Abstract
Latency-associated nuclear antigen (LANA) is encoded by the Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) open reading frame 73. LANA is expressed during latent KSHV infection of cells, including tumor cells, such as primary effusion lymphoma, KS and multicentric Castleman’s disease. Latently infected cells have multiple extrachromosomal copies of covalently closed circular KSHV genomes (episomes) that are stably maintained in proliferating cells. LANA’s best characterized function is that of mediating episome persistence. It does so by binding terminal repeat sequences to the chromosomal matrix, thus ensuring episome replication with each cell division and efficient DNA segregation to daughter nuclei after mitosis. To achieve these functions, LANA associates with different host cell proteins, including chromatin-associated proteins and proteins involved in DNA replication. In addition to episome maintenance, LANA has transcriptional regulatory effects and affects cell growth. LANA exerts these functions through interactions with different cell proteins.
Keywords: AIDS, HIV, human herpesvirus 8, Kaposi’s sarcoma, lymphoma, viral latency
Kaposi’s sarcoma-associated herpesvirus
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) or human herpesvirus 8, a γ-2 herpesvirus, is the most recently discovered human herpesvirus [1,2]. Viruses related to KSHV (other γ-2 herpesviruses) are found in many mammalian species (e.g., herpesvirus saimiri [HVS; New World monkeys], murine γ herpesvirus 68 [rodents] and rhesus rhadinovirus [Old World monkeys]) suggesting that these viruses are ancient and have co-evolved with their hosts (for reviews see [3,4]). The KSHV genome is co-linear with other γ-2 herpesviruses, and similar to the human γ-1 herpesvirus, Epstein–Barr Virus (EBV). These viruses share a common genomic organization and blocks of genes have analogous roles in the viral life cycle. KSHV encodes approximately 75 genes, of which some are novel and some are homologs of known cellular genes. Importantly, KSHV and EBV are important tumorigenic agents in immunosuppressed individuals, such as transplant recipients and HIV/AIDS patients.
KSHV is tightly associated with KS, primary effusion lymphoma (PEL) and multicentric Castleman’s disease (an aggressive lymphoproliferative disorder). KS remains the most prevalent tumor associated with HIV/AIDS patients [5]. Cell-mediated immunity plays a role in controlling the disease. Notably, KS lesions can regress with immune reconstitution by reducing immunosuppressive therapy in transplant recipients or by reconstitution of the immune system with HAART in AIDS patients [6–9].
A particularly aggressive form of KS occurs in African children and accounts for approximately 2–10% of all cancers in children in eastern and southern Africa [10–12]. KSHV seropositivity precedes KS and KSHV is found in all KS lesions in the presence or absence of HIV co-infection [8,13]. The seroprevalence of KSHV varies from approximately 50% of men who have sex with men to approximately 5% in the general population of the USA to 30–80% in endemic regions (for epidemiological studies see [8,11,13–18]). Serological assays are dependent upon the detection of different KSHV antigens, often including the protein encoded by open reading frame (ORF) 73, termed latency-associated nuclear antigen (LANA).
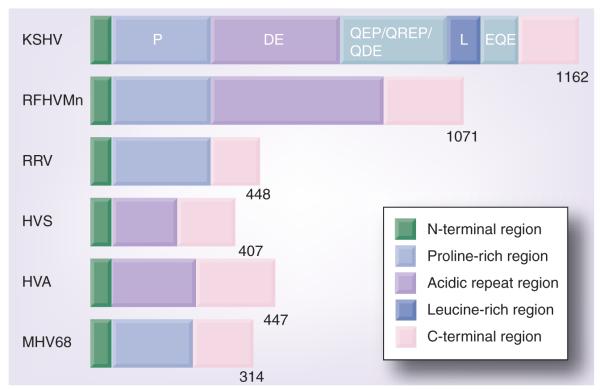
LANA is one of only a few KSHV-encoded proteins expressed during latency, which is the primary form of infection in both the normal host and in tumors [8,19,20]. LANA is approximately 1162 amino acids in length. A large repetitive region of acidic and glutamine-rich repeats comprise the middle of the protein separating the LANA N- and C-terminal regions (Figure 1). Heterogeneity in the length of the internal repeat region of LANA has been noted between KSHV isolates [21]. Some orthologs of LANA do not contain the internal repeats (e.g., murine γ-herpesvirus 68) (Figure 2), whereas other LANA orthologs, such as that of HVS, lack a proline-rich region [21–23]. The LANA ortholog of retroperitoneal fibromatosis herpesvirus, the homolog of KSHV that infects the pig tailed macaque, contains both a proline rich region and central acidic repeats, similar to LANA [24–26]. The N-terminal region of LANA contains a proline-rich region and associates with many chromatin associated proteins (Table 1). LANA amino acids 5–13 encode a chromosome binding motif that binds histones H2A/H2B [27]. The C-terminal region of LANA contains a unique leucine-rich domain and another repeat region, followed by unique sequence and associates with a number of host cell proteins and chromosomes (Table 1). The unique C-terminal LANA region self-associates to bind DNA and recognizes a specific DNA sequence within the terminal repeat (TR) region of KSHV. Many functions have been ascribed to the KSHV-encoded LANA, including episome persistence, transcriptional activity and growth effects on the cell.
Figure 1. Latency-associated nuclear antigen protein.
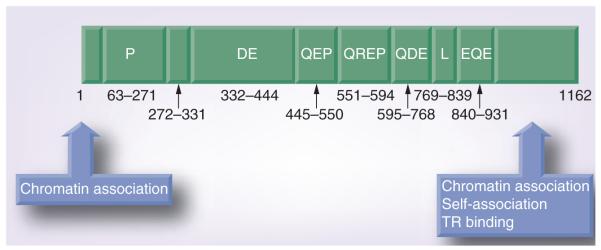
LANA consists of 1162 amino acids. Repetitive blocks of amino acids are noted (DE, QEP, QREP, QDE and EQE). Residues 63–271 contain a proline-rich region. Residues 769–839 contain a leucine-rich repeat region.
D: Aspartic acid; E: Glutamic acid; L: Leucine; P: Proline; Q: Glutamine; R: Arginine.
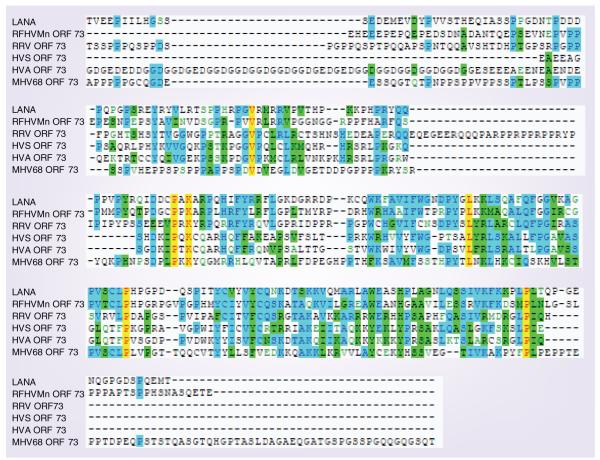
Figure 2. Schematic of latency-associated nuclear antigen and the ORF73 homologs.
Similar regions between homologs of ORF73s from different γ-2 herpesviruses are indicated and compared with KSHV LANA. KSHV LANA (Homo sapiens); (NP_572129), HVS (squirrel monkey; NP_040275), HVA (spider monkey; NP_048045), RRV (rhesus macaque; AAD21406), MHV68 (vole; NP_044913), RFHVMn (rhesus pig tailed macaque; ABH07415).
Adapted from [26].
Table 1.
Latency-associated nuclear antigen-associated proteins.
| Interactor | Name | LANA binding site† | Ref. |
|---|---|---|---|
| Transcription factors or chromatin-associated factors | |||
| BRD4 | Bromodomain protein 4 | 1133–1143 | [116,166] |
| CIR | CBF1 interacting co-repressor | 1–340 | [64] |
| CBP/p300 | cAMP response element binding protein | 340–431, 950–1162 | [128] |
| CREB2 | cAMP response element binding protein | 769–839 | [129] |
| DAXX | Death domain-associated protein | 320–344 | [177] |
| Dek | DEcoRVK | 986–1043 | [63] |
| H1 | Histone 1 | 1–1162 | [31] |
| H2a/H2b | Histones 2a and 2b | 5–13 | [27] |
| Hiflα | Hypoxia inducible factor 1α | 46–145 | [126] |
| Hp1α | Heterochromatic protein 1α | 1047–1062 | [65] |
| I-mfα | Inhibitor of MyoD family α | 995–1162 | [135] |
| Jκ | Immunoglobulin Jκ region recombination signal binding protein | 990–1162 | [136] |
| c-jun | Oncogene 17 | 1–1162 | [124] |
| KLIP | KSHV LANA interacting protein | 1–317 | [132] |
| KZLP | KRAB zinc finger binding protein | 1–1162 | [134] |
| MecP2 | Methyl cytosine binding protein 2 | 1–15, 936–1162 | [63,66] |
| Med25 | Mediator 25 | 1–340 | [117] |
| c-myc | Avian myelocytomatosis vial oncogene homolog | 1–1162 | [125,130] |
| p53 | p53 tumor suppressor | 441–1162 | [143] |
| Rb | Retinoblastoma protein | 803–990 | [133] |
| Rta | Replication and transcription activator | 990–1162 | [123] |
| Sap30α | Sin3α-associated polypeptide | 1–340 | [64] |
| Sin3a | Paired amphipathic helix protein Sin3a | 1–340 | [64] |
| Sp1 | Specificity protein 1 | 1–1162 | [119] |
| srf | Serum response factor | 1–1162 | [117] |
| Stat3 | Signal transducer and activator of transcription 3 | 933–1162 | [131] |
| tat | Transactivator protein | 762–1162 | [127] |
| Replication | |||
| NPM | Nucleophosmin | 1–1162 | [97] |
| Orc1 | Origin recognition complex 1 | 1–340, 762–1162, 1001–1068 | [86,178] |
| Orc2 | Origin recognition complex 2 | 762–1162, 1001–1068 | [86,178] |
| Orc3, 4 & 6 | Origin recognition complex 3, 4 & 6 | 762–1162 | [86,178] |
| Orc5 | Origin recognition complex 5 | 1–340, 762–1162 | [86,178] |
| SSRP1 | Structure-specific recognition protein 1 | 1–1162 | [95] |
| Kinases | |||
| Gsk3β | Glycogen synthase kinase 3β | 241–275, 1133–1147 | [154,158] |
| Pim1 | Proviral integration site MuLV | 762–1162 | [160] |
| Pim 3 | Proviral integration site MuLV | 1–1162 | [160] |
| Ubiquitin related | |||
| Cul5 | Culin 5 | 1085–1100 | [163] |
| Elongin C | Elongin C | 212–222 | [163] |
| FBW7/Sel10 | F box WD40 domain protein 7 | 1052–1082 | [179] |
| VHL | Von Hippel–Lindau tumor suppressor | 1–327 | [163] |
| Enzymes | |||
| DNMT1 | DNA methyltransferase 1 | 1–340 | [107] |
| DNMT3A | DNA methyltransferase 3A | 1–15 | [107] |
| DNMT3B | DNA methyltransferase 3B | 1–340 | [107] |
| Suv39h1 | Suppressor of varigation 39h1 | 275–467 | [180] |
| Ung2 | Uracyl DNA glycosylase 2 | 762–1162 | [178] |
| Miscellaneous proteins | |||
| Bub1 | Budding unhibited by benzimidazoles | 1–340, 842–1162 | [52] |
| CenpF | Centromere protein F | 1–340, 842–1162 | [52] |
| MNDA | Myeloid cell nuclear differentiation antigen | 22–274 | [181] |
| Numa | Nuclear mitotic apparatus protein | 762–1162 | [182] |
Numbers refer to amino acids of LANA (Genbank U75698) that have been mapped to interact with the stated protein. LANA: Latency-associated nuclear antigen.
LANA associates with chromosomes & KSHV TR DNA to maintain KSHV episomes
In tumors and latently infected cells, the KSHV genome (~200 kb) persists as a multiple copy (10–50 copies per cell), covalently closed circular extrachromosomal plasmid (episome). Only a small subset of KSHV genes are expressed in latent infection of PELs, including LANA, v-FLIP and v-cyclin, which are expressed from the same alternatively spliced transcript. Several viral miRNAs are also expressed in KSHV latently infected cells [28,29].
Episomes must overcome two major obstacles to persist in proliferating cells. Episomes must replicate prior to each cell division to avoid loss in copy number. In addition, episomes must segregate to newly formed nuclei after mitosis to avoid being destroyed in the cytoplasm. LANA overcomes both of these obstacles by mediating replication of KSHV DNA, and segregating viral episomes to daughter nuclei.
During mitosis, LANA partitions replicated viral episomes to daughter cells. This is accomplished by LANA’s ability to simultaneously bind host cell chromosomes and viral TR DNA sequences (Figure 3) [30–32]. Notably, the TR sequences account for approximately 25–30% of the KSHV genome, likely due to their role in episome persistence. The TR sequence of herpesviruses contains elements required for packaging the DNA into the nucleocapsid. Binding of a virally encoded gene product (LANA) to the TR to mediate episome persistence and replication is an additional and novel function for this element. Studies related to the epigenetic markers at the TR sequence may provide further insights into the relevance of this sequence in herpesviruses and into the role of host cell proteins that are recruited to it. Post-translational histone modifications provide scaffolds for protein assembly in many cellular processes including transcriptional activation and repression (chromatin remodeling), and DNA repair and replication. Histone modifications of heterochromatin, including H3K9me2, are found at the TRs of HVS [33]. Other histone marks implicated in transcriptional activation at the HVS TRs are dimethyl H3K4, and at both the HVS and KSHV TRs are H3K9ac [34,35]. Further evaluation of histone modifications at the TR would be of interest, as LANA-associated proteins include Hp1α and BRD4, which bind methylated and acetylated histones, respectively. Hence, the epigenetic modifications and the recruitment of cellular factors at the TR play a role in the life cycle of KSHV.
Figure 3. Model of latency-associated nuclear antigen binding to Kaposi’s sarcoma-associated herpesvirus episome and cellular chromosomes.
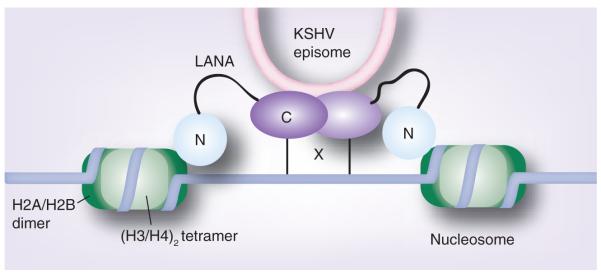
The C-terminal region of LANA (C) mediates self-association, binds to KSHV terminal repeat DNA in the KSHV episome, and binds to a putative protein (X) that associates with DNA (light gray line). The N-terminal domain of LANA (N) binds to core histones H2A/H2B, which are part of nucleosomes that also contain a H3/H4 histone tetramer.
KSHV: Kaposi’s sarcoma-associated herpesvirus; LANA: Latency-associated nuclear antigen.
Adapted from [51].
Chromatin-binding domains of LANA are essential for LANA-mediated episome persistence since mitotic chromosome binding is necessary for LANA tethering of episomes [30,31,35–38]. Both the N- and C-terminal regions of LANA bind chromatin (Figure 3). N-terminal LANA provides the dominant chromosome attachment region. Amino acids 1–32 of LANA diffusely distribute over chromatin and so LANA was speculated to interact with histones [31,38]. Further characterization of this interaction revealed that this N-terminal region of LANA binds to an acidic pocket at the interface of histones H2A and H2B [27,39]. Interestingly, this histone pocket also binds IL-33, suggesting that this may be a docking station that is underappreciated [40]. Full length LANA associates with chromosomes in the presence and absence of viral DNA sequences [41]. In the absence of episomes, the staining pattern of LANA exhibits broad staining of mitotic chromosomes, while in the presence of viral DNA LANA localizes to punctate foci at the sites of KSHV episomes [41]. This likely occurs as a result of C-terminal LANA binding to multiple high-affinity binding sites on KSHV TR DNA while simultaneously binding chromosomes.
Other DNA tumor viruses, including EBV and the papilloma viruses, encode nonhomologous genes with functions analogous to LANA [42,43]. For example, EBV has a similar system for episome segregation and replication fostered by the EBV encoded EBNA1 protein binding to the oriP DNA sequence of EBV [43–45]. The papilloma viruses utilize E1 and E2 proteins to facilitate episome maintenance and replication; E2 binds chromatin and tethers the genome to chromosomes [42,46–49]. Viral DNA and LANA colocalize on mitotic chromosomes [41]. However, in cells that are co-infected with EBV and KSHV, LANA and EBNA1 do not colocalize, suggesting differences in tethering mechanisms [50]. In mitotic cells, C-terminal LANA can localize to pericentromeric regions that do not associate with CenpA. This region of LANA is important for DNA binding and, similar to the N-terminal region of LANA, has a role in chromosome binding and episome persistence [37,51]. Colocalization of some LANA molecules with kinetechore-associated proteins CenpF and Bub1 has been noted [52]. EBNA1, however, does not associate with pericentromeric regions during mitosis but does appear as paired dots on sister chromatids, which coincide with EBV episomes [53,54]. Depending on the type, HPV E2 staining appears to resemble LANA as pericentromeric or EBNA1 as paired dots [47,48]. Human HPV E2 has been shown to associate with the spindle apparatus during mitosis; however, LANA appears to depend on chromatin associations for partitioning to daughter cells. Therefore, episomal DNA viruses have evolved mechanisms to facilitate association with chromosomes to ensure that the virus is not lost during segregation of replicated chromosomes with each cell division.
The C-terminal domain of LANA is the most conserved region among the LANA orthologs (Figure 2 & 4) [26]. The TR DNA-binding domain of LANA is in the C-terminal region of the protein encompassing amino acids 996–1139 [51,55–58]. C-terminal LANA self associates and this self association is essential for DNA binding [56,59,60]. The structure of LANA binding to the TR has not been solved. However, the DNA-binding domains of EBV EBNA1 and HPV E2 have been crystallized bound to their respective DNA-recognition sequences [42,61,62]. Remarkably there is structural homology between the EBNA1 and E2 DNA-binding domains, despite the absence of sequence homology. Software modeling studies predict structural similarities between the DNA-binding domain of LANA and EBNA1, and by extension, E2, suggesting either evolution from a common ancestor or convergent evolution [32].
Figure 4. Amino acid alignment of the C-terminal regions of latency-associated nuclear antigen (aa933–1162) and ORF73 homologs.
Yellow shaded regions indicate conserved amino acids; green shaded amino acids indicate conserved basic, acidic or hydrophobic residues; blue shaded regions Indicate conserved residues between two or more ORF73 homologs. Alignment was made using Vector NTi (Invitrogen, CA, USA).
The C-terminal domain of LANA also associates with chromatin at pericentromeric regions, juxtaposed to CENPA, and occasionally at peritelomeric regions, and this association with chromatin assists in episome persistence [37,51]. Interestingly, a number of LANA-associated proteins localize with pericentromeric regions of chromosomes or are heterochromatin-associated proteins. These include MecP2, Sin3A, HP1α, ORC2, ORC6 and SUV39H1 [63–67], some of which associate with C-terminal LANA. It is unclear if LANA associates with these interacting proteins in a cell-cycle dependent manner given the varied functions of these LANA-associated proteins (Table 1).
The association of the pericentromeric protein, MecP2, and the HVS ORF73 is important for episome persistence. Knockdown of Mecp2 using siRNA inhibited HVS episome persistence [68]. MeCP2 is a DNA-binding protein that recognizes methylated cytosine residues and is implicated in both transcriptional repression and activation [69]. While the C-terminal region of HVS ORF73 (LANA homolog) associates with MeCP2, the N-terminal and C-terminal regions of KSHV LANA were found to associate with MeCP2 [63,66]. MeCP2 and HVS ORF73 associate with pericentromeric regions of chromosomes. Stuber et al. found that MecP2 was scattered from chromocenters in mouse cells expressing LANA [70], although Matsumura et al. found that MecP2 relocalized LANA to chromocenters in mouse cells [63,66]. It is curious that MeCP2 can associate with DAXX (an N-terminal KSHV LANA-associated protein, see Table 1) and ATRX complexes of proteins, which have recently been implicated in remodeling of chromatin by incorporation of histone H3.3 at pericentric and telomeric loci of mouse embryonic stem cells [71]. Therefore, many cellular processes may be affected by LANA and its binding partners in KSHV-infected cells.
There is strong evidence that LANA is required for episome maintenance. Firstly, cells stably expressing LANA can maintain TR-containing plasmids as an episome, whereas cells that do not express LANA cannot [30,32,41,55,58,72–74]. Knockdown of LANA using siRNAs or genetic deletion of LANA from a bacterial artificial chromosome (BAC) containing the viral genome leads to a loss of episomes [75–78]. Genetic deletion of ORF73, the LANA homolog, from the related murine γ-herpesvirus 68 viral BAC, alters the pathophysiology in infected mice [79–82]. The absence of ORF73 leads to a lack of viral spread and latency in the splenic compartment. In addition, Paden et al. demonstrated that murine γ-herpesvirus 68 is integrated or linear in the absence of ORF73 in the B cell compartment, suggesting that LANA may be required for ligation and circularization of viral DNA, in addition to episome persistence [82]. Taken together, these data support the role of LANA in episome persistence.
The art of episomal maintenance and replication by viruses has many practical uses. For example, episomal vectors providing LANA or EBNA1 in cis or trans can be used for gene expression in the absence of integration [83]. This system, using EBNA1, has been used to express genes in fibroblasts to promote induced pluripotent stem cells, without the caveat of other expression systems that integrate into the host genome, indicating that episomal systems may prove useful for gene therapy [84]. Episomal systems have also been used for protein interaction studies [85]. Absence of LANA, and so absence of a mechanism for latency, may also prove useful in vaccine development [75].
LANA mediates DNA replication of KSHV episomes
LANA-mediated TR DNA replication is essential for episome persistence. In the absence of episome replication, KSHV DNA is rapidly lost from proliferating cells. Since only a few viral proteins are expressed in latent infection, and these do not include a viral-encoded DNA polymerase, host cell DNA replication machinery is used during latency to replicate episomal DNA. Cell replication machinery must assemble on the viral episome. Proteins associated with origins of DNA replication including ORC2, ORC3, ORC5 and MCM have been shown to be associate with glutathione-S-transferase-tagged fusion proteins of LANA or at the terminal repeat region by chromatin immuno-precipitation, suggesting that the terminal repeat region of KSHV may be an origin of replication [35,67,86]. Replication bubbles have been described for the EBV episome using 2D gel analyses or single molecule analysis of DNA, and the initially described origin of replication termed ‘oriP’ binds EBNA1. However, multiple origins of replication have now been described for EBV and KSHV, suggesting that multiple origins of replication may exist for DNA viral episomes [87–89]. Using Gardella gel analysis or the methyl-DNA sensitive restriction endonuclease DpnI and Southern blotting, it was shown that plasmids containing the TR sequence replicate in the presence of LANA but not in the absence of LANA [30,32,74,86,90]. Mutations in the N-terminal chromosome-associated region, encompassing amino acids 4–32 of LANA, indicate that these residues are critical for DNA replication as ascertained by DPNI sensitivity assays of TR-containing plasmids and Gardella gel analysis [36]. This could be related to priming the association of replication complexes, or for proper localization of the episome to replicate. Deletion of, or mutations within, amino acids 4–32 can greatly reduce or abolish episome persistence and replication as demonstrated by Gardella gel analyses and methylation sensitive restriction endonuclease digestion (DpnI) of TR-containing plasmids [36,90]. Similar to results described for EBNA1, the N-terminal region of LANA can be replaced by histone H1 and chromatin binding and replication of plasmids containing the terminal repeat are sustained [91,92].
C-terminal LANA binds TR DNA and this binding is essential for DNA replication and episome persistence. LANA binds specifically to a sequence within the TR [30,56,58,59,93]. LANA binds co-operatively to two adjacent binding sites located within each TR unit, a high-affinity site and a lower-affinity site. LANA binds to the high-affinity site with a kd of approximately 1.51 nm [59]. Near these two binding sites is a 32 bp GC-rich element and together they form the minimal LANA DNA replication element [55]. LANA binding to its TR-binding site is essential for DNA replication and episome persistence [56]. Similar to EBNA1, the LANA DNA binding domain located between residues 996–1139 must self associate to bind its recognition sequence [56,60]. Specific LANA residues important for TR binding within this region have been mapped by alanine substitution mutagenesis [51,94].
Using a biotinylated TR probe with the LANA recognition sequence, Hu et al, identified 30 proteins including SSRP1 [95]. SSRP1 is a member of the FACT complex, which is involved in histone–histone and histone–DNA interactions involving replication, transcription and DNA repair (for review see [96]). Another LANA-associated protein involved in latency, nucleophosmin (NPM), is also a part of the FACT complex [97]. RNAi mediated knockdown of SSRP1 diminished LANA-mediated replication of TR-containing plasmids, suggesting the FACT complex may be involved in LANA-mediated functions of replication. More studies regarding LANA and replication of KSHV episomes are required.
LANA & transcription
Several studies have examined gene expression levels by microarray analyses in KSHV-infected endothelial cells or hematopoietic cells. Infection with KSHV alters transcriptional signatures [98–106]. Other studies have focused on the effects of a single KSHV gene, such as LANA, on gene expression. Telomerase-immortalized endothelial cells, transduced with a retroviral vector containing LANA, were shown to repress approximately 80 genes as compared with empty vector control cells [107]. By chromatin immunoprecipitation, LANA was shown to bind to the promoters of some of the identified repressed genes including CDH13, CREG and CCND2. It was postulated that this repression was at the transcriptional level and due to the induction of methyltransferase activity recruitment by LANA. Cytosine methylation of CpG islands is felt to result in silencing of promoters, silencing of transposons, monoallelic expression of imprinted genes and X-chromosome inactivation (for reviews see [108–110]). CpG islands are found in promoters of many genes including some viral genes. Nuclear cytosine methyltransferase enzymes of eukaryotes include Dnmt1, Dnmt3a and Dnmt3b. Maintenance methylation is attributed to Dnmt1, which preferentially recognizes hemimethylated DNA during semiconservative replication [111,112]. Dnmt3a and DNMT3b have a role in the methylation of repeated sequences, such as transposons and pericentric repeats [113]. Histone-modifying enzymes and chromatin remodeling factors also associate with DNA methyltransferases. Dnmt3a, a de novo DNA methyltransferase, was increased in the chromatin-associated fraction in the presence of LANA [107]. Bacterially expressed glutathione-S-transferase-LANA and in vitro translated DNMTs were shown to interact and epitope-tagged Dnmt3a recruitment to the CCND2 promoter was enhanced in the presence of LANA. In addition, LANA was found to associate with DNMT3a by yeast two-hybrid analyses [63]. Methylation of the TGFβII receptor promoter element was also noted in the presence of LANA but not in the absence of LANA [114]. These data suggest that epigenetic factors, including DNA methylation and chromatin remodeling, may be involved in epigenetic reprogramming by LANA.
Transcriptional profiling of uninfected BJAB B lymphoma cells in the presence or absence of LANA indicates that LANA expression has an effect on transcription [102,115]. Several interferon responsive genes were induced approximately three-fold by LANA, including STAT1 and Staf-50. BJAB cells with a doxycycline inducible LANA were shown to affect the expression of approximately 186 genes [115]. Many of these genes are implicated in Rb/E2F-dependent pathways and WNT signaling. Several other studies demonstrate that LANA can induce or repress transcription using various promoter reporter constructs [102,107,115–120]. LANA was shown to activate its own promoter, SRE, SP1, ATF, CAAT and AP1 promoters, but repress an HIV LTR promoter. In terms of latency, LANA transactivates its own promoter [121]. LANA has also been shown to bind the KSHV lytic transactivator ORF50 (Rta), the ORF50 promoter, and to repress ORF50 expression as a mechanism to inhibit initiation of the viral lytic life cycle and so maintain latency [23,122,123].
LANA’s association with the Mediator complex suggests that LANA can recruit the polII transcriptional machinery to activate transcription [117]. Consistent with this observation is the association of LANA with transcriptional activators including CBP, CREB2, c-jun, KLIP, c-myc, Sp1, SRF, Stat3, Rb, Hif1α, KZLP and Tat (Table 1) [117,119,124–134]. Other transcriptional repressors like CIR, I-mfa, Sap30a, Sin3a and Jκ, also interact with LANA [63,64,134–136]. These interactions may have effects on viral persistence, transcription and growth transformation [63–65,137,138]. These data surrounding LANA’s ability to repress or induce transcription may be at least partially dependent upon the cell line used, concentration-dependent effects of LANA expression and the diversity of reporters examined. Somewhat lacking is a clear connectivity between the number of reported transcriptional activators or repressors with which LANA associates and roles they play in the viral life cycle.
LANA & effects on cell growth
Control of cell cycle checkpoints and inhibition of apoptosis are hallmarks of proliferating tumor cells including PELs and KSHV-infected KS spindle cells. The association of LANA with many proteins involved in cell cycle regulation suggests that LANA promotes cell survival. For example, LANA prolongs the lifespan of HUVECS [139]. Data gathered from protein interaction studies support the role of LANA in cellular transformation as certain LANA-associated proteins can affect cell growth. For example, the retinoblastoma protein associates with LANA [133]. Several studies implicate LANA in activating E2F-dependent reporters or E2F-dependent genes [115,140]. LANA induces the expression of Id1 (inhibitor of DNA binding) at the mRNA and protein levels [118]. Since Id proteins have effects on cell cycle regulation, this may be one mechanism of LANA-mediated proliferation of KSHV-infected cells. Collectively, these data implicate LANA in increasing the replicative function of cells.
In addition, LANA associates with, and has effects on, the prototypical tumor suppressor p53 [79,138,141–144]. LANA inhibits p53-dependent transcription and apoptosis. Significantly, mutations in the p53 gene were not found in primary KS samples and functional p53 activity is detected in PEL cell lines [145–148]. The presence of wild-type p53 in KSHV-infected PEL cells suggested that small molecule inhibitors of p53–MDM2 interactions, like nutlin3a, may affect PEL growth. Using gel filtration analyses to examine molecular complexes, nutlin3a interfered with the formation of MDM2–p53–LANA complexes. Nutlin3a induced p53-dependent gene transcription, and caused apoptosis of PEL cells [149,150]. These findings demonstrate that LANA modulates p53-dependent pathways to prevent cell cycle arrest and apoptosis.
Many cellular processes associated with prolonged cellular survival are affected by LANA. Expression of LANA in mice can result in lymphoma [151]. The LANA promoter was found to function in CD19+ B cells but not CD3+ T cells in the spleen and bone marrow of transgenic mice [152]. Further studies revealed that LANA enhanced B cell responses to antigen in mice expressing the LANA transgene in B cells [153]. Prolonged cellular proliferation is associated with telomerase expression and LANA is an activator of telomerase reverse transcriptase expression [119]. LANA affects the stabilization of the c-myc oncogene by reducing the level of phosphorylation at T58 of c-myc, and protecting the phosphoylation of c-myc at S62. This LANA function promotes c-myc transcriptional activity and growth transformation properties [125,130]. Other signaling pathways associated with cancer, such as Notch and WNT pathways, are affected by LANA. LANA influences WNT signaling by nuclear trapping of GSK3β and stabilizing β-catenin [154–157], although this mechanism was questioned in one report [158]. LANA’s effects on cell growth may be mediated by increases in survivin expression, an inhibitor of apoptosis [159]. RNAi-mediated knockdown of survivin affects the growth rate of KSHV-infected cells. LANA also associates with the oncogenes Pim1 and Pim3 [160]. These oncogenes are elevated in LANA-expressing cells. LANA activates the Pim1 promoter, and LANA is phosphorylated by Pim kinases [160,161]. Proliferation of LANA-expressing cells and control cells was downmodulated in cells expressing shRNAs to Pim1, suggesting that Pim1 can have affects on cell growth in the presence of LANA. However, Pim1 and Pim3 were found to associate with LANA in the lytic cycle and not in latent cells, implicating a complex role of these kinases in posttranslational modifications of LANA and in the viral life cycle [160]. LANA may stabilize HIF1α [126,142]. In so doing, LANA likely contributes to the Warburg effect, shifting the profile of metabolic pathways upon which KSHV-infected proliferating cells depend [162].
LANA has also been described to function as a component of the EC5S E3 ubiquitin ligase complex using unconventional suppressor of cytokine signaling box-like motifs to target p53 and von Hippel–Lindau for degradation, which leads to a favorable environment for cell growth [163]. LANA’s effects on p53 and von Hippel-Lindau protein stability have been questioned [158]. Notably, murine γ-herpesvirus 68 ORF73 has also been described to assemble an EC5S E3 ubiquitin ligase to regulate NF-κb [164].
BRD4 associates with LANA and the murine γ-herpesvirus 68 ORF73 [165,166]. Bromodomain proteins have conserved structures that recognize acetylated histones or acetylated proteins. BRD proteins have roles in cell cycle regulation and certain cancers exhibit fusion proteins (translocations) with BRD family members [116,167–172]. LANA represses BRD4-induced activation of the cyclin E promoter [116]. Interestingly, the EBNA1 gene of EBV and the E2 protein of HPV also associate with BRD4, in part to mediate chromosome association and episome persistence (E2) and transcriptional activation (EBNA1) [42,49,120,173–175]. In fact, a BRD4 peptide fused to tat, to promote nuclear localization, inhibits BRD4 and HPV E2 association and ablates chromosome association. Mutations in E2 that inhibit association with BRD4 also inhibit transcriptional activation [42,176]. Taken together, these data suggest a role for BRD4 in the life cycle of at least some DNA viruses with mechanisms of transcription, chromatin association, and episome persistence.
Conclusion
In summary, LANA’s functions are diverse within the viral life cycle of KSHV. LANA’s best characterized functions include its critical role in the maintenance of latency, episome replication and episome persistence. LANA serves as a hub for many host cell interacting proteins. These associated proteins have functions in DNA replication, transcriptional regulation and growth control, and lend insight into the active and multifunctional role of LANA in many cellular processes. Understanding LANA’s role in modifying or adapting host cell protein function will lead to a better understanding of viral latency and oncogenesis.
Future perspective
Viral proteins like LANA, involved in episomal maintenance and replication, are intriguing from an evolutionary perspective. The related γ-2 herpesviruses demonstrate species specificity, with some divergence of virus sequence and gene repertoire relating to their hosts, as well as differences in pathology. As seen with KSHV and other viral-driven tumors, during times of immunosuppression, progression of viral associated pathology, including malignancy, can occur. Understanding critical host cell pathways that are deregulated during immunosuppression that lead to KSHV tumors should reveal critical immune components for viral-mediated tumor suppression. Understanding how LANA deregulates many host cell proteins may also lead to a better understanding of cellular transcriptional and growth control processes. Most importantly, since LANA is central to KSHV latency, it serves as an opportune target to prevent and treat KSHV malignancies.
Executive summary.
Latency-associated nuclear antigen mediates Kaposi’s sarcoma-associated herpesvirus episome persistence
▪ By binding to chromosomes and Kaposi’s sarcoma-associated herpesvirus (KSHV) terminal repeats, latency-associated nuclear antigen (LANA) bridges KSHV episomes to chromatin, ensuring proper segregation of episomes to daughter cells.
▪ By binding terminal repeat DNA and interacting with components of the replication machinery, LANA assists in the replication of KSHV episomes.
Two LANA regions associate with chromosomes
▪ LANA N-terminal and C-terminal regions associate with chromosomes.
▪ The N-terminal region of LANA docks in the histone H2A/H2B pocket and is essential for episome persistence.
LANA is a DNA-binding protein & binds KSHV terminal repeat sequence
▪ LANA self-associates to bind specific DNA sequences in the GC-rich KSHV terminal repeat.
LANA associates with host cell proteins to modulate transcription, chromatin remodeling & cell growth
▪ LANA promotes both transcriptional silencing and gene expression in different contexts.
▪ LANA has effects on cell growth by inhibiting apoptosis and promoting proliferation.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA082036) and from the US Department of Defense (PR093491) (KMK). MEB thanks the UAB CFAR, UAB CCC, the Kaul Foundation, UAB SDRC (P30AR050498) and NINDS (P30NS047466) for support. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Russo JJ, Bohenzky RA, Chien MC, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc. Natl Acad. Sci. USA. 1996;93(25):14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113(6):1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 4.Jenner RG, Boshoff C. The molecular pathology of Kaposi’s sarcoma-associated herpesvirus. Biochim. Biophys. Acta. 2002;1602(1):1–22. doi: 10.1016/s0304-419x(01)00040-3. [DOI] [PubMed] [Google Scholar]
- 5.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25(5):691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bihl F, Mosam A, Henry LN, et al. Kaposi’s sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi’s sarcoma. AIDS. 2007;21(10):1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- 7.Bourboulia D, Aldam D, Lagos D, et al. Short- and long-term effects of highly active antiretroviral therapy on Kaposi sarcoma-associated herpesvirus immune responses and viraemia. AIDS. 2004;18(3):485–493. doi: 10.1097/00002030-200402200-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gao SJ, Kingsley L, Hoover DR, et al. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N. Engl. J. Med. 1996;335(4):233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 9.Qunibi WY, Barri Y, Alfurayh O, et al. Kaposi’s sarcoma in renal transplant recipients: a report on 26 cases from a single institution. Transplant Proc. 1993;25(1 Pt 2):1402–1405. [PubMed] [Google Scholar]
- 10.Kasolo FC, Mpabalwani E, Gompels UA. Infection with AIDS-related herpesviruses in human immunodeficiency virus-negative infants and endemic childhood Kaposi’s sarcoma in Africa. J. Gen. Virol. 1997;78(Pt 4):847–855. doi: 10.1099/0022-1317-78-4-847. [DOI] [PubMed] [Google Scholar]
- 11.Sarmati L. HHV-8 infection in African children. Herpes. 2004;11(2):50–53. [PubMed] [Google Scholar]
- 12.Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer Lett. 2011;305(2):150–162. doi: 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao SJ, Kingsley L, LI M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat. Med. 1996;2(8):925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 14.Boshoff C, Whitby D, Hatziioannou T, et al. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345(8956):1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 15.Inoue N, Spira T, Lam L, Corchero JL, Luo W. Comparison of serologic responses between Kaposi’s sarcoma-positive and -negative men who were seropositive for both human herpesvirus 8 and human immunodeficiency virus. J. Med. Virol. 2004;74(2):202–206. doi: 10.1002/jmv.20167. [DOI] [PubMed] [Google Scholar]
- 16.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 1998;338(14):948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 17.Simpson GR, Schulz TF, Whitby D, et al. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348(9035):1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 18.Spira TJ, Lam L, Dollard SC, et al. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J. Clin. Microbiol. 2000;38(6):2174–2180. doi: 10.1128/jcm.38.6.2174-2180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedes DH, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J. Clin. Invest. 1997;100(10):2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainbow L, Platt GM, Simpson GR, et al. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 1997;71(8):5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao SJ, Zhang YJ, Deng JH, Rabkin CS, Flore O, Jenson HB. Molecular polymorphism of Kaposi’s sarcoma-associated herpesvirus (Human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 1999;180(5):1466–1476. doi: 10.1086/315098. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou S, HO YM, Minson AC. Cloning and molecular characterization of the murine herpesvirus 68 genome. J. Gen. Virol. 1990;71(Pt 6):1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 23.Schafer A, Lengenfelder D, Grillhosl C, Wieser C, Fleckenstein B, Ensser A. The latency-associated nuclear antigen homolog of herpesvirus saimiri inhibits lytic virus replication. J. Virol. 2003;77(10):5911–5925. doi: 10.1128/JVI.77.10.5911-5925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose TM, Strand KB, Schultz ER, et al. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J. Virol. 1997;71(5):4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz ER, Rankin GW, Jr, Blanc MP, Raden BW, Tsai CC, Rose TM. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2000;74(10):4919–4928. doi: 10.1128/jvi.74.10.4919-4928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnside KL, Ryan JT, Bielefeldt-Ohmann H, et al. RFHVMn ORF73 is structurally related to the KSHV ORF73 latency-associated nuclear antigen (LANA) and is expressed in retroperitoneal fibromatosis (RF) tumor cells. Virology. 2006;354(1):103–115. doi: 10.1016/j.virol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, et al. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. ▪ Describes the crystal structure of the interface of latency-associated nuclear antigen (LANA) and histones, which may lead to the development of small-molecule inhibitors for viral latency.
- 28.Gottwein E, Mukherjee N, Sachse C, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450(7172):1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samols MA, HU J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005;79(14):9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballestas ME, Kaye KM. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 2001;75(7):3250–3258. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotter MA, 2nd, Robertson ES. The latency-associated nuclear antigen tethers the Kaposi’s sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264(2):254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 32.Grundhoff A, Ganem D. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 2003;77(4):2779–2783. doi: 10.1128/JVI.77.4.2779-2783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberter B, Ensser A. Histone modification pattern of the T-cellular Herpesvirus saimiri genome in latency. J. Virol. 2007;81(5):2524–2530. doi: 10.1128/JVI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J. Virol. 2010;84(6):2697–2706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stedman W, Deng Z, LU F, Lieberman PM. ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J. Virol. 2004;78(22):12566–12575. doi: 10.1128/JVI.78.22.12566-12575.2004. ▪ Origin of replication complexes are found in vivo at the Kaposi’s sarcoma-associated herpesvirus terminal repeats.
- 36.Barbera AJ, Ballestas ME, Kaye KM. The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 2004;78(1):294–301. doi: 10.1128/JVI.78.1.294-301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley-Clarke B, Ballestas ME, Komatsu T, Kaye KM. Kaposi’s sarcoma herpesvirus C-terminal LANA concentrates at pericentromeric and peri-telomeric regions of a subset of mitotic chromosomes. Virology. 2007;357(2):149–157. doi: 10.1016/j.virol.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 38.Piolot T, Tramier M, Coppey M, Nicolas JC, Marechal V. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 2001;75(8):3948–3959. doi: 10.1128/JVI.75.8.3948-3959.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chodaparambil JV, Barbera AJ, LU X, Kaye KM, Hansen JC, Luger K. A charged and contoured surface on the nucleosome regulates chromatin compaction. Nat. Struct. Mol Biol. 2007;14(11):1105–1107. doi: 10.1038/nsmb1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9(10):1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284(5414):641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 42.Abbate EA, Voitenleitner C, Botchan MR. Structure of the papillomavirus DNA-tethering complex E2:Brd4 and a peptide that ablates HPV chromosomal association. Mol. Cell. 2006;24(6):877–889. doi: 10.1016/j.molcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl Acad. Sci. USA. 1984;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugden B, Marsh K, Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol. Cell Biol. 1985;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 46.Bastien N, Mcbride AA. Interaction of the papillomavirus E2 protein with mitotic chromosomes. Virology. 2000;270(1):124–134. doi: 10.1006/viro.2000.0265. [DOI] [PubMed] [Google Scholar]
- 47.Dao LD, Duffy A, Van Tine BA, et al. Dynamic localization of the human papillomavirus type 11 origin binding protein E2 through mitosis while in association with the spindle apparatus. J. Virol. 2006;80(10):4792–4800. doi: 10.1128/JVI.80.10.4792-4800.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira JG, Colf LA, Mcbride AA. Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc. Natl Acad. Sci. USA. 2006;103(4):1047–1052. doi: 10.1073/pnas.0507624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You J, Croyle JL, Nishimura A, Ozato K, Howley PM. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell. 2004;117(3):349–360. doi: 10.1016/s0092-8674(04)00402-7. [DOI] [PubMed] [Google Scholar]
- 50.Szekely L, Kiss C, Mattsson K, et al. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J. Gen. Virol. 1999;80(Pt 11):2889–2900. doi: 10.1099/0022-1317-80-11-2889. [DOI] [PubMed] [Google Scholar]
- 51.Kelley-Clarke B, DE Leon-Vazquez E, Slain K, Barbera AJ, Kaye KM. Role of Kaposi’s sarcoma-associated herpesvirus C-terminal LANA chromosome binding in episome persistence. J. Virol. 2009;83(9):4326–4337. doi: 10.1128/JVI.02395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao B, Verma SC, Cai Q, et al. Bub1 and CENP-F can contribute to Kaposi’s sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores. J. Virol. 2010;84(19):9718–9732. doi: 10.1128/JVI.00713-10. ▪ Investigates LANA binding to proteins associated with the kinetochore.
- 53.Kanda T, Kamiya M, Maruo S, Iwakiri D, Takada K. Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. J. Cell Sci. 2007;120(Pt 9):1529–1539. doi: 10.1242/jcs.03434. [DOI] [PubMed] [Google Scholar]
- 54.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26(19):4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu J, Renne R. Characterization of the minimal replicator of Kaposi’s sarcoma-associated herpesvirus latent origin. J. Virol. 2005;79(4):2637–2642. doi: 10.1128/JVI.79.4.2637-2642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komatsu T, Ballestas ME, Barbera AJ, Kelley-Clarke B, Kaye KM. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology. 2004;319(2):225–236. doi: 10.1016/j.virol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Komatsu T, Barbera AJ, Ballestas ME, Kaye KM. The Kaposi’ s sarcoma-associated herpesvirus latency-associated nuclear antigen. Viral Immunol. 2001;14(4):311–317. doi: 10.1089/08828240152716565. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasan V, Komatsu T, Ballestas ME, Kaye KM. Definition of sequence requirements for latency-associated nuclear antigen 1 binding to Kaposi’s sarcoma-associated herpesvirus DNA. J. Virol. 2004;78(24):14033–14038. doi: 10.1128/JVI.78.24.14033-14038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garber AC, HU J, Renne R. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 2002;277(30):27401–27411. doi: 10.1074/jbc.M203489200. [DOI] [PubMed] [Google Scholar]
- 60.Schwam DR, Luciano RL, Mahajan SS, Wong L, Wilson AC. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 2000;74(18):8532–8540. doi: 10.1128/jvi.74.18.8532-8540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochkarev A, Barwell JA, Pfuetzner RA, Bochkareva E, Frappier L, Edwards AM. Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein, EBNA1, bound to DNA. Cell. 1996;84(5):791–800. doi: 10.1016/s0092-8674(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 62.Hegde RS, Androphy EJ. Crystal structure of the E2 DNA-binding domain from human papillomavirus type 16: implications for its DNA binding-site selection mechanism. J. Mol. Biol. 1998;284(5):1479–1489. doi: 10.1006/jmbi.1998.2260. [DOI] [PubMed] [Google Scholar]
- 63.Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 2002;76(22):11596–11604. doi: 10.1128/JVI.76.22.11596-11604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krithivas A, Young DB, Liao G, Greene D, Hayward SD. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 2000;74(20):9637–9645. doi: 10.1128/jvi.74.20.9637-9645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim C, Lee D, Seo T, Choi C, Choe J. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 2003;278(9):7397–7405. doi: 10.1074/jbc.M211912200. [DOI] [PubMed] [Google Scholar]
- 66.Matsumura S, Persson LM, Wong L, Wilson AC. The latency-associated nuclear antigen interacts with MeCP2 and nucleosomes through separate domains. J. Virol. 2010;84(5):2318–2330. doi: 10.1128/JVI.01097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma SC, Choudhuri T, Kaul R, Robertson ES. Latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 2006;80(5):2243–2256. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffiths R, Whitehouse A. Herpesvirus saimiri episomal persistence is maintained via interaction between open reading frame 73 and the cellular chromosome-associated protein MeCP2. J. Virol. 2007;81(8):4021–4032. doi: 10.1128/JVI.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chahrour M, Jung SY, Shaw C, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuber G, Mattsson K, Flaberg E, et al. HHV-8 encoded LANA-1 alters the higher organization of the cell nucleus. Mol. Cancer. 2007;6:28. doi: 10.1186/1476-4598-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc. Natl Acad. Sci. USA. 2010;107(32):14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garber AC, Shu MA, HU J, Renne R. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001;75(17):7882–7892. doi: 10.1128/JVI.75.17.7882-7892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skalsky RL, HU J, Renne R. Analysis of viral cis elements conferring Kaposi’s sarcoma-associated herpesvirus episome partitioning and maintenance. J. Virol. 2007;81(18):9825–9837. doi: 10.1128/JVI.00842-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Invest. 2004;113(1):124–136. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowler P, Marques S, Simas JP, Efstathiou S. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J. Gen. Virol. 2003;84(Pt 12):3405–3416. doi: 10.1099/vir.0.19594-0. [DOI] [PubMed] [Google Scholar]
- 76.Li Q, Zhou F, YE F, Gao SJ. Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology. 2008;379(2):234–244. doi: 10.1016/j.virol.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen KW, Dittmer DP, Damania B. Disruption of LANA in rhesus rhadinovirus generates a highly lytic recombinant virus. J. Virol. 2009;83(19):9786–9802. doi: 10.1128/JVI.00704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang YJ, Wang KY, Stein DA, et al. Inhibition of replication and transcription activator and latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus by morpholino oligomers. Antiviral Res. 2007;73(1):12–23. doi: 10.1016/j.antiviral.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forrest JC, Paden CR, Allen RD, 3rd, Collins J, Speck SH. ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J. Virol. 2007;81(21):11957–11971. doi: 10.1128/JVI.00111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jia Q, Freeman ML, Yager EJ, et al. Induction of protective immunity against murine gammaherpesvirus 68 infection in the absence of viral latency. J. Virol. 2010;84(5):2453–2465. doi: 10.1128/JVI.01543-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moorman NJ, Willer DO, Speck SH. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 2003;77(19):10295–10303. doi: 10.1128/JVI.77.19.10295-10303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paden CR, Forrest JC, Moorman NJ, Speck SH. Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J. Virol. 2010;84(14):7214–7224. doi: 10.1128/JVI.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Black J, Vos JM. Establishment of an oriP/EBNA1-based episomal vector transcribing human genomic beta-globin in cultured murine fibroblasts. Gene Ther. 2002;9(21):1447–1454. doi: 10.1038/sj.gt.3301808. [DOI] [PubMed] [Google Scholar]
- 84.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shioda T, Andriole S, Yahata T, Isselbacher KJ. A green fluorescent protein-reporter mammalian two-hybrid system with extrachromosomal maintenance of a prey expression plasmid: application to interaction screening. Proc. Natl Acad. Sci. USA. 2000;97(10):5220–5224. doi: 10.1073/pnas.97.10.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim C, Sohn H, Lee D, Gwack Y, Choe J. Functional dissection of latency-associated nuclear antigen 1 of Kaposi’s sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 2002;76(20):10320–10331. doi: 10.1128/JVI.76.20.10320-10331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Norio P, Schildkraut CL. Plasticity of DNA replication initiation in Epstein-Barr virus episomes. PLoS Biol. 2004;2(6):e152. doi: 10.1371/journal.pbio.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norio P, Schildkraut CL, Yates JL. Initiation of DNA replication within oriP is dispensable for stable replication of the latent Epstein-Barr virus chromosome after infection of established cell lines. J. Virol. 2000;74(18):8563–8574. doi: 10.1128/jvi.74.18.8563-8574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verma SC, Lan K, Choudhuri T, Cotter MA, Robertson ES. An autonomous replicating element within the KSHV genome. Cell Host Microbe. 2007;2(2):106–118. doi: 10.1016/j.chom.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu J, Garber AC, Renne R. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 2002;76(22):11677–11687. doi: 10.1128/JVI.76.22.11677-11687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hung SC, Kang MS, Kieff E. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proc. Natl Acad. Sci. USA. 2001;98(4):1865–1870. doi: 10.1073/pnas.031584698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shinohara H, Fukushi M, Higuchi M, et al. Chromosome binding site of latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 2002;76(24):12917–12924. doi: 10.1128/JVI.76.24.12917-12924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verma SC, Choudhuri T, Robertson ES. The minimal replicator element of the Kaposi’s sarcoma-associated herpesvirus terminal repeat supports replication in a semiconservative and cell-cycle-dependent manner. J. Virol. 2007;81(7):3402–3413. doi: 10.1128/JVI.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelley-Clarke B, Ballestas ME, Srinivasan V, et al. Determination of Kaposi’s sarcoma-associated herpesvirus C-terminal latency-associated nuclear antigen residues mediating chromosome association and DNA binding. J. Virol. 2007;81(8):4348–4356. doi: 10.1128/JVI.01289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu J, Liu E, Renne R. Involvement of SSRP1 in latent replication of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2009;83(21):11051–11063. doi: 10.1128/JVI.00907-09. ▪ A component of the replication machinery, SSRP1, is involved in replication of TR-containing plasmids in the presence of LANA.
- 96.Winkler DD, Luger K. The histone chaperone fact: structural insights and mechanisms for nucleosome reorganization. J. Biol. Chem. 2011;286(21):18369–18374. doi: 10.1074/jbc.R110.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarek G, Jarviluoma A, Moore HM, et al. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. PLoS Pathog. 2011;6(3):e1000818. doi: 10.1371/journal.ppat.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong YK, Foreman K, Shin JW, et al. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 2004;36(7):683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 99.Moses AV, Fish KN, Ruhl R, et al. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 1999;73(8):6892–6902. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. Host gene induction and transcriptional reprogramming in Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 2004;64(1):72–84. doi: 10.1158/0008-5472.can-03-2767. [DOI] [PubMed] [Google Scholar]
- 101.Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, Hayward GS. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2002;76(7):3395–3420. doi: 10.1128/JVI.76.7.3395-3420.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Renne R, Barry C, Dittmer D, Compitello N, Brown PO, Ganem D. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001;75(1):458–468. doi: 10.1128/JVI.75.1.458-468.2001. ▪ Demonstrates that LANA has transcriptional regulatory effects on cellular and viral genes.
- 103.Wang HW, Trotter MW, Lagos D, et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 2004;36(7):687–693. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 104.Wu W, Vieira J, Fiore N, et al. KSHV/HHV-8 infection of human hematopoietic progenitor (CD34+) cells: persistence of infection during hematopoiesis in vitro and in vivo. Blood. 2006;108(1):141–151. doi: 10.1182/blood-2005-04-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carroll PA, Brazeau E, Lagunoff M. Kaposi’s sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology. 2004;328(1):7–18. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen A, Henderson S, Lagos D, et al. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010;24(2):195–205. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shamay M, Krithivas A, Zhang J, Hayward SD. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi’s sarcoma-associated herpesvirus LANA. Proc. Natl Acad. Sci. USA. 2006;103(39):14554–14559. doi: 10.1073/pnas.0604469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones PL, Wolffe AP. Relationships between chromatin organization and DNA methylation in determining gene expression. Semin. Cancer Biol. 1999;9(5):339–347. doi: 10.1006/scbi.1999.0134. [DOI] [PubMed] [Google Scholar]
- 109.Nakao M. Epigenetics: interaction of DNA methylation and chromatin. Gene. 2001;278(1–2):25–31. doi: 10.1016/s0378-1119(01)00721-1. [DOI] [PubMed] [Google Scholar]
- 110.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286(5439):481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 111.Hermann A, Goyal R, Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004;279(46):48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 112.Vertino PM, Sekowski JA, Coll JM, et al. DNMT1 is a component of a multiprotein DNA replication complex. Cell Cycle. 2002;1(6):416–423. doi: 10.4161/cc.1.6.270. [DOI] [PubMed] [Google Scholar]
- 113.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 114.Di Bartolo DL, Cannon M, Liu YF, et al. KSHV LANA inhibits TGF-beta signaling through epigenetic silencing of the TGF-beta type II receptor. Blood. 2008;111(9):4731–4740. doi: 10.1182/blood-2007-09-110544. ▪ Describes a functional outcome of LANA expression as changes in the methylation pattern at the promoter of the TGFβII receptor.
- 115.An FQ, Compitello N, Horwitz E, Sramkoski M, Knudsen ES, Renne R. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J. Biol. Chem. 2005;280(5):3862–3874. doi: 10.1074/jbc.M407435200. [DOI] [PubMed] [Google Scholar]
- 116.Ottinger M, Christalla T, Nathan K, Brinkmann MM, Viejo-Borbolla A, Schulz TF. Kaposi’s sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 2006;80(21):10772–10786. doi: 10.1128/JVI.00804-06. ▪ LANA’s association with BRD4 is shown to affect cell cycle progression.
- 117.Roupelieva M, Griffiths SJ, Kremmer E, et al. Kaposi’s sarcoma-associated herpesvirus Lana-1 is a major activator of the serum response element and mitogen-activated protein kinase pathways via interactions with the Mediator complex. J. Gen. Virol. 2010;91(Pt 5):1138–1149. doi: 10.1099/vir.0.017715-0. [DOI] [PubMed] [Google Scholar]
- 118.Tang J, Gordon GM, Muller MG, Dahiya M, Foreman KE. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 2003;77(10):5975–5984. doi: 10.1128/JVI.77.10.5975-5984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verma SC, Borah S, Robertson ES. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 2004;78(19):10348–10359. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Viejo-Borbolla A, Ottinger M, Bruning E, et al. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 2005;79(21):13618–13629. doi: 10.1128/JVI.79.21.13618-13629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jeong JH, Orvis J, Kim JW, Mcmurtrey CP, Renne R, Dittmer DP. Regulation and autoregulation of the promoter for the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Biol. Chem. 2004;279(16):16822–16831. doi: 10.1074/jbc.M312801200. [DOI] [PubMed] [Google Scholar]
- 122.Dewire SM, Damania B. The latency-associated nuclear antigen of rhesus monkey rhadinovirus inhibits viral replication through repression of Orf50/Rta transcriptional activation. J. Virol. 2005;79(5):3127–3138. doi: 10.1128/JVI.79.5.3127-3138.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 2004;78(12):6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.An J, Sun Y, Rettig MB. Transcriptional coactivation of c-Jun by the KSHV-encoded LANA. Blood. 2004;103(1):222–228. doi: 10.1182/blood-2003-05-1538. [DOI] [PubMed] [Google Scholar]
- 125.Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi’s sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007;26(34):4979–4986. doi: 10.1038/sj.onc.1210299. [DOI] [PubMed] [Google Scholar]
- 126.Cai Q, Lan K, Verma SC, Si H, Lin D, Robertson ES. Kaposi’s sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate RTA expression during hypoxia: latency control under low oxygen conditions. J. Virol. 2006;80(16):7965–7975. doi: 10.1128/JVI.00689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hyun TS, Subramanian C, Cotter MA, 2nd, Thomas RA, Robertson ES. Latency-associated nuclear antigen encoded by Kaposi’s sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 2001;75(18):8761–8771. doi: 10.1128/JVI.75.18.8761-8771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim C, Gwack Y, Hwang S, Kim S, Choe J. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Biol. Chem. 2001;276(33):31016–31022. doi: 10.1074/jbc.M102431200. [DOI] [PubMed] [Google Scholar]
- 129.Lim C, Sohn H, Gwack Y, Choe J. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 2000;81(Pt 11):2645–2652. doi: 10.1099/0022-1317-81-11-2645. [DOI] [PubMed] [Google Scholar]
- 130.Liu J, Martin HJ, Liao G, Hayward SD. The Kaposi’s sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc. J. Virol. 2007;81(19):10451–10459. doi: 10.1128/JVI.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muromoto R, Okabe K, Fujimuro M, et al. Physical and functional interactions between STAT3 and Kaposi’s sarcoma-associated herpesvirus-encoded LANA. FEBS Lett. 2006;580(1):93–98. doi: 10.1016/j.febslet.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 132.Pan HY, Zhang YJ, Wang XP, Deng JH, Zhou FC, Gao SJ. Identification of a novel cellular transcriptional repressor interacting with the latent nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2003;77(18):9758–9768. doi: 10.1128/JVI.77.18.9758-9768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 2000;6(10):1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 134.Watanabe A, Higuchi M, Fukushi M, et al. A novel KRAB-Zinc finger protein interacts with latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus and activates transcription via terminal repeat sequences. Virus Genes. 2007;34(2):127–136. doi: 10.1007/s11262-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 135.Kusano S, Eizuru Y. Human I-mfa domain proteins specifically interact with KSHV LANA and affect its regulation of Wnt signaling-dependent transcription. Biochem. Biophys. Res. Commun. 2010;396(3):608–613. doi: 10.1016/j.bbrc.2010.04.111. [DOI] [PubMed] [Google Scholar]
- 136.Lan K, Kuppers DA, Robertson ES. Kaposi’s sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jkappa, the major downstream effector of the Notch signaling pathway. J. Virol. 2005;79(6):3468–3478. doi: 10.1128/JVI.79.6.3468-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim C, Seo T, Jung J, Choe J. Identification of a virus trans-acting regulatory element on the latent DNA replication of Kaposi’s sarcoma-associated herpesvirus. J. Gen. Virol. 2004;85(Pt 4):843–855. doi: 10.1099/vir.0.19510-0. [DOI] [PubMed] [Google Scholar]
- 138.Si H, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 2006;80(2):697–709. doi: 10.1128/JVI.80.2.697-709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Watanabe T, Sugaya M, Atkins AM, et al. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 2003;77(11):6188–6196. doi: 10.1128/JVI.77.11.6188-6196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wong LY, Matchett GA, Wilson AC. Transcriptional activation by the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen is facilitated by an N-terminal chromatin-binding motif. J. Virol. 2004;78(18):10074–10085. doi: 10.1128/JVI.78.18.10074-10085.2004. ▪ N-terminal LANA has a role in transcriptional activation.
- 141.Borah S, Verma SC, Robertson ES. ORF73 of herpesvirus saimiri, a viral homolog of Kaposi’s sarcoma-associated herpesvirus, modulates the two cellular tumor suppressor proteins p53 and pRb. J. Virol. 2004;78(19):10336–10347. doi: 10.1128/JVI.78.19.10336-10347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cai Q, Murakami M, Si H, Robertson ES. A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposi’s sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia. J. Virol. 2007;81(19):10413–10423. doi: 10.1128/JVI.00611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402(6764):889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 144.Katano H, Sato Y, Sata T. Expression of p53 and human herpesvirus-8 (HHV-8)-encoded latency-associated nuclear antigen with inhibition of apoptosis in HHV-8-associated malignancies. Cancer. 2001;92(12):3076–3084. doi: 10.1002/1097-0142(20011215)92:12<3076::aid-cncr10117>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 145.Boulanger E, Marchio A, Hong SS, Pineau P. Mutational ana lysis of TP53, PTEN, PIK3CA and CTNNB1/beta-catenin genes in human herpesvirus 8-associated primary effusion lymphoma. Haematologica. 2009;94(8):1170–1174. doi: 10.3324/haematol.2009.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carbone A, Cilia AM, Gloghini A, et al. Characterization of a novel HHV-8-positive cell line reveals implications for the pathogenesis and cell cycle control of primary effusion lymphoma. Leukemia. 2000;14(7):1301–1309. doi: 10.1038/sj.leu.2401802. [DOI] [PubMed] [Google Scholar]
- 147.Chen W, Hilton IB, Staudt MR, Burd CE, Dittmer DP. Distinct p53, p53:LANA, and LANA complexes in Kaposi’s Sarcoma--associated Herpesvirus Lymphomas. J. Virol. 2010;84(8):3898–3908. doi: 10.1128/JVI.01321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy MM, Lucas SB, Jones RR, et al. HHV8 and Kaposi’s sarcoma: a time cohort study. Mol. Pathol. 1997;50(2):96–100. doi: 10.1136/mp.50.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Petre CE, Sin SH, Dittmer DP. Functional p53 signaling in Kaposi’s sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 2007;81(4):1912–1922. doi: 10.1128/JVI.01757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarek G, Kurki S, Enback J, et al. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J. Clin. Invest. 2007;117(4):1019–1028. doi: 10.1172/JCI30945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J. Clin. Invest. 2006;116(3):735–742. doi: 10.1172/JCI26190. ▪ In vivo studies demonstrate that LANA expression in the B-cell compartment can result in lymphoma.
- 152.Jeong JH, Hines-Boykin R, Ash JD, Dittmer DP. Tissue specificity of the Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen (LANA/orf73) promoter in transgenic mice. J. Virol. 2002;76(21):11024–11032. doi: 10.1128/JVI.76.21.11024-11032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sin SH, Fakhari FD, Dittmer DP. The viral latency-associated nuclear antigen augments the B-cell response to antigen in vivo. J. Virol. 2010;84(20):10653–10660. doi: 10.1128/JVI.00848-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fujimuro M, Hayward SD. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J. Virol. 2003;77(14):8019–8030. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fujimuro M, Hayward SD. Manipulation of glycogen-synthase kinase-3 activity in KSHV-associated cancers. J. Mol. Med. 2004;82(4):223–231. doi: 10.1007/s00109-003-0519-7. [DOI] [PubMed] [Google Scholar]
- 156.Fujimuro M, Liu J, Zhu J, Yokosawa H, Hayward SD. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Virol. 2005;79(16):10429–10441. doi: 10.1128/JVI.79.16.10429-10441.2005. ▪ LANA dysregulates β-catenin in Kaposi’s sarcoma-associated herpesvirus latency.
- 157.Fujimuro M, Wu FY, Aprhys C, et al. A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpesvirus latency. Nat. Med. 2003;9(3):300–306. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- 158.Hagen T. Characterization of the interaction between latency-associated nuclear antigen and glycogen synthase kinase 3beta. J. Virol. 2009;83(12):6312–6317. doi: 10.1128/JVI.01671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu J, Verma SC, Murakami M, et al. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-Associated B-lymphoma cells and contributes to their proliferation. J. Virol. 2009;83(14):7129–7141. doi: 10.1128/JVI.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng F, Weidner-Glunde M, Varjosalo M, et al. KSHV reactivation from latency requires Pim-1 and Pim-3 kinases to inactivate the latency-associated nuclear antigen LANA. PLoS Pathog. 2009;5(3):e1000324. doi: 10.1371/journal.ppat.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajaj BG, Verma SC, Lan K, Cotter MA, Woodman ZL, Robertson ES. KSHV encoded LANA upregulates Pim-1 and is a substrate for its kinase activity. Virology. 2006;351(1):18–28. doi: 10.1016/j.virol.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 162.Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, Lagunoff M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl Acad. Sci. USA. 2010;107(23):10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cai QL, Knight JS, Verma SC, Zald P, Robertson ES. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2006;2(10):e116. doi: 10.1371/journal.ppat.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodrigues L, Filipe J, Seldon MP, et al. Termination of NF-kappaB activity through a gammaherpesvirus protein that assembles an EC5S ubiquitin-ligase. EMBO J. 2009;28(9):1283–1295. doi: 10.1038/emboj.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ottinger M, Pliquet D, Christalla T, Frank R, Stewart JP, Schulz TF. The interaction of the gammaherpesvirus 68 orf73 protein with cellular BET proteins affects the activation of cell cycle promoters. J. Virol. 2009;83(9):4423–4434. doi: 10.1128/JVI.02274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.You J, Srinivasan V, Denis GV, et al. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 2006;80(18):8909–8919. doi: 10.1128/JVI.00502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dey A, Nishiyama A, Karpova T, Mcnally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell. 2009;20(23):4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.French CA, Miyoshi I, Aster JC, et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am. J. Pathol. 2001;159(6):1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63(2):304–307. [PubMed] [Google Scholar]
- 170.Huang B, Yang XD, Zhou MM, Ozato K, Chen LF. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell Biol. 2009;29(5):1375–1387. doi: 10.1128/MCB.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maruyama T, Farina A, Dey A, et al. A Mammalian bromodomain protein, brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell Biol. 2002;22(18):6509–6520. doi: 10.1128/MCB.22.18.6509-6520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mochizuki K, Nishiyama A, Jang MK, et al. The bromodomain protein Brd4 stimulates G1 gene transcription and promotes progression to S phase. J. Biol. Chem. 2008;283(14):9040–9048. doi: 10.1074/jbc.M707603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jang MK, Kwon D, Mcbride AA. Papillomavirus E2 proteins and the host BRD4 protein associate with transcriptionally active cellular chromatin. J. Virol. 2009;83(6):2592–2600. doi: 10.1128/JVI.02275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin A, Wang S, Nguyen T, Shire K, Frappier L. The EBNA1 protein of Epstein-Barr virus functionally interacts with Brd4. J. Virol. 2008;82(24):12009–12019. doi: 10.1128/JVI.01680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mcphillips MG, Ozato K, Mcbride AA. Interaction of bovine papillomavirus E2 protein with Brd4 stabilizes its association with chromatin. J. Virol. 2005;79(14):8920–8932. doi: 10.1128/JVI.79.14.8920-8932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Senechal H, Poirier GG, Coulombe B, Laimins LA, Archambault J. Amino acid substitutions that specifically impair the transcriptional activity of papillomavirus E2 affect binding to the long isoform of Brd4. Virology. 2007;358(1):10–17. doi: 10.1016/j.virol.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 177.Murakami Y, Yamagoe S, Noguchi K, et al. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem. 2006;281(38):28113–28121. doi: 10.1074/jbc.M602026200. [DOI] [PubMed] [Google Scholar]
- 178.Verma SC, Bajaj BG, Cai Q, Si H, Seelhammer T, Robertson ES. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus recruits uracil DNA glycosylase 2 at the terminal repeats and is important for latent persistence of the virus. J. Virol. 2006;80(22):11178–11190. doi: 10.1128/JVI.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lan K, Verma SC, Murakami M, Bajaj B, Kaul R, Robertson ES. Kaposi’s sarcoma herpesvirus-encoded latency-associated nuclear antigen stabilizes intracellular activated Notch by targeting the Sel10 protein. Proc. Natl Acad. Sci. USA. 2007;104(41):16287–16292. doi: 10.1073/pnas.0703508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sakakibara S, Ueda K, Nishimura K, et al. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi’s sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 2004;78(14):7299–7310. doi: 10.1128/JVI.78.14.7299-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fukushi M, Higuchi M, Oie M, et al. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus interacts with human myeloid cell nuclear differentiation antigen induced by interferon alpha. Virus Genes. 2003;27(3):237–247. doi: 10.1023/a:1026391715071. [DOI] [PubMed] [Google Scholar]
- 182.Si H, Verma SC, Lampson MA, Cai Q, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation. J. Virol. 2008;82(13):6734–6746. doi: 10.1128/JVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]