of in vivo METH treatments resulting from experiments performed in mice1, rats2 or both* on CA1 hippocampal pyramidal cells. Both α4βδ GABAR expression levels and the polarity of the GABAergic current determine the ultimate effect of 3α,5β-THP on the tonic current and neuronal excitability.
| α4βδ expression in CA1 hippocampus | GABA current | Effect of GABA current* | Effect of 3α,5β-THP on the tonic GABAergic current | Effect of 3α,5β-THP on neuronal excitability | |
|---|---|---|---|---|---|
| CON* | Low | Hyperpolarizing | Inhibitory | Increase | Decrease |
| CHRONIC METH* | High | Depolarizing | Inhibitory | Increase | Decrease |
| METH WD* | High | Hyperpolarizing | Inhibitory | Decrease | Increase |
| METH WD α4−/−1 | None | No effect | |||
| METH WD + FLU2 | Low | Hyperpolarizing | Inhibitory | Increase | Decrease |
GABA was inhibitory in all cases regardless of the polarity of the current.
3.6. Paired pulse ratio of IPSC responses in rat hippocampus
In order to rule out the possibility that 3α,5β-THP was acting presynaptically to reduce GABA release during METH withdrawal, we assessed the ratio of IPSC responses to paired stimuli. Alterations in this parameter are routinely considered a measure of altered transmitter release (Dobrunz and Stevens, 1997; Galante and Marty, 2003). In fact, 3α,5β-THP had no effect on the paired pulse ratio recorded from CA1 pyramidal cells following METH withdrawal (2.2 ± 0.14, pre- 3α,5β-THP; 2.34 ± 0.28, 3α,5β-THP, n=4 rats/group; Fig. 5), suggesting that it was not acting presynaptically.
Fig. 5. 3α,5β-THP does not alter the paired pulse ratio of evoked IPSCs in rat hippocampus after a 24 h METH withdrawal.
IPSC responses to paired stimuli assessed before and after bath application of 30 nM 3α,5β-THP. The ratio of IPSC response was not altered by 3α,5β-THP, suggesting that the steroid does not act presynaptically 24 h after METH withdrawal. A, Mean ± SEM. B, Representative traces. P=0.11; n=4 rats/group.
3.7. Current clamp recordings in rat hippocampus
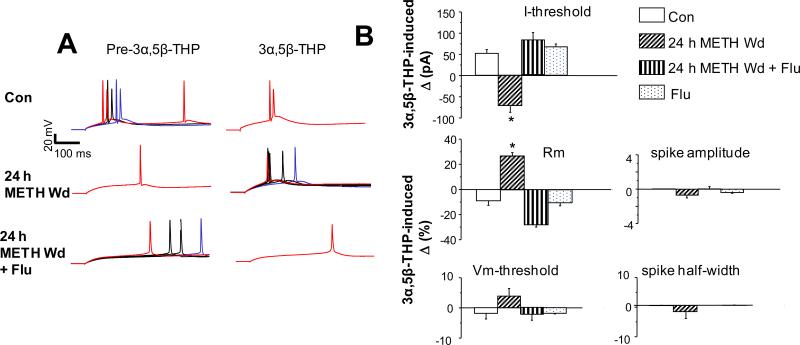
By reducing tonic inhibition, 3α,5β-THP should thereby increase neuronal excitability 24 h after withdrawal from METH (Summary Fig. 4). This was tested directly using whole cell patch clamp recording of pyramidal cells in current clamp mode. Under these conditions, 3α,5β-THP reduced the current necessary for triggering a spike by 71 ± 18 pA (P<0.05) and increased Rm by 29.6 ± 2% (P<0.05) in slices from rats undergoing a 24 h METH withdrawal (Fig. 6), compared to pre-3α,5β-THP levels. Both effects were opposite to the effect of 3α,5β-THP under control conditions, when the steroid increased the current necessary for triggering a spike by 51 ± 11 pA (P<0.05) and reduced Rm by 9.4 ± 3% (P<0.05), suggesting that its normal function is to increase inhibition, as has been widely reported (Stell et al., 2003). However, 24 h METH withdrawal did not significantly alter spike characteristics, suggesting a selective effect on GABARs. Administration of flumazenil 24 h after METH withdrawal or to control animals resulted in outcomes similar to control for all parameters assessed (n=4-5 rats/group).
Fig. 6. 3α,5β-THP increases excitability of rat CA1 hippocampal pyramidal cells after a 24 h METH withdrawal.
A, Representative traces and B, Mean ± SEM, 3α,5β-THP effects on neuronal excitability recorded from CA1 pyramidal cells (rat) in the hippocampal slice using whole cell recordings in current clamp mode. Membrane voltage was assessed in response to 20 pA increases in current injection. A, Red, current threshold (I-threshold) for action potential (AP) generation for the less excitable group (the recording with the higher current threshold: pre-3α,5β-THP or post-3α,5β-THP). Blue, I-threshold for AP generation for the more excitable group (i.e., the recording with the lower current threshold) before or after bath application of 30 nM 3α,5β-THP. Black, trace(s) in-between the two threshold currents. B, 3α,5β-THP increased Rm (ANOVA, F(3,14)=73, P<0.0001) and lowered I-threshold (ANOVA, F(3,14)=22, P<0.0001) only after 24 h METH Wd, an effect reversed by FLU; n=4-5 rats/group. *P<0.0001 vs. all other groups; n=4-5 rats/group.
3.8. Effect of METH in the mouse
3.8.1 Hippocampal α4 and δ expression in the mouse
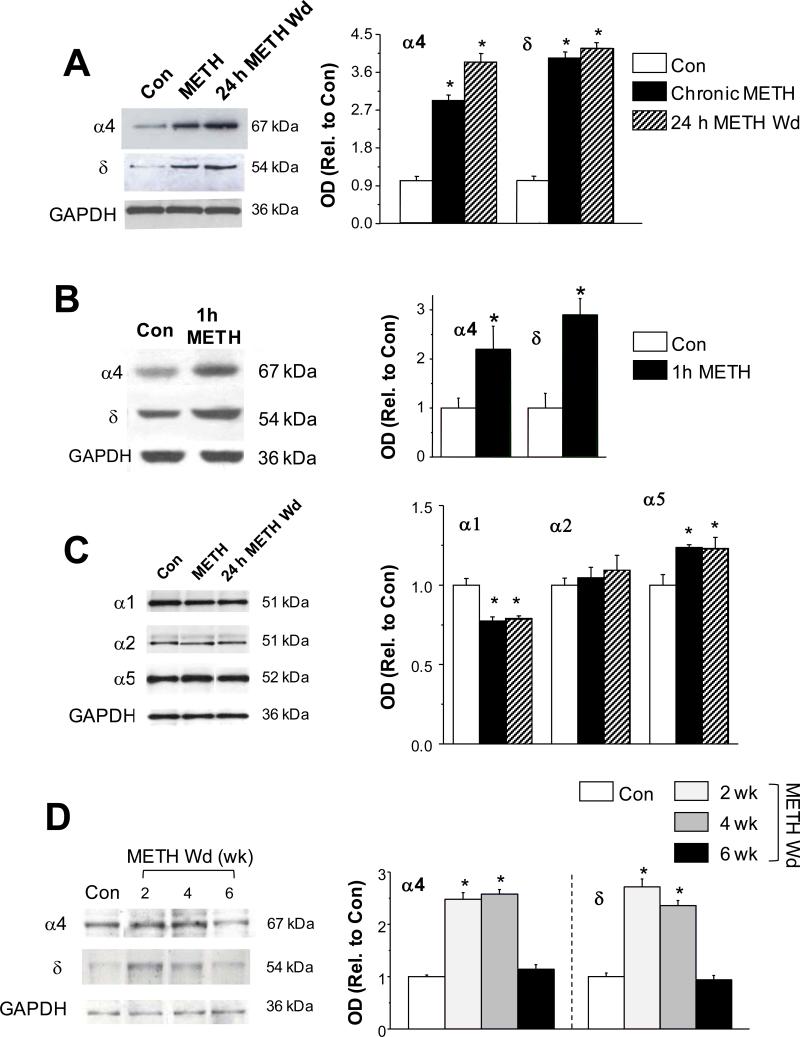
We tested effects of acute and chronic METH administered to mice on expression of α4 and δ GABAR subunits during short-term and longer withdrawal periods (24 h or 2-6 week) in order to compare +/+ with α4 −/− mice, which do not express δ (Sabaliauskas et al., 2012). Similar to the rat, both chronic METH and METH withdrawal (24 h) increased expression of α4 and δ GABAR subunits by 2-3-fold (P<0.05 vs. control, n=7 mice/group; Fig. 7A). This increase in α4 and δ expression was first seen 1h after a single METH injection (P<0.05, n=6 mice/group; Fig. 7B) suggesting that it was a rapid response to METH exposure. Smaller (~20%) increases in α5 expression were also seen after chronic METH and 24 h METH withdrawal, in association with a ~30% decrease in α1 expression (P<0.05 vs. control, Fig. 7C, n=4 mice/group), similar to results from rat hippocampus. In contrast, α2 expression was not altered by METH exposure. The increase in α4 and δ expression was persistent during the METH withdrawal period up to 4 weeks (Fig. 6D, P<0.05, n=4/group), but recovered to control levels by 6 weeks of METH withdrawal.
Fig. 7. METH increases α4, δ and α5 GABAR and expression in mouse CA1 hippocampus.
Western blots, mouse hippocampus, Left, Representative blots, Right, Mean ± SEM. A, α4, δ, Chronic METH and 24 h METH Wd: α4 (ANOVA, F(2,18)=53.1, P<0.0001) and δ (ANOVA, F(2,18)=100.3, P<0.0001); *P<0.05 vs. Con; n=7/group. B, α4, δ, 1h METH: α4 (student's t-test, t(10)=2.3, *P=0.044 vs. Con) and δ (student's t-test, t(10)=3.18, *P=0.01 vs. Con); n=6/group. C, α1, α2, α5, Chronic METH and 24 h METH Wd: α1 (ANOVA, F(2,9)=17.5, P=0.0008), α2 (ANOVA, F(2,9)=0.43, P=0.666), α5 (ANOVA, F(2,12)=6.03, P=0.037); *P<0.05 vs. Con; n=4/group. D, α4, δ, METH Wd – 2 – 6 wk: α4 (ANOVA, F(3,16)=88.2, P<0.0001) and δ (ANOVA, F(3,16)=77.7, P<0.0001); *P<0.05 vs. Con, METH Wd-6 wk; n=4/group.
3.8.2 Functional expression of α4βδ GABARs in the mouse
As seen in the rat, functional expression of α4βδ GABARs was confirmed by the robust responses of hippocampal pyramidal cells to 100 nM gaboxadol after METH withdrawal for up to 4 weeks, which was 8 to 9-fold greater than in control recordings (Fig. 8A,B). This response to gaboxadol was not seen in α4−/− hippocampus after 24 h METH withdrawal, confirming the selectivity of 100 nM gaboxadol for α4βδ GABARs, and recovered to control levels by 6 weeks after METH exposure. In addition, the noise generated in response to gaboxadol, reflecting α4βδ expression (δ-noise) was 50-100% greater after METH withdrawal (24 h – 4 weeks), an effect not seen in α4 −/− hippocampus, suggesting that the increase in tonic current produced by METH exposure is mediated by α4βδ GABARs (n=5 mice/group). These results also suggest that longer periods of METH withdrawal maintain high levels of α4βδ receptor expression.
Fig. 8. METH increases gaboxadol responsiveness of the tonic current in mouse CA1 hippocampus.
Whole cell voltage clamp recordings from CA1 pyramidal cells in the hippocampal slice after administration of METH or vehicle to +/+ or α4−/− mice. Robust responses to 100 nM GBX (left, arrow) were noted from 1d to 4 wk after METH Wd in +/+, but not α4−/−, mice, reflecting functional expression of α4βδ receptors. Right, arrow, 120 μM SR95531. A, Representative traces; B, Mean ± SEM, ANOVA, GBX-induced change in holding current, F(5,24)=27, P < 0.0001; δ-noise, F(5,24)=7.9, P=0.0002; *P<0.05 vs. Con, METH Wd-24 h α4−/−, METH Wd-6 wk; n=5 mice/group.
3.8.3. The inhibitory synaptic current in mouse hippocampus
As seen in the rat, sIPSC amplitude was increased by about 200% (P<0.05) and sIPSC frequency was increased by 150% (P<0.001) by chronic METH treatment (Fig. 9A-C). Increases in both parameters were maintained following cessation of drug treatment in the METH withdrawal state for 5 weeks (sIPSC amplitude) and 4 weeks (sIPSC frequency; n=4-5 rats/group), with full recovery after 6 weeks of METH withdrawal for both parameters (n=5 mice/group).
Fig. 9. Chronic METH increases the inhibitory synaptic current in mouse CA1 hippocampal pyramidal cells.
A, Whole cell voltage clamp recordings from CA1 hippocampal pyramidal cells. Representative traces illustrating the increase in sIPSC amplitude and frequency following chronic treatment of mice with METH or its withdrawal (METH Wd) from 24 h to 6 weeks (6 wk). B, Mean ± SEM, sIPSC amplitudes (ANOVA, F(7,58)=71.7, P<0.0001; *P<0.05 vs. Con, METH WD-6 wk; †P<0.05 vs. all other groups.) and averaged sIPSC frequencies (ANOVA, F(7,58)=20.25, P<0.0001; *P<0.001 vs. Con, METH WD-6 wk; †P<0.05 vs. all other groups); n=5/group. C, Probability plot for the distribution of sIPSC amplitudes; left, Con, chronic METH and 24 h METH withdrawal; right, METH withdrawal, 2 – 6 wk.
3.8.4. Polarity of GABAergic current after METH withdrawal in the mouse
In order to compare results in mouse hippocampus with those from the rat, the polarity of GABAergic current was first determined using gramicidin perforated patch recording in voltage clamp mode. As seen in rat hippocampus, bath application of 5 μM gaboxadol produced a downward deflection after chronic METH treatment, reflecting a depolarizing response, and an upward deflection 24 h after METH withdrawal, reflecting a hyperpolarizing response (Fig. 10A).
Fig. 10. 3α,5β-THP reduces tonic GABAergic current and increases excitability of mouse CA1 hippocampal pyramidal cells after METH withdrawal.
A, Gramicidin perforated patch recordings of the holding current in voltage clamp mode (−65 mV holding potential) in response to 100 nM GBX (arrow) after chronic METH (top) or a 24 h METH withdrawal (24 h METH Wd, bottom). GBX produced a depolarizing effect during chronic METH treatment, but a hyperpolarizing effect after 24 h METH Wd. Rep. of results from 3 cells, each. B, Whole cell voltage clamp recordings varying the Cl− gradient to produce depolarizing GABA currents (inward, upper trace) or hyperpolarizing GABA currents (lower 2 traces) from +/+ (upper 2 traces) or α4−/− (lower trace) hippocampus after METH Wd. 3α,5β-THP (30 nM, first arrow) increased depolarizing GABA current, but reduced hyperpolarizing GABA current in +/+ but not α4−/− hippocampus. (Second arrow, 120 μM SR95531 added to verify the direction of GABAergic current.) C, Mean ± SEM, ANOVA F(2,6) = 61.3, P=0.0001; *P<0.01 vs. all other groups; n=3 mice/group. D, Representative traces and E, Mean ± SEM, 3α,5β-THP effects on neuronal excitability recorded from CA1 pyramidal cells in the hippocampal slice using current clamp mode. Membrane voltage was assessed in response to 20 pA increases in current injection. D, Red, current threshold (I-threshold) for action potential (AP) generation for the less excitable group (i.e., higher current threshold). Blue, I-threshold for AP generation for the more excitable group (i.e., lower current threshold) before or after bath application of 30 nM 3α,5β-THP. Black, trace(s) in-between the two threshold currents. E, 3α,5β-THP effects on Rm (ANOVA, F(2,12)=40.91, P<0.0001) and I-threshold (ANOVA, F(2,12)=63, P<0.0001) were not seen in α4 −/− mice after 24 h METH Wd. *P<0.05 vs. Con, METH Wd-24 h α4−/− ; n=5 mice/group. F, G, Cell-attached recordings of spiking in voltage clamp mode before and after 30 nM 3α,5β-THP. 3α,5β-THP is excitatory after both 24 h and 12 d after METH Wd in +/+ but not α4 −/− mice. F, Representative traces, G, Mean ± SEM, ANOVA, F(3,8) = 64, P< 0.0001; *P<0.005 vs. Con, METH Wd-24 h α4−/− ; n=3 mice/group.
3.8.5. Effect of 3α,5β-THP on the tonic GABAergic current after METH withdrawal: Effect of varying the polarity of GABAergic current and α4 knock-out in the mouse
Our previous findings (Shen et al., 2007) suggest that 3α,5β-THP reduces current gated by α4βδ when it is hyperpolarizing, but potentiates this current when it is in the depolarizing direction. In order to determine the effect of the polarity of the GABAergic current on 3α,5β-THP's effect on this parameter after METH withdrawal, the Cl− gradient was altered by varying the pipet solution to yield depolarizing or hyperpolarizing GABA responses during recordings at a −50 mV holding potential. Under depolarizing conditions, 3α,5β-THP increased the tonic current, consistent with many reports. In contrast, 3α,5β-THP decreased the tonic hyperpolarizing current, an effect seen in CA1 hippocampus from +/+, but not from α4−/− mice (Fig. 10B,C; P<0.01, n=3/group). Thus, both a hyperpolarizing GABAergic current and the presence of α4βδ GABARs after METH withdrawal (1d-2 weeks) are required for 3α,5β-THP to reduce the tonic GABAergic current.
3.8.6. Current clamp recordings in mouse hippocampus
3α,5β-THP effects on neuronal excitability were assessed in mouse hippocampus using similar experiments as used for the rat hippocampus. As seen in the rat, 3α,5β-THP increased Rm by 40 ± 9% and reduced the current threshold for generating a spike after a 24 h METH withdrawal, an effect not seen in the α4 −/− mouse, implicating α4βδ GABARs (Fig. 10D,E). In contrast, 3α,5β-THP reduced Rm and increased the current threshold for generating a spike under control conditions. In the absence of 3α,5β-THP, as in the rat, METH withdrawal (24 h) reduced Rm by 28 ± 5% compared to control, an effect not observed in α4 −/− hippocampus, suggesting that increased expression of α4βδ receptors underlies this change in input resistance in response to chronic METH exposure. In contrast, spike amplitude was not altered across groups, suggesting no change in glutamate receptor density (n=5 mice/group).
3.8.7. Cell attached recordings in mouse hippocampus
In order to compare the effect of 3α,5β-THP on spontaneous neuronal activity during chronic METH treatment versus a 24 h METH withdrawal, when its effects on the tonic current are in opposite directions (Fig. 3), we employed cell attached recordings to assess spiking in voltage clamp mode. This technique allows the determination of 3α,5β-THP effects on neuronal excitability under physiological conditions where the intracellular milieu is undisturbed. Under these conditions, 3α,5β-THP reduced spiking by ~70 % during chronic METH treatment (Fig 10F,G), but increased spiking by ~120 % after METH withdrawal for either 24 h or 2 weeks , an effect seen only in the +/+ but not the α4 −/− hippocampus, implicating α4βδ GABARs. This suggests that 3α,5β-THP has opposite effects on neuronal excitability during chronic treatment with METH vs. METH withdrawal (1d – 2 weeks), and that this effect is dependent upon α4βδ GABARs (Summary Fig. 4, Table 1; n=3 mice/group).
3.9. Behavioral effects of 3α,5β-THP after a 24 h METH withdrawal
3.9.1. 3α,5β-THP increases anxiety after a 24 h METH withdrawal in rats
Because 3α,5β-THP increased neuronal excitability in the hippocampus during a 24 h METH withdrawal via its inhibition of the GABAergic tonic current, we tested the hypothesis that increasing activity of this limbic structure would increase anxiety at this time, in contrast to its typical anti-anxiety effect (Bitran et al., 1999). To this end, we assessed the effect of 3α,5β-THP, administered systemically (10 mg/kg, i.p.), on the acoustic startle response (ASR), an animal model of anxiety, which is regulated, in part, by the CA1 hippocampus (Caine et al., 1992). This protocol assesses the whole body movement in response to a 120 decibel acoustic stimulus.
Chronic METH treatment increased the ASR by more than 2-fold (Fig. 11A; P<0.0001, n=9 rats/group), suggesting that METH increases anxiety, consistent with its stimulant and anxiogenic effect in humans. The ASR recovered to control values after a 24 h withdrawal from METH, when the stimulant was no longer present. However, 3α,5β-THP increased the ASR in this group (Fig. 11A,B; P<0.0001), suggesting that this steroid increases anxiety 24 h after METH withdrawal. In contrast, 3α,5β-THP decreased the ASR after chronic METH exposure, but had no effect under control conditions or 24 h after METH withdrawal when flumazenil was administered. The lack of effect of this steroid in control rats is consistent with previous reports showing no effect of this class of neurosteroids on the ASR in untreated male rats (Reilly et al., 1999).
3.9.2. 3α,5α-THP and 3α,5β-THP increase anxiety after a 24 h METH withdrawal in +/+ mice
The ASR model of anxiety was also implemented in mice after chronic METH exposure and its withdrawal. In order to validate this test for adult, male mice, the parameters for the test were first verified because some studies have suggested that mice have a higher threshold for the auditory response to high frequency tones compared to rats (Ison et al., 2007). To this end, the ASR was compared in mice across a range of tone volumes (85 – 120 dB, tested at a 40 ms duration) and tone durations (10 – 90 ms, tested at a 120 dB intensity) varied in a random order. The ASR was maximal with a 120 dB, 40 ms tone (Fig. 12A, *P<0.05 vs. all other groups, n=10 mice/group), consistent with a number of other studies in adult mice (Robertson et al., 2005) and similar to findings in rats (Curzon et al., 2009).
Fig. 12. Both 3α,5α-THP and 3α,5β-THP increase anxiety after a 24 h METH withdrawal in mice.
A, ASR in untreated male mice across a range of tone volumes (85-120 decibels, db), left, and durations (10-90 ms), right. These data suggest that a 40 ms, 120 db signal is optimal for adult mice. Mean ± SEM, ANOVA, acoustic volume, F(7,72)=65.2, P<0.0001; acoustic signal duration, F(8,81)=4.20, P=0.0003; *P<0.05 vs. all other groups, n=10 mice/group. B, ASR in vehicle or 3α,5β-THP-injected mice. All animals were injected with 3α,5β-THP (10 mg/kg, i.p.) or oil vehicle 20 min. before testing using the ASR test of anxiety. Mean ± SEM, 2-factor ANOVA, F(4,98)=10.13, P<0.0001; *P<0.05 vs. all other vehicle-treatment groups; †P<0.05 vs. all other 3α,5β-THP -treatment groups; n=10-12 mice/group. C, 3α,5β-THP effects on the ASR after a 24 h METH withdrawal. Mean ± SEM, 1-factor ANOVA, F(4,51)=7.9, P<0.0001; *P<0.05 vs. Chronic METH, METH Wd-24 h, METH Wd-6 wk, †P<0.05 vs. all other groups; n=10-12 mice/group. 3α,5β-THP increased the startle response after a 24 h METH Wd (90% of animals). D, 3α,5α-THP effects on the ASR after a 24 h METH withdrawal. 3α,5α-THP (10 mg/kg, i.p.) or oil vehicle was injected 20 min before testing. Left, 3α,5α-THP produced an ASR greater than oil vehicle. (Mean ± SEM, student's t test, t(14)=2.41, *P=0.015 vs. vehicle-injected mice, n=8 mice/group). Right, 3α,5α-THP produced a 92% increase in the ASR compared to oil vehicle.
Thus, we used the same ASR test for the mice as used for the rats. As seen with the rats, chronic METH treatment significantly increased the average startle response by ~60% (P<0.05, n=10-12 mice/group; Fig. 12B), suggesting that it increased anxiety compared to the saline injected controls and 24 h METH withdrawal groups. 3α,5β-THPreduced the ASR by ~30% in the chronic METH treatment group, consistent with its typical anxiolytic effect (Bitran et al., 1999). However, 3α,5β-THP increased the ASR by ~70% 24 h after METH withdrawal (P<0.05, Fig. 12B,C), similar to findings in the rat, confirming that this steroid exerts anxiogenic effects after a 24 h METH withdrawal. This paradoxical anxiogenic effect of 3α,5β-THP was not seen when flumazenil was administered during the 24 h METH withdrawal or following a prolonged 6 week METH withdrawal. Both flumazenil treatment and long-term (6 week) METH withdrawal reduce α4βδ GABAR expression, thus implicating α4βδ GABARs.
Similar to its 3α,5β isomer, the stress steroid 3α,5α-THP (10 mg/kg, i.p.) increased the ASR by ~92% compared to vehicle-injected control mice following a 24 h METH withdrawal (Fig. 12D). This is in contrast to reports demonstrating either no effect or anxiolytic effects of this steroid in the ASR in rodents not exposed to stimulant drugs (Reilly et al., 2009; Toufexis et al., 2004). These results suggest that the two neurosteroid isomers have similar effects in producing anxiety during this 24 h METH withdrawal period when α4βδ GABAR expression is increased.
3.9.3. 3α,5β-THP does not alter anxiety after a 24 h METH withdrawal in α4 −/− mice
In order to determine the role of α4βδ GABARs in mediating the paradoxical anxiogenic effect of 3α,5β-THP after METH withdrawal, we implemented the ASR task in α4−/− mice following chronic treatment with METH or 24 h withdrawal. As seen in +/+ mice, chronic METH treatment increased the ASR compared to values obtained after a 24 h METH withdrawal, suggesting that anxiety levels are higher during chronic METH treatment (P<0.05, Fig. 13A, n=8-10 mice).
Fig. 13. 3α,5β-THP has no effect on anxiety after METH withdrawal in α4−/− mice.
All animals were injected with 3α,5β-THP (10 mg/kg, i.p.) or oil vehicle 20 min. before testing using the ASR test of anxiety. A, ASR in vehicle or 3α,5β-THP-injected mice. Mean ± SEM, 2-factor ANOVA, F(3,30)=3.74, P=0.02; METH, F(1,30)=8.3, P=0.007, *P<0.05 vs. all other groups; n=8-10 mice/group. B, 3α,5β-THP effects on the ASR across METH treatment groups. Mean ± SEM, t(13)=0.80, P=0.44; n=8-10 mice/group. 3α,5β-THP had no effect on the startle response after METH Wd in α4 −/− mice.
However, unlike the results in +/+ mice, 3α,5β-THP had no significant effects on the ASR in either chronic METH or 24 h METH withdrawal groups of α4−/− mice (Fig. 13A,B), suggesting that the anxiogenic effect of this steroid observed in +/+ mice after METH withdrawal is dependent upon α4βδ GABAR expression.
4. Discussion
4.1. Summary and implications
The results from the present study suggest that following withdrawal from chronic METH administration, the effect of the stress steroid 3α,5α-THP and its 3α,5β-THP isomer reverse to trigger anxiety, in contrast to their usual effect to either decrease (Bitran et al., 1999) or produce no effect on anxiety responses (Reilly et al., 2009) in male rodents. This surprising outcome was produced via a novel GABAR target, α4βδ, because 3α,5β-THP effects were not observed in α4 −/− mice. This receptor is unique in providing compensatory inhibitory tone under conditions of increased CNS excitability (Mtchedlishvili et al., 2010), as would be produced by stimulant action, but is inhibited by 3α,5β-THP when Cl− current is hyperpolarizing (Shen et al., 2007), as seen 24 h after METH withdrawal. Thus, its increased expression explains the seemingly incongruent symptoms of METH withdrawal, which include lethargy and sedation, punctuated by anxiety and irritability as a response to stress (London et al., 2004) when neurosteroid release is increased (Droogleever Fortuyn et al., 2004). Increased expression of α4βδ GABARs is also associated with mood changes at puberty (Shen et al., 2007) and a model of premenstrual dysphoric disorder (Smith et al., 2006), which suggest that its expression may play a role in general states of stress-triggered anxiety. Previous animal models of METH dependence have demonstrated conflicting reports of anxiety-like behavior during METH withdrawal (Kitanaka et al., 2010), but this is the first demonstration of an anxiety response triggered by a neurosteroid following withdrawal from METH.
METH and anxiety behavior
METH increased anxiety due to its stimulant effects, as verified in the present study, consistent with results from human and rodent studies (Pometlova et al., 2012; Salo et al., 2011). During the chronic METH protocol, anxiety was assessed within one hour after administration of the drug, when circulating levels of METH would still be significantly elevated (Riviere et al., 1999). Although α4βδ GABARs were increased by chronic METH treatment, the inhibition they provide was not sufficient to overcome the stimulant effects of METH on anxiety behavior (Summary diagram, Fig. 4).
After a 24 h METH withdrawal, the stimulant effects of the drug dissipate because METH has a half-life of ~98 min (Riviere et al., 1999). At this time anxiety was reduced to control levels (Summary diagram, Fig. 4), consistent with results from other studies (Grace et al., 2010) which also report no increase in the ASR after a 24 h METH withdrawal. However, 3α,5β-THP exerted excitatory effects at this time because it reduced the hyperpolarizing GABAergic current generated by α4βδ GABARs during the 24 h METH withdrawal as we have shown also occurs at puberty (Shen et al., 2007). Thus, 3α,5β-THP increased anxiety after a 24 h METH withdrawal, as did 3α,5α-THP. 3α,5α-THP belongs to a class of drugs, including THDOC (3α,21-dihydroxy-5α-pregnan-20-one) which are released by stress (Purdy et al., 1991; Mukai et al., 2008; Girdler et al., 2001; Drooglever-Fortuyn et al., 2004). 3α,5α-THP, like its 5β isomer, also increases anxiety at puberty when α4βδ GABARs are increased (Shen et al., 2007) and GABAergic current is hyperpolarizing. Thus, these findings suggest a potential mechanism for stress-triggered anxiety during METH withdrawal. In contrast to its effect during chronic METH and 24 h METH withdrawal, 3α,5β-THP had no effect on the ASR in control rats, consistent with studies which suggest that this class of neurosteroids does not alter the peak ASR in male rodents, despite its anxiolytic effect in females (Reilly et al., 2009; Toufexis et al., 2004)
4.2. METH withdrawal and α4βδ GABAR expression
Chronic METH exposure of both rats and mice produced marked increases in α4βδ GABAR expression, suggesting that this is a robust effect. This increase in α4βδ expression was first seen 1 h after METH administration in mice suggesting that this is a rapid response to METH exposure and not simply a result of chronic exposure to the drug. These α4βδ GABARs were verified to be functional receptors based on the responses of pyramidal cells to a low concentration of the GABA agonist gaboxadol, which is selective for δ-containing GABAR (Brown et al., 2003; Meera et al., 2010). (Although the magnitude of the change in receptor expression and tonic current are not identical, this may be due to a ceiling effect on the ability to detect a α4βδ expression with a Western blot.) This increased level of α4βδ GABAR expression was maintained during both acute and up to 4 weeks after METH withdrawal, suggesting that the compensatory increase in these receptors is long-lasting and may be relevant for METH abstinence. However, 6 weeks after METH withdrawal, α4βδ expression was reduced and 3α,5β-THP no longer generated an anxiety response.
4.3. 3α,5β-THP effects at α4βδ GABARs
One novel characteristic of α4βδ, but not other GABARs, is that their response to the 3α,5α and 3α,5β isomers of THP is dependent upon the polarity of GABAergic current: 3α,5α -THP, 3α,5β-THP and related steroids enhance depolarizing, shunting inhibition (Belelli D et al., 2002; Bianchi and Macdonald, 2003; Brown et al., 2002; Stell et al., 2003; Weir et al., 2004), such as found in dentate gyrus granule cells which normally have high expression of α4βδ (Staley and Mody, 1992). In contrast, 3α,5β-THP reduces hyperpolarizing current (Shen et al., 2007), such as found in mature CA1 hippocampus, where α4βδ are normally only expressed under special conditions, such as the onset of puberty (Shen et al., 2007) or METH withdrawal, as reported here (Summary figure 4). This surprising effect of the steroid was initially established in recombinant receptors where 3α,5β-THP reduced hyperpolarizing current but enhanced depolarizing current mediated by α4βδ GABARs (Shen et al., 2007) and did not alter the reversal potential, suggesting that conductances other than Cl− were not involved. This effect was dependent upon arginine 353 in the TM3-TM4 intracellular loop, a putative modulatory site for Cl−, and was due to acceleration of desensitization by 3α,5β-THP (Shen et al., 2007), consistent with reports of polarity-dependent desensitization of homologous δ-containing GABARs (Bianchi et al., 2002). Cl− has been shown to function in a modulatory capacity in GABARs and other membrane receptors in addition to role in ion conductance (Chen et al., 2000; Houston et al., 2009; Olsen and Snowman, 1982); our findings suggest that it may play a role in mediating steroid effects at α4βδ GABARs.
4.4. Polarity of hippocampal GABAergic current: Chronic METH versus METH withdrawal
During chronic METH treatment, α4βδ GABAR expression was increased, but 3α,5β-THP enhanced inhibition at this time, as predicted, due to the fact that the GABAergic current was depolarizing, but was still a shunting inhibition, during chronic stimulant exposure. In the CA1 hippocampus, where the GABAergic current is hyperpolarizing, the Cl− reversal potential lies close to the resting membrane potential (Staley and Proctor, 1999). Thus, small changes in Cl− equilibrium can shift the direction of the current, which is actively maintained by a selective K+- Cl− co-transporter, KCC2 (Payne, 1997). Other studies have shown that increased GABAR activation produced by tetanizing stimulation or depolarization can lead to accumulation of Cl− intracellularly and depolarizing GABA currents (Isomura et al., 2003), as has been seen in models of epilepsy (Fujiwara-Tsukamoto et al., 2007). In the Isomura study and ours, this reversal of GABAergic current may be due to increases in extracellular K+ levels produced by glutamate release, which are reported after METH exposure (Yamamoto et al., 1999). Increased extracellular K+ has effects on the K+-Cl− co-transporters to favor accumulation of intracellular Cl−, and may even reverse the transporter flux (Jarolimek et al., 1999). In addition, it could also be due to collapse of the Cl− gradient by sustained GABAR activity (Isomura et al., 2003), which would allow other anions, such as HCO3−, to generate an inward current in response to GABA agonists. Alternatively, alterations in expression of KCC2 could also explain this finding (Kahle et al., 2008).
4.5. METH effects in the CA1 hippocampus
METH is reported to have actions in the hippocampus, where it increases release of glutamate, depolarizes CA1 pyramidal neurons (Yamamoto et al., 1999) and enhances synaptic transmission (Hori et al., 2010). However, with chronic exposure, effects of the drug on glutamate release are reduced (Yamamoto et al., 1999) and long-term potentiation is impaired (Hori et al., 2010), suggesting that compensatory mechanisms develop. Interestingly, it has been shown that chronic exposure to METH reduces the input resistance of CA1 pyramidal cells (Hori et al., 2010). Our findings suggest one potential mechanism for this outcome is via increased expression of α4βδ GABAR. Because these extrasynaptic receptors localize along the dendritic shaft (Peng et al., 2002; Shen et al., 2007) where they generate a shunting, tonic inhibition in response to ambient levels of GABA, their primary effect is to reduce neuronal input resistance (Shen et al., 2010a) and increase spiking threshold. Indeed, withdrawal from chronic METH administration in the present study resulted in a significant decrease in neuronal input resistance compared to control, which increased the current necessary for triggering a spike, an effect which was prevented by down-regulation of α4 expression with flumazenil as well as by α4 knock-down. In contrast, flumazenil alone had no effect on input resistance or threshold for spiking.
4.6. Synaptic current
METH withdrawal also increased the phasic current, most likely as part of the compensatory response to the stimulant actions of METH. However, these IPSCs do not reflect increased expression of α4βγ2 GABAR sub-synaptically. This conclusion is suggested by the robust response of the synaptic current to benzodiazepine modulation, which would not be expected if α4βγ2 receptors were increased sub-synaptically (Hsu et al., 2003) because they are benzodiazepine-insensitive (Knoflach et al., 1996; Wafford et al., 1996), unlike the other sub-types of GABARs which express sub-synaptically in CA1 hippocampus. This increase in sIPSC frequency and amplitude may have served to mitigate the increase in anxiety when METH was present. However, increases in sIPSC frequency were maintained across a 4 week period of METH withdrawal, which may partially explain the reduced anxiety response during this withdrawal period compared to the anxiety associated with chronic METH treatment.
4.7. Plasticity of α4βδ GABARs
GABARs containing the δ subunit have a high degree of plasticity and are increased compensatorily, as is the tonic inhibitory current, in response to increased neuronal excitability produced by neuronal depolarization, NMDA receptor activation, traumatic brain injury and stroke (Mtchedlishvili et al., 2010; Payne et al., 2008; Santhakumar et al., 2010). It is likely that δ-containing GABARs play a neuroprotective role in this regard because excitotoxicity levels are increased in brain tissue from δ −/− animals (Santhakumar et al., 2010). Fluctuations in 3α,5α-THP levels, such as seen at puberty or across the ovarian cycle, also increase α4βδ GABAR expression (Brack and Lovick, 2007; Maguire et al., 2005; Shen et al., 2007), which may also be due, in some cases, to subtle changes in neuronal activity. The results from the present study provide an additional condition, chronic METH exposure, which triggers α4βδ GABAR expression. In contrast to its upregulation of α4, chronic METH and METH withdrawal decrease α1 expression, which suggests that expression of these two subunits has an inverse relationship, consistent with other reports (Kumar et al., 2002; Zhou et al., 2009). The mechanism for METH-induced increases in α4βδ expression is not known, but may be due to either heat shock protein chaperones or to brain-derived neurotrophic factor, both of which can be increased by METH exposure (Braun et al., 2011; Kiyatkin and Sharma , 2011; Numachi et al., 2000) and have been shown to regulate α4βδ expression (Joshi and Kapur, 2009; Pignataro et al., 2007).
4.8. Flumazenil effects at α4βδ GABARs
Flumazenil is a GABAR ligand, best known for its acute effect as a competitive benzodiazepine antagonist at GABARs containing either α1-3 or α5 and a γ2 subunit. In this regard, it binds to the benzodiazepine site where it prevents the potentiating effect of benzodiazepine agonists on GABA-gated current and the resultant sedation, but has no effect on its own (Olsen and Sieghart, 2009).
However, flumazenil can also produce longer term effects by altering expression of α4-containing GABARs that are separate and distinct from its acute effects and do not require the presence of a benzodiazepine. In fact, flumazenil (48 h) reduces α4 expression following its upregulation by ethanol withdrawal (Biggio et al., 2007) an effect which may be mediated via direct effects of the drug on the receptor. Unlike classic benzodiazepine agonists which do not bind to receptors containing α4 (Knoflach et al., 1996; Wafford et al., 1996) or δ subunits (Brown et al., 2002), flumazenil has been shown to bind to α4βδ GABARs (Wallner et al., 2006) where it likely produces allosteric effects. In the present study, flumazenil significantly reduced expression of the α4 subunit during 24 h METH withdrawal, suggesting that it may represent a pharmacological approach to the regulation of these receptors. Under control conditions, flumazenil had no effect on measures of neuronal function, consistent with its role as a benzodiazepine antagonist at GABAR which do not contain α4 or δ subunits. Flumazenil also increased expression of α1 after 24 h METH withdrawal, which may be simply be due to indirect effects due to decreases in α4 expression or to the presence of endogenous benzodiazepine-like compounds which have been reported (Costa and Guidotti, 1991). Flumazenil has been shown to produce a similar effect (increasing α1, while decreasing α6, which is homologous to α4) in cerebellum (Zheng et al., 1996), suggesting that this is a robust effect.
Early studies foreshadowed this regulatory role for flumazenil, where a single administration of the drug was able to reverse the effects of chronic benzodiazepine exposure which prevented GABA-induced enhancement of benzodiazepine binding (“uncoupling”) (Roca et al., 1990) and tolerance to benzodiazepines (Gonsalves and Gallager, 1988). Although the mechanism is not clear, these flumazenil-induced increases in benzodiazepine response are consistent with downregulation of GABAR containing the benzodiazepine-insensitive α4 subunit.
4.9. Other GABAR subtypes
Our findings suggest that α5 GABAR subunit expression is also increased by chronic METH exposure. This receptor is localized extrasynaptically and mediates a tonic inhibition in CA1 hippocampus (Bai et al., 2000). However, the observed METH-induced increase was modest (~20%), although it probably represents an additional compensatory response to the stimulant actions of METH. In addition, α5-GABARs likely do not mediate the anxiogenic effect of 3α,5β-THP during METH withdrawal because these receptors are relatively insensitive to 3α,5β-THP (Shen et al., 2007), and ablation of α4βδ GABARs was sufficient to prevent this paradoxical effect of both isomers of THP.
4.10. GABARs and addictive drugs
Recent studies have demonstrated the presence of α4 and δ-containing GABARs in the nucleus accumbens where their knock-down reduces the reinforcing effects of alcohol (Nie et al., 2011; Rewal et al., 2012). In addition, both the α1 and α2 GABARs play a role in the reward and stimulant aspects of addictive drugs. Benzodiazepines increase dopamine levels in the ventral tegmental area by potentiating α1-containing GABARs on interneurons which typically inhibit dopamine output in this region (Tan et al., 2010). α2-containing GABARs localize in the nucleus accumbens (Pirker et al., 2000), which plays a pivotal role in these effects of cocaine (Carelli, 2002), where they contribute to GABAergic synapses (Dixon et al., 2010). Studies with both knock-out and transgenic (α2(H101R)) mice have shown that α2 GABARs mediate cocaine-induced hyperactivity produced by benzodiazepine agonists (Morris et al., 2008) and are both necessary and sufficient for behavioral sensitivity to cocaine (Dixon et al., 2010). Additionally, α2 deletion prevents conditioned reinforcement to cocaine (Dixon et al., 2010), suggesting that GABARs provide an essential role in the behavioral effects of stimulant drugs.
4.11. Stress and drug relapse
Stress and anxiety are compelling states that can drive drug-seeking (Koob, 2009) and relapse (Back et al., 2010), which may represent an effort to lessen aversive withdrawal effects of the drug. In animal studies, stress increases the response rate for self-administration of METH (Shepard et al., 2004) and cocaine (Covington and Miczek, 2005), independently of corticosterone levels (Covington and Miczek, 2005), suggesting that an alternative stress-related mechanism exists, which may be explained by our findings. Triggering stress with the α2 adrenoreceptor blocker yohimbine can also induce reinstatement of cocaine seeking after extinction (Anker and Carroll, 2010) in rats of both sexes. Interestingly, 3α,5α-THP prevents this effect in female but not male rats, suggesting that our findings, showing that 3α,5α-THP triggers anxiety after METH withdrawal, may be an outcome relevant only for the male. Sex differences in 3α,5α-THP's effects on cocaine seeking have also been reported in the absence of a stressful stimuli (Anker et al., 2009), where it reduces this parameter only in females. Interestingly, cocaine increases hippocampal levels of 3α,5α-THP levels only in females (Quinones-Jenab et al., 2008), which may explain the sex difference in response to administration of exogenous steroid. Although the anxiogenic peptide CRF has been implicated in some withdrawal states (Koob, 2009), there is evidence that some of the effects of CRF are mediated via GABA (Waselus et al., 2005).
4.12. Conclusions
Although dopamine (Fleckenstein et al., 2007) and dopamine-GABA interactions (Janssen et al., 2009) have a role in striatal function and reward mechanisms, the GABAR is the major determinant of anxiety state (Rudolph et al., 1999). This study demonstrates a novel mechanism for stress-induced anxiety after METH withdrawal via increases in hippocampal α4βδ GABAR, which likely represent a homeostatic response to the stimulant action of the drug.
Highlights.
Methamphetamine increases hippocampal expression of α4βδ GABAA receptors
The stress steroid THP reduces GABAergic current during methamphetamine withdrawal
THP's effect was mediated by polarity-dependent effects at α4βδ GABAA receptors
THP paradoxically increases anxiety during methamphetamine withdrawal
This effect was prevented by flumazenil and α4 knock-out
Acknowledgements
The authors thank G. Homanics for supplying the α4 +/– mice and genotyping the litters, D. Dow-Edwards for the use of the startle apparatus and J. Celentano for reading the manuscript. We also thank N. Zeak, M. Elnashar, J. Molla, W. Russell, R. George and J. Wang who provided helpful technical assistance. The work in this study was supported by grants from the US National Institutes of Health (to S.S.S.), the US National Institute of Alcoholism and Alcohol Abuse (to S.S.S.) and the US National Institute of Mental Health (to S.S.S.) as well as a contract from Hythiam, Inc. (to S.S.S.).
Abbreviations
- ASR
acoustic startle response
- Δ
Change
- GABAR
GABAA receptor
- GBX
gaboxadol or THIP (a GABA agonist)
- I-threshold
The current threshold for triggering a spike
- METH
methamphetamine
- mIPSC
miniature inhibitory post-synaptic current
- Rm
input resistance
- sIPSC
spontaneous inhibitory post-synaptic current
- 3α,5α-THP
3α-OH-5α-pregnan-20-one or allopregnanolone
- 3α,5β-THP
3α-OH-5β-pregnan-20-one or pregnanolone
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, Desantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Molec Pharmac. 2000;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang W-N, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2009;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharm. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio F, Gorini G, Caria S, Murru L, Sanna E, Follesa P. Flumazenil selectively prevents the increase in α4-subunit gene expression and an associated change in GABAA receptor function induced by ethanol withdrawal. J NeuroChem. 2007;102:657–666. doi: 10.1111/j.1471-4159.2007.04512.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Brack KE, Lovick TA. Neuronal excitability in the periaqueductal grey matter during the estrous cycle in female Wistar rats. Neuroscience. 2007;144:325–335. doi: 10.1016/j.neuroscience.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Braun AA, et al. Neurotoxic (+)-methamphetamine treatment in rats increases brain-derived neurotrophic factor and tropomyosin receptor kinase B expression in multiple brain regions. Neuroscience. 2011;184:164–171. doi: 10.1016/j.neuroscience.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and pre-pulse inhibition in the rat. Pharacol Biochem Behav. 1992;43:1201–1208. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008;139:603–609. doi: 10.1016/j.pain.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24:267–277. doi: 10.1016/s0740-5472(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Curzon P, Zhang M, Radek RJ, Fox GB. The behavioral assessment of sensorimotor processes in the mouse: Acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. CRC Press; Boca Raton: 2009. pp. 20–31. [PubMed] [Google Scholar]
- Dixon CI, Morris HV, Breen G, Desrivieres S, Jugurnauth S, Steiner RC, Vallada H, Guindalini C, Laranjeira R, Messas G, Rosahl TW, Atack JR, Peden DR, Belelli D, Lambert JJ, King SL, Schumann G, Stephens DN. Cocaine effects on mouse incentive-learning and human addiction are linked to α2 subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2010;107:2289–2294. doi: 10.1073/pnas.0910117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, van Broekhoven F, Span PN, Backstrom T, Zitman FG, Verkes RJ. Effects of PhD examination stress on allopregnanolone and cortisol plasma levels and peripheral benzodiazepine receptors density. Psychoneuroendocrinol. 2004;29:1341–1344. doi: 10.1016/j.psyneuen.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Fadalti M, Petraglia F, Luisi S, Bernardi F, Casarosa E, Ferrari E, Luisi M, Saggese G, Genazzani AR, Bernasconi S. Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr Res. 1999;46:323–327. doi: 10.1203/00006450-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fowler JS, et al. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. NeuroImage. 2008;423:756–763. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Fukai T, Takada M. Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizure-like network activity. Eur J Neurosci. 2007;25:2713–2725. doi: 10.1111/j.1460-9568.2007.05543.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psych. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Gonsalves SF, Gallager DW. Persistent reversal of tolerance to anticonvulsant effects and GABAergic subsensitivity by a single exposure to benzodiazepine antagonist during chronic benzodiazepine administration. J Pharmacol Exp Ther. 1988;244:79–83. [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3α-OH-5α-pregnan-20-one levels after acute administration of delta 9-tetracannabinol, cocaine and morphine. Psychopharm. 2005;179:544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the α4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur.J.Neuroscience. 2003;17:1–8. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlikova H, Hill M, Kancheva L, Vrbikova J, Pouzar V, Cerny I, Kancheva R, Starka L. Serum profiles of free and conjugated neuroactive pregnanolone isomers in nonpregnant women of fertile age. J Clin Endocrinol Metab. 2006;91:3092–3099. doi: 10.1210/jc.2005-2785. [DOI] [PubMed] [Google Scholar]
- Hori N, Kadota MT, Watanabe M, Ito Y, Akaike N, Carpenter DO. Neurotoxic effects of methamphetamine on rat hippocampus pyramidal neurons. Cell Mol Neurobiol. 2010;130:849–856. doi: 10.1007/s10571-010-9512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Sugimoto M, Fujiwara-Tsukamoto Y, Yamamoto-Muraki S, Yamada J, Fukuda A. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J Neurophysiol. 2003;90:2752–2756. doi: 10.1152/jn.00142.2003. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE. Age-related hearing loss in C58BL/6J mice has both frequency-specific and non-frquency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Association for Research in Otolaryngology. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29:5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W, Lewen A, Misgeld U. A furosemide-sensitive K+-Cl− cotransporter counteracts intracellular Cl− accumulation and depletion in cultured rat midbrain neurons. J Neurosci. 1999;19:4695–4704. doi: 10.1523/JNEUROSCI.19-12-04695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABAA receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M. Effects of diabetes on methamphetamine-induced place preference in mice. Eur J Pharmac. 1996;318:251–256. doi: 10.1016/s0014-2999(96)00804-7. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: a review. Neurochem Res. 2008;33:204–219. doi: 10.1007/s11064-007-9409-7. [DOI] [PubMed] [Google Scholar]
- Kitanaka N, Kitanaka J, Tatsuta T, Tanaka K, Watabe K, Nishiyama N, Morita Y, Takemura M. Withdrawal from fixed-dose injection of methamphetamine decreases cerebral levels of 3-methoxy-5-hydroxyphylglycol and induces the expression of anxiety-related behavior in mice. Neurochem Res. 2010;35:749–760. doi: 10.1007/s11064-010-0132-4. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Expression of heat shock protein (HSP 72kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J Neural Transm. 2011;118:47–60. doi: 10.1007/s00702-010-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acidA receptors α4β2γ2 and α6β2γ2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry Supp. 2009;1:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuver A, Shen H, Smith SS. Regulation of the surface expression of α4βδ GABAA receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Borroni AM, Grover LM, Teyler TJ. Hyperpolarizing and depolarizing GABAA receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J.Neurophysiol. 1991;66:1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psych. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, Reis FM, Luisi M, Genazzani AR. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mancino MJ, Gentry BW, Feldman Z, Mendelson J, Oliveto A. Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: a pilot field study. Am J Drug Alcohol Abuse. 2011;37:131–136. doi: 10.3109/00952990.2010.543998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Simpson AK, Mahaut-Smith MP, Robinson HP. The interpretation of current-clamp recordings in the cell-attached patch-clamp configuration. Biophys J. 2005;88:739–750. doi: 10.1529/biophysj.104.049866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiology. 2011;106:2057–2011. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Rosahl TW, Stephens DN. α2-containing GABAA receptors are involved in mediating stimulant effects of cocaine. Pharacol Biochem Behav. 2008;90:9–18. doi: 10.1016/j.pbb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM. Increase of GABAA receptor-mediated tonic inhibtion in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38:464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Mukai H, Higashi T, Nagura Y, Shimada K. Studies on neurosteroids XXV. Influence of 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull. 2008;31:1646–1650. doi: 10.1248/bpb.31.1646. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Yoshida S, Toda S, Matsuoka H, Sato M. Two inbred strains of rats, Fischer 344 and Lewis, showed differential behavior and brain expression of corticosterone receptor mRNA induced by methamphetamine. Ann N Y Acad Sci. 2000;914:33–45. doi: 10.1111/j.1749-6632.2000.tb05181.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo MA, Salvestroni C, Gallo R, Guo AL, Gennazini AD, Artini PG, Petraglia F, Gennazani AR. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endo Invest. 1995;18:853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Payne HL, Ives JH, Sieghart W, Thompson CL. AMPA and kainate receptors mediate mutually exclusive effects on GABAA receptor expression in cultured mouse cerebellar granule neurones. J Neurochem. 2008;104:173–186. doi: 10.1111/j.1471-4159.2007.04989.x. [DOI] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci. 2007;27:12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pometlova M, Nohejlova-Deykun K, Slamberova R. Anxiogenic effect of low-dose methamphetamine in the test of elevated plus-maze. Prague Med Rep. 2012;113:223–230. doi: 10.14712/23362936.2015.20. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Leslie Morrow A. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–73. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat. PNAS. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Minerly AC, Niyomchia T, Akahvan A, Jenab S, Frye C. Progesterone and allopregnanolone are induced by cocaine in serum and brain tissues of male and female rats. Pharmacol Biochem Behav. 2008;89:292–7. doi: 10.1016/j.pbb.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol & Alcoholism. 2009;44:561–566. doi: 10.1093/alcalc/agp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α,5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacol Biochem Behav. 2004;78:505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Riviere GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala γ-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiol Aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roca DJ, Schiller GD, Friedman L, Rozenberg I, Gibbs TT, Farb DH. γ - aminobutyric acidA receptor regulation in culture: Altered allosteric interactions following prololonged exposure to benzodiazepines, barbiturates, and methylxanthines. Mol Pharmacol. 1990;37:710–719. [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the γ-aminobutyric acid receptor subunit α4 reduces functional δ-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Flower K, Kielstein A, Leamon MH, Nordahl TE, Galloway GP. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356–361. doi: 10.1016/j.psychres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 2010;167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4βδ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010a;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science (Supp Online Material) 2010b;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psych. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev. 2009:CD003021. doi: 10.1002/14651858.CD003021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3− transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit- containing GABAA receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. J Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose ethanol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antaqgonist Ro15-4513. Proc Natl Acad Sci. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of γ-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurology. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–711. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Sundin VS. Effects of exposure to an augmented acoustic environment on auditory function in mice: roles of hearing loss and age during treatment. Hear Res. 2000;142:79–88. doi: 10.1016/s0378-5955(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kitamura N, Lin XH, Ikeuchi Y, Hashimoto T, Shirakawa O, Maeda K. Differential changes in glutamatergic transmission via N-methyl-D-aspartate receptors in the hippocampus and striatum of rats behaviourally sensitized to methamphetamine. Int J Neuropsychopharmacol. 1999;2:155–163. doi: 10.1017/S1461145799001480. [DOI] [PubMed] [Google Scholar]
- Zheng TM, Caruncho HJ, Zhu WJ, Vicini S, Ikonomovic S, Grayson DR, Costa E. Chronic flumazenil alters GABAA receptor subunit mRNA expression, translation product assembly and channel function in neuronal cultures. J Pharmacol Exp Ther. 1996;277:525–533. [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006;27:131–136. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]