Abstract
Lipolysis is defined as the catabolism of triacylglycerols (TGs) stored in cellular lipid droplets. Recent discoveries of essential lipolytic enzymes and characterization of numerous regulatory proteins and mechanisms have fundamentally changed our perception of lipolysis and its impact on cellular metabolism. Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme for TG catabolism in most cells and tissues. This review focuses on recent advances in understanding the (patho)physiological impact due to defective lipolysis by ATGL deficiency on mitochondrial (dys)function. Depending on the type of cells and tissues investigated, absence of ATGL has pleiotropic roles in mitochondrial function.
Keywords: Adipose triglyceride lipase, Triacylglycerol, Lipotoxicity, Mitochondrial function
Abbreviation: ATGL, adipose triglyceride lipase; BAT, brown adipose tissue; DG, diacylglycerol; ER, endoplasmic reticulum; FFA, free fatty acids; PGC-1, peroxisome proliferator-activated receptor gamma co-activator-1; PPAR, peroxisome proliferator-activated receptor; TG, triacylglycerol; WAT, white adipose tissue; Wt, wild-type
Highlights
-
•
ATGL is the rate-limiting enzyme in TG hydrolysis in most organs and cells.
-
•
Depending on cell and tissue type ATGL has pleiotropic roles in mitochondrial function.
-
•
This review highlights the newest understanding of the impact of ATGL deficiency on mitochondrial (dys)function.
1. Introduction
Lipolysis provides free fatty acids (FFAs) depending on metabolic need (e.g. during fasting). FAs are essential substrates for energy production and lipid synthesis, including membrane lipids and lipid intermediates involved in cellular signaling. Despite their fundamental physiological importance, an excess of FFAs results in lipotoxicity. Lipotoxic effects impair membrane function and induce endoplasmic reticulum (ER) stress, mitochondrial dysfunction, inflammation, and cell death. Essentially all cells are able to detoxify FFAs by esterification within triacylglycerols (TGs). Since most cells have a limited capacity to accumulate excess fat, adipose tissue stores TGs during feeding and supplies FAs to other tissues during calorie restriction [1]. When the limit of adipose tissue expansion to store excess nutrients has been reached lipids accumulate in other tissues [2]. Thereby adipose tissue protects non-adipose tissues from ectopic lipid spillover, which otherwise would result in cell death and impaired organ function.
2. Role of ATGL in neutral lipolysis
Intracellular lipid catabolizing enzymes degrade neutral lipids such as TGs and cholesteryl esters and their metabolic intermediates, which are stored in cytosolic lipid droplets. Lipid droplet-associated cholesteryl esters are hydrolyzed by neutral cholesteryl ester hydrolase(s) including hormone-sensitive lipase [3]. In 2004, three groups independently published the discovery of an enzyme able to hydrolyze TGs and named it adipose triglyceride lipase (ATGL) [4], desnutrin [5], or calcium-independent phospholipase A2 (iPLA2) [6]. ATGL selectively performs the first step in TG hydrolysis, resulting in the formation of diacylglycerol (DG) and FFA. For maximal lipolytic activity ATGL requires CGI-58 as a co-activator [7]. G0S2 (predominantly expressed in adipose tissue and liver) has been identified as a selective inhibitor of ATGL [8]. It has been proposed recently that CGI-58 and G0S2 regulate ATGL via non-competing mechanisms [9].
ATGL is so far the only known lipase that hydrolyzes TGs selectively at the sn-2 position of the glycerol backbone [10]. Upon stimulation of the enzyme by its co-activator CGI-58, the stereo/regioselectivity of ATGL broadens to the sn-1 position, leading to increased FA release. sn-1,3 DG is the preferred substrate for the consecutive hydrolysis by hormone-sensitive lipase. ATGL exhibits 10-fold higher substrate specificity for TGs than for DGs [4] and shows low transacylase and phospholipase activity [6]. In contrast to hormone-sensitive lipase, ATGL lacks the ability to hydrolyze DGs, monoacylglycerols, cholesteryl esters, or retinyl esters [4].
ATGL is expressed in most tissues of the body with highest mRNA levels and enzyme activity found in white (WAT) and brown adipose tissue (BAT) (reviewed in Ref. [11]). The important role of ATGL in lipolysis became evident from observations in Atgl-deficient (−/−) mice, which accumulate TGs in essentially all organs [12]. Massive TG accumulation caused by impaired TG mobilization in cardiac myocytes results in cardiac failure and premature death of Atgl−/− mice, starting at the age of 12 weeks. Due to the reduced availability of FFAs as energy substrate, Atgl−/− mice show an increased utilization of carbohydrates as energy source, leading to improved glucose tolerance and insulin sensitivity. Atgl−/− male mice are fertile, whereas females lack the ability to suckle their offspring due to impaired milk production. Plasma FA, TG, total cholesterol, HDL-cholesterol and ketone body concentrations are decreased in both fed and fasted Atgl−/− mice [12]. Major findings in various Atgl–/– tissues and cells are summarized in Table 1.
Table 1.
Brief summary of major ATGL-related findings in various tissues and cells.
| Tissue | Mitochondrial dysfunction | Major findings |
|---|---|---|
| Cardiac muscle | Yes | Severe impairment of cardiac mitochondrial function in Atgl−/− hearts due to lack of FA as ligands for the PPARα–PGC-1 complex |
| Skeletal muscle | No | Unchanged energy status and mitochondrial oxidative capacity in Atgl−/− skeletal muscles |
| Liver | No | Upregulation of PGC-1α and β in Atgl−/− liver indicate unchanged or increased hepatic mitochondrial activity |
| White adipose tissue | ? | Reduced mitochondrial function and increased ER stress in Atgl−/− white adipose tissue; no significant changes in mRNA expression and biological processes |
| Brown adipose tissue | Yes | Defective PPARα and γ ligand production in Atgl−/− brown adipocytes results in reduced mitochondrial FA oxidation and oxidative phosphorylation |
| Brain | No | Sparse FA β-oxidation in the brain argues against an important role of ATGL in this process in the brain; no neurodegeneration in Atgl−/− mice |
| Small intestine | No | Reduced mRNA expression of genes involved in FA β-oxidation but unchanged PGC-1α mRNA and intact mitochondria in Atgl−/− small intestine |
| Macrophages | Yes | Induction of the mitochondrial apoptosis pathway, ER stress, and fragmented mitochondria in Atgl−/− macrophages |
| INS832/13 cells | No | Unchanged mitochondrial membrane potential, glucose and FA oxidation indicate intact mitochondrial function in shAtgl pancreatic cells |
3. ATGL deficiency and mitochondrial dysfunction
3.1. Cardiac muscle
Although TG accumulation occurs in essentially all organs, none of them is as much affected as the heart (Fig. 1). Although the overall morphology of mitochondria and the structure of mitochondrial cristae appear intact in electron micrographs (Fig. 1), mitochondrial size compared to that of wild-type (Wt) mice is increased [14]. The drastic cardiac phenotype with massive lipid accumulation and cardiomyopathy was initially thought to result in the premature death of Atgl−/− mice [12]. Mice lacking ATGL in all tissues except cardiac muscle (Atgl-ko/CM) have normal life expectancy and no physiological difficulties [13]. Haemmerle et al. have very recently shown that heart failure in Atgl−/− mice is not caused by lipid accumulation per se but is a net result of a severe impairment of cardiac mitochondrial function [14]. ATGL-mediated lipolysis of cellular TG depots, thereby delivering ligands for the PPARα–PGC-1 complex, is crucial for normal cardiac functioning. Severely reduced oxygen consumption under both basal and succinate-stimulated conditions in heart homogenates of Atgl−/− mice indicate mitochondrial defect regardless of the presence of glucose or palmitate, suggesting that the substrate energy source does not rescue the mitochondrial defect. The authors proposed the following mechanism: after entering the cell, exogenous FAs are activated and either enter mitochondria for oxidation or are re-esterified and stored within lipid droplets. ATGL-mediated hydrolysis of TG stores is critical to provide ligands for functional signaling by the PPARα–PGC-1 complex, which activates mitochondrial biogenesis and oxidative phosphorylation. Importantly, the lipolytic defect in the heart of Atgl−/− mice is able to be bypassed by PPARα agonist treatment. Fenofibrate and/or Wy16453 restores the cardiac expression of PGC-1α and PGC-1β, reverses systemic lipid accumulation, normalizes oxidative function in mitochondria, improves cardiac performance, and prevents lethal cardiomyopathy [14]. These data open the possibility of clinical application of PPARα or non-selective PPAR agonists for the treatment of patients with neutral lipid storage disease with myopathy.
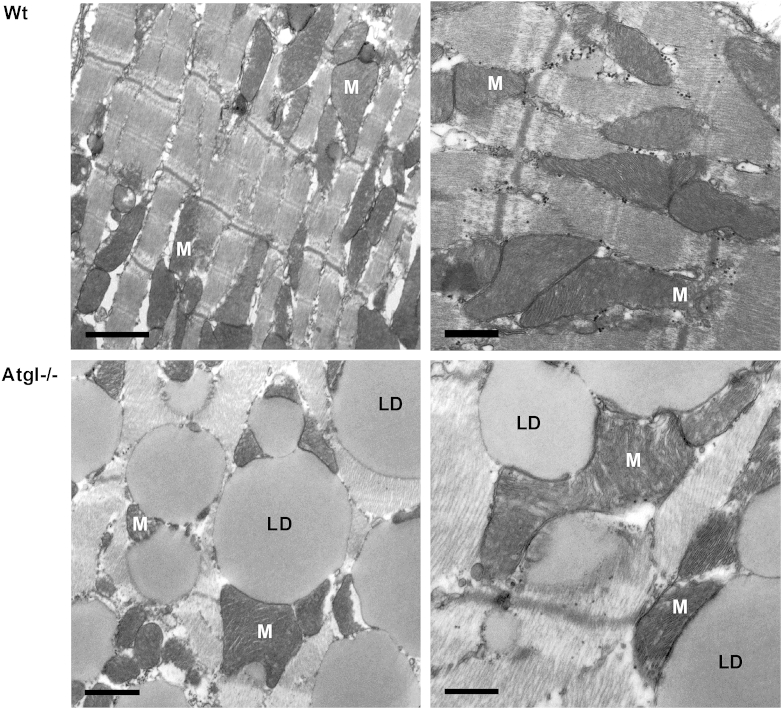
Fig. 1.
Transmission electron micrographs of heart sections from Wt and Atgl−/− mice. Heart tissue was dissected using a Zeiss OPI1 surgical microscope (Carl Zeiss, Oberkochen, Germany). Small tissue fragments were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 h, postfixed in 2% osmium tetroxide for 2 h at room temperature, dehydrated in graded series of ethanol, and embedded in a TAAB epoxy resin. Sections (70 nm thick) were contrasted with uranyl acetate and lead citrate. Images were taken using an FEI Tecnai G2 20 transmission electron microscope (FEI Eindhoven, Eindhoven, Netherlands) with a Gatan ultrascan 1000 CCD camera. Acceleration voltage used was 120 kV. Wt cardiac muscle sections (upper panels) show a intermyofibrillar network containing mitochondria (M). Atgl−/− cardiac muscle (lower panels) show a massive lipid droplet (LD) accumulation within the intermyofibrillar network. Overall morphology of mitochondria and the structure of mitochondrial cristae appeared normal. Mitochondrial size compared to that of Wt mice is increased [14]. Left panels: scale bars 1 μm; right panels: scale bars 0.5 μm.
3.2. Skeletal muscle
Compared with other non-adipose tissues, ATGL mRNA expression in murine skeletal muscle is quite high [4], suggesting an important role for ATGL in this metabolically active tissue. Intramyocellular TGs localized within cytoplasmic lipid droplets serve as a reservoir for lipoprotein-derived FAs [15], which are esterified into TGs [16,17] prior to mobilization by ATGL. Whole body ATGL deficiency in mice leads to 44% decrease in TG hydrolase activity and concomitant significant TG accumulation in myocytes, predominantly in oxidative fibres [12]. In line with these data, ATGL siRNA in myotubes reduces TG hydrolase activity and increases TG content, whereas retroviral-mediated ATGL overexpression induces the reciprocal response and increases the oxidation of FAs liberated from TGs and DGs [18]. Endurance exercise training increases skeletal muscle ATGL protein by 2-fold and reduces intramyocellular TG content in healthy young men [19]. These data confirm the importance of ATGL for lipid and energy metabolism in skeletal muscle.
The localization of lipid droplets in close proximity to the ER and mitochondrial network [20] might be crucial for the cellular economy of myocytes. Skeletal muscle is quantitatively the most prominent site for FA uptake [18]. Of particular importance is the activity of skeletal muscle during exercise, which increases the mitochondrial volume up to 50% after only few weeks in previously untrained subjects [21]. Similar data showing stimulating effect of exercise on mitochondrial biogenesis are observed in rats [22] and mice [23]. ATGL is essential for efficient lipolysis of intramyocellular TGs and supply of FAs for mitochondrial β-oxidation [13,24,25]. An impaired capacity for exercise-induced lipolysis despite even increased oxidative capacity is observed in skeletal muscles of Atgl−/− mice [24]. Being unable to efficiently hydrolyze TGs in the absence of ATGL carbohydrate metabolism is induced, resulting in elevated muscle and liver glycogen depletion. Reduced maximal running velocity and endurance capacity in Atgl−/− mice is not due to defective muscle contraction [24]. Intact cardiac function is essential for maintaining normal physiology, especially during exercise training. To exclude cardiac malfunction, the most prominent phenotype of Atgl−/− mice [12], Schoiswohl et al. have generated Atgl−/− mice that express ATGL only in cardiac muscle (Atgl-ko/CM) [13]. In contrast to whole body Atgl−/− mice, Atgl-ko/CM mice do not accumulate TGs in the heart and have a normal life expectancy. However, both mouse lines are unable to increase circulating FA levels during exercise. The reduced availability of FAs is compensated by an increased use of carbohydrates for energy conversion, leading to rapid depletion of liver glycogen stores during exercise [13]. To explain decreased force production and higher muscle relaxation times of Atgl−/− mice Nunes et al. measured mitochondrial oxidative phosphorylation [25]. Despite a highly increased intramyocellular lipid pool skeletal muscles from Atgl−/− show a comparable energy status and overall muscular in vivo mitochondrial oxidative capacity as Wt mice. According to these data mitochondrial dysfunction is likely not responsible for poor Atgl−/− muscle performance.
3.3. Liver
Inconsistent results are published on TG accumulation in livers of Atgl−/− mice with almost identical [26], 2.3-fold [12] or at least 3-fold increased hepatic TG concentrations [27] compared with Wt livers. Electron microscopy reveals lipid droplet accumulation in Atgl−/− livers with morphologically intact mitochondria surrounded by rough ER (Fig. 2). Using an adenovirus-mediated approach, Watt et al. [28] have very recently demonstrated that hepatic ATGL deficiency leads to hepatic steatosis without impairing hepatic insulin sensitivity. Intraperitoneal injections of tunicamycin induce hepatic steatosis in Atgl−/− but not in Wt mice [26]. Thus deficiency of liver ATGL causes progressive hepatic steatosis but inflammatory and fibrotic responses are reduced compared with those reported in other obesity models with similar degrees of steatosis [27]. Livers from Atgl−/− mice might be protected from hepatic ER stress and inflammation through alterations in FA composition. The authors conclude that ATGL-mediated TG hydrolysis might be a novel target in the treatment of hepatic ER stress [26], which is typically present in patients suffering from non-alcoholic fatty liver disease and steatohepatitis. Hepatic ATGL deficiency leads to lowering of mitochondrial β-oxidation without affecting very low-density lipoprotein secretion [29]. Mice deficient in liver ATGL exhibit 40–70% reduced mRNA expression of hepatic PPARα and its target genes involved in FA utilization and glucose production [29]. Consistent with these data, hepatic mitochondrial oxidation is down-regulated in high fat diet-fed mice administered Atgl shRNA adenovirus in comparison with controls [28]. Treatment with the PPARα agonist fenofibrate, however, cannot overcome the decreased expression of PPARα target genes in mice transduced with Atgl shRNA, suggesting that in the liver ATGL regulates PPARα through a ligand-independent mechanism. Knockdown of hepatic ATGL by adenoviral infection results in a higher respiratory quotient during food withdrawal, indicating that the main source of energy is glucose oxidation, resulting in improved glucose tolerance [28]. In contrast to cardiac ATGL deficiency, hepatic ATGL deficiency leads to an upregulation of PGC-1α and PGC-1β, indicating unchanged or even increased hepatic mitochondrial biosynthesis [14]. Liver-specific Atgl−/− mice have an increased number of lipolysosomes, suggesting that lysosomal TG degradation might reduce hepatic steatosis under conditions of ATGL deficiency [27].
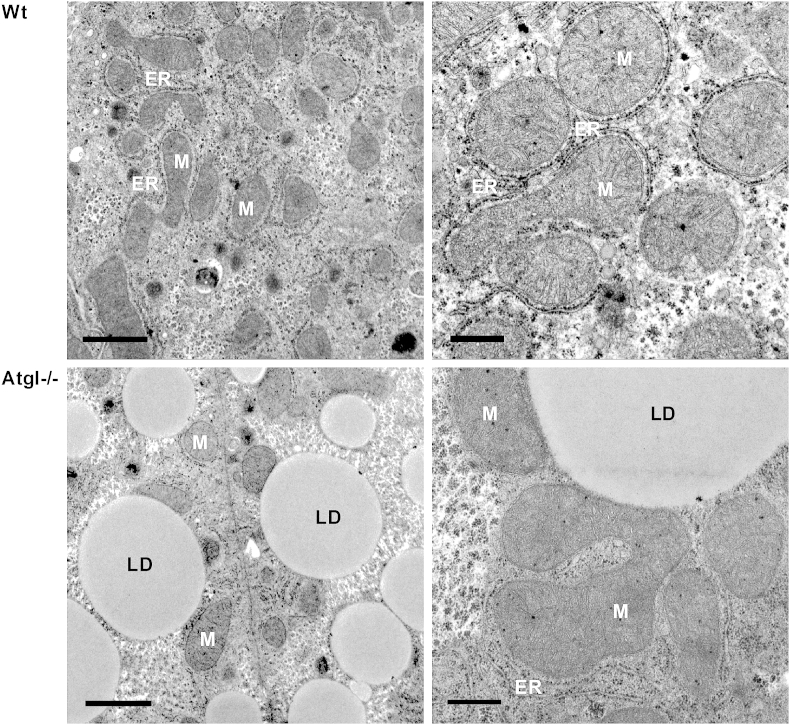
Fig. 2.
Transmission electron micrographs of liver sections from Wt and Atgl−/− mice. Wt liver cells (upper panels) show mitochondria (M) homogenous in appearance surrounded by rough endoplasmic reticulum (ER). Atgl−/− liver cells (lower panels) show a massive lipid droplet (LD) accumulation with morphologically intact mitochondria within the liver cells. Left panels: scale bars 1 μm; right panels: scale bars 0.5 μm.
In summary, absence of hepatic ATGL results in steatosis without inflammation [27], downregulation of PPARα target genes and consequently reduced β-oxidation [29]. Improved hepatic glucose tolerance but normal hepatic insulin signaling [28] and protection from tunicamycin-induced ER stress [26] argue for beneficial effects of ATGL deficiency on the liver.
3.4. Adipose tissues
Atgl−/− mice have enlarged fat depots, which is mostly pronounced in BAT [12]. In WAT, hormone-stimulated lipolysis is drastically reduced, indicating that ATGL is hormone-regulated via direct or indirect mechanisms. The transcriptional response to products of lipolysis in WAT and BAT reflects the functional differences of lipolysis in these tissues. In WAT mobilized FAs are released, whereas in BAT FAs activate uncoupling protein 1 and provide fuel for thermogenesis [30]. Thus Atgl−/− mice exhibit defective thermogenesis. ATGL may also affect PPARγ function since rosiglitazone-mediated PPARγ activation and lipid accumulation are associated with increased lipolysis and ATGL expression in rat adipose tissue [31]. Although increases in WAT mass and adipocyte volume have broad metabolic consequences including reduced mitochondrial function and increased ER stress [32,33], microarray studies in WAT of Atgl−/− mice revealed only moderate changes in the levels of transcripts associated with identifiable biological processes [34]. To our knowledge, no studies investigating mitochondrial function in Atgl−/− white adipocytes have been published so far. In contrast, microarray results from BAT indicate that ATGL deficiency results in decreased mRNA expression of genes involved in FA β-oxidation [34] and leads to downregulation of PPARα target genes in BAT [35]. Another study confirms that knockdown of ATGL significantly reduces the induction of the rate-limiting enzymes for mitochondrial FA β-oxidation [36]. Lipolysis is required for maximal induction of thermogenic genes by β-adrenergic receptor activation in BAT. Brown adipocytes lacking ATGL are fully responsive to exogenous PPARα and PPARδ ligands for increasing the expression of genes involved in FA oxidation and oxidative phosphorylation, indicating that the defect in gene regulation involves ATGL-dependent production of PPAR ligands. The authors conclude that in BAT ATGL is required for the maximal induction of genes involved in FA oxidation and mitochondrial electron transport [36].
3.5. Macrophages
Efficient FA oxidation and ATP synthesis depend on substrate availability, effective FA transport into the mitochondria, and efficient mitochondrial function. The marked downregulation of the PPAR target genes carnitine palmitoyltransferase 1a and long chain acyl-CoA dehydrogenase, the decrease of total acyl-CoA concentrations, and the reduction in essentially all middle and long chain carnitine esters [37] are indicators of mitochondrial dysfunction in Atgl−/− macrophages. Decreased concentrations of acetyl-CoA suggest defective mitochondrial β-oxidation, implicating that less NADH and FADH2 are available for oxidative phosphorylation to drive ATP synthesis. In fact, ATP levels are reduced in Atgl−/− macrophages, suggesting that the lipolytic defect affects the usage of FAs as energy fuel. As a consequence, decreased mitochondrial membrane potential, reduced mitochondrial O2 consumption, elevated cytosolic Ca2+, and intracellular reactive oxygen species concentrations are observed in Atgl−/− macrophages [38]. Mitochondria in Atgl−/− macrophages are clustered around lipid droplets, are less electron dense and smaller in size with aberrant cristae (Fig. 3), which provides evidence of considerable mitochondrial dysfunction [38], indicating a highly compromised mitochondrial function as a consequence of defective lipolysis.
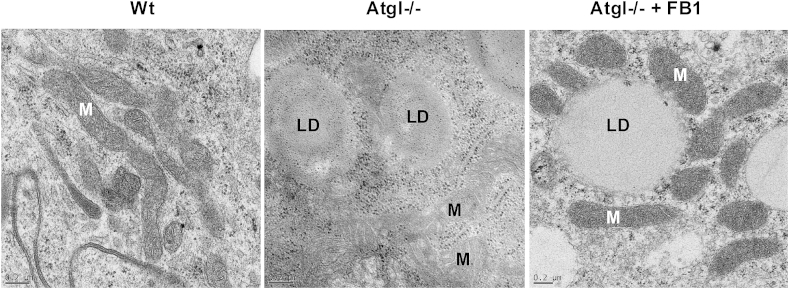
Fig. 3.
Transmission electron micrographs of macrophages from Wt and Atgl−/− mice. Wt macrophages show mitochondria (M) with intact cristae. Atgl−/− macrophages, which accumulate lipid droplets (LD), exhibit smaller, electron-light mitochondria with aberrant cristae. Treatment of Atgl−/− macrophages with the ceramide synthase inhibitor fumonisin B1 (FB1) normalizes mitochondria size and morphology [40]. Scale bars: 0.2 μm.
Fragmented mitochondria are defining features of apoptosis. Impaired mitochondria generate reactive oxygen species, which are capable of acting as second messengers that mediate multiple cellular responses, including apoptosis. Loading of macrophages with unesterified cholesterol in vitro induces programmed cell death via activation of the mitochondrial apoptosis pathway [39]. The absence of ATGL in macrophages, accompanied by TG accumulation, also results in an increase of typical markers of apoptosis [38] and ER stress [40]. In contrast with these results, TG accumulation in macrophages might also be cytoprotective [41,42]. Differences between the studies could be explained by additional mechanisms being activated in Atgl−/− macrophages. A highly increased concentration of the pro-apoptotic ceramide C16:0 might be responsible for induction of apoptosis and mitochondrial dysfunction in Atgl−/− macrophages [40]. The findings that Atgl−/− macrophages can be rescued from mitochondria fragmentation (Fig. 3), mitochondrial dysfunction and apoptotic cell death but not from ER stress by inhibition of ceramide synthesis by fumonisin B1 treatment indicate that C16:0 ceramide is essential and sufficient for apoptosis and mitochondrial dysfunction of Atgl−/− macrophages [40].
3.6. Other tissues
In the brain, intracellular FAs mediate the conversion into signaling lipid molecules, including eicosanoids, DGs, phospholipids, TGs, and fatty acyl carnitine species [43–45]. ATGL is expressed in various brain regions, including pyramidal cells of the hippocampus and the dentate gyrus, ependymal cells, and the choroid plexus [46]. Recent data from brains of Atgl−/− mice indicate that ATGL affects cerebral TG catabolism, in particular in regions in which brain metabolism interacts with the periphery [46]. Intracellular TG lipolysis by ATGL may be important to maintain FA supply and represents another mechanism of cerebral lipid management. Since FA β-oxidation in the brain occurs only at very low levels the impact of ATGL is expected to hardly influence this process. Atgl−/− mice show no sign of neurodegeneration, which might be due to the fact that 12–16 week old mice were used for the study [46].
ATGL has very recently been identified as an important TG hydrolase of the small intestine [47]. Absence of intestinal ATGL modulates PPARα signaling, thereby influencing the rate of FFA and cholesterol uptake from the intestinal lumen. Further data from this study revealed that intestinal ATGL deficiency has no influence on chylomicron production, suggesting that lipolytic products generated by ATGL are not used for intestinal lipoprotein production. The effects of intestinal ATGL deficiency on mitochondrial function are still elusive. However, as observed in liver and heart [14], genes involved in β-oxidation are down-regulated in the small intestine of fasted intestine-specific Atgl−/− mice, arguing for a decrease in β-oxidation rate [47]. Intact mitochondria albeit lipid droplet accumulation (Fig. 4) and unchanged mRNA expression of PGC-1α in Atgl−/− intestine (Obrowsky et al., unpublished observation) indicate normal mitochondrial biogenesis and function in Atgl−/− enterocytes. Downregulation of glutathion-S-transferases [47], which are important for the detoxification of xenobiotics and lipid peroxidation products [48,49], suggest that intestinal ATGL might play a role in the protection from oxidative stress.
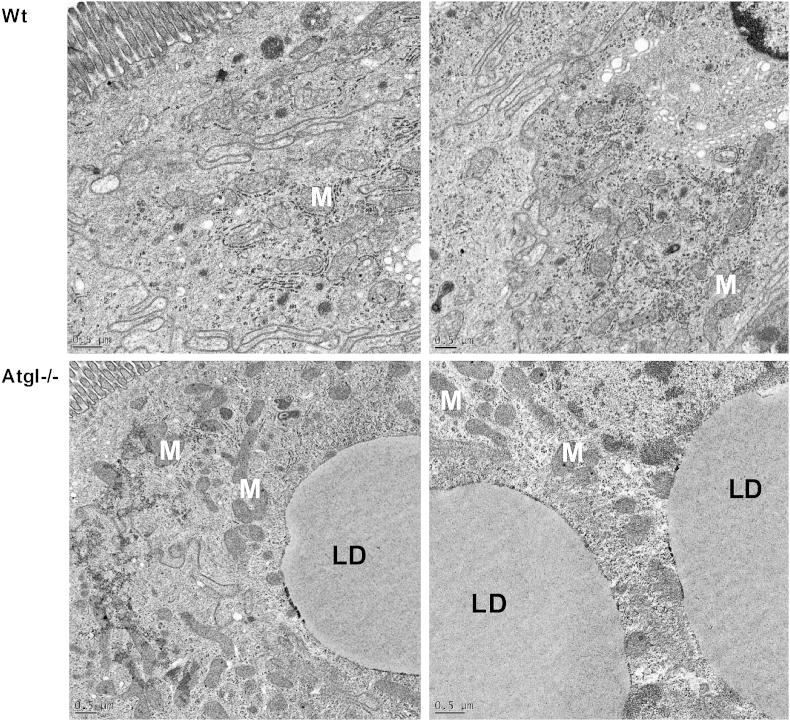
Fig. 4.
Transmission electron micrographs of small intestinal sections from Wt and intestine-specific Atgl−/− mice. Section from Wt (upper panels) and Atgl−/− (lower panels) small intestinal cells show intact mitochondria (M) albeit lipid droplet (LD) accumulation in Atgl−/− small intestine. Scale bars: 0.5 μm.
Knockdown of ATGL in pancreatic INS832/13 cells and its absence in pancreas of Atgl−/− mice results in ∼3-fold increased TG concentrations [50]. All findings published in this report (unchanged glucose oxidation, mitochondrial membrane potential, glucose usage, FA oxidation, and insulin content) illustrate that moderate increases in TG content within the pancreas protect against lipotoxicity, resulting in intact mitochondrial function.
4. Conclusion
Despite TG accumulation in all investigated Atgl−/− cells and tissues, current data on ATGL deficiency demonstrate that ATGL has pleiotropic roles in mediating mitochondrial function, depending on the type of cell or tissue. It is likely that ATGL-mediated lipolysis in cardiac muscle, BAT, and macrophages activates PPARα (and δ) through ligand-dependent and ligand-independent mechanisms to regulate mitochondrial gene expression and FA oxidation, thereby strongly influencing mitochondrial function. In the liver, ATGL deficiency leads to an upregulation of PGC-1α and PGC-1β, suggesting unchanged or even increased hepatic mitochondrial biosynthesis. According to studies performed so far defective lipolysis in skeletal muscle, WAT, small intestine, and pancreas has no impact on mitochondrial function. Numerous questions, including why ATGL exerts mitochondrial dysfunction in some but not all tissues, remain unanswered. Additional studies are necessary to answer the question whether tissue- or cell-specific targeting of ATGL might be a potential drug target for pharmaceutical intervention in humans.
Disclosure
DKr, SO and BR wrote the manuscript. DKo performed electron micrographs. All authors have approved the final article.
Acknowledgement
This work was supported by the grants P22832, SFB LIPOTOX F30 and DK-MCD W1226, which are funded by the Austrian Science Fund (FWF).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Virtue S., Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochim. Biophys. Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S. Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization. Expert Rev. Cardiovasc. Ther. 2011;9:329–340. doi: 10.1586/erc.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 5.Villena J.A., Roy S., Sarkadi-Nagy E., Kim K.H., Sul H.S. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 2004;279:47,066–47,075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins C.M., Mancuso D.J., Yan W., Sims H.F., Gibson B., Gross R.W. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004;279:48,968–48,975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 7.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J.G., Gorkiewicz G., Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin–Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang X., Lu X., Lombes M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X., Yang X., Liu J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle. 2010;9:2719–2725. doi: 10.4161/cc.9.14.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichmann T.O., Kumari M., Haas J.T., Farese R.V., Jr., Zimmermann R., Lass A., Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 2012;287:41,446–41,457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zechner R., Kienesberger P.C., Haemmerle G., Zimmermann R., Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E.F., Klingenspor M., Hoefler G., Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 13.Schoiswohl G., Schweiger M., Schreiber R., Gorkiewicz G., Preiss-Landl K., Taschler U., Zierler K.A., Radner F.P., Eichmann T.O., Kienesberger P.C., Eder S., Lass A., Haemmerle G., Alsted T.J., Kiens B., Hoefler G., Zechner R., Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 2010;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P.C., Zierler K., Schreiber R., Eichmann T., Kolb D., Kotzbeck P., Schweiger M., Kumari M., Eder S., Schoiswohl G., Wongsiriroj N., Pollak N.M., Radner F.P., Preiss-Landl K., Kolbe T., Rulicke T., Pieske B., Trauner M., Lass A., Zimmermann R., Hoefler G., Cinti S., Kershaw E.E., Schrauwen P., Madeo F., Mayer B., Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bessesen D.H., Rupp C.L., Eckel R.H. Trafficking of dietary fat in lean rats. Obes. Res. 1995;3:191–203. doi: 10.1002/j.1550-8528.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti M., Saltin B., Olsen D.B., van Hall G. High triacylglycerol turnover rate in human skeletal muscle. J. Physiol. 2004;561:883–891. doi: 10.1113/jphysiol.2004.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacchetti M., Saltin B., Osada T., van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J. Physiol. 2002;540:387–395. doi: 10.1113/jphysiol.2001.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt M.J., van Denderen B.J., Castelli L.A., Bruce C.R., Hoy A.J., Kraegen E.W., Macaulay L., Kemp B.E. Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol. Endocrinol. 2008;22:1200–1212. doi: 10.1210/me.2007-0485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Alsted T.J., Nybo L., Schweiger M., Fledelius C., Jacobsen P., Zimmermann R., Zechner R., Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. Endocrinol. Metab. 2009;296:E445–E453. doi: 10.1152/ajpendo.90912.2008. [DOI] [PubMed] [Google Scholar]
- 20.Shaw C.S., Jones D.A., Wagenmakers A.J. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem. Cell Biol. 2008;129:65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 21.Hoppeler H., Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med. Sci. Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- 22.Gollnick P.D., King D.W. Effect of exercise and training on mitochondria of rat skeletal muscle. Am. J. Physiol. 1969;216:1502–1509. doi: 10.1152/ajplegacy.1969.216.6.1502. [DOI] [PubMed] [Google Scholar]
- 23.Rowe G.C., El-Khoury R., Patten I.S., Rustin P., Arany Z. PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huijsman E., van de Par C., Economou C., van der Poel C., Lynch G.S., Schoiswohl G., Haemmerle G., Zechner R., Watt M.J. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. 2009;297:E505–E513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 25.Nunes P.M., van de Weijer T., Veltien A., Arnts H., Hesselink M.K., Glatz J.F., Schrauwen P., Tack C.J., Heerschap A. Increased intramyocellular lipids but unaltered in vivo mitochondrial oxidative phosphorylation in skeletal muscle of adipose triglyceride lipase-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2012;303:E71–E81. doi: 10.1152/ajpendo.00597.2011. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs C.D., Claudel T., Kumari P., Haemmerle G., Pollheimer M.J., Stojakovic T., Scharnagl H., Halilbasic E., Gumhold J., Silbert D., Koefeler H., Trauner M. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270–280. doi: 10.1002/hep.25601. [DOI] [PubMed] [Google Scholar]
- 27.Wu J.W., Wang S.P., Alvarez F., Casavant S., Gauthier N., Abed L., Soni K.G., Yang G., Mitchell G.A. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 28.Ong K.T., Mashek M.T., Bu S.Y., Mashek D.G. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. Faseb J. 2013;27:313–321. doi: 10.1096/fj.12-213454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls D.G., Rial E. A history of the first uncoupling protein, UCP1. J. Bioenerg. Biomembr. 1999;31:399–406. doi: 10.1023/a:1005436121005. [DOI] [PubMed] [Google Scholar]
- 31.Festuccia W.T., Laplante M., Berthiaume M., Gelinas Y., Deshaies Y. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49:2427–2436. doi: 10.1007/s00125-006-0336-y. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland L.N., Capozzi L.C., Turchinsky N.J., Bell R.C., Wright D.C. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1076–E1083. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- 34.Pinent M., Hackl H., Burkard T.R., Prokesch A., Papak C., Scheideler M., Hammerle G., Zechner R., Trajanoski Z., Strauss J.G. Differential transcriptional modulation of biological processes in adipocyte triglyceride lipase and hormone-sensitive lipase-deficient mice. Genomics. 2008;92:26–32. doi: 10.1016/j.ygeno.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadian M., Abbott M.J., Tang T., Hudak C.S., Kim Y., Bruss M., Hellerstein M.K., Lee H.Y., Samuel V.T., Shulman G.I., Wang Y., Duncan R.E., Kang C., Sul H.S. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mottillo E.P., Bloch A.E., Leff T., Granneman J.G. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 2012;287:25,038–25,048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandak P.G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., Tabas I., Levak-Frank S., Kratky D. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 2010;285:20,192–20,201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aflaki E., Radovic B., Chandak P.G., Kolb D., Eisenberg T., Ring J., Fertschai I., Uellen A., Wolinski H., Kohlwein S.D., Zechner R., Levak-Frank S., Sattler W., Graier W.F., Malli R., Madeo F., Kratky D. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J. Biol. Chem. 2011;286:7418–7428. doi: 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao P.M., Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J. Biol. Chem. 2000;275:23,807–23,813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 40.Aflaki E., Doddapattar P., Radovic B., Povoden S., Kolb D., Vujic N., Wegscheider M., Koefeler H., Hornemann T., Graier W.F., Malli R., Madeo F., Kratky D. C16 ceramide is crucial for triacylglycerol-induced apoptosis in macrophages. Cell Death Dis. 2012;3:e280. doi: 10.1038/cddis.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saraswathi V., Hasty A.H. Inhibition of long-chain acyl coenzyme A synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler. Thromb. Vasc. Biol. 2009;29:1937–1943. doi: 10.1161/ATVBAHA.109.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S., Schaffer J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farooqui A.A., Horrocks L.A., Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids. 2000;106:1–29. doi: 10.1016/s0009-3084(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 44.Rapoport S.I. In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. discussion 279–84. [DOI] [PubMed] [Google Scholar]
- 45.Lopaschuk G.D., Ussher J.R., Jaswal J.S. Targeting intermediary metabolism in the hypothalamus as a mechanism to regulate appetite. Pharmacol. Rev. 2010;62:237–264. doi: 10.1124/pr.109.002428. [DOI] [PubMed] [Google Scholar]
- 46.Etschmaier K., Becker T., Eichmann T.O., Schweinzer C., Scholler M., Tam-Amersdorfer C., Poeckl M., Schuligoi R., Kober A., Chirackal Manavalan A.P., Rechberger G.N., Streith I.E., Zechner R., Zimmermann R., Panzenboeck U. Adipose triglyceride lipase affects triacylglycerol metabolism at brain barriers. J. Neurochem. 2011;119:1016–1028. doi: 10.1111/j.1471-4159.2011.07498.x. [DOI] [PubMed] [Google Scholar]
- 47.Obrowsky S., Chandak P.G., Patankar J.V., Povoden S., Schlager S., Kershaw E.E., Bogner-Strauss J.G., Hoefler G., Levak-Frank S., Kratky D. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARalpha signaling. J. Lipid Res. 2013;54:425–435. doi: 10.1194/jlr.M031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins L.G., Hayes J.D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- 49.Siems W.G., Zollner H., Grune T., Esterbauer H. Metabolic fate of 4-hydroxynonenal in hepatocytes: 1,4-dihydroxynonene is not the main product. J. Lipid Res. 1997;38:612–622. [PubMed] [Google Scholar]
- 50.Peyot M.L., Guay C., Latour M.G., Lamontagne J., Lussier R., Pineda M., Ruderman N.B., Haemmerle G., Zechner R., Joly E., Madiraju S.R., Poitout V., Prentki M. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J. Biol. Chem. 2009;284:16,848–16,859. doi: 10.1074/jbc.M109.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]