Abstract
Activation of glial cells and neuro-glial interactions are emerging as key mechanisms underlying chronic pain. Accumulating evidence has implicated 3 types of glial cells in the development and maintenance of chronic pain: microglia and astrocytes of the central nervous system (CNS), and satellite glial cells of the dorsal root and trigeminal ganglia. Painful syndromes are associated with different glial activation states: (1) glial reaction (ie, upregulation of glial markers such as IBA1 and glial fibrillary acidic protein (GFAP) and/or morphological changes, including hypertrophy, proliferation, and modifications of glial networks); (2) phosphorylation of mitogen-activated protein kinase signaling pathways; (3) upregulation of adenosine triphosphate and chemokine receptors and hemichannels and downregulation of glutamate transporters; and (4) synthesis and release of glial mediators (eg, cytokines, chemokines, growth factors, and proteases) to the extracellular space. Although widely detected in chronic pain resulting from nerve trauma, inflammation, cancer, and chemotherapy in rodents, and more recently, human immunodeficiency virus-associated neuropathy in human beings, glial reaction (activation state 1) is not thought to mediate pain sensitivity directly. Instead, activation states 2 to 4 have been demonstrated to enhance pain sensitivity via a number of synergistic neuro-glial interactions. Glial mediators have been shown to powerfully modulate excitatory and inhibitory synaptic transmission at presynaptic, postsynaptic, and extrasynaptic sites. Glial activation also occurs in acute pain conditions, and acute opioid treatment activates peripheral glia to mask opioid analgesia. Thus, chronic pain could be a result of “gliopathy,” that is, dysregulation of glial functions in the central and peripheral nervous system. In this review, we provide an update on recent advances and discuss remaining questions.
Keywords: Astrocytes, ATP receptors, Chemokines, Cytokines, Human, Microglia, Rodents, Satellite glial cells, Spinal cord
1. Introduction
It is now well established that chronic pain, such as inflammatory pain, neuropathic pain, and cancer pain, is an expression of neural plasticity, both in the peripheral nervous system (PNS) as peripheral sensitization [11,78] and in the central nervous system (CNS) as central sensitization [111,139]. The most widely studied neuronal mechanisms are hyperexcitability and sensitization of primary sensory neurons (peripheral sensitization) and enhancement of excitatory synaptic transmission in spinal cord, brainstem, and cortical neurons (central sensitization), caused by transcriptional, translational, and post-translational regulation. Other neuronal mechanisms include disinhibition (reduced inhibitory synaptic transmission), descending pathway facilitation (eg, from the brainstem to the spinal cord), and long-term potentiation (LTP) in the cortex and spinal cord. These neuronal mechanisms have been strongly implicated in the development and maintenance of persistent pain in rodents [11,142,195,205,317]. Central sensitization and LTP are also involved in human pain conditions[134,285]. In parallel to the progress in these neuronal mechanisms is the increased recognition of the importance of non-neuronal cells, especially glial cells, in the initiation and maintenance of chronic pain. Of note, over the last 10 years, the field of pain research has witnessed a dramatic increase in the number of publications studying glia and pain. Numerous reviews have been published in high-impact journals to address this topic[24,52,68,80,160,164,200,209,247,273]. Here we provide a comprehensive and updated review of glia and pain by integrating recent advances in both the pain and glial research fields.
Glial cells in the CNS consist of 3 major groups: astrocytes, microglia, and oligodendrocytes [69]. Glial cells in the PNS consist of satellite glial cells (SGCs) in the dorsal root ganglia (DRGs) and trigeminal ganglia (TGs) and Schwann cells in the peripheral nerves. This review will cover 3 types of glia—microglia,astrocytes, and SGCs—as their roles in pain regulation are well documented.
1.1. Microglia
Microglia are macrophage-like cells in the CNS that originate from bone marrow-derived monocytes that migrate during perinatal development. They are heterogeneously distributed throughout the CNS. Under normal conditions, microglia are not as quiescent as many investigators originally thought, as it has been shown that microglia actively sense their environment with their ramified processes [93,175,199]. Notably, microglia dynamically interact with synapses to modulate their structures and functions in healthy brain [246]. During development, microglial processes can engulf synapses, and synaptic pruning by microglia, which involves the activation of the complement system, is necessary for normal brain development. [186,221].
Microglia are further activated after various insults such as nerve injury, by displaying morphological changes, such as a change from ramified to amoeboid shape [57] and upregulation of microglial markers (CCR3/CD11b, major histocompatibility complex II [MHC II], and ionized calcium-binding adaptor molecule-1 [IBA1]) [93,227] (Table 1). After peripheral nerve injury, microglia in the spinal cord undergo rapid proliferation [14,23,55,151], and this proliferation is already very prominent 2 days after spared nerve injury [227].
Table 1.
Distinct reaction of microglia, astrocytes, and satellite glial cells (SGCs) in different pain conditions, as examined by upregulation of the glial markers IBA1, CD11b, and glial fibrillary acidic protein (GFAP).
| Pain conditions | Microglia | Astrocytes | SGCs |
|---|---|---|---|
| Nerve injury | ↗ | ↗ | ↗ |
| Spinal cord injury | ↗ | ↗ | |
| Paw incision | ↗ | ↗ | |
| Inflammation | ↔/↗ | ↗ | ↗ |
| Joint arthritis | ↗ | ↗ | ↗ |
| Bone cancer | ↔/↗ | ↗ | ↗ |
| Skin cancer | ↔ | ↗ | |
| Chemotherapy | ↔/↗ | ↗ | ↗ |
| Diabetes | ↗ | ↗ | |
| HIV neuropathy | ↔ | ↗ | |
| Chronic opioid | ↗ | ↗ | |
| Acute opioid | ↔ | ↔ | ↗ |
Detailed, with related references, in Section 2.1.
Symbols: Right-upward diagonal arrow (↗) denotes upregulation; right&left horizontal arrow (↔) denotes no regulation; right-downward diagonal arrow (↘) denotes downregulation.
Numerous studies have demonstrated a critical role of microglia in the development of neuropathic pain [43,113,197,252], as well as acute inflammatory pain [229,311]. Minocycline, a nonselective inhibitor of microglia, has been shown to reduce neuropathic pain, inflammatory pain, and postoperative pain [13,86,100,197], but its role in reducing the established late-phase neuropathic pain is limited [197]. Importantly, recent progress has identified a large number of molecules that are induced in microglia after painful injuries, especially nerve trauma (Tables 1-4).
Table 4.
Regulation of the glial mediators cytokines, chemokines, growth factors, and proteases in microglia, astrocytes, and satellite glial cells (SGCs).
| Pain conditions | Microglia | Astrocytes | SGCs |
|---|---|---|---|
| Nerve injury | |||
| TNF-α | ↗ | ||
| IL-1β | ↗ | ↗ | ↗ |
| IL-6 | ↗ | ||
| IL-18 | ↗ | ||
| CCL2 | ↗ | ||
| BDNF | ↗ | ||
| bFGF | ↗ | ↗ | |
| MMP-2 | ↗ | ↗ | |
| tPA | ↗ | ↗ | |
| CatS | ↗ | ||
| TSP4 | ↗ | ||
| SCI | |||
| IL-1 β | ↗ | ||
| Inflammation | |||
| TNF-α | ↗ | ||
| IL-1 β | ↗ | ↗ | |
| IL-6 | ↗ | ||
| Bone cancer | |||
| TNF-α | ↗ | ||
| IL-1 β | ↗ | ||
| IL-6 | ↗ | ||
| Chronic opioid | |||
| TNF-α | ↗ | ||
| IL-1 β | ↗ | ||
| IL-6 | ↗ | ||
| Acute morphine | |||
| IL-1 β | ↗ |
Detailed, with related references, in Section 2.4.
Symbols: Right-upward diagonal arrow (↗) denotes upregulation; right&left horizontal arrow (↔) denotes no regulation; right-downward diagonal arrow (↘) denotes downregulation.
1.2. Astrocytes
Astrocytes are the most abundant cells in the CNS and were historically regarded as support cells. Work over the past decade indicates that astrocytes play multiple active roles in acute and chronic neuronal diseases such as seizure, stroke, and ischemia [133]. Unlike microglia and oligodendrocytes, astrocytes form physically coupled networks mediated by gap junctions, which, among other functions, facilitate intercellular transmission of Ca2+ signaling and exchange of cytosolic contents, and display oscillations in ion permeability through astrocytic networks. Gap junction communication is mediated by homo- and heteromeric associations of hemichannels, such as connexin-43 (Cx43), the predominant connexin expressed in astrocytes [27]. Although astrocytes are typically immune labeled by glial fibrillary acidic protein (GFAP), GFAP immunoreactivity labels only major branches and processes of astrocytes. The actual territory occupied by an astrocyte is much larger than that revealed by GFAP immunostaining. Of note, each astrocyte forms a non-overlapping territory or domain [106,133], which collectively resemble a lattice framework, appearing crystalline in nature. Although the implications of this organization are not fully understood, it becomes lost when astrocytes transition to reactive states [181]. In addition, astrocytes have extensive contacts with both synapses and cerebral blood vessels, and control the increase in blood flow evoked by synaptic activity. The astrocyte-mediated blood flow increase is fundamental to the bloodoxygen-level-dependent (BOLD) signal detected by functional magnetic resonance imaging (fMRI) [106].
It is estimated that a single astrocyte can enwrap 140,000 synapses and 4 to 6 neuronal somata, and can contact 300 to 600 neuronal dendrites in rodents. [22,69,180]. A close contact with neurons and synapses makes it possible for astrocytes not only to support and nourish neurons but also to regulate the external chemical environment during synaptic transmission.
The growing appreciation for active roles of astrocytes has led to the proposal of a “tripartite synapse” theory, based on the facts that (1) glia respond to neuronal activity with an elevation of their internal Ca2+ concentration and trigger the release of chemical transmitters from glia themselves, and (2) glial transmitters cause feedback regulation of neuronal activity and synaptic strength. According to this theory, astrocytic processes are active components of synapses, in addition to pre- and post-synaptic components [7]. Although active contribution to synaptic activity remains a possibility, several recent studies have challenged the theory of the tripartite synapse, by demonstrating that alterations in astrocytic Ca2+do not modulate synaptic transmission[4,172,193]. In reviewing these conclusions, however, it is important to note that most of the classical studies of the tripartite synapse are based on electrophysiological analysis of acute slices prepared from rodent pups. Since the expression of membrane proteins as well as neural circuits undergo significant changes during development [16,61], it is possible that the concept of receptor-mediated Ca2+signaling as a key feature defining astrocytic participation in higher neural function will be expanded to include other intracellular signaling pathways. Of note, glutamate-dependent neuroglial Ca2+signaling differs between the young and adult rodent brain [223]. Thus, alternative pathways for astrocytic modulation of synaptic transmission exist: 1 of the essential housekeeping duties of astrocytes is to maintain potassium hemostasis. Recently, it has been shown that receptor-mediated increases in astrocytic Ca2+can modulate neural network activity by active uptake of extracellular K+ [263]. Because the extracellular concentration of K+is an important determinant of the resting membrane potential and thereby of neuronal activity, active uptake of K+represents a simple yet powerful tool for rapid modulation of neural networks.
Studies using astroglial toxins (eg, flurocitrate and a-aminoadipate), astroglial aconitase inhibitor (sodium fluoroacetate), or inhibitors of the astroglial enzyme glutamine synthetase (eg, methionine sulfoximine) in adult animals suggest that astrocytes are important both for the induction and maintenance of inflammatory and for neuropathic pain [30,31,69,83,110,161,184,200,272]. Proliferation of spinal cord astrocytes has been demonstrated in models of neuropathic pain, such as rhizotomy [151] and spinal nerve ligation [248]. Conversely, inhibiting astrocyte proliferation in the spinal cord was shown to reduce neuropathic pain [248].
1.3. Satellite glial cells
Satellite glial cells (SGCs) are prominent glial cells in the PNS. They are found not only in sensory glia (DRGs and TGs) but also in sympathetic and parasympathetic ganglia. Like Schwann cells, SGCs are derived from neural crest cells. SGCs are characterized by thin cellular sheaths that surround the individual neurons. They exhibit many similarities to astrocytes: (1) both express the glial markers GFAP, S100, and glutamine synthetase; and (2) both form gap junctions [89]. The number of SGCs in DRGs and TGs is muchlower than that of astroctyes in the spinal cord. Unlike astrocytes, each SGC contacts only 1 neuron. Strikingly, the gap of extracellular space between the SGC sheath and the associated neuronal plasma membrane measures only 20 nm, allowing for close interactions and effective signaling between neurons and SGCs [89]. Emerging evidence suggests that SGCs are activated after painful injuries and play an active role in the development of persistent pain [29,54,91,107,150]. SGCs also exhibit enhanced coupling in persistent inflammatory and neuropathic pain [54,295].
2. Different activation states of glia after painful stimuli and injuries
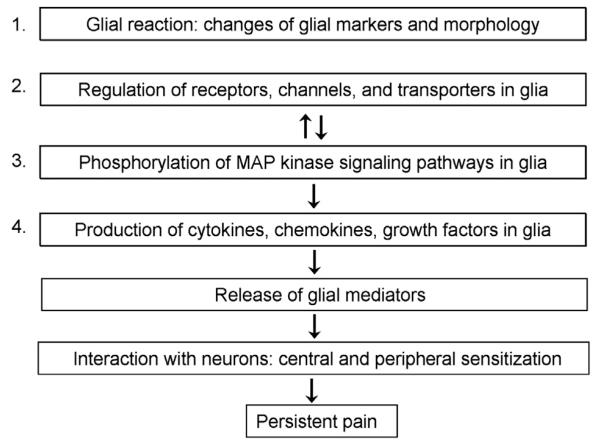
After painful stimuli and injuries, glia exhibit variable alterations in functions and morphologies, including the following: (1) ionic changes (eg, intracellular Ca2+rises in astrocytes); (2) posttranslational regulation (eg, phosphorylation of mitogen-activated protein kinases [MAPK]); (3) translational and transcriptional modulation (eg, modulation of surface molecules, glial markers, pro- and anti-inflammatory mediators); (4) morphological changes (eg, hypertrophy); and (5) proliferation. These changes are associated with different activation states of glia (Fig. 1). Below we discuss activation states that are frequently measured in the pain research field.
Fig. 1.
Different activation states of glia. Glia exhibit different activation states after painful injuries. (1) Glial reaction refers to upregulation of glial markers and morphological changes of glia (gliosis); (2) upregulation of glial receptors such as adenosine triphosphate (ATP) receptors, chemokine receptors, and Toll-like receptors, which will lead to the third activation state: (3) activation of intracellular signaling pathways, such as mitogen-activated protein kinase (MAPK) pathways. Phosphorylation of MAPKs will lead to the next activation state: (4) upregulation of glial mediators, such as cytokines, chemokines, and growth factors. Upon release, these glial mediators can interact with neurons to elicit pain via central and peripheral sensitization. Unlike glial reaction (state 1), the other activation states (states 2-4) have been shown to induce pain.
2.1. Glial reaction: Changes in glial markers and/or morphology
Most studies define glial activation as upregulation of the glial markers such as CCR3/CD11b, IBA1, and GFAP, which are often, but not always, associated with morphological changes (eg, hypertrophy or process retraction/extension). Thus, we refer to this glial activation state as glial reaction.
Observations that nerve injury induces microglial responses date back to the 1970s [3]. Microglial reaction (microgliosis) in the spinal cord has been intensively investigated after peripheral nerve injury. Nerve trauma induces very robust microglial reaction, such as hypertrophy and upregulation of the microglial markers CD11b, IBA1, and CD68 in the spinal cord and brainstem[118,252,300] (Fig. 2). IBA1 is probably the most widely used marker for microglial reaction in the pain field, partly because the IBA1 antibody from Wako Chemicals works better than other antibodies of microglial markers. As expected, microglial reaction is also very robust after spinal cord injury [86,102]. Furthermore, chronic opioid exposure, streptozotocin-induced diabetic neuropathy, and surgical incision result in microglial reaction [49,192,275,310]. However, microglial reaction is less evident after bone cancer [98] and chemotherapy-induced neuropathy [297,307], depending on the doses of chemotherapy drugs and severity of nerve damage after tumor growth (as shown by ATF-3 expression in DRG neurons) [23,303]. Intra-articular but not intraplantar injection of complete Freund’s adjuvant (CFA) induces microglial reaction[222], because of deep tissue (joint) injury and possible axonal injury (Table 1). Interestingly, in young rats (P10), nerveinjury-evoked spinal microglial reaction is not so evident, in parallel with the absence of nerve injury-induced neuropathic pain in these young animals [170,256]. Furthermore, prior neonatal injury can “prime” the spinal microglial response to adult injury, resulting in enhanced microglial reactivity [13]. Microglia can also be primed by previous insult in adults, leading to enhanced pain intensity and duration of the second insult [87].
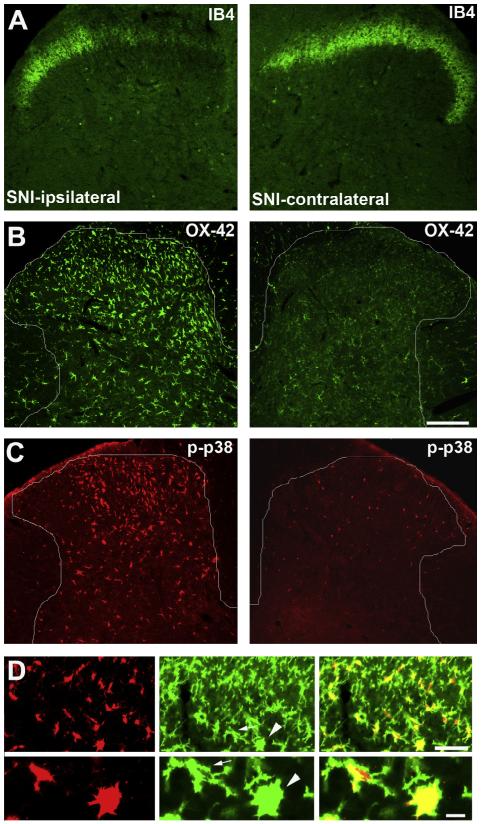
Fig. 2.
Activation of microglia in the spinal cord dorsal horn 3 days after spared nerve injury (SNI) in rats. (A) IB4 staining in the spinal cord dorsal horn ipsilateral and contralateral to the injury side. Note a loss of IB4 staining in the dorsal horn region innervated by the injured nerve branches. (B and C) CD11b (OX-42) and phosphorylated p38 (p-p38) immunostaining in the dorsal horn ipsilateral and contralateral to the injury side. Note overlapping expression patterns of OX-42 and p-p38 in the injury side. (D) Double staining of p-p38 (red) and OX-42 (green) in the ipsilateral dorsal horn. Lower panel presents high-magnification images of 2 microglial cells (indicated by arrow and arrowhead) from the upper panel. Note that p-p38 is completely co-localized with OX-42. Scale, 100 lm. Images are modified from Wen et al. [276], with permission.
Compared to microglial reaction, astrocyte reaction in the spinal cord is more general and evident after painful injuries [69]. Robust astrocyte reaction is induced not only by nerve trauma and spinal cord injury [75,76,173,316], but also by chronic opioid exposure [214], intraplantar or intra-articular CFA injection[70,83,198,222], bone [98] and skin cancer [67], chemotherapy, and human immunodeficiency virus (HIV)-induced neuropathy[297]. In addition, it appears that astrocytic reaction is more persistent than microglial reaction. It has been shown that GFAP and CD11b upregulation peaks at 150 and 14 days after nerve injury, respectively, although CD11b upregulation remains after 150 days[298]. GFAP upregulation is also prominent 9 months after spinal cord injury [85,173]. Although most studies have focused on glial reaction in the spinal cord and brainstem, astrocyte reaction has also been found in the forebrain, such as the anterior cingulate cortex, which contributes to affective pain [28]. One caveat is that immunohistochemistry of some GFAP antibodies may detect conformational or solubility changes or post-translational modifications of the protein but not actual changes in protein expression, because of different fixation conditions [15,56]. Thus, it is ideal to validate the results of GFAP immunohistochemistry with different antibodies and different methods such as Western blot and quantitative polymerase chain reaction (PCR).
Less is known about SGC reaction (GFAP upregulation) after painful injuries. SGC reaction is induced not only by nerve injury[150,295] but also by inflammation [235,236] in DRGs and TGs. Nerve injury further results in SGC proliferation [107]. SGCs reaction after nerve injury and DRG compression is very rapid, becoming evident within 4 hours. This reaction peaks at 1 week but declines after 3 weeks. This time course of SGC reaction suggests a possible role of SCGs in the induction and early maintenance of neuropathic pain [150,295]. Administration of glial toxin to DRGs has been shown to reduce neuropathic pain [150]. Also, there is increased coupling between SGCs after nerve injury [185,295] and inflammation [54]. Interestingly, even acute opioid treatment after a subcutaneous injection results in marked SGC reaction in DRGs at 2 hours when morphine analgesia declines [18] (Table 1).
2.2. Phosphorylation of MAPKs and Src in glia
The MAPK family includes 3 major members: extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK2, respectively), p38, and c-Jun N-terminal kinases (JNK)). ERK5 is a new family member and was shown to be activated in spinal microglia after nerve injury [177]. MAPK pathways play an important role in intracellular signaling in neurons and glia, and both are required for the genesis of persistent pain [109,178]. Interestingly, different MAPKs exhibit distinct activation (phosphorylation) patterns in glial cells after painful injuries [109] (Table 2).
Table 2.
Phosphorylation of mitogen-activated protein kinases (MAPKs; ERK, p38, JNK, ERK5) in microglia, astrocytes, and satellite glial cells (SGCs) in different pain conditions.
| Pain conditions | Microglia | Astrocytes | SGCs |
|---|---|---|---|
| Nerve injury | |||
| P-ERK | ↗ | ↗ | ↗ |
| P-p38 | ↗ | ↗ | |
| P-JNK | ↗ | ||
| P-ERK5 | ↗ | ||
| SCI | |||
| P-ERK | ↗ | ||
| P-p38 | ↗ | ||
| Paw incision | |||
| P-p38 | ↗ | ||
| Inflammation | |||
| P-ERK | ↗ | ↗ | |
| P-p38 | ↗ | ||
| P-JNK | ↗ | ||
| Bone cancer | |||
| PERK | ↗ | ↗ | |
| P-p38 | ↗ | ||
| P-JNK | ↗ | ||
| Skin cancer | |||
| P-JNK | ↗ | ||
| Diabetes | |||
| P-ERK | ↗ | ||
| P-p38 | ↗ | ||
| Chronic opioid | |||
| P-ERK | ↗ | ||
| P-p38 | ↗ |
Detailed, with related references, in Section 2.2.
SCI = spinal cord injury.
Symbols: Right-upward diagonal arrow (↗) denotes upregulation; right&left horizontal arrow (↔) denotes no regulation; right-downward diagonal arrow (↘) denotes downregulation.
Numerous studies have shown increased phosphorylation (activation) of p38 (P-p38) in spinal cord microglia after nerve injury[118,136,251] (Fig. 2), spinal cord injury [46,86], formalin-induced acute inflammatory pain [229], surgery-evoked postoperative pain[192,275], and chronic opioid exposure [47]. Nerve injury also activates microglial p38 in the trigeminal nucleus [194]. It has been shown that the b isoform of p38 (p38b) is expressed in microglia[228]. In addition, P-p38 is induced in neurons and SGCs of DRGs following nerve injury [118,179] and also in SGCs of TGs after inflammation in the temporomandibular joint [65].
P-JNK is induced in spinal astrocytes after nerve injury [316], CFA-induced persistent inflammatory pain [70], bone cancer[267], and melanoma-induced skin cancer [67]. Consistently, nerve injury also activates the upstream activator of JNK, the transforming growth factor-activated kinase-1 (TAK1), and the downstream effector of JNK, c-Jun in spinal astrocytes [125,316]. Among several JNK isoforms (JNK 1,2,3), JNK1 was shown to be expressed in spinal astrocytes [70].
P-ERK induction in glia after injury is highly dynamic: induction in spinal microglia corresponds to the early-phase (first week), and gradually transitions to astrocytes in the late phase after nerve injury and bone cancer [268,314]. CFA also induces P-ERK in spinal astrocytes in the late phase [279]. Furthermore, nerve injury evokes P-ERK in SGCs of DRGs [314], and temporomandibular joint inflammation elicits P-ERK in SGCs of TGs [65].
MAPKs are activated by proinflammatory mediators [109] and inactivated by phosphatases, such as MAPK phosphatase (MKP1,2,3). For example, P-p38 expression in spinal microglia after nerve injury can be suppressed by MKP3 [171]. Activation of CB2 in microglia was shown to upregulate MKP1 and MKP3, leading to a reduction of P-ERK in microglia [202]. Inflammation induces rapid upregulation of MKP1, MKP2, and MKP3 in SGCs of TGs [65], which may regulate the resolution of inflammatory pain.
Mounting evidence indicates that activation of MAPKs in spinal cord glial cells is essential for the development of persistent pain[109]. Thus, intrathecal injection(s) of selective inhibitors of MEK (ERK kinase), p38, and JNK, as well as antisense knockdown of ERK5, attenuated inflammatory, neuropathic, and cancer pain in rats and mice [109]. Systemic injection of p38 inhibitor also reduced spinal nerve ligation-induced mechanical allodynia in mice[113]. Upregulation of spinal MKP-3 via gene therapy attenuates neuropathic pain by suppressing P-p38 [171].
The importance of MAPK pathways for neuropathic pain has also been demonstrated in human beings. In HIV patients with neuropathic pain, P-ERK, P-p38, and P-JNK levels in the dorsal horns are significantly increased, compared to those in HIV patients without neuropathic pain [211]. In a double-blind, placebo-controlled clinical trial, oral delivery of a selective p38 inhibitor, dilmapimod (SB-681323) attenuated neuropathic pain in patients with nerve trauma, radiculopathy, or carpal tunnel syndrome [6].
Nerve injury also induces phosphorylation of Src family kinases (Src, Lyn, Fyn) in spinal microglia [126,250]. Intrathecal infusion of a Src inhibitor (PP2) reduced nerve ligation-elicited neuropathic pain. Of interest, PP2 suppressed the activation of ERK but not p38 in spinal microglia [126].
2.3. Regulation of receptors, channels, and transporters in glia
As shown in Table 3, multiple receptors, channels, and transporters are expressed in glial cells and are regulated in different pain conditions. Although these molecules are not secreted, they play active roles in glial intracellular signaling by activating the MAPK pathways and inducing the synthesis, release, and uptake of the secreted molecules (Table 4).
Table 3.
Regulation of receptors, channels, transporters, enzymes, and transcriptional factors in microglia, astrocytes, and satellite glial cells (SGCs) in different pain conditions.
| Pain conditions | Microglia | Astrocytes | SGCs |
|---|---|---|---|
| Nerve injury | |||
| P2X4 | ↗ | ↔ | |
| P2X7 | ↗ | ||
| P2Y6 | ↗ | ||
| P2Y12 | ↗ | ↗ | |
| TLR2 | ↔ | ||
| TLR3 | ↔ | ||
| TLR4 | ↗ | ||
| C1q, 3, 4, 5 | ↗ | ↔ | |
| CX3CR1 | ↗ | ↔ | |
| CCR2 | ↗ | ||
| IFN-γ R | ↗ | ||
| Cx43 | ↗ | ↗ | |
| Kir4.1 | ↗ | ↘ | |
| TRPM2 | ↔ | ↔ | |
| GLT-1 | ↘ | ||
| GLAST | ↘ | ||
| COX-1 | ↗ | ||
| COX-2 | ↗ | ||
| NF-kB | ↗ | ↗ | |
| NOX-2 | ↗ | ||
| STAT3 | ↗ | ↗ | |
| c-Jun | ↗ | ||
| CB2 | ↗ | ||
| SCI | |||
| Cx43 | ↗ | ||
| Inflammation | |||
| TLR3 | ↔ | ||
| TLR4 | ↗ | ||
| Cx43 | ↗ | ||
| TRPM2 | ↗ | ||
| GRK2 | ↘ | ||
| ALX | ↘ | ||
| Joint arthritis | |||
| CX3CR1 | ↗ | ||
| Bone cancer | |||
| CX3CR1 | ↗ | ||
| HIV | |||
| TLR2 | ↗ | ||
| TLR9 | ↘ | ||
| Chronic opioid | |||
| P2X4 | ↗ | ||
| P2X7 | ↗ | ||
| TLR2 | ↗ | ||
| TLR4 | ↔ |
Detailed, with related references, in Section 2.3.
Symbols: Right-upward diagonal arrow (↗) denotes upregulation; right&left horizontal arrow (↔) denotes no regulation; right-downward diagonal arrow (↘) denotes downregulation.
ATP modulates glial activation via activating P2X (ion channels) and P2Y receptors (GPCR-coupled), and these ATP receptors gate microglial signaling for neuropathic pain [244,253]. Peripheral nerve injury upregulates P2X4, P2X7, P2Y6, and P2Y12 in spinal microglia; and, furthermore, neuropathic pain is reduced after pharmacological inhibition, antisense knockdown, or genetic deletion of P2X4, P2X7, P2Y6, or P2Y12 [135-137,215,243,244,252,253]. Mice lacking the P2x4 gene display diminished inflammatory pain and blunted neuropathic pain [249]. P2Y12 is also induced in SGCs of TG after nerve injury, and injection of a P2Y12R antagonist into TG reduces trigeminal neuropathic pain [124]. Moreover, chronicopioid treatment upregulates P2X4 and P2X7 in spinal microglia, and opioid tolerance is prevented after spinal knockdown of P2X4 or P2X7 [99,108,310]. However, a recent study demonstrated that opioid-induced hyperalgesia but not tolerance is mediated by opioid receptor-dependent expression of P2X4 in microglia [60].
Toll-like receptors (TLRs) are known to regulate innate immunity and have been strongly implicated in glial activation[152,174]. Lipopolysaccride (LPS), an agonist of TLR4, is highly potent in activating microglia. It also activates TLR4 in astrocytes[152]. Of note, spinal microglial reaction and neuropathic pain after nerve injury are reduced in Tlr2 knockout mice [130] and Tlr4 mutant mice [238]. Arthritic pain in the late phase is also reduced in Tlr4 knockout mice [33]. Strikingly, male but not female mice with Tlr4 mutation exhibit reduced neuropathic pain [168], suggesting sex differences in TLR4 and microglial signaling. Chronic morphine was shown to induce glial responses via activation of TLR4 [271]. Of note, opioid-inactive isomers were shown to induce spinal proinflammatory responses via activation of TLR4[104]. Pharmacological blockade of TLR4 signaling in vivo attenuated development of analgesic tolerance, hyperalgesia, and opioid withdrawal behaviors in rats [105]. In contrast, Ferrini et al. showed that chronic morphine-induced hyperalgesia is intact in Tlr4 mutant mice [60].
Microarray analysis reveals that the complement components (eg, C1q, C3, C4, C5) are among the most regulated transcripts in the spinal cord following nerve injury. In particular, these compliment components are upregulated in spinal microglia. Induction of C5aR in spinal microglia has been implicated in neuropathic pain sensitization [82].
Increasing evidence suggests that chemokine receptors contribute to the pathogenesis of chronic pain via modulating glial activation and neural plasticity [1,35,68,280]. CX3CL1 (fractalkine) and CCL2 (MCP-1) are 2 of the most well-studied chemokines for pain modulation. Although a chemokine normally activates multiple receptors, CX3CR1 appears to be the only known receptor for CX3CL1 and is exclusively expressed in microglia. Thus, Cx3cr-1GFP mice have been used for studying the localization and activation of microglia [175]. Nerve injury and joint inflammation induce a robust upregulation of CX3CR1 in spinal microglia, and spinal blockade of CX3CR1 with a neutralizing antibody inhibited inflammatory and neuropathic pain [165,222,257,315]. Consistently, mice lacking Cx3cr1 exhibited reduced inflammatory and neuropathic pain [219]. Compared to selective microglial expression of CX3CR1, CCR2, a major receptor for CCL2, is expressed in both neurons and microglia [2,72,81,84,121,305]. Nerve injury-induced spinal microglial reaction is abolished in Ccr2 knockout mice[300], whereas intrathecal CCL2 causes microgliosis in the spinal cord [239,300]. Neuropathic pain is impaired in Ccr2 knockout mice or after spinal injection of CCR2 antagonist [2,300,305]. Activation of CCR2 by CCL2 also rapidly modulates DRG neuronal sensitivity and spinal cord synaptic plasticity [72,81,315].
Like LPS, interferon-γ (IFN-γ) is a strong activator of microglia, by means of inducing microglial reaction, P2X4 upregulation, and Lyn phosphorylation [250]. Nerve injury upregulates INF-γ receptors in spinal microglia, and nerve injury-induced microglial reaction and mechanical allodynia are abrogated in Ifnγ receptor knockout mice [250].
Astrocytes and SGCs are characterized by forming gap junction-coupled networks, leading to the transmission of Ca2+signaling through networks [7,54]. Connexins are the major structural components of gap junctions, and Cx30 and Cx43 are known to be expressed by astrocytes [27]. Cx43 is upregulated in astrocytes after nerve lesion, spinal cord injury, and inflammation[27,62,77,83,145]. Inhibition of gap junction function by carbenoxolone (CBX), a nonselective gap junction inhibitor, reduces inflammatory and neuropathic pain [140,218]. In addition to modulating gap junction communication, recent studies also proposed a paracrine signaling of Cx43 to release key astrocytic mediators such as ATP and glutamate [123,148,240,262]. Unopposed Cx43 hemichannels are ideal for modulating ATP release pathways, as the biophysical properties of these hemichannels enable them to conduct high levels of ATP efflux [17,42,190]. Of note, SCI-induced ATP release in the spinal cord is diminished after Cx43 blockade [45]. In double knockout mice lacking Cx30/Cx43, the development of neuropathic pain (heat hyperalgesia and mechanical allodynia) is prevented, and spinal astroglial reaction is reduced [27]. Nerve injury was shown to upregulate Cx43 in SGCs of TGs. Of interest, reducing Cx43 expression in SGCs via RNAi reduced neuropathic pain in nerve-injured rats but induced pain-like behaviors in normal rats, suggesting different roles of SGCs-Cx43 in pain modulation in non-injured vs injured animals [183]. Notably, the gap junction blocker CBX also inhibits pannexin-1 (PNX1), which is expressed in astrocytes and modulates ATP release [74]. The role of PNX1 in pain control needs further investigation.
The following ion channels have also been implicated for glial signaling in pain. The K+channel subunit Kir 4.1 is expressed in SGCs, and silencing this K+subunit with RANi leads to pain hypersensitivity [261]. The water channel aquaporin-4 (AQP4) is induced in spinal cord astrocytes after spinal cord injury [173], and mice lacking Aqp4 display decreased pain sensitivity (hypoalgesia) [9]. TRPM2 is expressed in microglia and contributes to spinal cord microglial activation. Inflammatory and neuropathic pain are impaired in Trpm2 knockout mice [95].
The glutamate transporters such as GLT-1 and GLAST are expressed in astrocytes (Table 3) and regulate the clearance of glutamate from synaptic clefts and extracellular space, leading to altered glutamatergic transmission and neuronal plasticity[203,204]. Nerve injury and chronic morphine elicit a sustained down-regulation, after an initial upregulation, of glutamate transporter-1 (GLT1) and glutamate and aspartic acid transporter (GLAST) in the spinal cord [158,224,288]. Inhibition of glutamate transporters results in an elevation in spinal extracellular glutamate and spontaneous pain [147,278]. Consistently, GLT-1 gene delivery to the spinal cord attenuates inflammatory and neuropathic pain [157], supporting a role of astroglial glutamate transporters in the resolution of chronic pain.
Several enzymes are also actively involved in glial signaling in pain. Cyclooxygenase-1 and -2 (COX-1 and COX-2, respectively) are induced in microglia after surgical incision and nerve injury to facilitate postoperative and neuropathic pain [306,312,313]. NADPH oxidase 2 (Nox2) expression is induced in dorsal horn microglia after L5 spinal nerve transection, and Nox2-deficient mice showed decreases in oxidative stress, microglial reaction, and proinflammatory cytokine expression in the spinal cord, as well as neuropathic pain[131]. Of interest, G-protein-coupled receptor kinase (GRK2) in microglia was implicated in the transition from acute to chronic inflammatory pain. Spinal microglia/macrophage GRK2 expression is reduced after inflammation, leading to the activation of microglia and persistent pain via p38 and interleukin-1β (IL-1β) signaling [283].
Furthermore, nerve injury upregulates the transcriptional factors in spinal cord glia, including c-Jun in astrocytes [316], signal transducers and activators of transcription 3 (STAT3) in microglia [53] and astrocytes [248], and nuclear factor-κB (NF-κB) in microglia [227] and astrocytes [167], to enhance and maintain neuropathic pain.
Finally, painful injuries also induce upregulation of anti-inflammatory receptors in glia for the resolution of acute pain. Inflammation increases lipoxin receptor ALX expression in spinal cord astrocytes, and lipoxin A4 reduces inflammatory pain via inhibiting JNK phosphorylation in astrocytes [231]. Lipoxin A4 also attenuates morphine tolerance via modulating glial activation and cytokine expression [117]. Nerve injury increased cannabinnoidreceptor CB2 expression in spinal microglia [299], and CB2 agonists suppressed microglial reaction and neuropathic pain [282]. Of interest Cb2 knockout mice displayed increased microglial and astrocytic reactivity in the spinal cord and enhanced neuropathic pain, whereas transgenic mice overexpressing Cb2 showed attenuated glial reactivity and neuropathic pain [196].
2.4. Regulation of cytokines, chemokines, growth factors, and proteases in glia
A key issue regarding glial control of pain is to understand how glial mediators are produced and released. As shown in Table 4, glia produce both large molecules (cytokines, chemokines, growth factors, and proteases) and small molecules (glutamate, ATP, D-serine, and prostaglandin E2(PGE2). These glial mediators can modulate neuronal and synaptic activity and pain sensitivity.
Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1β, and IL-6 are among the most well-studied glial mediators. They are upregulated in spinal cord glia after nerve in-jury, inflammation, bone cancer, and chronic opioid exposure, and they contribute to the development of and maintenance of inflammatory, neuropathic, and caner pain and morphine tolerance[52,213,230,273]. TNF-α is primarily produced by microglia and plays an essential role in the generation of central sensitization and persistent pain [92,289,301,308], in addition to its well-documented role in modulating peripheral sensitization [119,207,216]. IL-1β is induced in astrocytes after bone cancer, inflammation, and nerve injury [83,274,279,303]. IL-1β can also be produced by microglia and neurons in the spinal cord [36,51,92]. Inhibition of spinal and brain IL-1β signaling reduces inflammatory, neuropathic, and cancer pain [83,163,232,274,303] and enhances morphine analgesia [120,270]. IL-18 is highly related to IL-1β, and both require caspase-1 and inflammasomes for active cleavage[146]. Nerve injury induces IL-18 expression in spinal microglia[34,167]. Furthermore, painful injuries induce cytokine expression in peripheral glia. For example, nerve injury and CFA inflammation increase IL-1β expression in SGCs of DRGs and TGs [127,234]. Of note, acute morphine upregulates IL-1β only in peripheral glia (SGCs) in DRGs but not in central glia (microglia and astrocytes) in the spinal cord [18].
Chemokines are expressed in glial cells, particularly in astrocytes in the CNS [68], as well as in neurons [84]. In primary cultures of astrocytes, TNF-α induced rapid expression of CCL2, CXCL10, and CXCL1 [72]. Spinal injection of TNF-α-activated astrocytes results in persistent mechanical allodynia via releasing CCL2 [71]. Spinal nerve ligation also induces CCL2 in spinal astrocytes, and intrathecal administration of an MCP-1 neutralizing antibody reduces neuropathic pain [72]. CCL2 expression is further increased in astrocytes of the medullary dorsal horn and contributes to trigeminal neuropathic pain [305]. Consistently, mice with CCL2 overexpression in astrocytes display pain hypersensitivity [162].
Growth factors are known to be induced in spinal glia by nerve injury. In particular, nerve ligation upregulates brain-derived neurotrophic factor (BDNF) in spinal microglia, via activation of P2X4 and p38 [244,254]. Spinal injection of ATP-activated microglia is sufficient to induce mechanical allodynia via releasing BDNF, and, conversely, neuropathic pain is suppressed by spinal blockade of the BDNF receptor TrkB [43]. Furthermore, treatment of microglial cultures with morphine increases BDNF release, which does not require μ-opioid receptor and TLR [60]. BDNF is also induced in DRG neurons after nerve injury and can be released from primary afferents in the spinal cord [66,143]. Unlike BDNF, basic fibroblast growth factor (bFGF or FGF-2) is induced in reactive astrocytes of the spinal cord in the late phase (3 weeks) of nerve injury [110]. Intrathecal infusion of bFGF produces persistent activation of spinal astrocytes (upregulation of P-JNK and GFAP) and sustained mechanical allodynia [110]. By contrast, intrathecal administration of a bFGF-neutralizing antibody attenuates established neuropathic pain [156]. Therefore, bFGF maintains chronic pain via activation of astrocytes.
Proteases are also upregulated in spinal glia after nerve injury. Notably, spinal nerve ligation induces matrix metalloprotease-2 (MMP-2) in spinal cord astrocytes and DRG SGCs in the late phase of neuropathic pain to maintain neuropathic pain, via activation of IL-1β and ERK [127]. Nerve injury further induces cathepsin S in spinal microglia [37] and tissue type plasminogen activator (tPA) in spinal astrocytes [138] to enhance neuropathic pain.
A recent study showed that nerve injury increases the expression of thrombospondin-4 (TSP4), an extracellular matrix glycoprotein, in spinal cord astrocytes. This increase is not only correlated but also required for the development neuropathic pain [132]. TSP4 release from astrocytes can promote synaptogenesis. Of great interest, the α2δ-1 calcium channel subunit, a possible target of gabapentin, was shown to be a neuronal receptor of TSP4. Thus, gabapentin may inhibit neuropathic pain via modulating synaptogenesis [58]. Astrocytes also produce small molecule mediators such as D-serine, ATP, and glutamate to enhance pain states [69]. Interestingly, inhibition of glycinergic transmission, which is known to occur in chronic pain, results in D-serine release from astrocytes to generate tactile allodynia [166]. D-serine is known as an agonist of glycine site of N-methyl-D-aspartate (NMDA) receptors [176].
In addition to the pro-inflammatory and pronociceptive mediators, glial cells may also produce anti-inflammatory and antinociceptive mediators, such as IL-4, IL-10, and TGF-β [92] for the recovery and resolution of pain [41,92,94,114,164]. Enhancement of endogenous production of interleukin-10 via gene therapy has been shown to produce long-term relief in neuropathic pain[212]. Of interest, a possible off-target effect of high doses of siR-NAs is to induce IFN-α in spinal astrocytes for eliciting antinociceptive effects [237].
3. Neuronal–glial and glial–glial interactions in persistent pain
Because pain is conveyed only by neurotransmission in the neural circuits, glia must interact with neurons to modulate pain sensitivity. Here we focus on neuronal–glial (neuronal–glial) (Section 3.1) and glial–glial (Section 3.2) interactions in the CNS under persistent pain conditions (Fig. 3). We also discuss neuroglial interactions in the PNS after painful injuries and acute morphine treatment (Section 3.3) (Fig. 4).
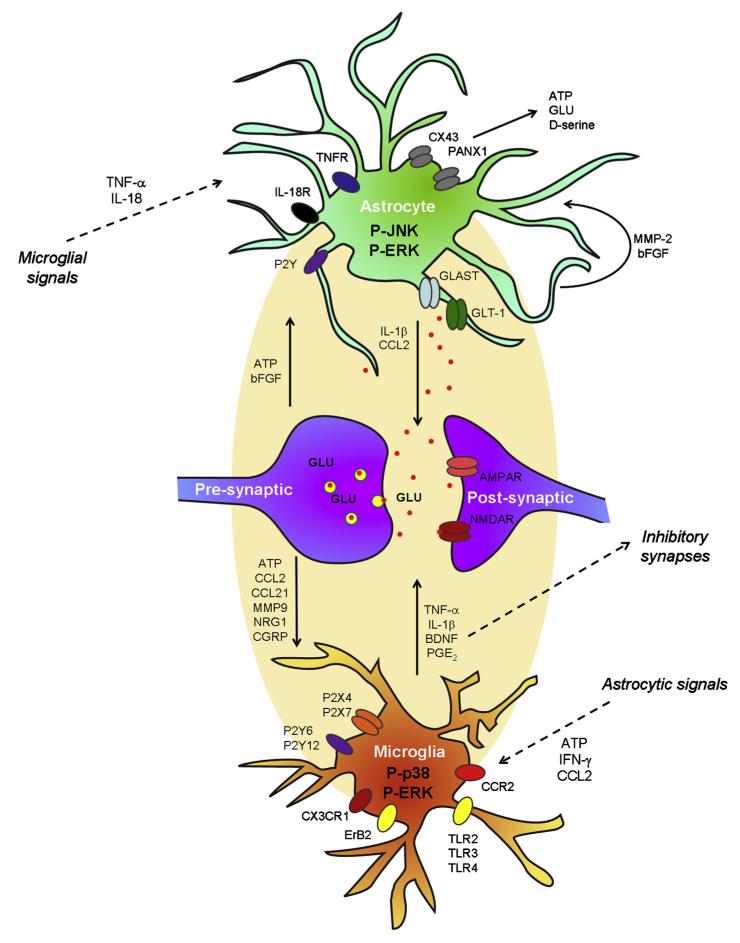
Fig. 3.
Schematic of neuronal–glial and glial–glial interactions in the spinal cord in persistent pain. Spontaneous discharge after a painful injury (eg, nerve injury) results in the release of ATP, chemokines (CCL2, CCL21, CX3CL1), MMP-9, NRG1, and CRGP from primary afferent central terminals, leading to activation of microglia in the dorsal horn. Spinal microglia express the receptors for ATP (P2X4, P2X7, P2Y6, P2Y12), and chemokines (CX3CR1, CCR2), and NRG1 (ErB2). Activation of these receptors induces phosphorylation of p38 and ERK (early phase) in microglia, leading to the production and release of the proinflammatory cytokines (TNF-α, IL-1β, IL-18) and the growth factor BDNF, and the consequent sensitization of dorsal horn neurons. Astrocytes can be activated by microglial mediators (TNF-α and IL-18), as well as astrocytic mediators (matrix metalloprotein-2 (MMP-2) and bFGF). Subsequent phosphorylation of JNK and P-ERK in astrocytes results in the production and release of chemokines (eg, CCL2) and cytokines (eg, interleukin-1β [IL-1β]). Astrocytes also produce adenosine triphosphate (ATP) and glutamate after the activation of the hemichannels (Cx43 and PNX1). After nerve injury, downregulation of astrocytic GLT1 results in decrease in astrocytic uptake of glutamate. Release of astrocytic mediators (CCL2, interleukin-1β [IL-1β], glutamate) can elicit NMDAR-mediated central sensitization. Release of adenosine triphosphate (ATP) and CCL2 from astrocytes can further maintain microglial activation.
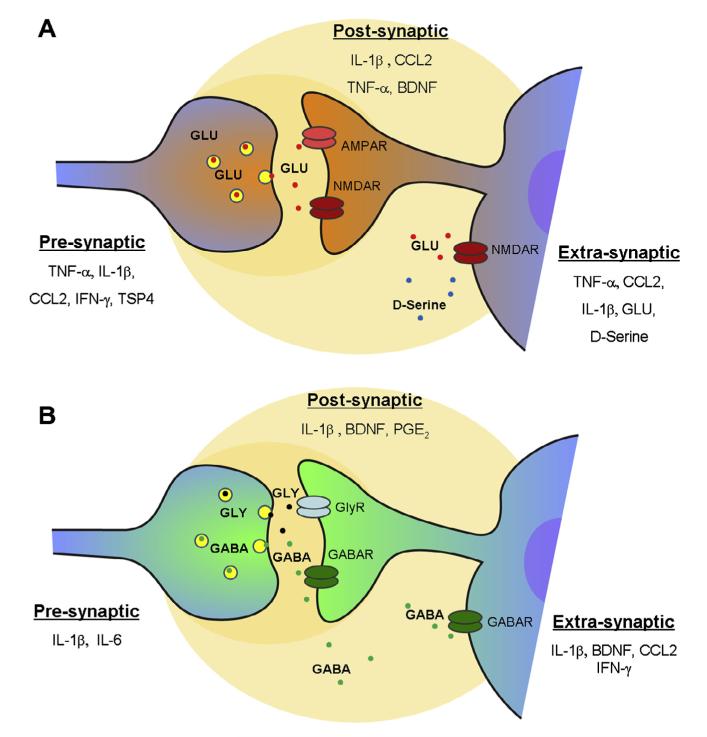
Fig. 4.
Glial mediators modulate excitatory and inhibitory synaptic transmission in the spinal cord. (A) Modulation of excitatory synaptic transmission at presynaptic, postsynaptic, and extrasynaptic sites by glial mediators. Presynaptically, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), CCL2, interferon-γ (IFN-γ), and TSP4 increase glutamate release to enhance EPSC frequency. Postsynaptically, IL-1β TNF-α, and CCL2 increase AMPAR activity. Extrasynaptically, TNF-α, IL-1β, CCL2, and D-serine increase NMDAR-NR2B activity and enhance NMDA-induced currents. Astrocyte-released glutamate can further induce NR2B-mediated inward currents in surrounding neurons. (B) Modulation of inhibitory synaptic transmission at presynaptic, postsynaptic, and extrasynaptic sites. Presynaptically, IL-1β and IL-6 decrease GABA and glycine release to decrease IPSC frequency. Postsynaptically, IL-1β decreases GABA/GlyR activity and IPSC amplitude. Prostaglandin E2(PGE2) inhibits evoked glycine current. Extrasynaptically, IL-1β, CCL2, and IFN-γ suppress GABA-and/or glycine-induced currents. TNF-α inhibits action potentials in inhibitory neurons. In lamina I neurons, BDNF produces disinhibition by altering chloride reverse potential.
3.1. Neuronal–glial interactions: Signals from neurons to glia
It is generally believed that injury-induced spontaneous discharge from primary afferents drives neuropathic pain[149,155,286]. Several lines of evidence suggest that nerve injury-released signaling molecules from primary afferent central terminals trigger microglial activation (Fig. 3). A brief, lowfrequency electrical stimulation of the peripheral C-fibers was shown to induce spinal microglial reaction without causing noticeable nerve injury [97]. Sustained nerve blockade via bupivacaine microspheres prevented nerve injury-induced microglial responses (CD11b expression and P-p38 induction) [276,287]. However, inhibition of C-fiber activity alone in the sciatic nerve with resininferatoxin may not be sufficient to prevent spared nerve injury-induced microglial activation [226], suggesting possible contribution of large A-fibers. Consistently, deletion of vesicular glutamate transporter-2 (vGluT2) in Nav1.8-expressing nociceptors did not prevent nerve injury-induced spinal microglial reaction, suggesting that glutamate release from nociceptors may not be sufficient to drive microglial reaction [208]. Although spontaneous activity is important for the initiation of microglial activation, it is not so critical for the maintenance of microglial activation [276].
Chemokines, such as CCL2, CCL21, and CX3CL1, are ideal for mediating neuronal–microglial interactions, given the distinct expression of their ligands and receptors. Nerve injury induces CCL2 and CCL21 expression in DRG neurons [19,281,298]. Stimulation of the dorsal root results in activity-dependent CCL2 release in the spinal cord [239,255]. Chemokines may activate microglia via P2X4 signaling: CCL2 induces the surface trafficking of P2X4[242], and CCL21 increases the expression of P2X4 [19]. Activation of P2X4 resulted in BDNF expression and release from microglia via p38 activation [245].
Proteases have also been implicated in microglial activation. Nerve injury induces rapid and transient upregulation of MMP-9 in DRG neurons, which is essential for the early-phase development of neuropathic pain [115]. Activity-dependent release of MMP-9 from primary sensory neurons was implicated in microglial activation, in part through IL-1b cleavage [127]. Cathepsin S is also involved in microglial–neuronal–microglial signaling. Nerve injury-evoked release of cathepsin S from microglia results in further activation of microglia, through the cleavage and release of CX3CL1 from primary sensory neurons [35,37].
The growth factor neuregulin-1 (NRG1) plays an active role in microglial activation. Although NRG1 is expressed in DRG neurons, its receptor, erbB2, is expressed in microglia. NRG1 was shown tostimulate microglial proliferation, chemotaxis, and IL-1b release via erbB2 [26]. Blockade of the erbB2 receptor or sequestration of endogenous NRG1 reduces nerve injury-induced microglial proliferation, p38 activation, and neuropathic pain [26]. NRG1 also induces microglial proliferation via phosphorylation of ERK and AKT [23]. In addition, release of the neuropeptide CGRP from primary sensory neurons is not only involved in neurotransmission but also contributes to microglial activation after chronic morphine exposure [269].
p38 MAPK serves as a key signaling molecule in microglia by integrating various input to microglia [112]. Microglia p38 is activated by ATP [79], TNF-α [230], and IL-1b [225]. After nerve injury, p38 is phosphorylated following the activation of multiple receptors, such as ATP receptors (P2X4 and P2Y12) [136,245] and chemokine receptors (CCR2 and CX3CR1) [2,315]. Microglial p38 is also activated by CGRP in chronic morphine-induced tolerance [269]. Notably, minocycline inhibits microglial activation by inhibiting spinal microglial p38 activation after inflammation and chronic morphine treatment [48,100]. Upon activation, p38 induces the synthesis and release of microglial mediators TNF-α, IL-1β, and BDNF [277]. Although p38 is critical for the synthesis and release of inflammatory mediators, it has a limited role in morphological changes (microgliosis) and proliferation of microglia, which could be mediated by another MAPK family member, ERK [25].
Neuronal signals are also important for the activation of astroytes. For example, neuronal activity appears to drive astrocyte activation after nerve injury [287] and inflammation [264]. Basic fibroblast growth factor (bFGF or FGF-2) is induced in primary sensory neurons after nerve injury and has an active role in neuronastrocyte signaling. As a well-known activator of astrocytes, bFGF elicits mitosis, growth, differentiation, and gliosis of astrocytes[110]. Nerve injury not only induces bFGF in DRG neurons [116] but also produces a delayed bFGF upregulation in astrocytes for maintaining neuropathic pain [110].
3.2. Glial–glial interactions
Astrocytic reaction is often preceded by microglial reaction, and microglial activation is known to drive astrocyte activation [197]. TNF-α, a key signal molecule produced by microglia, causes rapid JNK activation in astrocytes [72]. Of interest, nerve injury elicits IL-18 and IL-18R expression in spinal microglia and astrocytes, respectively, and IL-18 released from microglia was shown to activate IL-18R in astrocytes to upregulate NF-κB and facilitate neuropathic pain [167].
On the other hand, astrocytes can also release signaling molecules to activate microglia. After spinal cord injury, Cx43 is upregulated and gains a new function of paracrine signaling, leading to the release of ATP and glutamate [123,148,240,262]. Increases inextracellular ATP have been documented in a wide range of peripheral and central nervous system injuries, such as sciatic nerve entrapment [159], traumatic brain injury [50,64], and spinal cord injury [191,265]. ATP is critical for nerve injury-evoked microglial activation via activation of P2X4, P2X7, P2Y6, and P2Y12 receptors[244,253]. Of note, CCL2 is induced not only in primary sensory neurons but also in astrocytes [72,281]. Although DRG-CCL2 induces microglial activation, astrocytic-CCL2 may maintain microglial activation. IFN-γ, a strong microglial activator and neuropathic pain inducer [250], is also produced by astrocytes [196].
Finally, microglia and astrocytes could be self-activated via autocrine or paracrine signals. For example, bFGF is upregulated in spinal astrocytes after nerve injury to maintain astrocyte activation [110]. Nerve injury-induced astrocytic MMP-2 upregulation in the late phase can maintain astrocytic activation and neuropathic pain through IL-1β cleavage (activation) and phosphorylation of ERK in astrocytes [127].
3.3. Neuronal–glial interactions in dorsal root and trigeminal ganglia in the PNS
SGCs in DRGs and TGs are tightly associated with sensory neurons via gap junction; and gap junction communication between SGCs and SGC and neurons is greatly enhanced in persistent pain conditions [54,90,91]. Nerve injury-induced SGC activation requires neuronal activity and local inflammation [144,287]. Purinergic signaling is critically involved in neuronal–glial communication in DRGs [29,304]. For example, activity-dependent ATP release from neuronal soma activates P2X7 in SGCs [304], leading to TNF-α release from SGCs, which can in turn act on surrounding neurons to increase their excitability [119,216] (Fig. 4). ATP can also be released from SGCs to activate P2X3 receptor, which is expressed in primary sensory neurons and plays an important role in peripheral sensitization [40,217].
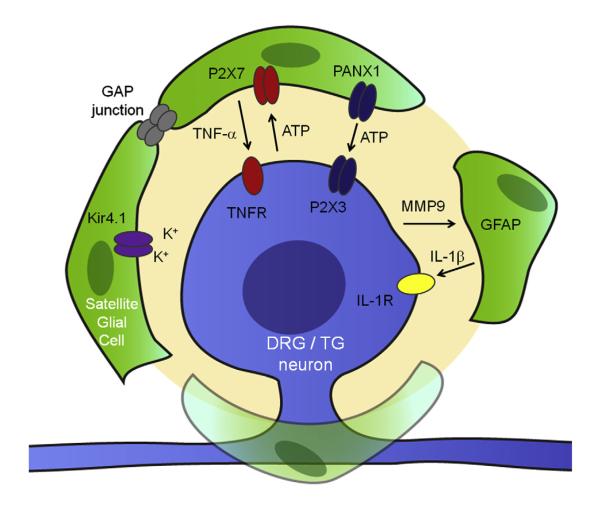
Of note, MMP-9 mediates neuron-SGC interaction in DRGs after acute morphine treatment, which can mask morphine analgesia[153] (Fig. 5). Systemic morphine administration was shown to elicit rapid MMP-9 upregulation in DRG neurons in the recovery phase of morphine analgesia (2 hours), which requires activation of μ-opioid receptors[153]. Notably, morphine analgesia is enhanced and prolonged in Mmp9 knockout mice [153]. Acute morphine also upregulates GFAP and IL-1β in SGCs of DRGs, and both require MMP-9 [18]. MMP-9 release from neurons results in IL-1β cleavage and release, which in turn activates IL-1β receptors in sensory neurons to elicit action potentials [20]. IL-1β is known to increase the excitability of sensory neurons via enhancing sodium currents and suppressing potassium currents [20,233,236]. Of interest, IL-1β has also been shown to mask morphine-induced analgesia [103,120]. Thus, targeting peripheral neuronal–glial interactions, in addition to previously recognized central neuronal–glial interactions, can also enhance opioid analgesia.
Fig. 5.
Schematic representation of neuronal-glial interactions in dorsal root and trigeminal ganglia of the peripheral nervous system (PNS). Spontaneous neuronal discharge after painful injury results in adenosine triphosphate (ATP) release in neuronal somata, leading to the activation of P2X7 and subsequent release of tumor necrosis factor-α (TNF-α) in satellite glial cells (SGCs). Persistent nociceptive activity or activation of opioid receptors by morphine also results in matrix metalloproteinase-9 (MMP-9) release from primary sensory neurons, causing the cleavage (activation) and release of interleukin-1β (IL-1β) in SGCs. TNF-α and IL-1β bind respective TNFR and IL-1R on sensory neurons to elicit hyperexcitability. SGCs can also release ATP via hemichannels (Cx43 and PNX1) or gap junction communication to activate P2X3 in neurons for triggering peripheral sensitization. In addition, SGCs express Kir 4.1 to maintain homeostasis of extracellular K+levels of sensory neurons, and injury-induced downregulation of Kir4.1 in SGCs will disrupt this K+homeostasis and generate neuronal hyperexcitability.
4. Glial mediators modulate excitatory and inhibitory synaptic transmission
A key issue regarding glial control of pain is how glial mediators regulate synaptic transmission. Strikingly, glial mediators can modulate spinal cord synaptic transmission at very low concentrations. Although neurotransmitters (eg, glutamate, GABA, glycine, and substance P) normally regulate neuronal and synaptic activity at micromolar concentrations, glial mediators (cytokines, chemokines, and growth factors) can change synaptic activity at nanomolar concentrations in vitro [43,72,128]. In particular, glial mediators can modulate both excitatory and inhibitory synaptic transmission (Fig. 5). Although most studies used young (3- to 5-week-old) and adult animals (rats and mice) for recording spinal neuronal activities [43,72,128,189,296], some studies used neonatal animals [73,81,259]. It is well known that the gene expression profiles of primary sensory and spinal cord neurons, glial responses, as well as spinal cord pain circuits undergo dramatic changes in the first 2 weeks after birth [16,61,172]. Thus, caution must be taken to interpret the data from neonatal animals.
4.1. Modulation of excitatory synaptic transmission
Glial mediators can modulate excitatory synaptic transmission via pre-, post-, and extrasynaptic mechanisms (Fig. 5). The effects of proinflammatory cytokines and chemokines on excitatory postsynaptic currents (EPSCs) have been examined in lamina II neurons using ex vivo spinal cord slice preparations [293]. Although the EPSC frequency change may result from presynaptic mechanisms (due to glutamate release from presynaptic terminals), the EPSC amplitude increase is caused by enhanced signaling of glutamate receptors (AMPA subtype) in post-synaptic sites.
Incubation of spinal cord slices with TNF-α, IL-1β, and CCL2 very rapidly (within minutes) increased spontaneous EPSC (sEPSC) frequency [72,128,296]. Chronic exposure of cultured dorsal horn neurons to IFN-γ also increased sEPSC frequency [260] (Fig. 5A), supporting a possible presynaptic modulation. TNF-α increases sEPSC frequency via activation of TRPV1 in presynaptic terminals, as this sEPSC increase is abolished in Trpv1 knockout mice. Single-cell PCR analysis indicates that TNF-α-responding lamina II interneurons are exclusively excitatory ones, because they all express vesicular glutamate transporter-2 (vGluT2). These lamina II neurons also receive input from TRPV1-expressing C-fibers and make synapses to lamina-I projection neurons [241], forming aspinal circuit to mediate TNF-α-induced pain. A recent study also demonstrated that TSP4, produced by astrocytes, increased sEPSC frequency [132].
Glial mediators such as IL-1β and CCL2 also increase the amplitudes of sEPSCs, via AMPA-mediated postsynaptic mechanisms (Fig. 5A). TNF-α is known to induce the trafficking and surface expression of AMPA receptors in hippocampal neurons [12,220]. After spinal cord injury, TNF-α induces rapid trafficking of GluR2-lacking AMPARs to the plasma membrane in spinal cord motor neurons [59]. Of note, inflammation induces a TNF-α-dependent surface trafficking of GluR1-AMPARs in the dorsal horn [32]. Although TNFR1 is the predominant receptor mediating the effects of TNF-α, both TNFR1 and TNFR2 are required for the induction of central sensitization [301,309].
Pro-inflammatory cytokines and chemokines further induce central sensitization via extrasynaptic mechanisms (Fig. 5A). NMDA currents in lamina II neurons, induced by bath application of NMDA to spinal cord slices, are enhanced by IL-1β, TNF-α, or CCL2 [72,128]. TNF-α increases NMDA receptor (NMDAR) activity through phosphorylation of ERK in dorsal horn neurons [292]. IL-1β induces phosphorylation of the NR1 subunit in spinal cord neurons [302]. Astrocytic D-serine enhances NMDA currents via binding the glycine site of NMDA receptors [201]. Interestingly, astrocytic glutamate release can be detected as slow inward currents, via patch-clamp recordings in nearby neurons. Slow inward currents are mediated by extrasynaptic NR2B receptors and induced in spinal dorsal horn neurons after inflammation [10].
4.2. Modulation of inhibitory synaptic transmission
Reduction or loss of inhibitory synaptic transmission (disinhibition) in the spinal cord pain circuit has been strongly implicated in the genesis of central sensitization and chronic pain[8,44,169,294]. Disinhibition after peripheral nerve injury involves a trans-synaptic reduction in the expression of the potassium-chloride co-transporter KCC2 and subsequent disruption of anion homeostasis (chloride homeostasis) in spinal lamina I neurons. In some cases, the shift in the transmembrane anion gradient can convert normally inhibitory anionic synaptic currents to be excitatory [44].
Glial mediators such as BDNF, cytokines, chemokines, and PGE2 can also modulate inhibitory synaptic transmission via pre-, post-, and extrasynaptic mechanisms (Fig. 5B). Presynaptically, IL-1β and IL-6 were shown to inhibit the frequency of spontaneous postsynaptic currents (sIPSCs) in spinal lamina II neurons [128]. Postsynaptically, IL-1β reduces the sIPSC amplitude [128]. PGE2 inhibits glycinergic neurotransmission in the dorsal horn via post-synaptic GlyR3 and the cAMP/PKA pathway [5,96].
At extrasynaptic sites, GABA and glycine currents, induced by bath application of GABA and glycine, can be suppressed by IL-1α and IL-6 [128]. BDNF acts on spinal lamina I neurons to reverse GABA inhibition by altering chloride reverse potential [43]. Furthermore, ATP or morphine-stimulated microglia result in a depolarizing shift in the anion reversal potential by releasing BDNF[43,60]. Like nerve injury, administration of ATP-stimulated microglia or pharmacological disruption of chloride transport in vivo alter the phenotype of spinal lamina I output neurons, leading to neuropathic pain phenotypes [129]. TNF-α was also shown to suppress action potentials in GAD67+ inhibitory neurons in spinal cord slices [296]. Moreover, CCL2 and IFN-γ inhibit GABA-induced responses in spinal cord neurons [81,259].
It remains to be investigated how anti-inflammatory cytokines (eg, IL-4, IL-10, TGF-β) regulate synaptic plasticity. It appears that IL-10 can suppress TNF-α-induced synaptic plasticity (unpublished observations). In particular, the anti-inflammatory lipid mediators such as resolvin E1 (RvE1) and neuroprotectin (NPD1) blocked TNF-α-induced synaptic plasticity (sEPSC frequency increase)[292]. NPD1 and RvD2 further reversed inflammation-induced synaptic plasticity and tetanic stimulation-induced spinal long-term potentiation (LTP) [189].
Finally, the proinflammatory cytokines TNF-α, IL-1β, and IL-6 also elicit long-term neuronal plasticity in the pain circuit by inducing the phosphorylation of the transcription factor cAMP response element-binding protein (CREB), leading to the transcription of CREB-mediated pronociceptive genes (eg, cyclooxygenase-2 [COX-2], neurokinin-1 [NK-1]) in spinal cord neurons[111,128,206]. Of note, TNF-α is sufficient to induce spinal LTP after nerve injury [154], and tetanic stimulation-induced spinal LTP is abolished in TNFR1 or TNFR2 knockout mice [188].
4.3. Concluding remarks
In the past decade great progress has been made to demonstrate critical roles of glial cells, such as microglia, astrocytes, and SGCs in the genesis of persistent pain. As evidence emerges, the list of glial-derived signaling molecules and mediators continues to grow (Tables 1-4). Glia can communicate with neurons by “listening” and “talking” to neurons. It is increasingly appreciated that chronic pain can manifest not only by neural plasticity but also by dysfunction of glial cells. Under the normal physiological conditions, astrocytes and SGCs provide trophic support to neurons and maintain the homeostasis of K+, glutamate, and H2O in CNS and PNS [258]. Astrocytes and SGCs could also “insulate” the neural circuit of pain by forming a structural barrier and keep the circuit silent by releasing inhibitory mediators [172]. Nerve injury-induced chronic pain is associated not only with neuropathy but also with “gliopathy.” Astrocytes lose their ability to maintain the homeostasis of K+and glutamate, leading to neuronal heperexcitability, as a result of higher extracellular levels of glutamate and K+. Dysfunction of astrocytic water channel (AQP4) will also result in edema in the CNS and PNS [258]. As a result of gliopathy, glia can no longer insulate the pain circuit; instead they serve as an amplifier of pain, by producing proinflammatory and pronociceptive mediators.
Painful injuries evoke rapid reaction of SGCs in the PNS, followed by microglial and astrocytic reaction in the CNS. Most studies on glia and pain focus on microglia and astrocytes in the spinal cord. Upon activation, presumably initiated by neuronal signals, glia synthesize and release proinflammatory and pronociceptive mediators (eg, proinflammatory cytokines and chemokines and growth factors) to enhance pain states, via activation of key signaling pathways, such as the MAP kinase pathways. Activation of hemichannels (eg, Cx43 and PNX1) and P2X7 results in the release of ATP and glutamate from astrocytes. Importantly, glial mediators (eg, TNF-α, IL-1β, IL-6, CCL2, BDNF) can powerfully modulate excitatory and inhibitory synaptic transmission at comparably lower concentrations. Glial mediators (ATP, CCL2, IFN-γ , bFGF, MMP-2) also result in further activation of glial cells via paracrine or autocrine regulation. Last but not the least, glia may also produce antiinflammatory and antinociceptive mediators for the resolution of acute pain. Further inquiry is needed to determine whether failure in the production of these resolution mediators leads to the transition from acute pain to chronic pain.
5. Remaining questions and future directions
5.1. Is glial activation associated with pain?
Despite the growing importance of glial cells in pain regulation, “glial activation” is not well defined. Most studies in the field use glial reaction (upregulation of the glial markers IBA1, CD11b, andGFAP to define the activation of microglia (IBA1/CD11b), astrocytes (GFAP), and SGCs (GFAP) (Table 1). Although the upregulation of these markers is associated with pain behaviors, especially in the induction phase, there are several caveats related to these markers. First, dissociation between microglial marker expression and pain behaviors has been reported by different groups [21,38,307]. Compared to microglial markers (IBA1 and CD11b), the astrocytic marker GFAP is better correlated with pain behaviors, especially after inflammation, bone cancer, chemotherapy, and HIV neuropathy[98,211,297,307]. Second, we should not exclude microglial activation if there is no change in IBA1 expression. Glial activation can also manifest as quick responses, such as Ca2+changes and phosphorylation of signaling molecules (eg, MAPKs) that could occur within minutes after a stimulation or insult. Indeed, sensory whisker stimulation was shown to evoke rapid increases, within several seconds, in astrocytic cytosolic Ca2+in the barrel cortex of adult mice [266]. Third, even under the activation states with upregulation of glial markers and hypertrophy, microglia could still have different functional states by exhibiting either pro-inflammatory (neurotoxic, M1) and anti-inflammatory (neuroprotective, M2) phenotypes [93,284]. Finally, and importantly, glial reactivity and morphological changes do not directly modulate pain. Neuronal activity and pain sensitivity are controlled by the glial mediators (cytokines, chemokines, ATP, BDNF, glutamate). Thus, the regulation of glial signaling molecules and glial mediators after painful injuries (Tables 2–4) could be better associated with pain states than glial reactivity.
5.2. Can we target glia for pain therapy?
How can we design drugs to target glial activity for pain control? Do we really need glia-selective drugs? Indeed, it is extremely difficult to design drugs that target only glial cells without affecting neurons. Furthermore, elimination of glial cells with glia-selective toxins may cause detrimental effects, given the supportive and protective roles of glia. Instead, there are alternative strategies: (1) to target the MAPK signaling pathways (ERK, p38, JNK), hemichannels (eg, Cx43 and PNX1), or P2X7 to suppress the release of glial mediators; (2) to target the upstream activators of glia, such as P2X4, P2Y6/12, MMP-9/2, and cathepsin S; and (3) to target the downstream mediators released by glia, such as TNF-α, IL-1β, IL-6, or BDNF.
We should learn lessons from recent failures in 2 clinical trials: 1 trial with a glial modulator, propentofylline, which showed no efficacy in reducing neuropathic pain in patients with post-herpetic neuralgia [141]; another trial with a CCR2 antagonist AZD2423, which showed no significant effects, compared to placebo, in post-traumatic neuralgia patients [122]. The failures may result from multiple reasons, including lack of translation from rodents to human beings, different ways of pain measurement in rodents and human beings (evoked pain vs spontaneous pain), and different pain conditions tested in rodents and human beings (nerve trauma-induced pain hypersensitivity in several weeks vs post-herpetic/traumatic neuralgia after many years). Of note, propentofylline is a well-known inhibitor of phosphodiesterase, and therefore could alter cAMP levels in glial and non-glial cells [63]. Propentofylline is also an adenosine uptake inhibitor [63]. Compared to the complete lack of effect of propentofylline, AZD2423 (150 mg) showed some trends toward reduction in paroxysmal pain and paresthesia/dysesthesia, indicating that a CCR2 antagonist may have some possible effects for some sensory components of pain [122]. Notably, the variability between and within individuals was very high, in part because of the nature of a multicenter trial. It is also a concern that inhibition of glial responses in the CNS cannot be validated in this trial, because of the lack of effective imaging technique for detecting glial responses (see Section 6.3).
Theoretically, it should be more effective for a drug to target both neurons and glia for pain relief. For example, p38 is activated both in spinal cord microglia and DRG neurons, and systemic p38 inhibitor has been shown to alleviate neuropathic pain in a clinical trial [6]. Recent studies have demonstrated that the anti-inflammatory and pro-resolution lipid mediators such as resolvins (RvD1, RvD2, RvE1), protectins/neuroprotectins (PD1/NPD1), and lipoxins (LXA4) could potently reduce inflammatory and postoperative pain, at very low doses [101,114,231]. Peri-surgical application of PD1/NPD1 effectively protects nerve trauma-induced neuropathic pain and spinal cord glial activation in mice [291]. RvE1 and PD1 further inhibit glial activation in cultures [290,291]. The receptors of these mediators, such as ChemR23 (RvE1) and ALX (RvD1 and LXA4) are widely expressed in neurons, glia, and immune cells[39,114,210,231]. Thus, these lipid mediators not only inhibit glial activation and inflammation but also inhibit TRP channels (eg, TRPA1/V1) and reverse synaptic plasticity in neurons[114,188,189]. Given the potency and safety, these endogenous lipid mediators, or their analogs, or small-molecule agonists of theirreceptors, could be developed for preventing and treating chronic pain, via targeting both neuronal and non-neuronal (immune and glial) mechanisms.
5.3. How much do we know about human glia?
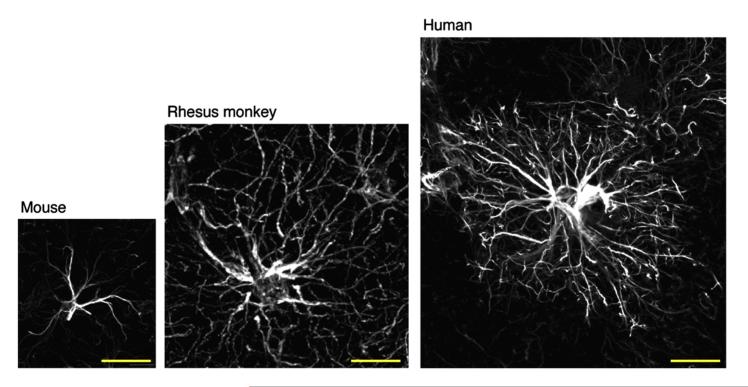
Little is known about the role of human glia in pain control. Indeed, astrocytes from mice, monkeys, and human beings are quite different in their sizes [180,182] (Fig. 6). The human brain appears to contain subtypes of GFAP-positive astrocytes that are not represented in rodents. In human cortex, astrocytes are more than 2-fold larger in diameter and extend 10-fold more GFAP-positive primary processes than their rodent counterparts (Fig. 6). The domain of a single human astrocyte has been estimated to contact up to 2 million synapses [133,180]. Remarkably, human glial progenitor cells (GPCs), after being implanted into neonatal immunodeficient mice, are gap junction-coupled to host astroglia, propagate Ca2+signals 3-fold faster than their hosts, and exhibit enhanced LTP and learning capability [88]. Hence, human astrocytes could play a more sophisticated role in chronic pain than rodent astrocytes. Importantly, astrocyte reaction, but not microglial reaction, is associated with chronic pain in HIV-infected patients [211]. Activation of the MAPK pathways is also correlated with neuropathic pain in these patients [211]. Future research should focus on the following: studying the responses of human glia in cultures and human glia transplantation in mice; investigating the changes in human glia in painful disease conditions in post mortem tissues; and imaging real-time glial activation in patients with chronic pain.
Fig. 6.
Glial fibrillary acidic protein (GFAP) immunostaining of mouse, rhesus monkey, and human astrocytes in cortex. Note striking differences in the sizes of mouse, monkey, and human astrocytes. Also note differences in the number and lengths of branches of astrocytes from mouse, monkey, and human being. Sizes of astrocytes increase with increasing complexity of brain function. Scale, 50 μm. Images are reproduced from Kimelberg and Nedergaard [133], with permission.
Acknowledgments
This work was supported by National Institutes of Health Grants NS67686 and DE17794 (to R.R.J.) and DE22743 (to R.R.J. and M.N.).
Footnotes
Conflict of interest statement
The authors declare no conflict of interest in regard to this work.
References
- [1].Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–34. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–52. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adrian EK, Jr, Williams MG, George FC. Fine structure of reactive cells in injured nervous tissue labeled with 3H-thymidine injected before injury. J Comp Neurol. 1978;180:815–39. doi: 10.1002/cne.901800412. [DOI] [PubMed] [Google Scholar]
- [4].Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short-and long-term plasticity are not modulated by astrocyte Ca2+signaling. Science. 2010;327:1250–4. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- [5].Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- [6].Anand P, Shenoy R, Palmer JE, Baines AJ, Lai RY, Robertson J, Bird N, Ostenfeld T, Chizh BA. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain. 2011;15:1040–8. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- [7].Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- [8].Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–30. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- [9].Bao F, Chen M, Zhang Y, Zhao Z. Hypoalgesia in mice lacking aquaporin-4 water channels. Brain Res Bull. 2010;83:298–303. doi: 10.1016/j.brainresbull.2010.08.015. [DOI] [PubMed] [Google Scholar]
- [10].Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol. 2010;588:831–46. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- [13].Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135:404–17. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624–33. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bell PB, Jr, Rundquist I, Svensson I, Collins VP. Formaldehyde sensitivity of a GFAP epitope, removed by extraction of the cytoskeleton with high salt. J Histochem Cytochem. 1987;35:1375–80. doi: 10.1177/35.12.2445810. [DOI] [PubMed] [Google Scholar]
- [16].Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ. Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci. 2001;21:6077–85. doi: 10.1523/JNEUROSCI.21-16-06077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–7. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berta T, Liu T, Liu YC, Xu ZZ, Ji RR. Acute morphine activates satellite glial cells and up-regulates IL-1beta in dorsal root ganglia in mice via matrix metalloprotease-9. Mol Pain. 2012;8:18. doi: 10.1186/1744-8069-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, Inoue K. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30:1864–73. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blackbeard J, Wallace VC, O’Dea KP, Hasnie F, Segerdahl A, Pheby T, Field MJ, Takata M, Rice AS. The correlation between pain-related behaviour and spinal microgliosis in four distinct models of peripheral neuropathy. Eur J Pain. 2012;16(10):1357–67. doi: 10.1002/j.1532-2149.2012.00140.x. [DOI] [PubMed] [Google Scholar]
- [22].Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–92. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234:271–82. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- [24].Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–42. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- [25].Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–68. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30:5437–50. doi: 10.1523/JNEUROSCI.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic Cx43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–70. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen FL, Dong YL, Zhang ZJ, Cao DL, Xu J, Hui J, Zhu L, Gao YJ. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res Bull. 2012;87:60–6. doi: 10.1016/j.brainresbull.2011.09.022. [DOI] [PubMed] [Google Scholar]
- [29].Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci U S A. 2008;105:16773–8. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chiang CY, Sessle BJ, Dostrovsky JO. Role of astrocytes in pain. Neurochem Res. 2012;37(11):2419–31. doi: 10.1007/s11064-012-0801-6. [DOI] [PubMed] [Google Scholar]
- [31].Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–76. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. PAIN®. 2010;149:243–53. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. PAIN®. 2011;152:2881–91. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chu YX, Zhang YQ, Zhao ZQ. Involvement of microglia and interleukin-18 in the induction of long-term potentiation of spinal nociceptive responses induced by tetanic sciatic stimulation. Neurosci Bull. 2012;28:49–60. doi: 10.1007/s12264-012-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Clark AK, Staniland AA, Malcangio M. Fractalkine/CX3CR1 signalling in chronic pain and inflammation. Curr Pharm Biotechnol. 2011;12:1707–14. doi: 10.2174/138920111798357465. [DOI] [PubMed] [Google Scholar]
- [36].Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–82. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:10655–60. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- [39].Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Jr, Serhan CN, Smith LE. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–8. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- [41].Correa F, Hernangomez-Herrero M, Mestre L, Loria F, Docagne F, Guaza C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav Immun. 2011;25:736–49. doi: 10.1016/j.bbi.2011.01.020. [DOI] [PubMed] [Google Scholar]
- [42].Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–40. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- [44].Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- [45].Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152–60. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [46].Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213:257–67. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–43. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- [48].Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–23. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- [49].Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–54. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- [50].Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- [51].DeLeo JA, Colburn RW, Rickman AJ. Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res. 1997;759:50–7. doi: 10.1016/s0006-8993(97)00209-6. [DOI] [PubMed] [Google Scholar]
- [52].DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. PAIN®. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- [53].Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem. 2008;107:50–60. doi: 10.1111/j.1471-4159.2008.05566.x. [DOI] [PubMed] [Google Scholar]
- [54].Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–8. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- [55].Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. PAIN®. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- [56].Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP—thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–51. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- [57].Eriksson NP, Persson JK, Svensson M, Arvidsson J, Molander C, Aldskogius H. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res. 1993;96:19–27. doi: 10.1007/BF00230435. [DOI] [PubMed] [Google Scholar]
- [58].Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–92. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ferrini F, Trang T, Mattioli TA, Laffray S, Del’guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De KY. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(–) homeostasis. Nat Neurosci. 2013;16(2):183–92. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- [62].Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929:105–16. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- [63].Frampton M, Harvey RJ, Kirchner V. Propentofylline for dementia. Cochrane Database Syst Rev. 2003:CD002853. doi: 10.1002/14651858.CD002853. [DOI] [PubMed] [Google Scholar]
- [64].Franke H, Grummich B, Hartig W, Grosche J, Regenthal R, Edwards RH, Illes P, Krugel U. Changes in purinergic signaling after cerebral injury— involvement of glutamatergic mechanisms? Int J Dev Neurosci. 2006;24:123–32. doi: 10.1016/j.ijdevneu.2005.11.016. [DOI] [PubMed] [Google Scholar]
- [65].Freeman SE, Patil VV, Durham PL. Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience. 2008;157:542–55. doi: 10.1016/j.neuroscience.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gao YJ, Cheng JK, Zeng Q, Xu ZZ, Decosterd I, Xu X, Ji RR. Selective inhibition of JNK with a peptide inhibitor attenuates pain hypersensitivity and tumor growth in a mouse skin cancer pain model. Exp Neurol. 2009;219:146–55. doi: 10.1016/j.expneurol.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–93. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. PAIN®. 2010;148:309–19. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58:1871–80. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18:2467–76. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- [74].Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A. 2010;107:22659–64. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp Neurol. 1994;129:237–43. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- [76].Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- [77].Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–25. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- [78].Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, Li YY, Zhang T, Liu XG. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2X4 receptors and p38 MAPK in microglia. Glia. 2009;57:583–91. doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- [80].Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–31. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–34. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- [82].Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci. 2007;27:8699–708. doi: 10.1523/JNEUROSCI.2018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Guo W, Wang H, Zou S, Dubner R, Ren K. Chemokine signaling involving chemokine (C-C motif) ligand 2 plays a role in descending pain facilitation. Neurosci Bull. 2012;28:193–207. doi: 10.1007/s12264-012-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–72. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hains LE, Loram LC, Weiseler JL, Frank MG, Bloss EB, Sholar P, Taylor FR, Harrison JA, Martin TJ, Eisenach JC, Maier SF, Watkins LR. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–14. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–53. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [90].Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: Implications for chronic pain. Brain Res. 2012;1487:183–91. doi: 10.1016/j.brainres.2012.03.070. [DOI] [PubMed] [Google Scholar]
- [91].Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–83. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- [92].Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- [93].Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- [94].Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Haraguchi K, Kawamoto A, Isami K, Maeda S, Kusano A, Asakura K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J Neurosci. 2012;32:3931–41. doi: 10.1523/JNEUROSCI.4703-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Muller U. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–7. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- [97].Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. PAIN®. 2009;144:110–8. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- [99].Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–40. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- [101].Huang L, Wang CF, Serhan CN, Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. PAIN®. 2011;152:557–65. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–13. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–89. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–93. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- [107].Jasmin L, Vit JP, Bhargava A, Ohara PT. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol. 2010;6:63–71. doi: 10.1017/S1740925X10000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ji RR. Targeting microglial purinergic signaling to improve morphine analgesia. PAIN®. 2010;150:377–8. doi: 10.1016/j.pain.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–69. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- [112].Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Science Signaling (Science Sig), part of Science. 2004:reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- [113].Ji RR, Suter MR. P38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ji RR, Xu ZZ, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30:336–40. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ji RR, Zhang Q, Zhang X, Piehl F, Reilly T, Pettersson RF, Hokfelt T. Prominent expression of bFGF in dorsal root ganglia after axotomy. Eur J Neurosci. 1995;7:2458–68. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- [117].Jin H, Li YH, Xu JS, Guo GQ, Chen DL, Bo Y. Lipoxin A4 analog attenuates morphine antinociceptive tolerance, withdrawal-induced hyperalgesia, and glial reaction and cytokine expression in the spinal cord of rat. Neuroscience. 2012;208:1–10. doi: 10.1016/j.neuroscience.2012.02.009. [DOI] [PubMed] [Google Scholar]
- [118].Jin SX, Zhuang ZY, Woolf CJ, Ji RR. P38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–55. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–62. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kalliomaki J, Attal N, Jonzon B, Bach FW, Huizar K, Ratcliffe S, Eriksson B, Janecki M, Danilov A, Bouhassira D. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. PAIN®. 2013;154:761–7. doi: 10.1016/j.pain.2013.02.003. [DOI] [PubMed] [Google Scholar]
- [123].Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Mol Pain. 2012;8:23. doi: 10.1186/1744-8069-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Katsura H, Obata K, Miyoshi K, Kondo T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia. 2008;56:723–33. doi: 10.1002/glia.20648. [DOI] [PubMed] [Google Scholar]
- [126].Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–90. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Keller AF, Beggs S, Salter MW, De KY. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- [131].Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010;107:14851–6. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kim DS, Li KW, Boroujerdi A, Peter YY, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Luo ZD. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J Neurosci. 2012;32:8977–87. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–53. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci. 2004;24:964–71. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett. 2011;504:57–61. doi: 10.1016/j.neulet.2011.08.058. [DOI] [PubMed] [Google Scholar]
- [136].Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60(10):1529–39. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- [138].Kozai T, Yamanaka H, Dai Y, Obata K, Kobayashi K, Mashimo T, Noguchi K. Tissue type plasminogen activator induced in rat dorsal horn astrocytes contributes to mechanical hypersensitivity following dorsal root injury. Glia. 2007;55:595–603. doi: 10.1002/glia.20483. [DOI] [PubMed] [Google Scholar]
- [139].Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–66. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- [140].Lan L, Yuan H, Duan L, Cao R, Gao B, Shen J, Xiong Y, Chen LW, Rao ZR. Blocking the glial function suppresses subcutaneous formalin-induced nociceptive behavior in the rat. Neurosci Res. 2007;57:112–9. doi: 10.1016/j.neures.2006.09.014. [DOI] [PubMed] [Google Scholar]
- [141].Landry RP, Jacobs VL, Romero-Sandoval EA, DeLeo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340–50. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- [142].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–77. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Li JY, Xie W, Strong JA, Guo QL, Zhang JM. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Reg Anesth Pain Med. 2011;36:56–62. doi: 10.1097/AAP.0b013e318203087f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Li WE, Nagy JI. Activation of fibres in rat sciatic nerve alters phosphorylation state of connexin-43 at astrocytic gap junctions in spinal cord: evidence for junction regulation by neuronal-glial interactions. Neuroscience. 2000;97:113–23. doi: 10.1016/s0306-4522(00)00032-4. [DOI] [PubMed] [Google Scholar]
- [146].Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. PAIN®. 2009;147:277–86. doi: 10.1016/j.pain.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. PAIN®. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [148].Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–95. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Liu CN, Wall PD, Ben Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. PAIN®. 2000;85:503–21. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- [150].Liu FY, Sun YN, Wang FT, Li Q, Su L, Zhao ZF, Meng XL, Zhao H, Wu X, Sun Q, Xing GG, Wan Y. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012;1427:65–77. doi: 10.1016/j.brainres.2011.10.016. [DOI] [PubMed] [Google Scholar]
- [151].Liu L, Rudin M, Kozlova EN. Glial cell proliferation in the spinal cord after dorsal rhizotomy or sciatic nerve transection in the adult rat. Exp Brain Res. 2000;131:64–73. doi: 10.1007/s002219900273. [DOI] [PubMed] [Google Scholar]
- [152].Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–44. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Liu YC, Berta T, Liu T, Tan PH, Ji RR. Acute morphine induces matrix metalloproteinase-9 up-regulation in primary sensory neurons to mask opioid-induced analgesia in mice. Mol Pain. 2012;8:19. doi: 10.1186/1744-8069-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, Li YY, Liu XG. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–15. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- [155].Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- [156].Madiai F, Goettl VM, Hussain SR, Clairmont AR, Stephens RL, Jr, Hackshaw KV. Anti-fibroblast growth factor-2 antibodies attenuate mechanical allodynia in a rat model of neuropathic pain. J Mol Neurosci. 2005;27:315–24. doi: 10.1385/JMN:27:3:315. [DOI] [PubMed] [Google Scholar]
- [157].Maeda S, Kawamoto A, Yatani Y, Shirakawa H, Nakagawa T, Kaneko S. Gene transfer of GLT-1, a glial glutamate transporter, into the spinal cord by recombinant adenovirus attenuates inflammatory and neuropathic pain in rats. Mol Pain. 2008;4:65. doi: 10.1186/1744-8069-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Matsuka Y, Ono T, Iwase H, Mitrirattanakul S, Omoto KS, Cho T, Lam YY, Snyder B, Spigelman I. Altered ATP release and metabolism in dorsal root ganglia of neuropathic rats. Mol Pain. 2008;4:66. doi: 10.1186/1744-8069-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- [161].Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–8. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- [162].Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, DeMartino JA, MacIntyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–14. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [163].Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- [166].Miraucourt LS, Peirs C, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through astrocyte-derived D-serine. PAIN®. 2011;152:1340–8. doi: 10.1016/j.pain.2011.02.021. [DOI] [PubMed] [Google Scholar]
- [167].Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–87. doi: 10.1523/JNEUROSCI.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- [169].Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–31. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, Fitzgerald M. Spinal microglia and neuropathic pain in young rats. PAIN®. 2007;128:215–24. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- [171].Ndong C, Landry RP, DeLeo JA, Romero-Sandoval EA. Mitogen activated protein kinase phosphatase-1 prevents the development of tactile sensitivity in a rodent model of neuropathic pain. Mol Pain. 2012;8:34. doi: 10.1186/1744-8069-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Nedergaard M, Verkhratsky A. Artifact versus reality—how astrocytes contribute to synaptic events. Glia. 2012;60:1013–23. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- [174].Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–29. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- [176].Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–7. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- [177].Obata K, Katsura H, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Noguchi K. Roles of extracellular signal-regulated protein kinases 5 in spinal microglia and primary sensory neurons for neuropathic pain. J Neurochem. 2007;102:1569–84. doi: 10.1111/j.1471-4159.2007.04656.x. [DOI] [PubMed] [Google Scholar]
- [178].Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–53. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- [179].Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–76. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–53. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [183].Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–73. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29:11161–71. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Pannese E, Ledda M, Cherkas PS, Huang TY, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol (Berl) 2003;206:337–47. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- [186].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- [188].Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF- mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–85. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin d2 is a potent endogenous inhibitor for transient receptor potential subtype v1/a1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin d1, d2, and e1. J Neurosci. 2011;31:18433–8. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–64. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- [191].Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology. 2010;112:1250–8. doi: 10.1097/ALN.0b013e3181d3d978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–73. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. PAIN®. 2006;121:219–31. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- [195].Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- [196].Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, Pintado B, Gutierrez-Adan A, Sanguino E, Bellora N, Manzanares J, Zimmer A, Maldonado R. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J Neurosci. 2008;28:12136–45. doi: 10.1523/JNEUROSCI.3402-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- [198].Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- [199].Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–3. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [200].Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–76. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ren WH, Guo JD, Cao H, Wang H, Wang PF, Sha H, Ji RR, Zhao ZQ, Zhang YQ. Is endogenous D-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J Neurochem. 2006;96:1636–47. doi: 10.1111/j.1471-4159.2006.03677.x. [DOI] [PubMed] [Google Scholar]
- [202].Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- [204].Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- [205].Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- [207].Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–38. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci U S A. 2010;107:22296–301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- [210].Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Shi YQ. Chronic-pain-associated astrocytic reaction in the spinal cord of HIV-infected patients. J Neurosci. 2012;32(32):10833–40. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sloane EM, Soderquist RG, Maier SF, Mahoney MJ, Watkins LR, Milligan ED. Long-term control of neuropathic pain in a non-viral gene therapy paradigm. Gene Ther. 2009;16:470–5. doi: 10.1038/gt.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- [214].Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–6. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- [215].Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander MH, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–9. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–62. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- [217].Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- [218].Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- [219].Staniland AA, Clark AK, Wodarski R, Sasso O, Maione F, D’Acquisto F, Malcangio M. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem. 2010;114:1143–57. doi: 10.1111/j.1471-4159.2010.06837.x. [DOI] [PubMed] [Google Scholar]
- [220].Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–28. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- [222].Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. PAIN®. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- [223].Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94:742–52. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- [226].Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–68. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92:1508–20. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- [229].Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–44. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- [230].Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–13. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- [231].Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204:245–52. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–39. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- [233].Takeda M, Kitagawa J, Takahashi M, Matsumoto S. Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. PAIN®. 2008;139:594–602. doi: 10.1016/j.pain.2008.06.015. [DOI] [PubMed] [Google Scholar]
- [234].Takeda M, Takahashi M, Matsumoto S. Contribution of activated interleukin receptors in trigeminal ganglion neurons to hyperalgesia via satellite glial interleukin-1beta paracrine mechanism. Brain Behav Immun. 2008;22:1016–23. doi: 10.1016/j.bbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- [235].Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–92. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- [236].Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. PAIN®. 2007;129:155–66. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [237].Tan PH, Gao YJ, Berta T, Xu ZZ, Ji RR. Short small-interfering RNAs produce interferon-alpha-mediated analgesia. Br J Anaesth. 2012;108:662–9. doi: 10.1093/bja/aer492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–72. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [240].Theriault E, Frankenstein UN, Hertzberg EL, Nagy JI. Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J Comp Neurol. 1997;382:199–214. [PubMed] [Google Scholar]
- [241].Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–36. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Toyomitsu E, Tsuda M, Yamashita T, Tozaki-Saitoh H, Tanaka Y, Inoue K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012;8:301–10. doi: 10.1007/s11302-011-9288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–56. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234:354–61. doi: 10.1016/j.expneurol.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–28. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–9. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [248].Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(4):1127–39. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:8032–7. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- [252].Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- [253].Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–32. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [254].Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–8. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Van SJ, Reaux-Le GA, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Melik PS. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31:5865–75. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Vega-Avelaira D, McKelvey R, Hathway G, Fitzgerald M. The emergence of adolescent onset pain hypersensitivity following neonatal nerve injury. Mol Pain. 2012;8:30. doi: 10.1186/1744-8069-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–60. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- [258].Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012:4. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vikman KS, Duggan AW, Siddall PJ. Interferon-gamma induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. PAIN®. 2007;133:18–28. doi: 10.1016/j.pain.2007.02.010. [DOI] [PubMed] [Google Scholar]
- [260].Vikman KS, Hill RH, Backstrom E, Robertson B, Kristensson K. Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. PAIN®. 2003;106:241–51. doi: 10.1016/S0304-3959(03)00262-8. [DOI] [PubMed] [Google Scholar]
- [261].Vit JP, Ohara PT, Bhargava A, Kelley K, Jasmin L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci. 2008;28:4161–71. doi: 10.1523/JNEUROSCI.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–47. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca(2)(+)-dependent uptake of extracellular K(+) Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Wang H, Guo W, Yang K, Wei F, Dubner R, Ren K. Contribution of primary afferent input to trigeminal astroglial hyperactivity, cytokine induction and NMDA receptor phosphorylation. Open Pain J. 2010:144–52. doi: 10.2174/1876386301003010144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- [266].Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–23. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- [267].Wang XW, Hu S, Mao-Ying QL, Li Q, Yang CJ, Zhang H, Mi WL, Wu GC, Wang YQ. Activation of c-jun N-terminal kinase in spinal cord contributes to breast cancer induced bone pain in rats. Mol Brain. 2012;5:21. doi: 10.1186/1756-6606-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Wang XW, Li TT, Zhao J, Mao-Ying QL, Zhang H, Hu S, Li Q, Mi WL, Wu GC, Zhang YQ, Wang YQ. Extracellular signal-regulated kinase activation in spinal astrocytes and microglia contributes to cancer-induced bone pain in rats. Neuroscience. 2012;217:172–81. doi: 10.1016/j.neuroscience.2012.04.065. [DOI] [PubMed] [Google Scholar]
- [269].Wang Z, Ma W, Chabot JG, Quirion R. Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFkappaB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. PAIN®. 2010;151:194–205. doi: 10.1016/j.pain.2010.07.006. [DOI] [PubMed] [Google Scholar]
- [270].Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–9. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [271].Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–91. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. PAIN®. 1997;71:225–35. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- [273].Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- [274].Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–95. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Wen YR, Suter MR, Ji RR, Yeh GC, Wu YS, Wang KC, Kohno T, Sun WZ, Wang CC. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110:155–65. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- [276].Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji RR. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology. 2007;107:312–21. doi: 10.1097/01.anes.0000270759.11086.e7. [DOI] [PubMed] [Google Scholar]
- [277].Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR. Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc. 2011;110:487–94. doi: 10.1016/S0929-6646(11)60074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–60. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- [279].Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. PAIN®. 2010;148:237–46. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–8. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–7. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED. Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels. PAIN®. 2012;153:1091–106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, Kelley KW, Heijnen CJ, Kavelaars A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. PAIN®. 2010;150:550–60. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Willemen HL, Huo XJ, Mao-Ying QL, Zijlstra J, Heijnen CJ, Kavelaars A. MicroRNA-124 as a novel treatment for persistent hyperalgesia. J Neuroinflamm. 2012;9:143. doi: 10.1186/1742-2094-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN®. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–57. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Xin WJ, Weng HR, Dougherty PM. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol Pain. 2009;5:15. doi: 10.1186/1744-8069-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar 5 ventral root transection in rat. PAIN®. 2006;123:306–21. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- [290].Xu ZZ, Berta T, Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmun Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Xu ZZ, Liu XJ, Berta T, Park C, Lu N, Serhan CN, Ji RR. Neuroprotectin/protectin-1 protects neuropathic pain in mice following nerve trauma. Ann Neurol. 2013 doi: 10.1002/ana.23928. http://dx.doi.org/10.1002/ana.23928.[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:1. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- [294].Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–33. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- [295].Zhang H, Mei X, Zhang P, Ma C, White FA, Donnelly DF, LaMotte RH. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia. 2009;57:1588–99. doi: 10.1002/glia.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–55. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J Pain. 2012;13:293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–83. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- [299].Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–4. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- [300].Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. PAIN®. 2011;152:419–27. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. PAIN®. 2008;135:232–9. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. PAIN®. 2005;118:125–36. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [304].Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864–9. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Zhang ZJ, Dong YL, Lu Y, Cao S, Zhao ZQ, Gao YJ. Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain. J Neuroinflamm. 2012;9:136. doi: 10.1186/1742-2094-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 2011;176:447–54. doi: 10.1016/j.neuroscience.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, Liu XG. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain Behav Immun. 2010;24:874–80. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- [310].Zhou D, Chen ML, Zhang YQ, Zhao ZQ. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci. 2010;30:8042–7. doi: 10.1523/JNEUROSCI.5377-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–90. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. PAIN®. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- [313].Zhu X, Eisenach JC. Cyclooxygenase-1 in the spinal cord is altered after peripheral nerve injury. Anesthesiology. 2003;99:1175–9. doi: 10.1097/00000542-200311000-00026. [DOI] [PubMed] [Google Scholar]
- [314].Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. PAIN®. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- [315].Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–51. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–60. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]