Abstract
Background
Despite widespread use of multivitamin supplements, their effect on cognitive health – a critical issue with aging – remains inconclusive. To date, there have been no long-term clinical trials to study multivitamin use and cognitive decline in older persons.
Objective
To evaluate whether long-term multivitamin supplementation affects cognitive health in later-life.
Design
Randomized, double-blind, placebo-controlled trial of a multivitamin from 1997 to June 1, 2011. The cognitive function sub-study began in 1998; we completed up to four repeated cognitive assessments by telephone interview over 12 years.
Setting
The Physicians’ Health Study II.
Patients
5,947 male physicians aged ≥ 65 years.
Intervention
Daily multivitamin, or placebo.
Measurements
A global composite score averaging 5 tests of global cognition, verbal memory, and category fluency. The secondary endpoint was a verbal memory score combining 4 tests of verbal memory, a strong predictor of Alzheimer disease.
Results
There was no difference in the mean cognitive change over time between the multivitamin and placebo groups, or in the mean level of cognition at any of the four assessments. Specifically, for the global composite score, the mean difference in cognitive change over follow-up was −0.01 (95% confidence interval [CI] −0.04, 0.02) standard units, comparing treatment versus placebo. Similarly, there was no difference in cognitive performance between the treated and placebo groups on the secondary outcome, verbal memory (e.g., mean difference in cognitive change over follow-up=−0.005, 95% CI −0.04, 0.03).
Limitations
Doses of vitamins may be too low, or population may be too well-nourished to benefit from multivitamin.
Conclusions
In male physicians aged ≥ 65 years, long-term use of a daily multivitamin did not provide cognitive benefits.
Trial Registration
http://www.clinicaltrials.gov identifier: NCT00270647
Keywords: multivitamin, cognitive function, randomized clinical trial, men
Introduction
As our population ages, it is important to identify preventive strategies for cognitive decline, a step on the pathway to dementia (1). Multivitamins are the most commonly used dietary supplement in the United States, taken by more than one-third of Americans (2). In addition to preventing vitamin and mineral deficiency, multivitamin supplements were found to reduce the risk of cancer in the Physician’s Health Study II (PHS II) trial (3), and are frequently marketed to prevent a variety of chronic conditions (4).
A typical daily multivitamin contains a combination of nutrients that could help prevent cognitive decline (5, 6). For instance, vitamins C, E and beta-carotene may protect the brain from oxidative damage (7). B-vitamins are involved in the synthesis of neurotransmitters, DNA, and neuronal membrane, and prevent the accumulation of homocysteine, a risk factor for cognitive decline (8). Vitamin A plays a role in neuronal survival and synaptic plasticity in the hippocampus (9). Each of these vitamins – alone or in combination – could delay onset of cognitive decline, including at the lower doses common in multivitamin supplements (10, 11).
Yet randomized trials have been inconsistent regarding potential benefits of multivitamin supplementation on cognitive health (12). Some have found no effect of multivitamins on cognition (13, 14), while others found modest benefits (15–17). However, these trials have important limitations, including fairly short treatment duration and modest sample size.
The PHSII is a large-scale, randomized, double-blind, placebo-controlled trial testing long-term effects of a common multivitamin in the prevention of chronic disease. In this manuscript, we present the results of the cognitive substudy of the PHSII.
METHODS
Design Overview
PHS II is a randomized, double-blind, placebo-controlled, 2×2×2×2 factorial trial testing beta-carotene, vitamin E, ascorbic acid, and a multivitamin for their role in the prevention of chronic diseases among 14,641 male physicians aged ≥50 years. Cognitive function was a pre-specified secondary outcome of PHS II.
Setting and Participants
Recruitment occurred in two phases. First, in July 1997 invitations to enroll in PHS II were mailed to eligible PHS I participants who had been part of an earlier trial of aspirin and beta-carotene in 22,071 physicians aged 40 to 84 years in 1982 (18, 19). In the second phase beginning in July 1999, invitation letters were mailed to a new group of male physicians identified from a list provided by the American Medical Association. Men were ineligible if they had a history of cirrhosis, active liver disease, were taking anticoagulants, or reported a serious illness that may interfere with study participation. Men were also required to forego the current use of multivitamins or individual supplements containing more than 100% of the RDA of vitamin E, vitamin C, beta-carotene, or vitamin A during PHS II follow-up. In total, 14,641 were randomized into PHS II, including 7,641 men from PHS I who agreed to enroll in PHS II along with 7,000 new physician participants. Original PHS I participants kept their original beta-carotene assignments, and beta-carotene assignment was not related to participation in PHS II (20). All participants provided written informed consent, and the Institutional Review Board at Brigham and Women’s Hospital approved the PHS II research protocol.
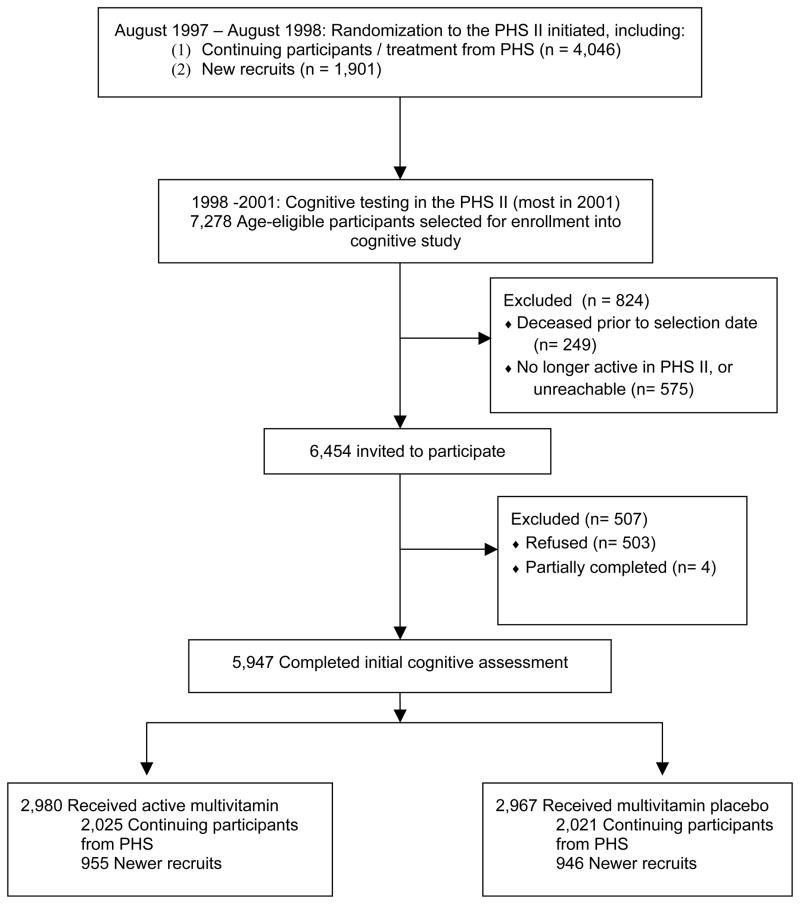
In 1998, we initiated a substudy of cognitive function among men aged ≥65 years. Of the 7,278 men eligible for the substudy of cognitive function, 249 were deceased prior to the selection date and 575 were no longer active PHS II subjects or were unreachable. Of the 6,454 PHS II participants contacted (503 refused, 4 partially completed), 5,947 (92%) completed an initial cognitive assessment, which included 4,046 original PHS I participants and 1,901 new PHS II participants (Figure 1). The participation rates for the initial cognitive interview were similar comparing multivitamin versus placebo groups, and by PHS II group (range: 92–93% of those contacted).
Figure 1.
Flow diagram of participants in the cognitive substudy of the Physicians’ Health Study II (PHS II).
After the initial cognitive assessment, there were up to three additional waves of follow-up: a second wave beginning in 2002, a third wave beginning in 2006, and a fourth beginning in 2010. There was a mean duration of about two years between the first and second assessments, four years between the second and third assessments, and about four years between the third and fourth assessments. High follow-up was maintained; 96%, 92% and 90% of those contacted at waves two, three, and four, respectively, completed cognitive testing. However, the fourth assessment was not attempted in many participants due to the completion of the trial (i.e., 2,700 [45%]out of the initial 5,947 who completed the initial interview were invited to participate at the fourth assessment prior to the trial close on June 1, 2011).
Randomization and Interventions
The design of PHS II, including randomization procedures, has been previously described (21). The interventions included beta-carotene (50 mg Lurotin or its placebo, alternate days; BASF Corporation, Florham Park, New Jersey), vitamin E (400 IU synthetic α-tocopherol or its placebo, alternate days; BASF Corporation)), ascorbic acid (500mg synthetic ascorbic acid or its placebo, daily; BASF Corporation), or a multivitamin (Centrum Silver or its placebo, daily; Pfizer [formerly Wyeth, American Home Products, and Lederle], see Appendix 1 for details).
Outcomes and Follow-up
Cognitive assessments were administered using a validated telephone interview (22–24). The cognitive battery included: 1) the Telephone Interview for Cognitive Status (TICS) (25), a telephone adaptation of the Mini-Mental State Examination (26); 2) immediate and 3) delayed recalls of the East Boston Memory Test (EBMT) (27), to assess verbal memory; 4) the delayed recall of a 10-word list in the TICS to test verbal memory; and 5) a category fluency task (28). The primary pre-specified outcome of the cognitive sub-study was a composite score of global cognition (i.e., an average of all cognitive tests). We created the composite global score by standardizing results of each cognitive test using z-scores and averaged the z-scores (see Appendix 2 for methods). Because verbal memory is strongly associated with risk of Alzheimer’s disease (1, 29–31), we assessed a secondary outcome of a verbal memory composite score, calculated by averaging the z-scores from the immediate and delayed recalls of the East Boston Memory Test and TICS 10-word list.
In a previous validation study of the telephone cognitive testing, the correlation between the global composite score from the telephone interview versus an extensive in-person assessment was 0.81 (23). There was also high reliability of TICS performance between 50 women who were given the test twice, 31 days apart (test-retest correlation = 0.7) (23).
Every six months for the first year, then annually thereafter, participants received monthly calendar packs containing a multivitamin or placebo (taken daily). Participants completed annual questionnaires on compliance, risk factors, and study outcomes. The beta- carotene arm of the PHS II continued as planned through May 2003, for which results on cognitive function have been reported (20). Treatment and follow-up of the vitamin E and C components continued through August 2007, with benefits reported for cancer (32) and findings of no effect for cardiovascular disease (33). The multivitamin intervention continued through June 1, 2011, the scheduled end of the multivitamin component of the PHS II, with findings reported to date for cancer (3) and cardiovascular disease (34).
Statistical Analysis
Characteristics at randomization by treatment group were compared using Wilcoxon’s rank-sum tests for continuous variables and chi-square tests for proportions.
We preliminarily examined mean performance at each cognitive assessment in the treatment versus placebo group, using repeated measures analysis of means, which allows examination of each time point, accounting for correlation between assessments. For our primary, pre-specified analysis, we examined mean change in cognitive function over up to four cognitive assessments. We treated mean scores and mean change in scores at each assessment as repeated continuous outcomes, and modeled the treatment effect with a time by treatment interaction. Because trajectories of test scores were non-linear, we used general linear models of response profiles, modeling time with indicator variables rather than linearly (35). This approach imposes minimal structure on outcome trends over time, and permits valid estimation of effects in non-linear data. The non-linearity of cognitive data due to “learning effects” is common in studies of cognitive function (i.e., there was a mean increase in scores from the first to second assessment) (36). We fitted all models by maximum likelihood, incorporating longitudinal correlations within participants, using unstructured covariance structures; for statistical testing, we used Wald tests. For all statistical analyses, we used PROC MIXED in SAS (SAS release 9.2; SAS Institute Inc, Cary, NC).
In secondary analyses, we tested for effect modification by possible risk factors of cognitive decline by including interaction terms in our models for cognitive change (age, smoking, alcohol consumption, body mass index, history of diabetes, hypertension, high cholesterol, folate intake (with and without supplements), intake of fruits and vegetables, and history of depression. We also evaluated the differences in cognitive change comparing those assigned to active multivitamins compared with those assigned to placebo for all other arms of the trial (i.e., placebo for beta-carotene, vitamin C, vitamin E and the multivitamin), although the sample size for the placebo-only group was small (n = 372).
RESULTS
Characteristics at randomization were equally distributed between the multivitamin and placebo groups (all P>0.05) (Table 1). The average time from randomization to initial cognitive assessment was 2.5 years (range: 0.18–5.3 years), although this time was shorter for the newly recruited PHS II participants (mean time from randomization to initial assessment was 1.1 years for new participants, and 3.2 years for original PHS I participants). The average total duration of follow-up from randomization to the final cognitive evaluation was 8.5 years (range: 0.3–14.2 years). 83.5% of the multivitamin group and 84.2% in the placebo group reported taking at least two-thirds of their study pills.
Table 1.
Self-reported Characteristics at Randomization According to Multivitamin Treatment Assignment of 5,947 Participants Aged ≥ 65 Years Participating in the Cognitive Substudy of the Physicians’ Health Study II*
| Characteristics | Multivitamin | |
|---|---|---|
| Active | Placebo | |
| Participants, No. | 2,980 | 2,967 |
| Age, mean, (SD), y | 71.6 (6.0) | 71.6 (5.9) |
| Age, y | ||
| 65–74 | 2,129 (71.4) | 2,146 (72.3) |
| 75–85 | 790 (26.5) | 757 (25.5) |
| ≥85 | 61 (2.1) | 64 (2.2) |
| BMI, mean (SD) | 25.8 (3.2) | 25.7 (3.3) |
| Alcohol intake | ||
| Rarely or never | 559 (18.9) | 521 (17.6) |
| ≥1 drink/mo | 2,404 (81.1) | 2,436 (82.4) |
| Cigarette smoking | ||
| Never | 1,453 (48.8) | 1427 (48.1) |
| Former | 1,417 (47.6) | 1431 (48.3) |
| Current | 107 (3.6) | 106 (3.6) |
| Vigorous Exercise ≥ 1/wk | ||
| No | 1,214 (41.2) | 1,196 (40.9) |
| Yes | 1,733 (58.8) | 1,728 (59.1) |
| Hypertension | ||
| No | 1,387 (46.7) | 1,359 (45.9) |
| Yes | 1,585 (53.3) | 1,602 (54.1) |
| High cholesterol | ||
| No | 1,744 (59.2) | 1,685 (57.6) |
| Yes | 1,203 (40.8) | 1,239 (42.4) |
| Type 2 diabetes | ||
| No | 2,724 (91.5) | 2,737 (92.4) |
| Yes | 253 (8.5) | 225 (7.6) |
| History of MI | ||
| No | 2,818 (94.6) | 2,799 (94.4) |
| Yes | 162 (5.4) | 167 (5.7) |
| History of stroke | ||
| No | 2,897 (97.2) | 2,899 (97.7) |
| Yes | 83 (2.8) | 68 (2.3) |
| History of angina | ||
| No | 2,702 (90.7) | 2,674 (90.1) |
| Yes | 278 (9.1) | 293 (9.9) |
| History of depression | ||
| No | 2,704 (91.1) | 2,687 (91.0) |
| Yes | 263 (8.9) | 265 (9.0) |
| Fruit and Vegetable Intake, servings/d†, mean (SD) | 4.9 (2.6) | 4.9 (2.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MI, myocardial infarction; SD, standard deviation.
Data are No. (%) unless otherwise indicated. All variables defined as of PHSII randomization. The numbers do not always sum to group totals due to missing information for some variables. P > 0.05 for all comparisons between multivitamin and placebo groups.
Among 5,575 with available dietary data on fruit and vegetable intake.
At the first cognitive assessment, performance was not different between the multivitamin and placebo groups (Table 2) (For raw scores at each cognitive assessment, see Appendix 3). For instance, the global composite score for the multivitamin group was 0.01 (SD=0.7) standard units, and the global composite score for the placebo group was −0.005 (SD=0.7) standard units. When performance was examined at each follow-up assessment, there were no differences between mean global composite score of cognitive function for men taking a daily multivitamin versus placebo at any point (Table 3). For example, the mean difference in global composite score between multivitamin and placebo groups at the fourth assessment (after an average of 8.5 years of follow-up) was 0.02 standard units (95% CI: −0.04, 0.08). Likewise, for our secondary outcome of verbal memory, no differences were observed between groups at any of the assessments. For example, at the fourth assessment, the mean difference for the multivitamin compared to placebo group was 0.01 standard units (95% CI: −0.05, 0.07). Similarly, in secondary analyses, the multivitamin group did not show any differences in mean performance versus the placebo group on the TICS or category fluency.
Table 2.
Mean (SD) Cognitive Test Scores at Initial Assessment*
| Cognitive Tests | Multivitamin group (n=2,980) | Placebo Group (n=2,967) |
|---|---|---|
| Global Composite (z-score) | 0.01 ± 0.7 | −0.005 ± 0.7 |
| Verbal Composite (z-score) | 0.00 ± 0.7 | −0.005 ± 0.7 |
| Telephone Interview of Cognitive Status | 34.3 ± 2.7 | 34.3 ±2.7 |
| East Boston Memory Test | ||
| Immediate Recall | 9.7 ± 1.9 | 9.7 ± 1.9 |
| Delayed Recall | 9.4 ± 2.1 | 9.3 ± 2.2 |
| Delayed Recall of 10 word list | 2.6 ±2.0 | 2.6 ± 2.0 |
| Category Fluency | 20.1 ± 6.0 | 20.0 ± 6.1 |
Abbreviations: SD, standard deviation.
Data are given as mean ± SD. Initial cognitive testing was conducted a mean of 2.5 years (range: 0.18 – 5.3 years) after randomization.
Table 3.
Cognitive Function at Each Cognitive Assessment, by Multivitamin Status
| Cognitive Test | Treatment Assignment
|
Difference in Score, Mean (95% CI)‡, Multivitamin – Placebo | |||
|---|---|---|---|---|---|
| Multivitamin Group | Placebo Group | ||||
| No. of Subjects | Mean (SE)† | No. of Subjects | Mean (SE) | ||
| Global score* | |||||
| 1 | 2978 | 0.01 (0.01) | 2964 | −0.00 (0.01) | 0.01 (−0.02, 0.05) |
| 2 | 2657 | 0.02 (0.01) | 2639 | 0.01 (0.01) | −0.01 (−0.05, 0.03) |
| 3 | 2091 | −0.13 (0.02) | 2015 | −0.15 (0.02) | 0.02 (−0.03, 0.06) |
| 4 | 1165 | −0.26 (0.02) | 1159 | −0.28 (0.02) | 0.02 (−0.04, 0.08) |
| Verbal memory scorea | |||||
| 1 | 2978 | 0.00 (0.01) | 2964 | −0.00 (0.01) | 0.01(−0.03, 0.05) |
| 2 | 2657 | 0.03 (0.01) | 2639 | 0.03 (0.02) | −0.00 (−0.05, 0.04) |
| 3 | 2091 | −0.08 (0.02) | 2015 | −0.10 (0.02) | 0.02 (−0.03, 0.07) |
| 4 | 1165 | −0.16 (0.02) | 1159 | −0.18 (0.02) | 0.01 (−0.05, 0.07) |
| TICS score | |||||
| 1 | 2980 | 34.3 (0.05) | 2967 | 34.3 (0.05) | 0.04 (−0.09, 0.18) |
| 2 | 2657 | 34.5 (0.05) | 2639 | 34.5 (0.06) | 0.10 (−0.05, 0.24) |
| 3 | 2091 | 34.0 (0.07) | 2015 | 34.0 (0.07) | 0.00 (−0.18, 0.19) |
| 4 | 1165 | 33.2 (0.09) | 1159 | 33.1 (0.09) | 0.12 (−0.14, 0.38) |
| Category fluency score | |||||
| 1 | 2978 | 20.1 (0.11) | 2964 | 20.0 (0.11) | 0.02 (−0.29, 0.33) |
| 2 | 2657 | 20.0 (0.11) | 2639 | 20.2 (0.12) | −0.21 (−0.53, 0.12) |
| 3 | 2091 | 18.8 (0.12) | 2015 | 18.7 (0.13) | 0.04 (−0.31, 0.40) |
| 4 | 1165 | 18.5 (0.15) | 1159 | 18.3 (0.15) | 0.22 (−0.21, 0.65) |
Abbreviations: CI, confidence interval; TICS, Telephone Interview of Cognitive Status.
Global score is a composite of TICS, immediate and delayed recalls of the East Boston Memory Test, category fluency, and delayed recall of the TICS 10-word list. Verbal memory score is a composite score of the immediate and delayed recalls of both the TICS 10-word list and the East Boston Memory Test.
Least squares mean and standard errors.
Differences from longitudinal models of mean cognitive performance.
For our primary, pre-specified outcome of change in cognitive function over follow-up, no differences were observed according to treatment group for any outcome (Table 4). For example, the average difference in change over the follow-up period between the multivitamin and placebo groups was −0.01 standard units (95% CI: −0.04, 0.02) for the global score, and, in secondary analyses was −0.005 (95% CI: −0.04, 0.03) for the verbal memory score, 0.02 (95% CI: −0.11, 0.15) for the TICS, and −0.07 (95% CI: −0.35, 0.20) for category fluency. To help interpret these mean differences, we contrasted the effect of the multivitamin to the effect of time: across the study population, on the global score, there was a mean decrease of −0.045 standard units per year; on the verbal score, there was a mean decrease of −0.044 standard units; on the TICS, the yearly mean decrease was −0.16 points. Therefore, the means differences we observed were smaller than the decline we would expect with one year of aging.
Table 4.
Mean Differences in Cognitive Decline Between Multivitamin and Placebo Groups, from Initial Assessment
| Cognitive Test | Difference in cognitive decline†, Mean (95% CI) Multivitamin group – Placebo group | p- value |
|---|---|---|
| Global score* | ||
| From initial cognitive assessment to | ||
| 2nd cognitive assessment | −0.02 (−0.05, 0.02) | 0.28 |
| 3rd cognitive assessment | 0.01 (−0.04, 0.05) | 0.79 |
| 4th cognitive assessment | 0.01 (−0.05, 0.06) | 0.77 |
| Average over follow-up | −0.01 (−0.04, 0.02) | 0.53 |
| Verbal memory scorea | ||
| From initial cognitive assessment to | ||
| 2nd cognitive assessment | −0.02 (−0.06, 0.02) | 0.43 |
| 3rd cognitive assessment | 0.01 (−0.03, 0.06) | 0.57 |
| 4th cognitive assessment | 0.01 (−0.05, 0.07) | 0.84 |
| Average over follow-up | −0.005 (−0.04, 0.03) | 0.80 |
| TICS score | ||
| From initial cognitive assessment to | ||
| 2nd cognitive assessment | 0.04 (−0.10, 0.18) | 0.59 |
| 3rd cognitive assessment | −0.04 (−0.21, 0.13) | 0.64 |
| 4th cognitive assessment | 0.07 (−0.18, 0.32) | 0.59 |
| Average over follow-up | 0.02 (−0.11, 0.15) | 0.79 |
| Category fluency score | ||
| From initial cognitive assessment to | ||
| 2nd cognitive assessment | −0.22 (−0.52, 0.09) | 0.165 |
| 3rd cognitive assessment | 0.05 (−0.30, 0.40) | 0.77 |
| 4th cognitive assessment | 0.22 (0.21, 0.65) | 0.31 |
| Average over follow-up | −0.07 (−0.35, 0.20) | 0.59 |
Abbreviations: CI, confidence interval; TICS, Telephone Interview of Cognitive Status
Global score is a composite of TICS, immediate and delayed recalls of the East Boston Memory Test, category fluency, and delayed recall of the TICS 10-word list. Verbal memory score is a composite score of the immediate and delayed recalls of both the TICS 10-word list and the East Boston Memory Test.
From longitudinal models of least-squares means of change in cognitive performance from the initial assessment, and averaged over follow-up. The number of subjects in each assessment is shown in Table 3.
We examined effect modification by key risk factors for cognitive decline (Table 5). There was no evidence that the differences in the magnitude of cognitive decline across the treated versus placebo groups were influenced by any of these factors.
Table 5.
Mean Difference in Cognitive Decline in Global Score between Multivitamin and Placebo Groups: Effect Modification by Risk Factors for Cognitive Decline
| Characteristics* | Difference in Cognitive Decline†, Mean (95% CI) Multivitamin group – Placebo group | P for interaction‡ |
|---|---|---|
| Age at first cognitive assessment (years) | ||
| < 74 | −0.02 (−0.06, 0.02) | 0.50 |
| ≥74 | 0.00 (−0.05, 0.05) | |
| Cognitive performance at first assessment | ||
| Below median | 0.01 (−0.04, 0.06) | 0.26 |
| Above median | −0.03 (−0.06, 0.01) | |
| Cigarette smoking | ||
| Never | 0.01 (−0.04, 0.05) | 0.25 |
| Ever | −0.03 (−0.07, 0.02) | |
| Alcohol Consumption | ||
| Rare or Never | −0.04 (−0.11, 0.03) | 0.38 |
| ≥1 drink/mo | −0.00 (−0.04, 0.03) | |
| Body mass index | ||
| <30 | −0.01 (−0.04, 0.03) | 0.57 |
| ≥30 | −0.04 (−0.15, 0.07) | |
| Diabetes | ||
| Yes | −0.04 (−0.15, 0.08) | 0.64 |
| No | −0.01 (−0.04, 0.02) | |
| Hypertension | ||
| Yes | −0.01 (−0.05, 0.03) | 0.99 |
| No | −0.01 (−0.05, 0.04) | |
| High cholesterol | ||
| Yes | −0.02 (−0.07, 0.03) | 0.56 |
| No | −0.00 (−0.04, 0.04) | |
| Folate (w/o supplements)§ | ||
| <279 mcg/d | 0.01 (−0.08, 0.10) | 0.67 |
| ≥279 mcg/d | −0.01 (−0.04, 0.03) | |
| Folate (w/supplements)§ | ||
| <279 mcg/d | 0.02 (−0.08, 0.11) | 0.67 |
| ≥279 mcg/d | −0.01 (−0.04, 0.03) | |
| Fruit and vegetable intake (servings/d) | ||
| <4 | −0.02 (−0.07, 0.02) | 0.31 |
| 4–7 | −0.02 (−0.06, 0.03) | |
| 7+ | 0.05 (−0.03, 0.13) | |
| History of depression | ||
| Yes | −0.08 (−0.18, 0.02) | 0.142 |
| No | −0.00 (−0.04, 0.03) | |
Abbreviations: CI, confidence interval.
Characteristics as of randomization unless otherwise noted.
From longitudinal models of least-squares means of change in cognitive performance from the initial assessment, and averaged over follow-up.
P-value for interaction from testing effect modification in longitudinal models.
Cutoff for low folate intake was determined from intakes found to be significantly associated with elevated homocysteine in the Framingham study (64).
In analyses comparing men assigned to multivitamin treatment versus placebo across all the treatment arms (i.e., not taking any other active vitamin supplement), we observed a suggestion of higher scores at the first cognitive assessment for the multivitamin group (e.g., global composite score at 1st assessment = 0.01 for multivitamin group, and −0.03 for pure placebo group), although the differences were not significant for any cognitive test (p>0.05 for all tests). We also found significantly worse cognitive decline in the multivitamin compared to the pure placebo group for the global and verbal composite scores, but not for the TICS or category fluency task (e.g., mean difference in decline in global score = −0.08 standard units [95% CI: −0.14, −0.01] and mean difference in decline in verbal memory score=−0.10 standard units [95% CI: −0.17, −0.02]). However, these results must be interpreted cautiously given the small number of participants in the pure placebo group (n=372), and the likely random increase in cognitive performance at the first cognitive assessment for the multivitamin group.
COMMENT
In this long-term, randomized, placebo-controlled trial with over a decade of treatment among 5,947 men aged 65 and older, those assigned to a daily multivitamin had similar overall cognitive performance as those taking a placebo.
Previous Observational Studies of Multivitamins and Cognition
Few observational studies have examined multivitamin usage and cognition. There is some epidemiologic research suggesting that moderate doses of antioxidant vitamins (similar to those found in a multivitamin supplement) are associated with slower rate of cognitive decline (37). For example, in 2,889 subjects from the Chicago Health and Aging Project with mean follow-up of 3.2 years, higher total vitamin E and vitamin E intake from foods (mean intake of vitamin E was 90 IU, 17% of participants used vitamin E supplements) was associated with slower cognitive decline (10). Nonetheless, findings for antioxidant vitamins are not consistent; an analysis of 16,010 women in the Nurses’ Health Study found no association between total antioxidant intake or antioxidant intake from foods alone and cognitive decline over four years (38).
Observational studies of B vitamins and cognitive status have also been inconsistent (39). Some studies have shown better cognitive performance among people with higher blood levels of folate (40, 41) or other B vitamins (42–44), while other studies have shown no association (45–47). Studies of dietary intake and supplements have also been variable. One cohort study of 321 subjects with mean follow-up of 3 years found that dietary folate (mean intake = 440 μg), vitamin B-6 (mean intake = 3.98 mg), and vitamin B-12 (mean intake = 9.57 μg) from food and supplement sources were related to better performance on a spatial copying task, but not other memory-related tests (48). Another study found that vitamin B-12 intake was not related to cognitive decline in 3,718 subjects with median follow-up of 5.5 years, except for a potential benefit limited to the oldest participants (49).
Trials of Multivitamins or Combinations of Vitamins and Cognition
Results from previous randomized controlled trials (RCTs) of multivitamin supplements and cognition have not found clear benefits in well-nourished populations. In a recent meta-analysis of 10 smaller, shorter-term RCTs of multivitamin supplements, there was no effect on seven different cognitive domains except for immediate free recall memory, which was not a specific a priori hypothesis (12). Trials testing high doses of individual vitamin supplements have generally had null results for cognition as well, including large-scale trials of antioxidant supplements (50–54), as well as B vitamins (55–58).
Yet, one issue with many of the trials is that supplementation may be administered too late or for an inadequate duration to prevent cognitive decline, a process which begins years before symptoms are detected. In a cognitive substudy of the SU.VI.MAX trial (n=4,447), investigators assessed cognition 6 years after the conclusion of an 8-year trial of antioxidant supplementation, and found better performance for the supplement group on a test of episodic memory(17). However, results were not significant for the five other cognitive outcomes tested, and thus findings are difficult to interpret. Stronger evidence comes from a previous report of the beta-carotene component from the PHS II trial; those randomized to beta-carotene had significantly better performance on global cognitive and verbal memory after an average 18 years of supplementation, suggesting that very long-term vitamin supplementation – or exposure at younger ages before significant neuropathology has accumulated – may be required to maintain brain health (20, 59).
Strengths and Limitations
A limitation of this study is that our population of male physician participants may have been too well nourished to observe benefits of supplementation. When cognitive benefits have been observed in other trials of nutriceuticals, these benefits are usually within groups with inadequate dietary intakes of the relevant vitamin (51, 60). Future studies are needed to clarify whether multivitamin supplementation may be more beneficial in those with less optimal nutritional status or vitamin deficiencies. This is of particular interest in an aging population, since older persons are often at risk for nutritional deficiencies due to reduced micronutrient intake, altered absorption and metabolic requirements of vitamins (61).
This population is also unique in that the participants are all highly-educated men, so it is possible that effects of multivitamins could have been different in a study population with varying levels of educational attainment. That said, our large sample size gave us sufficient power to detect effects of the multivitamin supplement on changes in cognition, and we have identified numerous risk factors for cognitive decline in previous studies using PHSII data, including beta-carotene treatment and type 2 diabetes (20, 62).
Furthermore, cognitive testing began on average 2.5 years (range: 0.18 – 5.3 years) after randomization. This prevented evaluating change in performance from randomization, and it is possible we missed acute benefits of multivitamins during initial follow-up. However, risk factors for cognitive decline were similarly distributed among treatment groups at randomization, and cognition was similar at the initial cognitive assessment (including among newly recruited participants, with a mean of just one year from randomization to initial cognitive testing), and therefore it is likely that cognitive function was comparable between the two groups at randomization. Given the long period of time over which cognitive changes occur, it is unlikely that we missed any meaningful changes due to multivitamin supplementation in the time between randomization and initial cognitive testing.
Finally, the formulation of the multivitamin used in PHS II has changed since PHS II began, reflecting evolving perspectives and priorities in nutrition. For example, vitamin D increased from 400 to 500 IU, vitamin A (% as beta-carotene) decreased from 5000 IU (50%) to 2500 IU (40%), and 250 μg lutein and 300 μg lycopene were added. However, the formulation of the multivitamin used throughout PHS II (Appendix 1) has remained the same throughout the duration of PHS II.
Strengths of this trial include the large population of men with a long duration of randomized treatment. Additional strengths include completion of four repeated cognitive assessments over nearly a decade with high rates of follow-up, and a validated neuropsychological test battery covering a variety of cognitive domains, based on the same cognitive domains used in the National Institute on Aging’ Uniform Data Set Neuropsychological Test Battery (63). The PHS II also benefited from high levels of compliance with multivitamin treatment, with two-thirds of men still compliant with their treatment regimen after more than a decade of follow-up.
Conclusion
In this large, randomized, placebo controlled trial among 5,947 men aged 65 and over, we observed no benefit of a daily multivitamin in slowing cognitive decline after more than a decade of treatment and follow-up. These data do not provide support for the potential use of multivitamin supplements in the prevention of cognitive decline. However, it is important to consider other health effects of multivitamin supplementation, including modest protection against overall cancer risk in PHS II with long-term use (3) as well as any potential effects on other important health outcomes yet to be evaluated. Moreover, further research is needed in other populations, such as those with nutrient deficiencies, to determine whether there are cognitive benefits specific to daily multivitamin use.
Supplementary Material
Acknowledgments
Funding/Support: Supported by grants CA 097193, CA 34944, CA 40360, HL 26490, HL 34595, AG 15933, and T32-AG000158 from the National Institutes of Health (Bethesda, MD), and an investigator-initiated grant from BASF Corporation (Florham Park, NJ). Study agents and packaging were provided by BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle) (New York, NY), and study packaging was provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ).
Role of the Sponsors: NIH, BASF, Pfizer, and DSM Nutritional Products Inc had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Data and Safety Monitoring Board: Voting members over the course of the PHS II trial included LawrenceCohen, Rory Collins, Theodore Colton, I. Craig Henderson, Andrea LaCroix, Ross Prentice, and Nanette Wenger (chair); ex-officio members included Mary Francis Cotch, Jeffrey Cutler, Frederick Ferris, Jerome Fleg, Peter Greenwald, Natalie Kurinij, Howard Parnes, Marjorie Perloff, Eleanor Schron, and Alan Zonderman.
Additional Contributions: We are deeply indebted to the 14,641 physician participants for their long-standing dedication and conscientious collaboration. We also acknowledge the long-term contributions of Charles Hennekens, MD, DrPH, Florida Atlantic University, to the Physicians’ Health Study, and the exemplary contributions of the staff of the Physicians’ Health Study at Brigham and Women’s Hospital.
Reproducible Research Statement: Study protocol: Available from Dr. Sesso (hsesso@partners.org) and Dr. Gaziano (jmgaziano@partners.org). Statistical code: not available. Data set: not available.
Relevant Financial Disclosures:
Dr. Manson reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from BASF and Cognis Corporations for the Women’s Antioxidant and Folic Acid Cardiovascular Study and from PronovaBioPharma and Pharmavite for the VITamin D and OmegA-3 TriaL, and funding from the non-profit Aurora Foundation.
Dr. Glynn reported receiving investigator-initiated research funding from the National Institutes of Health, Bristol-Meyers Squibb, AstraZeneca, and Novartis, and signed a consulting agreement with Merck to give an invited talk.
Dr. Buring reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from Natural Source Vitamin E Association and Bayer Healthcare for the Women’s Health Study.
Dr. Gaziano reported receiving investigator-initiated research funding from the National Institutes of Health, the Veterans Administration, and the BASF Corporation to assist in the establishment of this trial cohort, assistance with study agents and packaging from BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle), and assistance with study packaging provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins).
Dr. Sesso reported receiving investigator-initiated research funding from the National Institutes of Health, the Tomato Products Wellness Council, and Cambridge Theranostics, Ltd.
Dr. Grodstein received investigator-initiated research funding from the National Institutes of Health, the California Dried Plum Board, the California Strawberry Commission, and an unrestricted gift for research from the California Walnut Commission.
References
- 1.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57(6):839–44. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–6. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–80. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg IH. Challenges and opportunities in the translation of the science of vitamins. Am J Clin Nutr. 2007;85(1):325S–7S. doi: 10.1093/ajcn/85.1.325S. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DO, Haskell CF. Vitamins and cognition: what is the evidence? Drugs. 2011;71(15):1957–71. doi: 10.2165/11594130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71(1):1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 7.Sardesai VM. Role of antioxidants in health maintenance. Nutr Clin Pract. 1995;10(1):19–25. doi: 10.1177/011542659501000119. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71(2):614S–20S. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- 9.Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54(4):489–95. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Archives of neurology. 2002;59(7):1125–32. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 11.Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, et al. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11(3):230–7. [PubMed] [Google Scholar]
- 12.Grima NA, Pase MP, Macpherson H, Pipingas A. The effects of multivitamins on cognitive performance: a systematic review and meta-analysis. J Alzheimers Dis. 2012;29(3):561–9. doi: 10.3233/JAD-2011-111751. [DOI] [PubMed] [Google Scholar]
- 13.Wolters M, Hickstein M, Flintermann A, Tewes U, Hahn A. Cognitive performance in relation to vitamin status in healthy elderly German women-the effect of 6-month multivitamin supplementation. Prev Med. 2005;41(1):253–9. doi: 10.1016/j.ypmed.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 14.McNeill G, Avenell A, Campbell MK, Cook JA, Hannaford PC, Kilonzo MM, et al. Effect of multivitamin and multimineral supplementation on cognitive function in men and women aged 65 years and over: a randomised controlled trial. Nutr J. 2007;6:10. doi: 10.1186/1475-2891-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summers WK, Martin RL, Cunningham M, DeBoynton VL, Marsh GM. Complex antioxidant blend improves memory in community-dwelling seniors. J Alzheimers Dis. 2010;19(2):429–39. doi: 10.3233/JAD-2010-1229. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson H, Ellis KA, Sali A, Pipingas A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement: A randomized controlled trial. Psychopharmacology (Berl) 2012;220(2):351–65. doi: 10.1007/s00213-011-2481-3. [DOI] [PubMed] [Google Scholar]
- 17.Kesse-Guyot E, Fezeu L, Jeandel C, Ferry M, Andreeva V, Amieva H, et al. French adults’ cognitive performance after daily supplementation with antioxidant vitamins and minerals at nutritional doses: a post hoc analysis of the Supplementation in Vitamins and Mineral Antioxidants (SU.VI.MAX) trial. Am J Clin Nutr. 2011;94(3):892–9. doi: 10.3945/ajcn.110.007815. [DOI] [PubMed] [Google Scholar]
- 18.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 19.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 20.Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians’ Health Study II. Arch Intern Med. 2007;167(20):2184–90. doi: 10.1001/archinte.167.20.2184. [DOI] [PubMed] [Google Scholar]
- 21.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125–34. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 22.Hee Kang J, Grodstein F. Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology. 2003;60(10):1591–7. doi: 10.1212/01.wnl.0000065980.33594.b7. [DOI] [PubMed] [Google Scholar]
- 23.Evans DA, Grodstein F, Loewenstein D, Kaye J, Weintraub S. Reducing case ascertainment costs in U.S. population studies of Alzheimer’s disease, dementia, and cognitive impairment-Part 2. Alzheimers Dement. 2011;7(1):110–23. doi: 10.1016/j.jalz.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, Leurgans SE, Foroud TM, Sweet RA, Graff-Radford N, Mayeux R, et al. Telephone assessment of cognitive function in the late-onset Alzheimer’s disease family study. Arch Neurol. 2010;67(7):855–61. doi: 10.1001/archneurol.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt J, Spencer M, Folstein MF. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111–7. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57(3–4):167–78. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 28.Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 29.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–24. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 30.Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64(6):862–71. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 31.Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52(5):485–90. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 32.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751–60. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzmaurice GMLN, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley & Sons, Inc; 2004. pp. 103–39. [Google Scholar]
- 36.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 37.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, Barberger Gateau P, Berr C, Bonnefoy M, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11(2):132–52. [PubMed] [Google Scholar]
- 38.Devore EE, Kang JH, Stampfer MJ, Grodstein F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am J Clin Nutr. 2010;92(5):1157–64. doi: 10.3945/ajcn.2010.29634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raman G, Tatsioni A, Chung M, Rosenberg IH, Lau J, Lichtenstein AH, et al. Heterogeneity and lack of good quality studies limit association between folate, vitamins B-6 and B-12, and cognitive function. J Nutr. 2007;137(7):1789–94. doi: 10.1093/jn/137.7.1789. [DOI] [PubMed] [Google Scholar]
- 40.Kim JM, Kim SW, Shin IS, Yang SJ, Park WY, Kim SJ, et al. Folate, vitamin b(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry investigation. 2008;5(1):36–40. doi: 10.4306/pi.2008.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. The American journal of medicine. 2005;118(2):161–7. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82(3):627–35. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 43.Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009;72(4):361–7. doi: 10.1212/01.wnl.0000341272.48617.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. The American journal of clinical nutrition. 2007;86(5):1384–91. doi: 10.1093/ajcn/86.5.1384. [DOI] [PubMed] [Google Scholar]
- 45.Kang JH, Irizarry MC, Grodstein F. Prospective study of plasma folate, vitamin B12, and cognitive function and decline. Epidemiology. 2006;17(6):650–7. doi: 10.1097/01.ede.0000239727.59575.da. [DOI] [PubMed] [Google Scholar]
- 46.Garcia AA, Haron Y, Evans LR, Smith MG, Freedman M, Roman GC. Metabolic markers of cobalamin deficiency and cognitive function in normal older adults. Journal of the American Geriatrics Society. 2004;52(1):66–71. doi: 10.1111/j.1532-5415.2004.52012.x. [DOI] [PubMed] [Google Scholar]
- 47.Teunissen CE, Blom AH, Van Boxtel MP, Bosma H, de Bruijn C, Jolles J, et al. Homocysteine: a marker for cognitive performance? A longitudinal follow-up study. The journal of nutrition, health & aging. 2003;7(3):153–9. [PubMed] [Google Scholar]
- 48.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. The American journal of clinical nutrition. 2005;82(3):627–35. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 49.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Archives of neurology. 2005;62(4):641–5. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 50.Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta. 2012;1822(5):631–8. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166(22):2462–8. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 52.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 53.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe K, Clemons TE, McBee WL, Lindblad AS. Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology. 2004;63(9):1705–7. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nachum-Biala Y, Troen AM. B-vitamins for neuroprotection: narrowing the evidence gap. Biofactors. 2012;38(2):145–50. doi: 10.1002/biof.1006. [DOI] [PubMed] [Google Scholar]
- 56.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. The American journal of medicine. 2010;123(6):522–7. e2. doi: 10.1016/j.amjmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88(6):1602–10. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haller J. The vitamin status and its adequacy in the elderly: an international overview. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition. 1999;69(3):160–8. doi: 10.1024/0300-9831.69.3.160. [DOI] [PubMed] [Google Scholar]
- 62.Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56(6):1028–36. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 63.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.