Abstract
A previous study by the authors has shown that isoflurane (ISO), a commonly used volatile anesthetic, has an excitatory effect on bronchopulmonary C-fibers (PCFs). Since selective stimulation of PCFs by action on local 5-HT3 receptor could evoke an apnea, this current study addresses whether inhalation of ISO would facilitate the PCF 5-HT3 receptor-mediated apneic response and, if so, how. In anesthetized and spontaneously breathing rats, inhalation of 5% ISO markedly inhibited the apneic response to intra-atrium injection of phenylbiguanide (PBG, 25 µg/kg), a 5-HT3 receptor agonist, which was contrary to the hypothesis. Extracellular recording of the nodose ganglion neurons in anesthetized, paralyzed and ventilated rats revealed that ISO attenuated the PBG-elicited excitation of pulmonary C neurons. Furthermore, using the patch clamp technique, it was found that ISO depressed the PBG-induced inward current of the pulmonary C neurons labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) instilled previously into the lungs. These results suggest that ISO inhibits PCF 5-HT3 channel functions, and thereby attenuates PCF excitatory response to PBG, likely contributing to the diminution of the PBG-induced apnea by ISO in rats.
Keywords: volatile anesthetic, 5-HT3 receptor, pulmonary sensory neurons, breathing
Bronchopulmonary C-fibers (PCFs) innervating the lungs and airways constitute 75–90% of the sensory fibers in the pulmonary branches of the vagus nerve [1, 9, 14, 18] and play an important role in modulating the respiratory rhythm [3, 4, 14, 24]. It has been well-documented that right atrial bolus injection of phenylbiguanide (PBG), a 5-HT3 receptor agonist, can stimulate PCFs to produce an apnea [5, 17]. In our recent study [26], we found that isoflurane (ISO), a volatile anesthetic widely used in clinics, could increase the excitability of PCFs in rats, which is supported by another study performed in dogs [21]. Additionally, ISO is able to potentiate the function of 5-HT3 receptors in Xenopus oocytes of cloned mice [16] and neuroblastoma cells in mice [10]. Taken together, these findings question whether ISO exposure would facilitate the PCF excitatory response to PBG to prolong the PBG-induced apnea and, if so, whether this ISO facilitatory effect was achieved by potentiating 5-HT3 receptor function in PCFs.
Experiments were conducted on pathogen-free Sprague-Dawley male rats (80–150 g). Relatively young rats were chosen because the rate of success of extracellularly recording C-neurons within the nodose ganglia was much higher in these rats as compared to older ones [26]. The experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Lovelace Respiratory Research Institute (LRRI) sponsored Institutional Animal Care and Use Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, USA.
Animal preparation and experiment methods were the same as mentioned in a recent study by the authors [26]. Study Series I tested whether inhalation of ISO could prolong the apnea induced by right atrial bolus injection of PBG in anesthetized and spontaneously breathing rats. The test compared the apneic responses to PBG (25 µg/kg) before and during 5% ISO exposure, and this concentration of ISO was used in the previous studies in dogs [21] and rats [26]. PBG was injected ~ 6 s after ISO exposure because a relatively stable breathing frequency (f) response (following the peak response) was reached at this time in the previous study [26]. It was further determined in Study Series II whether ISO could increase the pulmonary C-type neural response to PBG. The animals were anesthetized, paralyzed and artificially ventilated [26]. The experimental protocols were the same as described above, with the exception that the electrical activity of the pulmonary C-type neuron (C neuron) in nodose ganglia were extracellularly recorded. The midcervical vagus nerve was electrically stimulated, and a PBG-responsive neuron with a conduction velocity of less than 2 m/s was defined as a pulmonary C neuron [8, 15]. We then examined whether ISO could potentiate the PBG-induced inward current in pulmonary C neurons in Study Series III. The neurons isolated from nodose ganglia were cultured in vitro [26], and those with the following features were defined as pulmonary C neurons: labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI), which was instilled previously into lungs serving as a retrograde tracer; small size (diameter < 25 µm), with a spherical shape; no visible cellular processes; and an electrophysiologically excitatory response to PBG [6, 13, 26]. The effect of ISO (1 mM) on the pulmonary C neuron responsiveness was assessed by comparing the PBG (10 µM)-induced inward current before and 3 min after ISO perfusion. The ISO concentration in solution (1 mM) was closely equivalent to 5% ISO used in the in vivo experiment [26].
With respect to data acquisition and analysis, baseline TE and associated cardiovascular values were averaged 1 min before PBG administration as controls, and the values from the PBG-evoked apneic responses were presented as percentage changes from the control (Δ%). The firing rate and duration of pulmonary C neurons in vivo and the magnitude of PBG-induced currents on the pulmonary C neurons in vitro were presented as absolute values. Paired-t tests were used to test the effects of ISO on PBG-induced cardiorespiratory or neural response. The software Statistica 6.0 (StatSoft, Inc., Tulsa, OK) was used for statistical analysis. All data are presented as means ± standard error (SE). The differences were considered significant at a P value < 0.05.
There are three major findings: 1) ISO shortens, rather than elongates, PBG-induced apnea in anesthetized rats; 2) ISO inhibits PBG-elicited bursting in pulmonary C neurons; and 3) ISO inhibits PBG-induced inward current in pulmonary C neurons.
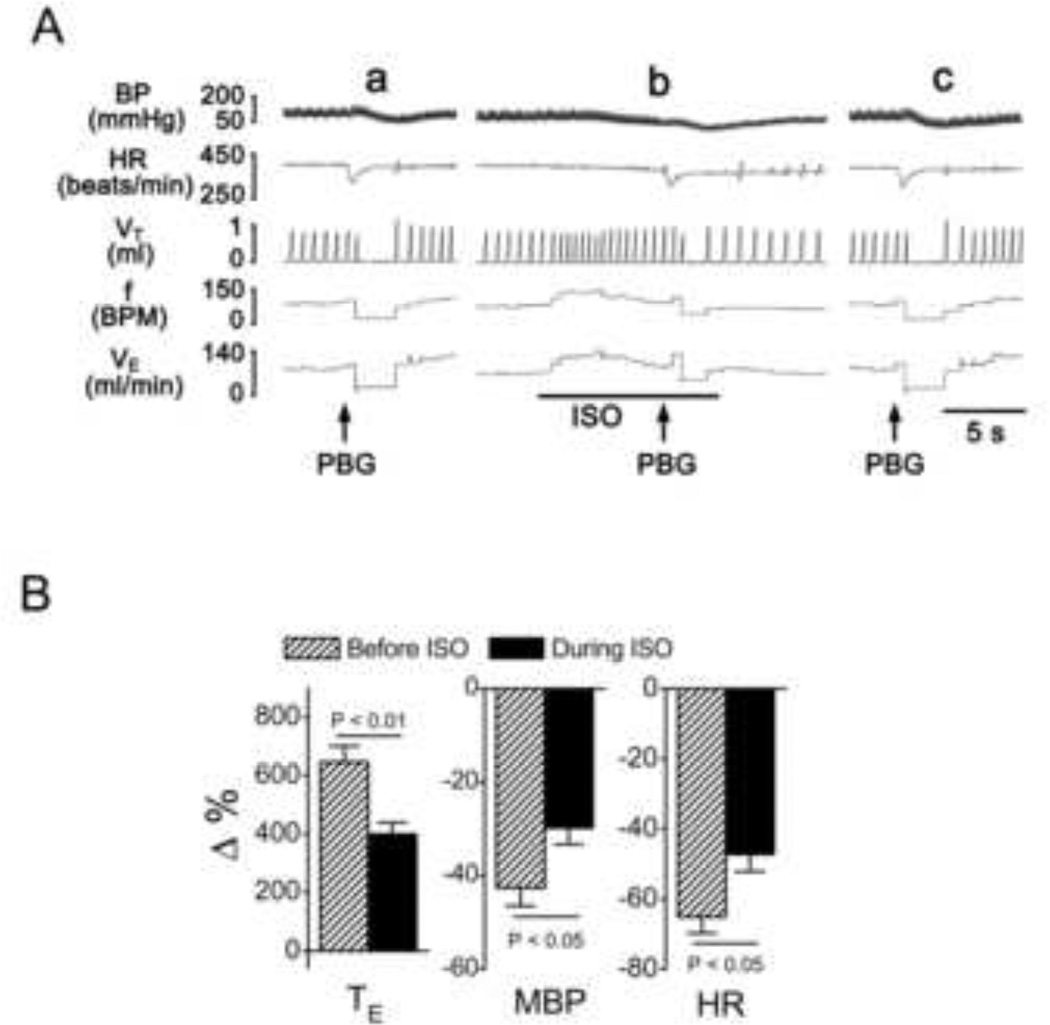
First, we found that ISO shortened, rather than prolonged, the apnea induced by intraatrial injection of PBG in the anesthetized and spontaneously breathing rats, which is the opposite of our hypothesis. As shown in Fig. 1, PBG produced an apnea with the expiratory duration (TE) prolonged from 0.41 ± 0.02 s to 3.1 ± 0.32 s (6.5-fold prolongation, P < 0.01), accompanied by bradycardia and hypotension, similar to previous reports [8, 20, 23]. Unexpectedly, ISO exposure in this study significantly inhibited the apneic response to PBG with TE increased from 0.43 ± 0.03 s to 2.1 ± 0.34 s (3.9-fold prolongation, P < 0.01) and attenuated the PBG-induced bradycardia and hypotension. In addition, ISO alone evoked a brief tachypnea that lasted approximately 7 s with the peak tachypnea response (breath frequency increased by 26%) occurring 4 ± 0.3 s after exposure and was associated with elevated minute ventilation (VE) and unchanged tidal volume (VT), which is similar to the previous recent report [26].
Fig. 1.
The effect of isoflurane (ISO) on PBG-induced apnea. A: Representative recordings showing apneic responses to PBG before (a), during (b) and 20 min after 5% ISO (c). Traces from top to bottom are arterial blood pressure, BP; heart rate, HR; tidal volume, VT; respiratory frequency (BPM = breaths/min), f; and minute ventilation, VE. B: Group data showing the effect of ISO on the PBG-induced cardiorespiratory changes. TE, expiratory duration; MBP, mean arterial blood pressure; HR, heart rate. N = 8, means ± SE.
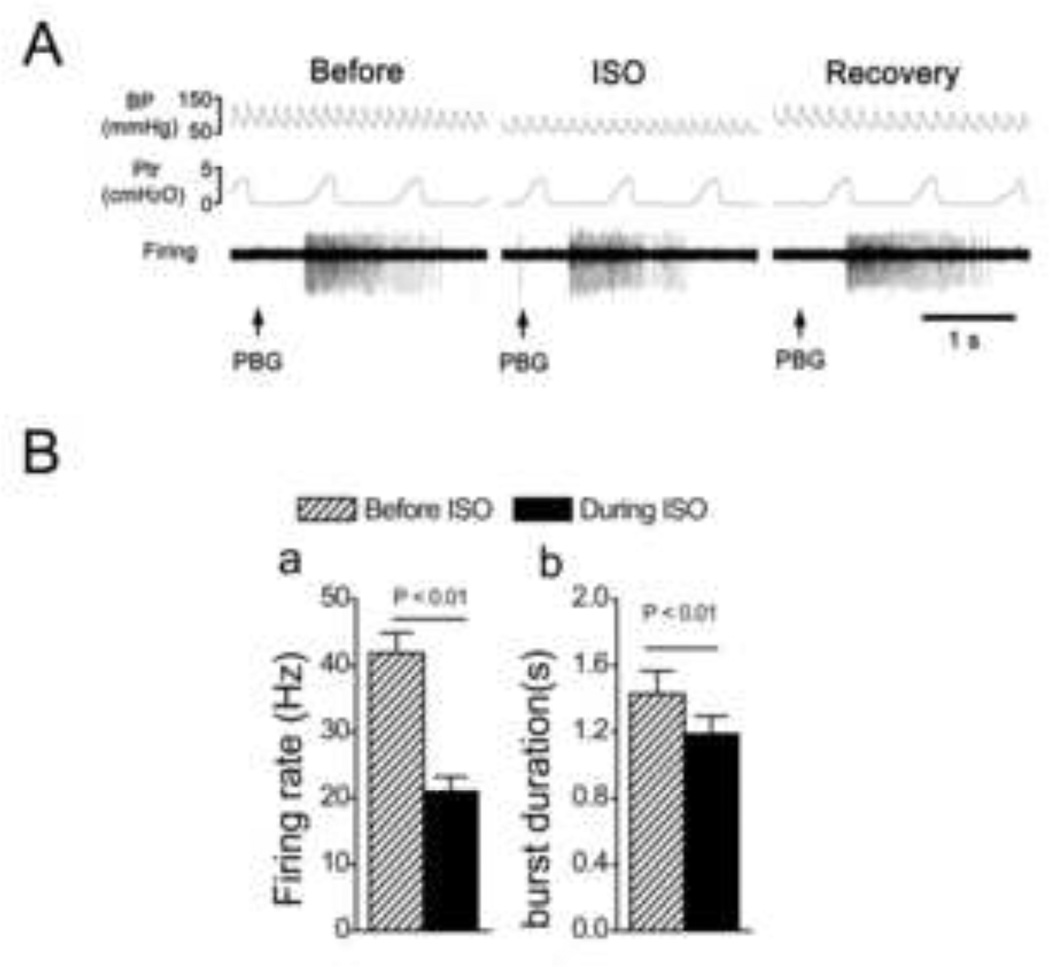
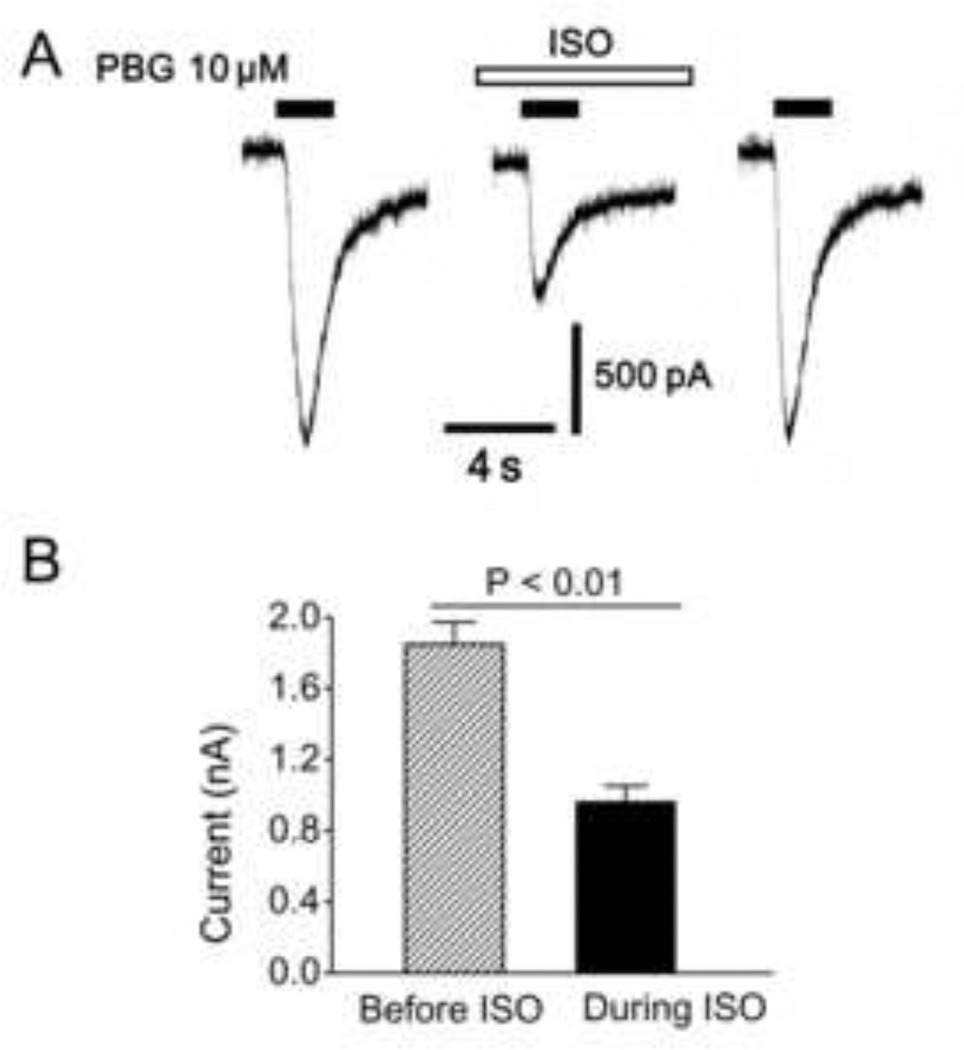
To elucidate the mechanisms underlying the inhibitory effect of ISO on PBG-induced apnea, the following two studies were performed. We extracellularly recorded pulmonary C neuron activity, and assessed the effect of ISO on the neural response to PBG. The results showed that 1), ISO per se slightly, but significantly increased neural firing rate (0.38 ± 0.05 vs. 1.02 ± 0.20 impulses/s, P < 0.05), and 2), the neural firing rate and duration of PBG-induced burst were depressed by ISO exposure (Fig. 2), which is consistent with an ISO inhibitory impact on the PBG-induced apnea. Subsequently, the patch clamp technique was used to further explore how ISO attenuated the PBG-induced bursting response by recording the PBG-induced current in pulmonary C neurons before and during ISO perfusion. Of the recorded 43 neurons labeled with DiI, 12 responded to PBG by generating an inward current; another 31 did not, and these were not studied further. As shown in Fig. 3, ISO solution infusion depressed the PBG-induced inward current in these pulmonary C neurons, indicating an inhibitory effect of ISO on the function of 5-HT3 receptors in these cells. ISO alone elicited a sustained inward current with the amplitude of 84 ± 6 pA. Differing from these data, ISO reportedly potentiated the function of 5-HT3 receptors in Xenopus oocytes of cloned mice [16] and neuroblastoma cells in mice [10]. The discrepancy may be due to the differences of neuron/cell types, tissues, and animal species used in the research and these studies, which is supported by other reports [19]. The latter revealed that the 5-HT3 receptor molecule has interspecies differences in both tissue distribution and functional profile. Future study will be performed to explore the mechanism by which ISO depresses the function of 5-HT3 receptors on pulmonary C neurons.
Fig. 2.
The effects of isoflurane (ISO) on the PBG-induced bursts in pulmonary C-type neurons. A: A representative recording showing that the bursting response to intra-atrium injection of PBG was attenuated by ISO in a pulmonary C-type neuron (the conduction velocity = 1.01 m/s). Traces from top to bottom are arterial blood pressure, BP; tracheal pressure, Ptr; and the cell’s activity, firing. B: Group data showing that ISO decreased the firing rate (a) and duration (b) of the PBG-induced bursts. N = 13, means ± SE.
Fig. 3.
A representative recording (A), and group data (B), showing isoflurane (ISO, 1 mM) decreases the PBG-induced current in rat pulmonary C-tpye neurons. N = 12. Data are presented as means ± SE.
A recent study by the authors [26] reported that ISO could depolarize the pulmonary C neurons and thereby activate PCFs, at least partly, via inhibiting transient A-type potassium current (IA) channels and delayed rectifier potassium current (IK) channels. The excitatory effect on PCFs can exaggerate PCF-mediated respiratory response to PBG, which is shown in a previous study [25] where fentanyl, another PCF stimulant [27], facilitated PBG-induced tachypnea. It is believed that the excitatory effect on PCFs by ISO should be overwhelmed by its inhibitory effect on 5-HT3 receptors, resulting in a shortened (rather than prolonged) PBG-induced apnea by ISO.
One concern in this study is that the PCFs-mediated apnea response is a reflex requiring central pathway involvement [12]. The data cannot rule out the possibility that the central inhibitory effect of ISO [2] may also have contributed to the shortened PBG-induced apnea by ISO.
In summary, we determined that ISO could inhibit PCFs 5-HT3 channel function, and thereby attenuate PCF excitation by PBG, which likely contributed to the depressed apneic response to PBG in rats. Clinically, 5-HT3 receptors may mediate some of the unpleasant side effects associated with general anesthesia, such as the postoperative nausea and vomiting, which can be alleviated with 5-HT3 receptor antagonists [7]. Also, stimulation of the vagus nerve is thought to play a key role in nausea and vomiting, especially those induced by chemotherapy and radiotherapy [22]. The finding that ISO can inhibit the function of 5-HT3 receptors on PCFs may be helpful to explain why ISO had a lower postoperative nausea and vomiting rate than other volatile anesthetics used in the clinic, like desflurane and sevoflurane [11].
Highlights.
Isoflurane shortens Phenylbiguanide (PBG)-induced apnea in anesthetized rats
Isoflurane inhibits PBG-elicited bursting in pulmonary C neurons
Isoflurane inhibits PBG-induced inward current in pulmonary C neurons.
Acknowledgments
Funding: This study is supported by RO1 HL107462 from the National Heart, Lung, and Blood Institute, Bethesda, MD, and American Lung Association Biomedical Research Grant RG-191095-N, New York, NY
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canet J, Sanchis J, Zegri A, Llorente C, Navajas D, Casan P. Effects of halothane and isoflurane on ventilation and occlusion pressure. Anesthesiology. 1994;81:563–571. doi: 10.1097/00000542-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 4.Coleridge HM, Coleridge JCG. Reflexes evoked from the tracheobronchial tree and lungs. In: Cherniack N, Widdicombe JG, editors. Handbook of Physiology, section 3, Respiratory System. part 1. Vol. II. Bethesda: American Physiological Society; 1986. pp. 395–429. [Google Scholar]
- 5.Dutta A, Deshpande S. Cardio-respiratory reflexes evoked by phenylbiguanide in rats involve vagal afferents which are not sensitive to capsaicin. Acta Physiol (Oxf) 2010;200:87–95. doi: 10.1111/j.1748-1716.2010.02105.x. [DOI] [PubMed] [Google Scholar]
- 6.Gu Q, Lim ME, Gleich GJ, Lee LY. Mechanisms of eosinophil major basic protein-induced hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol. 2009;296:L453–L461. doi: 10.1152/ajplung.90467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haigh CG, Kaplan LA, Durham JM, Dupeyron JP, Harmer M, Kenny GN. Nausea and vomiting after gynaecological surgery: a meta-analysis of factors affecting their incidence. British journal of anaesthesia. 1993;71:517–522. doi: 10.1093/bja/71.4.517. [DOI] [PubMed] [Google Scholar]
- 8.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respiration physiology. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 9.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst. 1982;5:165–176. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins A, Franks NP, Lieb WR. Actions of general anaesthetics on 5-HT3 receptors in N1E-115 neuroblastoma cells. Br J Pharmacol. 1996;117:1507–1515. doi: 10.1111/j.1476-5381.1996.tb15314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsen KL, Persson E, Wennberg E, Stenqvist O. Anaesthesia, recovery and postoperative nausea and vomiting after breast surgery. A comparison between desflurane, sevoflurane and isoflurane anaesthesia. Acta anaesthesiologica Scandinavica. 2000;44:489–493. doi: 10.1034/j.1399-6576.2000.440423.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol. 2002;93:1419–1428. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:17–24. doi: 10.1002/ar.a.10005. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. American journal of physiology. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- 16.Machu TK, Harris RA. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in Xenopus laevis oocytes. The Journal of pharmacology and experimental therapeutics. 1994;271:898–905. [PubMed] [Google Scholar]
- 17.Matsumoto S, Kanno T, Yamasaki M, Nagayama T, Shimizu T. Pulmonary C-fibers elicit both apneusis and tachypnea in the rabbit. Respiration physiology. 1992;87:165–181. doi: 10.1016/0034-5687(92)90057-4. [DOI] [PubMed] [Google Scholar]
- 18.Mei N, Condamin M, Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209:423–431. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- 19.Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Molecular pharmacology. 1995;48:407–416. [PubMed] [Google Scholar]
- 20.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. Journal of neurophysiology. 2007;98:3627–3637. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- 21.Mutoh T, Tsubone H, Nishimura R, Sasaki N. Effects of volatile anesthetics on vagal C-fiber activities and their reflexes in anesthetized dogs. Respiration physiology. 1998;112:253–264. doi: 10.1016/s0034-5687(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 22.Perdue C. Understanding nausea and vomiting in advanced cancer. Nursing times. 2005;101:32–35. [PubMed] [Google Scholar]
- 23.Tyers MB. 5-HT3 receptors and the therapeutic potential of 5-HT3 receptor antagonists. Therapie. 1991;46:431–435. [PubMed] [Google Scholar]
- 24.Wilson CG, Bonham AC. Effect of cardiopulmonary C fibre activation on the firing activity of ventral respiratory group neurones in the rat. J Physiol. 1997;504(Pt 2):453–466. doi: 10.1111/j.1469-7793.1997.453be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhang C, Zhou M, Xu F. Activation of opioid mu-receptors, but not delta- or kappa-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats. Respiratory physiology & neurobiology. 2012;183:211–217. doi: 10.1016/j.resp.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhuang J, Zhang C, Xu F. Isoflurane depolarizes bronchopulmonary C neurons by inhibiting transient A-type and delayed rectifier potassium channels. Respiratory physiology & neurobiology. 2013 doi: 10.1016/j.resp.2013.01.006. in press. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang J, Zhang Z, Zhang C, Xu F. 8-OH-DPAT Abolishes the Pulmonary C-Fiber-Mediated Apneic Response to Fentanyl Largely via Acting on 5-HT1A Receptors in the Nucleus Tractus Solitarius. Am J Physiol Regul Integr Comp Physiol. 2012;303:449–458. doi: 10.1152/ajpregu.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]