Abstract
Only quinones with a 2-methoxy group can act simultaneously as the primary (QA) and secondary (QB) electron acceptors in photosynthetic reaction centers from Rb. sphaeroides. 13C HYSCORE measurements of the 2-methoxy in the semiquinone states, SQA and SQB, were compared with QM calculations of the 13C couplings as a function of dihedral angle. X-ray structures support dihedral angle assignments corresponding to a redox potential gap (ΔEm) between QA and QB of ~180 mV. This is consistent with the failure of a ubiquinone analog lacking the 2-methoxy to function as QB in mutant reaction centers with a ΔEm ≈ 160–195 mV.
Type II reaction centers (RCs) from photosynthetic bacteria and oxygenic organisms contain two quinones that function in series as electron acceptors.1 In many cases, the two quinones are chemically identical and yet forward electron transfer from the primary quinone, QA, to the secondary quinone, QB, is thermodynamically favorable by 60–75 meV.2 In RCs from Rhodobacter (Rb.) sphaeroides, which utilizes ubiquinone, reconstitution studies show that only quinones with a 2-methoxy group are able to function as both QA and QB. This was most clearly demonstrated using two synthetic analogs of ubiquinone in which one or the other of the two methoxy groups was replaced by a methyl (2-methoxy-3,5-dimethyl-6-tetraisoprenyl-1,4-benzoquinone and 3-methoxy-2,5-dimethyl-6-tetraisoprenyl-1,4-benzoquinone, abbreviated 2-MeO-Q and 3-MeO-Q, respectively). Both can fully reconstitute QA function, but only 2-MeO-Q was able to support QB activity; 3-MeO-Q showed no QB activity.3
The tuning of cofactor redox potentials by the protein is universal and can be extreme. It is often readily accounted for by the local electrostatic potential provided by the protein.4 This is sufficient for electron transfer in photosystem II RCs where plastoquinone, which has no methoxy groups, binds and functions in both quinone sites,5 but it cannot account for the unique requirement of a 2-methoxy group to simultaneously restore QA and QB activity in Rb. sphaeroides RCs. An additional factor evidently resides with the methoxy group itself, and the methoxy dihedral angle has been suggested to have a strong influence on the redox midpoint potential (Em) of benzoquinones.6 When the methoxy group is out of the plane of the quinone ring, the main influence is the electron withdrawing nature of the electronegative oxygen, but when the methoxy is in plane, the oxygen p orbitals can conjugate with the π-system of the quinone causing electron donation to the ring. Previous computational studies showed that rotating one methoxy group alters the electron affinity by up to 0.25 eV,7 in very good agreement with our own calculations.8 Earlier results showed that rotation of both methoxy groups of 2,6-dimethoxy-1,4-benzoquinone altered the electron affinity by up to 0.4 eV.9
Applying this to the reaction center quinones is hampered by a lack of adequate information on the methoxy orientations in QA and QB, as the numerous available x-ray structures of RCs yield a wide range of values.10 To address this we recently carried out hyperfine sublevel correlation (HYSCORE) measurements of the semiquinone radicals (SQA and SQB) in RCs containing ubiquinone 13C-labeled at the two methoxy groups and single methyl of the ring. We identified the hyperfine coupling constants, aiso, of the 2-methoxy groups in QA and QB, and compared these measured values to quantum mechanically calculated aiso values as a function of the 2-methoxy dihedral angle. The angles determined were then compared to the computed relationship between the dihedral angle and the resulting electron affinity.8
Comparison of 13C couplings (aiso) for the 2-methoxy group in SQA (1.3 MHz) and SQB (5.7 MHz, adjusted to the same unpaired spin density (0.11) on C2)8 defines four possible combinations for the dihedral angle θ (CmOmC2C1) in the two SQs (Table 1, Fig. S2).
Table 1.
Estimated angles of the 2-methoxy conformation in SQA and SQB and corresponding differences in electron affinity (EA) and redox potential (Em).
| θA(°) | θB (°) | ΔEA(eV) | ΔEm(mV) | |
|---|---|---|---|---|
| (i) | 45 | 75 | 0.04 | 40 |
| (ii) | 45 | 135 | −0.13 | −130 |
| (iii) | 155 | 75 | 0.18 | 180 |
| (iv) | 155 | 135 | 0.05 | 50 |
In our previous analysis we discussed two of the pairs in Table 1, i.e. (i) and (iv), in which the 2-methoxy dihedral angles of both quinones are on the same side of the perpendicular. This comparison yielded a contribution by the different 2-methoxy angles to the ΔEm between QA and QB of ~50 mV.8 This is a substantial fraction of the experimental ΔEm of 60–75 mV.2 However, it is not large enough to account for the complete absence of electron transfer with 3-MeO-Q, which lacks the 2-methoxy group.3
Consideration of the methoxy angle pairs (ii) and (iii) in Table 1 shows significantly larger Em differences than for pairs (i) and (iv). Pair (ii) indicates that the 2-methoxy group makes an unfavorable contribution of −130 mV to the ΔEm, while pair (iii) shows a favorable contribution of +180 mV. The latter value is consistent with the complete failure to support QB function without a 2-methoxy group, e.g., 3-MeO-Q, but some independent evidence for this assignment is needed.
Confirmation that the 2-methoxy group makes a substantially larger contribution to the Em gap between QA and QB comes from mutants of the QA site that lower the Em of QA (Figs 1 and 2). Mutation of isoleucine M265 to threonine (mutant M265IT) decreases the Em of QA by 100–120 mV by a mechanism that does not involve the methoxy groups.11 This greatly increases ΔEm, the driving force for electron transfer from QA− to QB. However, in polar mutants of M265, 3-MeO-Q is still completely inactive as QB (Fig. 1).
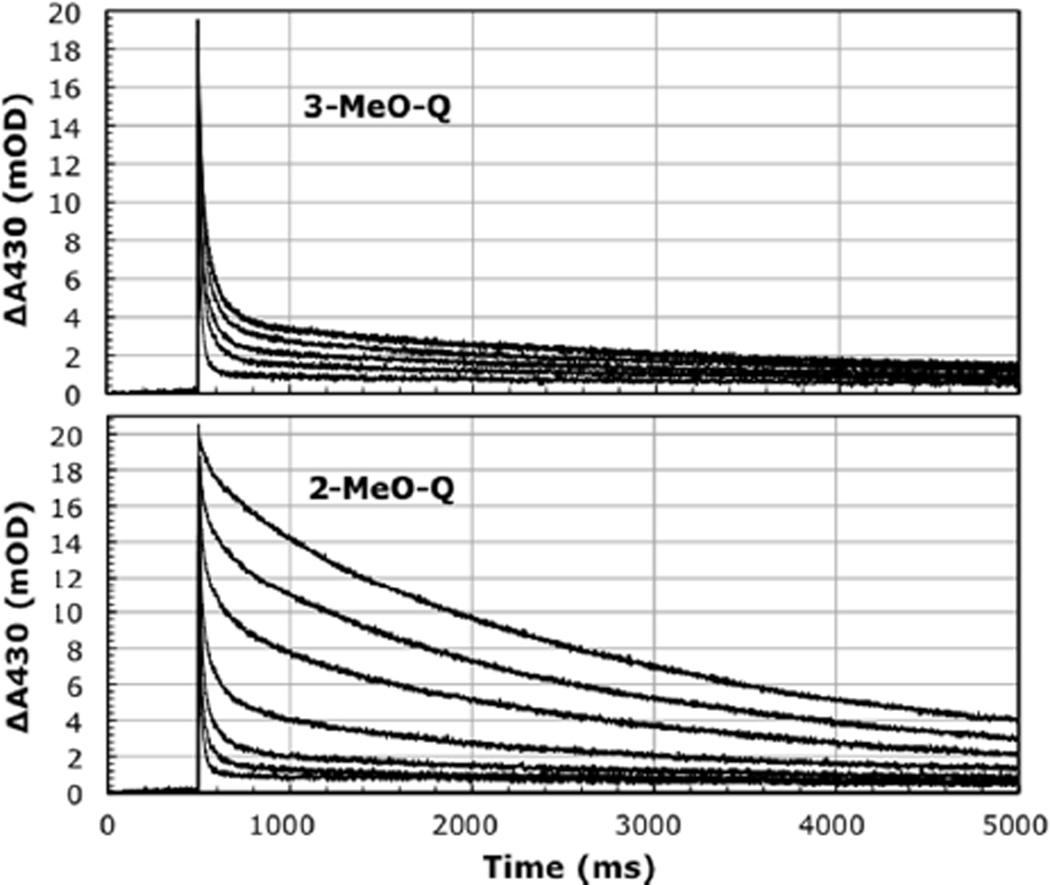
Figure 1.
Kinetics of the back reaction for 2- and 3-MeO-Q reconstituted in M265IT RCs with ubiquinone as QA (1Q-RCs). Top: 3-MeO-Q concentrations: 0, 0.5, 1, 2, 4, 16 µM. Bottom: 2-MeO-Q concentrations: 0, 0.5, 1, 2, 4, 8 µM. Approx. 1 µM M265IT RCs, 10 mM Tris, pH 7.8, 0.1% LDAO.
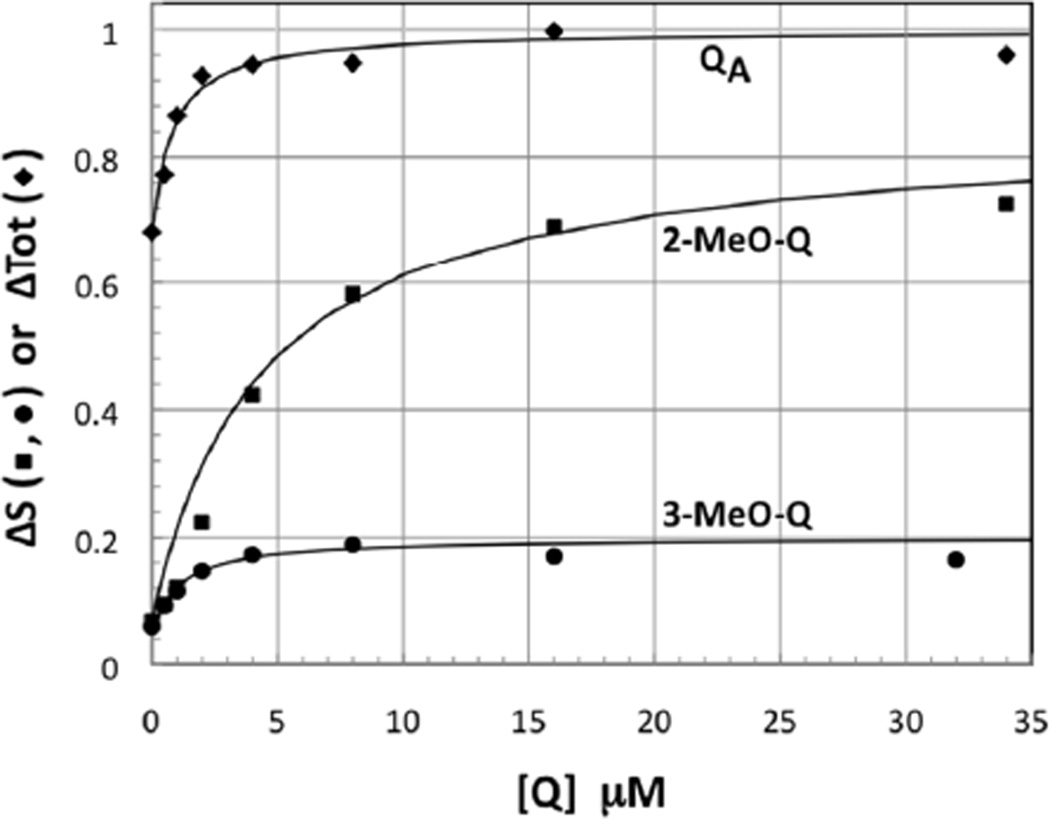
Figure 2.
Titration curves for initial (QA activity) and slow phase (ΔS) amplitudes. The fitted curves are for Kd values of 1 µM (QA), 4 µM (2-MeO-Q, ΔS) and 1 µM (3-MeO-Q, ΔS).
The kinetics of the back reaction in Fig.1 (charge recombination) reflect the activity of QA (initial amplitude) and QB (fraction slow phase, ΔS) (Fig. 2). 2-MeO-Q fully reconstitutes both. However, 3-MeO-Q shows no true ΔS restoration - the small increase in slow phase seen reflects reconstitution of QA, which is partially depleted in these mutant preparations. Some extraneous Q-10 is also present and functions as QB when QA is restored by 3-MeO-Q (note that the apparent affinity for ΔS is the same as for QA, Kd ~1µM).
Taking into account the 60–75 mV favorable ΔEm for ubiquinone in wild type RCs, the failure of 3-MeO-Q in M265IT mutant RCs indicates that its Em in the QB site is more than 160–195 mV lower than that of ubiquinone. It is reasonable to assume that other influences on the Em are not significantly affected by the substitution of one methoxy group with a methyl.
In a survey of over 20 x-ray structures at resolutions of at least 2.8Å (range 1.8–2.8Å), the average values for the methoxy dihedral angles of QA and QB were as shown in Table 2.10 Note that the dihedral angles for the 2-methoxy groups of QA and QB are quite distinct, while those for the 3-methoxy group are similar. The 2-methoxy angles are most consistent with pair (iii) derived from the 13C HYSCORE data and QM calculations (Table 1), giving support for this assignment. This would provide a calculated contribution of ~180 mV to the redox potential gap between the quinones.
Table 2.
Average values for the methoxy dihedral angles (degrees) of QA and QB from X-ray structures.
| Quinone | 2-MeO | 3-MeO |
|---|---|---|
| QA | 139±25° | 77±8° |
| QB | 90±9° | 88±20° |
Other factors, e.g., electrostatics, hydrogen bonds, etc, undoubtedly contribute (either positively or negatively) to the net difference in midpoint potentials, but the data presented here clearly indicate a large, favorable role for the 2-methoxy group in setting the functional redox potential gap between QA and QB. The HYSCORE and computational analysis show that this effect is implemented through different dihedral angles for QA and QB. These are presumably determined by interactions with the environment of the binding sites. For QB the methoxy dihedral angles are likely restricted by hydrogen bond(s) to the 2-methoxy oxygen from the peptide NH of Gly-L225 and/or Thr-L226, accounting for a fairly narrow distribution (Table 2); for QA the constraints are by steric interactions with non-polar groups, although a weak hydrogen bond from Ala-M249 is also possible.10
Supplementary Material
Acknowledgments
Funding
This work was supported by NSF Grant MCB-0818121 (C.A.W.), DE-FG02-08ER15960 Grant from Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, U.S. Department of Energy, and NIH Grant GM062954 (S.A.D.), and NCRR/NIH Grants S10-RR15878 and S10-RR025438 for pulsed EPR instrumentation. P.J.O'M. acknowledges the use of computer resources granted by the EPSRC UK national service for computational chemistry software (NSCCS). A.T.T. gratefully acknowledges support as a NIH trainee of the Molecular Biophysics Training Program (5T32-GM008276).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental details and Figures S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written with contributions from all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interests
REFERENCES
- 1.(a) Heathcote P, Fyfe PK, Jones MR. Reaction centres: the structure and evolution of biological solar power. Trends in Biochemical Sciences. 2002;27:79–87. doi: 10.1016/s0968-0004(01)02034-5. [DOI] [PubMed] [Google Scholar]; (b) Cardona T, Sedoud A, Cox N, Rutherford AW. Charge separation in Photosystem II: A comparative and evolutionary overview. Biochim. Biophys. Acta, Bioenergetics. 2012;1817:26–43. doi: 10.1016/j.bbabio.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mancino LJ, Dean DP, Blankenship RE. Kinetics and thermodynamics of the P870+QA−->P870+QB− reaction in isolated reaction centers from the photosynthetic bacterium Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta, Bioenergetics. 1984;764:46–54. [Google Scholar]; (b) Shinkarev VP, Wraight CA. Electron and Proton Transfer in the Acceptor Quinone Complex of Reaction Centers of Phototrophic Bacteria. In: Deisenhofer J, Norris JR, editors. The Photosynthetic Reaction Center. Vol. 1. San Diego: Academic Press; 1993. pp. 193–255. [Google Scholar]
- 3.Wraight CA, Vakkasoglu AS, Poluektov Y, Mattis A, Takahashi E, Nihan D, Lipshutz BH. The 2-methoxy group of ubiquinone is essential for function of the acceptor quinines in reaction centers from Rba. sphaeroides. Biochim. Biophys. Acta, Bioenergetics. 2008;1777:631–636. doi: 10.1016/j.bbabio.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gunner MR, Alexov E, Torres E, Lipovaca S. The importance of the protein in controlling the electrochemistry of heme metalloproteins: Methods of calculation and analysis. J. Biol. Inorg. Chem. 1997;2:126–134. [Google Scholar]; (b) Rabenstein B, Ullmann GM, Knapp E-W. Electron Transfer between the Quinones in the Photosynthetic Reaction Center and Its Coupling to Conformational Changes. Biochemistry. 2000;39:10487–10496. doi: 10.1021/bi000413c. [DOI] [PubMed] [Google Scholar]; (c) Zhu Z, Gunner MR. The energetics of quinine dependent electron and proton transfers in Rhodobacter sphaeroides photosynthetic reaction centers. Biochemistry. 2005;44:82–96. doi: 10.1021/bi048348k. [DOI] [PubMed] [Google Scholar]
- 5.McComb JC, Stein RR, Wraight CA. Investigations on the influence of headgroup substitution and isoprene side-chain length in the function of primary and secondary quinones of bacterial reaction centers. Biochim. Biophys. Acta, Bioenergetics. 1990;1015:156–171. doi: 10.1016/0005-2728(90)90227-u. [DOI] [PubMed] [Google Scholar]
- 6.Prince RC, Dutton PL, Bruce JM. Electrochemistry of ubiquinones, menaquinones and plastoquinones in aprotic solvents. FEBS Lett. 1983;160:273–276. [Google Scholar]
- 7.Nonella M. A quantum chemical investigation of structures, vibrational spectra and electron affinities of the radicals of quinone model compunds. Photosynth. Res. 1998;55:253–259. [Google Scholar]
- 8.Taguchi AT, O'Malley PJ, Wraight CA, Dikanov SA. Conformational differences between the methoxy groups of QA and QB site ubiquinones in bacterial reaction centers: A key role for methoxy group orientation in modulating ubiquinone redox potential. Biochemistry. 2013;52:4648–4655. doi: 10.1021/bi400489b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson HH, Kahn SD. Interplay of substitutent conformation and electron affinity in quinone models of quinone reductases. J. Am. Chem. Soc. 1990;112:4728–4731. [Google Scholar]
- 10.Wraight CA, Gunner MR. The Acceptor Quinones of Purple Photosynthetic Bacteria – Structure and Spectroscopy. In: Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. The Purple Phototrophic Bacteria. The Netherlands: Springer; 2009. pp. 379–405. [Google Scholar]
- 11.(a) Takahashi E, Wells TA, Wraight CA. Protein control of the redox potential of the primary acceptor quinone in reaction centers from Rhodobacter sphaeroides. Biochemistry. 2001;40:1020–1028. doi: 10.1021/bi001055g. [DOI] [PubMed] [Google Scholar]; (b) Rinyu L, Martin EW, Takahashi E, Maróti P, Wraight CA. Modulation of the free energy of the primary quinone acceptor (QA) in reaction centers from Rhodobacter sphaeroides: Contributions from the protein and protein-lipid(cardiolipin) interactions. Biochim. Biophys. Acta, Bioenergetics. 2004;1655:93–101. doi: 10.1016/j.bbabio.2003.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.