Abstract
Developmentally regulated genes in Drosophila, which are conserved through evolution, are potential candidates for key functions in biological processes such as cell cycle, programmed cell death, and cancer. We report cloning and characterization of the human homologue of the Drosophila seven in absentia gene (HUMSIAH), which codes for a 282 amino acids putative zinc finger protein. HUMSIAH is localized on human chromosome 16q12-q13. This gene is activated during the physiological program of cell death in the intestinal epithelium. Moreover, human cancer-derived cells selected for suppression of their tumorigenic phenotype exhibit constitutively elevated levels of HUMSIAH mRNA. A similar pattern of expression is also displayed by the p21waf1. These results suggest that mammalian seven in absentia gene, which is a target for activation by p53, may play a role in apoptosis and tumor suppression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsen H. M., Abderrahim H., Cann H. M., Dausset J., Le Paslier D., Cohen D. Construction and characterization of a yeast artificial chromosome library containing seven haploid human genome equivalents. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4256–4260. doi: 10.1073/pnas.87.11.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
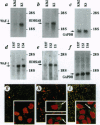
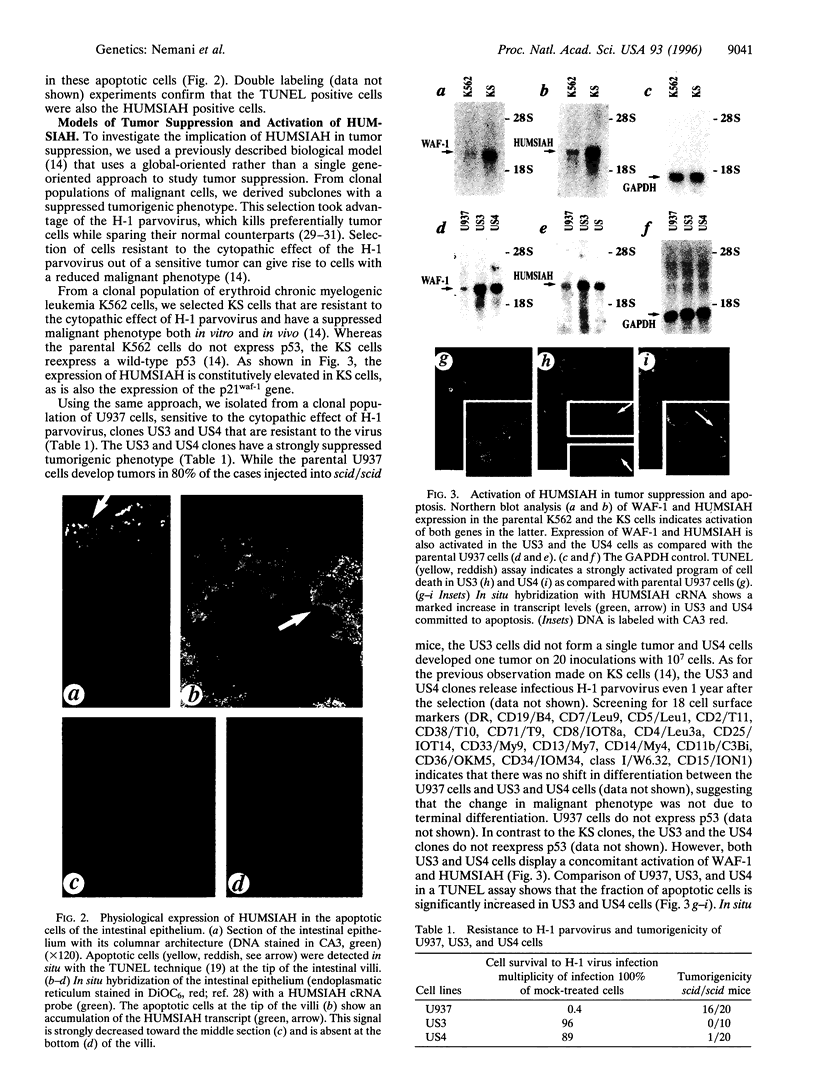
- Amson R. B., Nemani M., Roperch J. P., Israeli D., Bougueleret L., Le Gall I., Medhioub M., Linares-Cruz G., Lethrosne F., Pasturaud P. Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: activation of the vertebrate homologue of the drosophila seven in absentia gene. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):3953–3957. doi: 10.1073/pnas.93.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austruy E., Candon S., Henry I., Gyapay G., Tournade M. F., Mannens M., Callen D., Junien C., Jeanpierre C. Characterization of regions of chromosomes 12 and 16 involved in nephroblastoma tumorigenesis. Genes Chromosomes Cancer. 1995 Dec;14(4):285–294. doi: 10.1002/gcc.2870140407. [DOI] [PubMed] [Google Scholar]
- Berns K. I. Parvovirus replication. Microbiol Rev. 1990 Sep;54(3):316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E., Kumar S., Krainer A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993 Dec 25;21(25):5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bièche I., Lidereau R. Genetic alterations in breast cancer. Genes Chromosomes Cancer. 1995 Dec;14(4):227–251. doi: 10.1002/gcc.2870140402. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Rubin G. M. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990 Nov 2;63(3):561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- Cherif D., Julier C., Delattre O., Derré J., Lathrop G. M., Berger R. Simultaneous localization of cosmids and chromosome R-banding by fluorescence microscopy: application to regional mapping of human chromosome 11. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6639–6643. doi: 10.1073/pnas.87.17.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della N. G., Bowtell D. D., Beck F. Expression of Siah-2, a vertebrate homologue of Drosophila sina, in germ cells of the mouse ovary and testis. Cell Tissue Res. 1995 Feb;279(2):411–419. doi: 10.1007/BF00318499. [DOI] [PubMed] [Google Scholar]
- Della N. G., Senior P. V., Bowtell D. D. Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina). Development. 1993 Apr;117(4):1333–1343. doi: 10.1242/dev.117.4.1333. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Simon M. A., Rubin G. M. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992 Feb 6;355(6360):559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- Gaul U., Mardon G., Rubin G. M. A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell. 1992 Mar 20;68(6):1007–1019. doi: 10.1016/0092-8674(92)90073-l. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Kennedy D., Ramsdale T., Mattick J., Little M. An RNA recognition motif in Wilms' tumour protein (WT1) revealed by structural modelling. Nat Genet. 1996 Mar;12(3):329–331. doi: 10.1038/ng0396-329. [DOI] [PubMed] [Google Scholar]
- Koken M. H., Linares-Cruz G., Quignon F., Viron A., Chelbi-Alix M. K., Sobczak-Thépot J., Juhlin L., Degos L., Calvo F., de Thé H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995 Apr 6;10(7):1315–1324. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Linares-Cruz G., Rigaut J. P., Vassy J., De Oliveira T. C., De Cremoux P., Olofsson B., Calvo F. Reflectance in situ hybridization (RISH): detection, by confocal reflectance laser microscopy, of gold-labelled riboprobes in breast cancer cell lines and histological specimens. J Microsc. 1994 Jan;173(Pt 1):27–38. doi: 10.1111/j.1365-2818.1994.tb03425.x. [DOI] [PubMed] [Google Scholar]
- Macleod K. F., Sherry N., Hannon G., Beach D., Tokino T., Kinzler K., Vogelstein B., Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995 Apr 15;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Maw M. A., Grundy P. E., Millow L. J., Eccles M. R., Dunn R. S., Smith P. J., Feinberg A. P., Law D. J., Paterson M. C., Telzerow P. E. A third Wilms' tumor locus on chromosome 16q. Cancer Res. 1992 Jun 1;52(11):3094–3098. [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Mousset S., Rommelaere J. Minute virus of mice inhibits cell transformation by simian virus 40. Nature. 1982 Dec 9;300(5892):537–539. doi: 10.1038/300537a0. [DOI] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Raap A. K., van de Corput M. P., Vervenne R. A., van Gijlswijk R. P., Tanke H. J., Wiegant J. Ultra-sensitive FISH using peroxidase-mediated deposition of biotin- or fluorochrome tyramides. Hum Mol Genet. 1995 Apr;4(4):529–534. doi: 10.1093/hmg/4.4.529. [DOI] [PubMed] [Google Scholar]
- Sheng W. W., Soukup S., Bove K., Gotwals B., Lampkin B. Chromosome analysis of 31 Wilms' tumors. Cancer Res. 1990 May 1;50(9):2786–2793. [PubMed] [Google Scholar]
- Telerman A., Tuynder M., Dupressoir T., Robaye B., Sigaux F., Shaulian E., Oren M., Rommelaere J., Amson R. A model for tumor suppression using H-1 parvovirus. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8702–8706. doi: 10.1073/pnas.90.18.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toolan H. W., Ledinko N. Inhibition by H-1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology. 1968 Jul;35(3):475–478. doi: 10.1016/0042-6822(68)90226-2. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]