Abstract
Background
HIV-specific cellular immune responses are associated with control of viremia and delayed disease progression. An effective therapeutic vaccine could mimic these effects and reduce the need for continued antiretroviral therapy. DermaVir, a topically administered pDNA-nanomedicine expressing HIV (CladeB) virus-like particles consisting of 15 antigens, induces predominantly central memory T-cell responses.
Methods
Treated HIV-infected adults (HIV RNA<50, CD4>350) were randomized to placebo or escalating DermaVir doses (0.1 or 0.4 mg pDNA at weeks 1/7/13 in the low and intermediate-dose groups, 0.8 mg at weeks 0/1/6/7/12/13 in the high-dose group), n=5–6 evaluable subjects/group. Immunogenicity was assessed by a 12-day cultured IFN-γ ELISPOT assay, at baseline and at 9/17/37 weeks, using one Tat/Rev and three overlapping Gag peptide pools (p17/p24/p15).
Results
Groups were comparable at baseline. The study intervention was well tolerated, without dose-limiting toxicities. Most responses were highest at week 17 (4 weeks after last vaccination), when Gag p24 responses were significantly greater among intermediate-dose compared to control subjects (median [IQR]: 67,600 [5,633 – 74,368] vs. 1,194 [9 – 1,667] net spot-forming units/million cells, p = 0.032. In the intermediate-dose group, there was also a marginal Gag p15 response increase from baseline to week 17 (2859 [1867 – 56933], p=0.06), and this change was significantly greater than in the placebo group (0 [−713 – 297], p=0.016).
Conclusions
DermaVIr administration was associated with a trend toward greater HIV-specific, predominantly central memory T-cell responses. The intermediate DermaVir dose tended to show the greatest immunogenicity, consistent with previous studies in different HIV-infected patient populations.
Keywords: DermaVir, CTL responses, HIV therapeutic vaccines, HIV-specific immune response
BACKGROUND
Combination antiretroviral therapy (cART) decreases HIV-related morbidity and mortality1,2 and in some settings, reduces HIV transmission3–5. Nonetheless, even during successful cART-mediated suppression of HIV viremia, replication-competent viruses persist in certain tissues and long-lived cells6, and exaggerated systemic inflammation can be readily demonstrated7–11. This in turn is related to a host of end-organ damage syndromes and to accelerated mortality, particularly in the context of limited CD4+ T cell reconstitution. Development of HIV-specific cellular immune reactivity, either spontaneously or in response to therapeutic immunization, is accompanied by amelioration of this cascade of events in humans and in non-human primate SIV infection models12–24. Thus, induction of such immune responses may confer physiological and clinical benefits in chronic HIV infection, including potential reductions of antiretroviral exposure with its attendant toxicities. Furthermore, identification of an effective strategy to elicit robust HIV-specific immune responses may improve understanding of the biological correlates of protection against HIV acquisition.
DermaVir is the first in vivo dendritic cell-targeting therapeutic vaccine developed for treatment of HIV-infected persons. The vaccine employs a plasmid DNA (pDNA) immunogen to express 15 HIV antigens, in a synthetic pDNA nanomedicine formulation shown to deliver antigen effectively to lymph node DCs and to induce significant expansions of the HIV-specific precursor/memory T cell pool25–32. The effects of DermaVir in humans have been analyzed in two previous studies. In a phase I study, nine HIV-infected subjects on suppressive cART who received a single dose (0.1, 0.4 or 0.8 mg pDNA) of DermaVir demonstrated significant increases in HIV-specific memory T cell responses after immunization, and these responses were detectable up to a year after vaccine administration33. In a subsequent phase I-II study, 36 cART-naïve HIV-infected patients were assigned to three different doses of DermaVir or matching placebo. Subjects receiving the intermediate dose of 0.4 mg showed a median plasma HIV decrease from baseline, and compared to placebo, of 0.5 log10 copies/mL34. Thus, DermaVir appears to induce immune responses in humans reminiscent of those seen in preclinical non-human primate models35. A5176 was designed to extend these observations to a larger group of HIV-infected subjects receiving suppressive cART.
METHODS
Study design and study population
A5176 was a multicenter, randomized, placebo-controlled clinical trial comparing three doses of DermaVir and a placebo arm among chronically HIV-infected subjects of both sexes, aged 18–50 years, receiving stable cART, with confirmed plasma HIV RNA <50 copies/mL, CD4+ T-cell count >350 cells/μL, nadir CD4+ T-cell count >250 cells/μL, and negative hepatitis B surface antigen and hepatitis C antibody. Other exclusion criteria are listed in supplemental table 1. Participants were recruited from: University of California Davis Medical Center (Sacramento, CA); Chicago Children’s Hospital (Chicago, IL); Metro Health Medical Center (Cleveland, OH); University Hospitals of Cleveland Case Medical Center (Cleveland, OH); and University of Pittsburgh (Pittsburgh, PA). The protocol was approved by the the participating institutions’ IRBs of. All subjects provided written informed consent.
Study intervention
Subjects were sequentially enrolled into one of three dosing cohorts and randomized to receive either DermaVir (six subjects per cohort) or placebo (two subjects per cohort). DermaVir or placebo were administered over either two or four skin sites on the left and right upper back and both upper ventral thighs using the DermaPrep device33. The selected area was disinfected and exfoliated to disrupt the stratum corneum of the skin and induce mild erythema. This procedure enhances DermaVir nanoparticle endocytosis s by activated Langerhans cells and delivery of pDNA-encoded antigens to naïve T-lymphocytes in the regional lymph nodes26. After skin preparation, a patch covering approximately 80 cm2 was applied over the area, and the vaccine solution was placed on the skin underneath the patch with a needleless syringe. The patch was kept for 3 hours after vaccination. Subjects assigned to the active arm received the following doses of DermaVir: In the low-dose cohort, three vaccinations (0.1 mg DNA/subject, 0.8 mL total over two skin sites of 80 cm2 each, 0.4 mL/site) on weeks 1, 7, and 13; in the intermediate-dose cohort, three vaccinations (0.4 mg DNA/subject, 3.2 mL total over four skin sites of 80 cm2 each, 0.8 mL/site) on study weeks 1, 7, and 13; in the high-dose cohort E, six vaccinations (0.4 mg DNA/subject, 3.2 mL total over four skin sites of 80 cm2 each, 0.8 mL/site) on study weeks 0, 1, 6, 7, 12, and 13. Subjects in the placebo arm received a matching glucose placebo, administered as described above. Each cohort was enrolled sequentially, from low to high dose. Enrollment into each higher dose cohort required that at least 6 of the 8 subjects in the previous dose cohort had been followed on study for ≥14 days after their second study vaccination, and that no subject had experienced a primary safety endpoint, as defined below.
Assessment of safety study endpoints
The primary endpoint was the occurrence of grade 3 or higher clinical or laboratory adverse events possibly or definitely related to study treatment from first day of study treatment to 28 days after last study vaccination. Events were defined and graded according to the current DAIDS AE Grading Table (found at: http://rsc.tech-res.com/safetyandpharmacovigilance). Relationship to study treatment was determined by the protocol core team, including site clinicians, blinded to treatment arm. Reactions felt to be solely due to adhesive and not the vaccine itself were not used to determine the primary safety endpoint. Anti-double stranded DNA was measured by ELISA to exclude potential autoimmune reactions to the immunization.
Assessment of immunogenicity endpoints
We report the results of a previously described36 extended culture IFN-γ ELISPOT assay as the main immunogenicity readout. The ELISPOT readout was chosen over other immunogenicity endpoints because of its reproducibility and sensitivity across HIV vaccine studies37. This assay detects predominantly T-cell precursors with high proliferating capacity (PHPC), a memory T-cell population that has been shown to be induced by DermaVir immunization and to correlate inversely with plasma viremia in untreated HIV infection36.
Briefly, cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed, rested overnight, and suspended in R10 complete culture medium to a final concentration of 5×105 cells/mL. Cases in which cell viability at the beginning of the culture was below 80% were excluded from the analysis. The cut-off of 80% was chosen to remove the artifactual effect of poor viability at the start of the PHPC culture (24 hours after thawing) on the PHPC readout. Cells were plated in 48-well tissue culture plates with the HIV peptide pools, phytohemaglutinin (PHA)/staphylococcal enterotoxin B (SEB) as positive control, or medium alone as negative control, and cultured at 37°C in 5% CO2 for 12 days. On days 3 and 7, 500 μL of supernatant per well were removed and replaced with fresh complete culture medium supplemented with 10 IU/mL recombinant human IL-2. The HIV peptides were obtained from the NIH AIDS Research and Reference Reagent Program, and were 15 amino acids in length overlapping by 11, representing the entire HIV-1 subtype B sequence of Gag (divided into 3 pools, p17, p24, and p15), Tat, and Rev. At the end of the 12-day culture, cells were harvested, and a standard overnight ELISPOT assay was performed as previously described with minor modifications38. Briefly, cells were plated in triplicate into 96-well anti-human INF-γ antibody pre-coated ELISPOT plates (BD Biosciences, San Diego CA) at 2.5×104 cells/well and stimulated with each of the HIV peptide pools (5μg/ml), PHA/SEB (5μg/ml), or medium alone. After incubation for 24 hours at 37°C in 5% CO2, plates were washed and developed following the manufacturer’s protocol (BD Biosciences, San Diego CA). Spots were counted using an automated ELISPOT reader. Results were expressed as net IFNγ spot-forming units(SFU)/million PBMCs (the difference between mean SFU/million PBMCs in stimulated wells and mean SFU/million PBMCs in medium-only wells) multiplied by the proliferation index (number of stimulated cells/number of control-stimulated cells at the end of the culture), to account for the expected expansion of T cells during prolonged culture.
Because HIV-specific T-cell responses were frequently detected at baseline, we defined development of a new response in two ways: 1) As any post-vaccination response that was >1 log10 above the log10 baseline response, regardless of study arm; and 2) as an area under the response curve (AUC) to a given peptide antigen pool that was greater than the maximum AUC to the same stimulant observed in the placebo arm, which meant that this definition applied only to those subjects assigned to a DermaVir dosing group. All timepoints were considered, and the point of greatest response magnitude was derived from the data.
Other immunogenicity assays included HIV-specific CD4+ and CD8+ T-cell proliferation by CFSE dye dilution, HIV-specific intracellular cytokine (IFN-γ and IL-2) staining by flow cytometry, and conventional overnight ELISPOT. As previously reported36,39, these assays were less sensitive than the cultured ELISPOT assay, and will not be reported here.
Statistical analysis
Vaccine site reactions, clinical events, signs and symptoms, laboratory toxicities and adverse events grade 2 or higher from first day of study treatment to 28 days after the last study vaccination were tabulated by study group considering all subjects who started study treatment. Time-averaged area under the curve was computed, using the linear trapezoidal method, only for subjects who started study treatment with non-missing cultured ELISPOT data until week 37 (i.e. AUC/37). Differences among study groups were analyzed by Fisher’s exact test or Wilcoxon’s rank sum test, as appropriate. Dose-response trends were assessed by the Jonckheere-Terpstra test. Changes from baseline were assessed by the sign test. All p-values presented were two-sided and nominal, unadjusted for multiple comparisons. Analyses were done using SAS, version 9.2 (SAS Institute, Cary, North Carolina) and StatXact 8 PROCs (Cytel, Cambridge, Massachusetts).
RESULTS
Twenty-six subjects were enrolled. One subject in the intermediate-dose group withdrew voluntarily before receiving any study vaccination and was excluded from all analyses. Another subject was non-compliant with study visits and was discontinued at week 8. Both were replaced. Groups were well balanced with respect to baseline characteristics, except for a slightly younger age in the placebo group. Detailed demographic characteristics are shown in table 1. Median age was 39 years, median baseline CD4 count was 645 cells/μL, 92% of participants were men, and 64% were white. All had plasma HIV RNA <50 copies/mL at study entry.
Table 1.
Characteristics of study participants. Data are shown as n (%), unless otherwise specified
| Study group
|
|||||
|---|---|---|---|---|---|
| Placebo, n = 7 (Placebo × 3 or 6 doses) | Low-dose, n=6) (DermaVir, 0.1 mg × 3 doses) | Intermediate- dose, n=6 (DermaVir, 0.4 mg × 3 doses) | High-dose, n = 6 (DermaVir, 0.4 mg × 6 doses) | Overall | |
|
| |||||
| Age, years, median (IQR) | 34 (30, 35) | 43.5 (32, 48) | 41 (38, 43) | 45.5 (34, 47) | 39 (32, 45) |
| Male gender | 6 (86) | 5 (83) | 6 (100) | 6 (100) | 23 (92) |
| Race/Ethnicity | |||||
| White, non-hispanic | 3 (43) | 4 (67) | 4 (67) | 5 (83) | 16 (64) |
| Black, non-hispanic | 4 (57) | 1 (17) | 1 (17) | 1 (17) | 7 (28) |
| Hispanic, any race | 0 (0) | 1 (17) | 1 (17) | 0 (0) | 2 (8) |
| History of IV drug abuse | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| CD4+ T cell count, cells/μL, median (IQR) | 674 (611, 762) | 664 (608, 718) | 799 (592, 1123) | 551 (517, 704) | 645 (565, 762) |
Safety endpoints
Overall, study interventions were well tolerated. No subject experienced a grade ≥3 treatment-related adverse event (primary endpoint). There were no deaths. The overall proportions of subjects experiencing any adverse event between the first treatment date and 28 days after the last dose were 57% in the placebo arm and 67%, 100%, and 50%, in each of the low-, intermediate- and high-dose DermaVir groups, respectively (p = 0.31). Differences were also not significant comparing the placebo to the combined Dermavir arms (p=0.64). General body complaints (aches/pain/discomfort, asthenia/fatigue/malaise, fever) were most common and occurred in 7 subjects, followed by cutaneous symptoms (pruritus, rash) and blood chemistry abnormalities in 5 subjects each. A summary of adverse events is shown in table 2. The proportion of treatment-related adverse events was comparable between the DermaVir and placebo arms (0/7, 3/6, 1/6 and 0/6 in the placebo arm and the low-, intermediate- and high-dose DermaVir groups, respectively, p = 0.063). All adverse events felt to be possibly, probably or definitely related to study agent and all abnormal blood chemistries were mild (grade 1–2) and no subject had to modify or discontinue the study treatment due to an adverse event. None of the subjects with available data developed detectable anti-double stranded DNA antibodies.
Table 2.
Numbers of subjects experiencing adverse events of grade 2 severity occurring from first day of study treatment to 28 days after the last study vaccination. Data are shown as number of subjects (percent). All events were included in the categories shown. Some subjects had more than one event. The highest severity of a given event for each subject was recorded
| Study group
|
||||||
|---|---|---|---|---|---|---|
| Placebo, n = 7 (Placebo × 3 or 6 doses) | Low-dose, n=6) (DermaVir, 0.1 mg × 3 doses) | Intermediate- dose, n=6 (DermaVir, 0.4 mg × 3 doses) | High-dose, n = 6 (DermaVir, 0.4 mg × 6 doses) | Overall | P- value | |
|
| ||||||
| Any event | 4 (57.1) | 4 (66.7) | 6 (100) | 3 (50) | 17 (68) | 0.306† |
| General/systemic | 1 (14.3) | 2 (33.3) | 2 (33.3) | 2 (33.3) | 7 (28) | |
| Ache/pain/discomfort | 0 (0) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 4 (16) | |
| Asthenia/fatigue/malaise | 1 (14.3) | 1 (16.7) | 1 (16.7) | 0 (0) | 3 (12) | |
| Fever | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 1 (4) | |
| Skin abnormalities | 2 (28.6) | 3 (50) | 0 (0) | 0 (0) | 5 (20) | |
| Pruritus | 1 (14.3) | 2 (33.3) | 0 (0) | 0 (0) | 3 (12) | |
| Rash | 1 (14.3) | 1 (16.7) | 0 (0) | 0 (0) | 2 (8) | |
| Laboratory abnormalities* | 2 (28.6) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 10 (40) | |
| Neurologic abnormalities** | 1 (14.3) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 4 (16) | |
| Lymphadenopathy/edema | 0 (0) | 1 (16.7) | 1 (16.7) | 0 (0) | 2 (8) | |
Events included hypoglycemia, hypo/hyperphosphatemia, increased creatinine, increased SGPT, and hyperbilirubinemia
Events included sleep disturbances, headache, and memory loss
Testing for a difference among all groups. Pairwise comparisons between groups were also not statistically significant
Changes in CD4+ and CD8+ T-cells
CD4+ T and CD8+ T-cell counts were measured at weeks 0, 3, 9, 15, 17, 24, 37, and 61, and did not change significantly during the study in any of the dosing groups (p ≥0.688 and ≥0.219, respectively). Median (IQR) change in CD4+ T-cell count from baseline to week 61 was +13 (−132, +229) cells/μL in the combined DermaVir groups vs. +151 (−126, +237) in the placebo group. Differences between the DermaVir arm (either combined or each group separately) and the placebo arm were not statistically significant at any of the measured timepoints, and there was no evidence for a dose-related trend in CD4+ T-cell change across the dosing groups of Dermavir (Jonckheere-Terpstra test p-values for all timepoints >0.49).
Changes in plasma HIV RNA
All subjects had plasma HIV RNA below the limit of detection at baseline, and all maintained virologic suppression through week 24, except for three subjects who had episodes of transient viremia (one subject in each of the DermaVir groups, with 65 copies at week 9, 274 copies at week 24, and 933 copies at week 61, respectively).
Extended culture ELISPOT (PHPC) responses
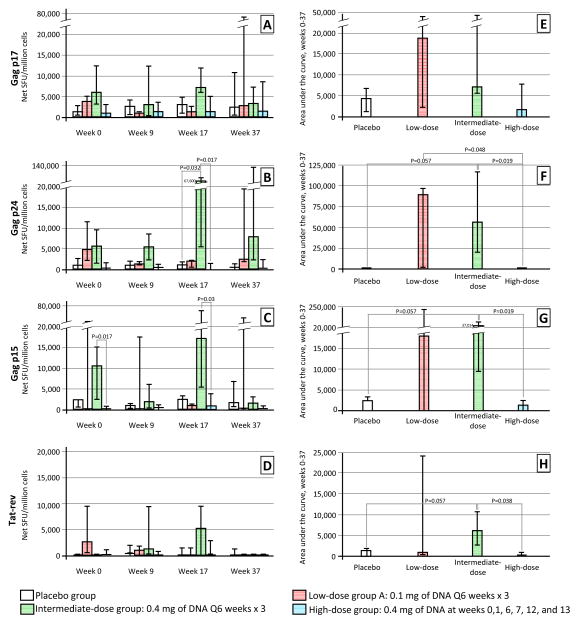
Cultured ELISPOT responses are shown in Figure 1. There was considerable baseline variability in all groups. Overall, there was a trend toward higher responses in the DermaVir groups, particularly the low- and intermediate-dose groups, compared to placebo. Responses in the high-dose group were not significantly different than the placebo group at any of the studied timepoints in response to any of the peptide pools used (all P>0.05), and responses in this group did not correlate significantly with baseline CD4+ T cell count. At week 17 (four weeks after last study vaccination), responses to Gag p24 were significantly greater among subjects in the intermediate-dose group compared to control subjects (median [IQR]: 67,600 [5,633 – 74,368] vs. 1,194 [9 – 1,667] net SFU/million cells, p = 0.032. Responses to both Gag p24 and to Gag p15 among intermediate-dose subjects were also significantly greater than among high-dose subjects at week 17 (67,600 [5,633 – 74,368] vs. 485 [172 – 1,440], p = 0.017 and 17,067 [5,495 – 67,600] vs. 642 [74 – 4,086] net SFU/million cells, p = 0.03.
Figure 1.
PHPC responses to escalating doses of Dermavir in A5176, shown as spot-forming units (SFU)/million cells (A, B, C, D) or as the area under the curve of responses from week to week 37 (E, F, G, H). Bars represent the median values and error bars the interquartile range.
The increased anti-HIV T cell response frequency in the intermediate-dose group was consistent across stimulus conditions (figure 1): with the exception of responses to Gag p15 at week 37, responses in the intermediate-dose group were higher than those in the placebo group and the other two DermaVir dosing groups at all post-vaccine timepoints (figure 1), although the difference was only significant at week 17.
Because baseline reactivity showed substantial variation, we tested whether there was evidence for a change from baseline in cultured ELISPOT responses in any of the groups (not shown). Response to Gag p15 increased significantly among participants in the high-dose group from baseline to week 17 (median [IQR] change 149 [44 – 3049] net SFU/million, p=0.031), but this increase was not significantly different from placebo (p=0.247). Participants in the intermediate-dose group experienced a marginally significant increase from baseline at the same time point in response to Gag p15 (2859 [1867 – 56933], p=0.06), and this change was significantly greater than in the placebo group (0 [−713 – 297], p=0.016). We observed a similar trend toward a greater absolute week 0–37 AUC in the intermediate-dose group compared to placebo in response to Gag p24, Gag p15 and Tat-Rev (figure 1), although these differences were marginal (all p = 0.057). In this analysis, the intermediate-dose group also showed significantly greater responses to each of those peptide pools compared to the high-dose group (p = 0.019, 0.019 and 0.038, respectively).
Finally, we compared the proportions of subjects who achieved a response to the study intervention in each of the groups. When a response was defined as a 1-log10 net SFU increase from baseline to each of the Gag peptide pools, 20 to 60% of the DermaVir recipients were classified as responders, compared to 20% of the placebo recipients, although the differences were not statistically significant (table 3). A similar trend was observed when we defined a DermaVir-induced response as an area under the curve in any DermaVir dosing group that was higher than the AUC in the placebo group. In the latter analysis, 100% of subjects in the low- and intermediate-dose groups achieved a response, compared to only 33% of subjects in the high-dose group (table 3).
Table 3.
Summary of new HIV-specific T cell responses on study. Two definitions of a new response were used. In definition 1, subjects were considered responders if they had any post-vaccination cultured ELISPOT titer that was >1 log10 above the log10 ELISPOT response at baseline, regardless of study arm. In definition 2, a response was an area under the response curve (AUC) to a given peptide antigen pool that was greater than the maximum AUC to the same stimulant observed in the placebo arm. Because the latter definition was intended to account only for those responses that exceeded the greatest background responses seen in the absence of DermaVir exposure, this meant that subjects in the placebo group could not be classified as responders under this approach. Similarly, this definition required no missing data up the 37-week timepoint. Data are shown as number (percent) of responders per group
| Peptide pool
|
||||||
|---|---|---|---|---|---|---|
| Gag p17 | Gag p24 | Gag p15 | Tat-Rev | Any peptide pool | P-value | |
|
Responder definition 1
| ||||||
| Study group
| ||||||
| Placebo (N =5) | 1 (20) | 1 (20) | 1 (20) | 4 (80) | 4 (80) | |
| Low-dose (N = 5) | 2 (40) | 3 (60) | 2 (40) | 1 (20) | 4 (80) | 1* |
| Intermediate-dose (N = 5) | 2 (40) | 2 (40) | 1 (20) | 3 (60) | 4 (80) | 1* |
| High-dose (N = 6) | 3 (50) | 2 (33.3) | 2 (33.3) | 1 (16.7) | 3 (50) | 0.545* |
| Combined DermaVir (N = 16) | 7 (43.8) | 7 (43.8) | 5 (31.3) | 5 (31.3) | 11 (68.8) | 1* |
|
| ||||||
|
Responder definition 2
| ||||||
| Study group
| ||||||
| Low-dose (N = 3) | 2 (66.7) | 3 (100) | 2 (66.7) | 1 (33.3) | 3 (100) | 0.167** |
| Intermediate-dose (N = 4) | 2 (50) | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 0.076** |
| High-dose (N = 6) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 2 (33.3) | |
| Combined DermaVir (N = 13) | 6 (46.2) | 8 (61.5) | 7 (53.8) | 6 (46.2) | 9 (69.2) | |
Comparing each group to the placebo group, Fisher’s exact test
Comparing to the high-dose group, Fisher’s exact test. P value is not defined for the comparison of the low- and intermediate-dose groups (both 100%).
DISCUSSION
An effective therapeutic vaccine capable of inducing durable HIV-specific CD8+ T-cell responses with potent cytotoxic activity and reducing viremia could theoretically confer many of the benefits of cART without some of its limitations. Evidence in favor of this approach comes from observations in non-human primate models indicating that cytotoxic T-cell responses are crucial in maintaining spontaneous and DermaVir-induced control of viremia15,35, by the recognized persistence of vigorous CD8+ T-cell responses in human long-term non-progressors40–42, and by the over representation of protective HLA alleles thought to be capable of presenting highly conserved HIV epitopes among spontaneous controllers of HIV disease progression43. Previous human studies have shown trends toward enhanced immune responses and virologic control after administration of various CTL-targeting therapeutic vaccination strategies33,44–52. Further, in vitro studies indicate that Gag peptide stimulation of CD8+ T-cells results in efficient killing of autologous HIV-infected CD4+ targets reactivated from latency, suggesting that induction of CTL responses may be a component in a successful reservoir eradication strategy53.
In A5176, we administered escalating doses of DermaVir, a therapeutic vaccine candidate, or matched placebo, to 25 HIV-infected patients with chronic HIV infection receiving suppressive cART. The study interventions, including repeated doses of DermaVir, were well tolerated, without dose-limiting toxicities. Adverse events were generally mild, and there was no significant difference in adverse event frequency among study groups (table 2).
DermaVir administration tended to be associated with greater cultured ELISPOT (PHPC) responses to all three Gag peptide pools compared to placebo, particularly among patients who received the intermediate dose of DermaVir. These DermaVir-induced T-cell responses were greatest at week 17, 4 weeks after the last immunization dose. Responses to Tat/Rev, on the other hand, were infrequently observed and of low magnitude (figure 1), perhaps due to the smaller number of potential high-affinity epitopes in these small proteins30. While the large number of comparisons included in the analysis could conceivably have led to spurious results, these findings are consistent with a previous study in treatment-naïve patients, in which the 0.4 mg dose was associated with a 0.5 log reduction in plasma HIV RNA and with the most robust cultured ELISPOT responses at 24 weeks34. The greater immunogenicity of the intermediate dose may reflect high-dose antigen-induced hyporesponsiveness, as shown in animal models54.
The nature of the T-cell responses elicited in this study deserves separate comment. Although T cell subsets were not sorted or characterized in this study, the cultured ELISPOT assay detects predominantly antigen-specific, long-lived central memory T-cells55,56, and robust central memory T-cell responses have been associated with spontaneous control of HIV viremia57. Thus, DermaVir could enhance the type of cellular immune responses needed for control of plasma viremia, which are almost universally lost early in the course of HIV infection.
Nonetheless, the magnitude of DermaVir-induced responses in this study was variable and reached statistical significance relative to placebo only at week 17 for the intermediate-dose group and only in response to certain peptide pools. A number of limitations in the study design may have contributed to these findings. First, the small sample sizes in each of the dosing groups hindered detection of significant differences, despite responses in the DermaVir groups that were thousands of net SFUs above those in the placebo group (figure 1). Second, in this as in most HIV vaccine studies, predicting and monitoring the breadth and magnitude of meaningful HIV-specific responses remains a major challenge, because the range of immunodominant epitopes varies dramatically from person to person as a result of the complex sequence of mechanisms that enable the establishment of immunodominance58. Indeed, an analysis of the high-affinity epitopes present in Dermavir predicted that this immunogen can potentially present up to 933 high-affinity MHCI-restricted epitopes and up to 2330 high-affinity MHCII-restricted epitopes30. By comparison, the peptide pools used in our monitoring assay cover only approximately 25% of the entire repertoire potentially presented by DermaVir. Therefore, a more closely matched set of peptides may be needed to test the full range of immunogenicity induced by this candidate preparation. Equally important will be to assess the HLA background of future study participants, and to map in greater detail the epitopes predicted to be bound with highest affinity.
This short-term study was not designed to assess efficacy, as neither clinical nor virological endpoints were included in the study design, but possible roles for this therapeutic vaccine strategy can nonetheless be envisaged. If, as shown previously34, DermaVir is capable of reducing plasma HIV RNA durably and safely in the absence of cART, this approach could allow delayed initiation or even interruption of antiretroviral therapy in some individuals, or it could be used as a bridge strategy for patients awaiting new antiretroviral options in the setting of multidrug resistance or intractable toxicity. Therapeutic immunization could also be a component of a future multi-pronged approach to eradicate HIV infection.
In summary, DermaVir is a safe candidate therapeutic vaccine that may induce HIV-specific central memory T-cell responses in a some HIV-infected persons receiving suppressive cART. Larger studies, potentially including DermaVir immune intensification on cART prior to activation of latently infected cells followed by an analytic treatment interruption, are necessary to quantify the entire repertoire of potential responses more accurately, to explore the clinical, virological, and immunological effects of repeated administration, and to define the potential therapeutic role of DermaVir in the HIV armamentarium.
Supplementary Material
Acknowledgments
The authors wish to thank Xiao-Li Huang, Weimin Jiang, Ping Zhang from University of Pittsburgh for their technical assistance with the cultured ELISPOT assay.
Footnotes
Conflict of Interest and Source of Funding: FL is CEO of Virostatics srl; JL is CEO of Genetic Immunity; RP is a consultant and has received financial compensation from Genetic Immunity. NIAID: AI-36219 & AI-069501 (BR), AI-68636 & AI-68634 (RM, JS), 201IC001. SSSS, Inc (ACTG Immunol. Support Lab, DMA, XDL, RBP). Nat’l Office for Res. and Technol (Hungary): HIKC05 & DVCLIN01 (JL).
ClinicalTrails.gov identifier: NCT00270205
References
- 1.FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. World AIDS Report 2011. Geneva: 2011. [Google Scholar]
- 3.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997 Nov 14;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 7.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011 Oct 15;204(8):1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010 Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009 Aug 24;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Motal UM, Gillis J, Manson K, et al. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virology. 2005 Mar 15;333(2):226–238. doi: 10.1016/j.virol.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009 Jan;9(1):55–69. doi: 10.1517/14712590802571928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich TC, Valentine LE, Yant LJ, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007 Apr;81(7):3465–3476. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hel Z, Nacsa J, Tryniszewska E, et al. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002 Nov 1;169(9):4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 17.Mudd PA, Watkins DI. Understanding animal models of elite control: windows on effective immune responses against immunodeficiency viruses. Curr Opin HIV AIDS. 2011 May;6(3):197–201. doi: 10.1097/COH.0b013e3283453e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson C, Makitalo B, Thorstensson R, et al. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS. 1998 Dec 3;12(17):2261–2270. doi: 10.1097/00002030-199817000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010 Dec 10;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds MR, Weiler AM, Weisgrau KL, et al. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008 Oct 27;205(11):2537–2550. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull EL, Lopes AR, Jones NA, et al. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J Immunol. 2006 May 15;176(10):6130–6146. doi: 10.4049/jimmunol.176.10.6130. [DOI] [PubMed] [Google Scholar]
- 24.Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, Desrosiers RC. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999 Oct;73(10):8356–8363. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisziewicz J, Kelly L, Lori F. Topical DermaVir vaccine targeting dendritic cells. Curr Drug Deliv. 2006 Jan;3(1):83–88. doi: 10.2174/156720106775197574. [DOI] [PubMed] [Google Scholar]
- 26.Lisziewicz J, Trocio J, Whitman L, et al. DermaVir: a novel topical vaccine for HIV/AIDS. J Invest Dermatol. 2005 Jan;124(1):160–169. doi: 10.1111/j.0022-202X.2004.23535.x. [DOI] [PubMed] [Google Scholar]
- 27.Lori F. DermaVir: a plasmid DNA-based nanomedicine therapeutic vaccine for the treatment of HIV/AIDS. Expert Rev Vaccines. 2011 Oct;10(10):1371–1384. doi: 10.1586/erv.11.118. [DOI] [PubMed] [Google Scholar]
- 28.Lori F, Trocio J, Bakare N, Kelly LM, Lisziewicz J. DermaVir, a novel HIV immunisation technology. Vaccine. 2005 Mar 18;23(17–18):2030–2034. doi: 10.1016/j.vaccine.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Lorincz O, Toke ER, Somogyi E, et al. Structure and biological activity of pathogen-like synthetic nanomedicines. Nanomedicine. 2011 Aug 10; doi: 10.1016/j.nano.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somogyi E, Xu J, Gudics A, et al. A plasmid DNA immunogen expressing fifteen protein antigens and complex virus-like particles (VLP+) mimicking naturally occurring HIV. Vaccine. 2011 Jan 17;29(4):744–753. doi: 10.1016/j.vaccine.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Lisziewicz J, Gabrilovich DI, Varga G, et al. Induction of potent human immunodeficiency virus type 1-specific T-cell-restricted immunity by genetically modified dendritic cells. J Virol. 2001 Aug;75(16):7621–7628. doi: 10.1128/JVI.75.16.7621-7628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toke ER, Lorincz O, Somogyi E, Lisziewicz J. Rational development of a stable liquid formulation for nanomedicine products. Int J Pharm. 2010 Jun 15;392(1–2):261–267. doi: 10.1016/j.ijpharm.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Lisziewicz J, Bakare N, Calarota SA, et al. Single DermaVir Immunization: Dose-dependent Expansion of Precursor/Memory T Cells Against All HIV Antigens in HIV-1 Infected Individuals. PLoS One. 2012:7. doi: 10.1371/journal.pone.0035416. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Lunzen J, Pollard R, Stellbrink HJ, et al. DermaVir for initial treatment of HIV-infected subjects demonstrates preliminary safety, immunogenicity and HIV-RNA reduction versus placebo immunization. XVIII International AIDS Conference, Abstract A-240-0111-12561; 2010; Vienna. [Google Scholar]
- 35.Lisziewicz J, Trocio J, Xu J, et al. Control of viral rebound through therapeutic immunization with DermaVir. AIDS. 2005 Jan 3;19(1):35–43. doi: 10.1097/00002030-200501030-00004. [DOI] [PubMed] [Google Scholar]
- 36.Calarota SA, Foli A, Maserati R, et al. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J Immunol. 2008 May 1;180(9):5907–5915. doi: 10.4049/jimmunol.180.9.5907. [DOI] [PubMed] [Google Scholar]
- 37.Gill DK, Huang Y, Levine GL, et al. Equivalence of ELISpot assays demonstrated between major HIV network laboratories. PLoS One. 2010;5(12):e14330. doi: 10.1371/journal.pone.0014330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang XL, Fan Z, Kalinyak C, Mellors JW, Rinaldo CR., Jr CD8(+) T-cell gamma interferon production specific for human immunodeficiency virus type 1 (HIV-1) in HIV-1-infected subjects. Clin Diagn Lab Immunol. 2000 Mar;7(2):279–287. doi: 10.1128/cdli.7.2.279-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goonetilleke N, Moore S, Dally L, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006 May;80(10):4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein MR, van Baalen CA, Holwerda AM, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995 Apr 1;181(4):1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantaleo G, Demarest JF, Schacker T, et al. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinaldo C, Huang XL, Fan ZF, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infeted long-term nonprogressors. J Virol. 1995 Sep;69(9):5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinges WL, Richardt J, Friedrich D, et al. Virus-specific CD8+ T-cell responses better define HIV disease progression than HLA genotype. J Virol. 2010 May;84(9):4461–4468. doi: 10.1128/JVI.02438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schooley RT, Spritzler J, Wang H, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010 Sep 1;202(5):705–716. doi: 10.1086/655468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Routy JP, Boulassel MR, Yassine-Diab B, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010 Feb;134(2):140–147. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and indces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008 Feb;15(2):284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Lunzen J. AIDS Vaccine. Vol. 2011. Bangkok, Thailand: 2011. Results from Phase IIB Placebo Controlled Study of Bionor Pharma’s Vacc-4x Show Excellent Safety Profile, Statistically Significant Viral Load Reduction in Patients with HIV who Suspend Antiretroviral Therapy. [Google Scholar]
- 48.Vardas E, Stanescu I, Leinonen M, et al. AIDS. Vol. 2010. Vienna, Austria: 2010. Indicators of Therapeutic Vaccine Effect Using Multi-HIV B Clade DNA in Treatment-Naïve Subtype C HIV-1 Infected Subjects; p. Abstract MOPD102. [Google Scholar]
- 49.Garcia F, Climent N, Assoumou L, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011 Feb 15;203(4):473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kloverpris H, Karlsson I, Bonde J, et al. Induction of novel CD8+ T-cell responses during chronic untreated HIV-1 infection by immunization with subdominant cytotoxic T-lymphocyte epitopes. AIDS. 2009 Jul 17;23(11):1329–1340. doi: 10.1097/QAD.0b013e32832d9b00. [DOI] [PubMed] [Google Scholar]
- 51.Van Gulck E, Vlieghe E, Vekemans M, et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS. 2012 Feb 20;26(4):F1–F12. doi: 10.1097/QAD.0b013e32834f33e8. [DOI] [PubMed] [Google Scholar]
- 52.Jacobson JM, Pat Bucy R, Spritzler J, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis. 2006 Sep 1;194(5):623–632. doi: 10.1086/506364. [DOI] [PubMed] [Google Scholar]
- 53.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012 Mar 23;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehl S, Barchet W, Oehen S, et al. Donor cell persistence and activation-induced unresponsiveness of peripheral CD8+ T cells. Eur J Immunol. 2000 Mar;30(3):883–891. doi: 10.1002/1521-4141(200003)30:3<883::AID-IMMU883>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 55.Todryk SM, Pathan AA, Keating S, et al. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent and distal priming. Immunology. 2009 Sep;128(1):83–91. doi: 10.1111/j.1365-2567.2009.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keating SM, Bejon P, Berthoud T, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005 Nov 1;175(9):5675–5680. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 57.Ndhlovu ZM, Proudfoot J, Cesa K, et al. Elite controllers with low to absent effector CD8+ T cell responses maintain highly fucntional, broadly directed central memory responses. J Virol. 2012 Apr 18;86 doi: 10.1128/JVI.00531-12. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedrich D, Jalbert E, Dinges WL, et al. Vaccine-induced HIV-specific CD8+ T cells utilize preferential HLA alleles and target-specific regions of HIV-1. J Acquir Immune Defic Syndr. 2011 Nov 1;58(3):248–252. doi: 10.1097/QAI.0b013e318228f992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.