Abstract
Ca2+ influx through voltage-activated Ca2+ channels and its feedback regulation by Ca2+-activated K+ (BK) channels is critical in Ca2+-dependent cellular processes, including synaptic transmission, growth and homeostasis. Here we report differential roles of cacophony (CaV2) and Dmca1D (CaV1) Ca2+ channels in synaptic transmission and in synaptic homeostatic regulations induced by slowpoke (slo) BK channel mutations. At Drosophila larval neuromuscular junctions (NMJs), a well-established homeostatic mechanism of transmitter release enhancement is triggered by experimentally suppressing postsynaptic receptor response. In contrast, a distinct homeostatic adjustment is induced by slo mutations. To compensate for the loss of BK channel control presynaptic Sh K+ current is upregulated to suppress transmitter release, coupled with a reduction in quantal size. We demonstrate contrasting effects of cac and Dmca1D channels in decreasing transmitter release and muscle excitability, respectively, consistent with their predominant pre- vs. post-synaptic localization. Antibody staining indicated reduced postsynaptic GluRII receptor subunit density and altered ratio of GluRII A and B subunits in slo NMJs, leading to quantal size reduction. Such slo-triggered modifications were suppressed in cac;;slo larvae, correlated with a quantal size reversion to normal in double mutants, indicating a role of cac Ca2+ channels in slo-triggered homeostatic processes. In Dmca1D;slo double mutants, the quantal size and quantal content were not drastically different from those of slo, although Dmca1D suppressed the slo-induced satellite bouton overgrowth. Taken together, cac and Dmca1D Ca2+ channels differentially contribute to functional and structural aspects of slo-induced synaptic modifications.
Keywords: Synaptic transmission, cacophony (CaV2), Dmca1D (CaV1), slowpoke (BK), synaptic homeostasis, EJPs, mEJPs, spontaneous vesicle release, larval neuromuscular junction (NMJ)
INTRODUCTION
Homeostasis of neuronal excitability and synaptic strength has been well demonstrated in a number of defined neural circuits in invertebrate species (Turrigiano et al., 1995; Marder et al., 1996; Stewart et al., 1996) and in vertebrates (Plomp et al., 1992; Turrigiano, 2004 for review). However, the underpinning molecular mechanisms still await further exploration. In Drosophila larval neuromuscular junctions (NMJs), a striking phenomenon was reported in an earlier study, in which nearly-intact excitatory junctional potential (EJP) sizes are observed despite the fact that the number of synaptic boutons or releasing sites are greatly decreased by Fasciclin II mutations (Stewart et al., 1996). Similar upregulation of transmitter release is observed when the miniature EJP (mEJP) amplitude, the quantal size, is diminished by mutations (Peterson et al., 1997; DiAntonio et al., 1999) and pharmacological blockade of glutamate receptors (Frank et al., 2006), or by forced expression of K+ channels in postsynaptic muscle cells (Paradis et al., 2001). A bone morphogenic protein (BMP) -mediated signaling mechanism has been discovered in follow-up investigations (Frank et al., 2009) to mediate this homeostatic adjustment that is triggered trans-synaptically to increase the number of vesicles released or the quantal content. This line of research has established a clear example of synaptic homeostasis in a genetic model system, in which cellular mechanisms of identified or novel signaling pathway can be further studied (Frank et al., 2006; Dickman and Davis 2009; Frank et al., 2009; Müller et al., 2012).
One conclusion derived from the above studies is that this homeostatic regulation depends on increased presynaptic Ca2+ influx (Frank et al., 2006, 2009; Müller et al., 2012). We have previously reported a surprising homeostatic regulation of synaptic strength of a different nature in slo mutants, in which synaptic transmission appears largely intact at physiological Ca2+ concentrations, despite the dysfunction in Ca2+-activated K+ channels (BK), a major feedback repolarizing force to terminate Ca2+ influx for transmitter release (Lee et al., 2008). The homeostatic adjustments to maintain nearly normal EJP sizes involve modifications of both pre- and post-synaptic properties. Specifically, presynaptic Shaker (Sh) K+ current is upregulated to compensate for the reduced repolarizing BK currents. Suppression of Sh K+ current in slo mutants by 4-AP immediately leads to explosive EJPs. Moreover, a change in postsynaptic glutamate receptor subunit compositions leads to reduced quantal size. These two adjustments contribute to the restoration of transmission levels in slo mutants (Lee et al., 2008).
In a separate study, we described a striking overgrowth of satellite boutons in slo larval NMJs (Lee and Wu, 2010), in which distinct patterns of genetic interactions of slo BK channels with two types of Ca2+ channels, separately encoded by cac and Dmca1D, dictate the expression of this satellite synaptic outgrowth. Therefore, it is of interest to determine whether such distinct couplings of Cac and Dmca1D channels with BK channels also play separate roles in homeostatic regulations of synaptic transmission in slo mutants (Lee et al., 2008). In the present study, physiological alterations in single and double mutants of cac, Dmca1D, and slo demonstrate distinct patterns of functional interactions between slo-encoded BK channels with cac- and Dmca1D-encoded Ca2+ channels. The findings implicate differential involvements of Cac and Dmca1D channels in homeostatic regulation of synaptic function and structure.
METHODS
Fly stocks
The fly stocks used in this study have been previously described (Lee et al., 2008; Lee and Wu, 2010) and include: wild type (WT) Canton-S (CS) and when specified in some cases, Oregon-R (OR); K+ channel mutants, slowpoke (slo) (slo1, slo98, slo1/slo4, and slo98/slo4); Ca2+ channel mutants, cacophony (cac) (cacS and cacNT27) and Dmca1D (Dmca1DAR66); double mutant combinations, cac;;slo (cacS;;slo1, cacS;;slo98, and cacS;;slo4) and Dmca1D;slo (Dmca1DAR66;slo1 and Dmca1DAR66;slo98). Data collected from different alleles of slo and their combinations with cacs and Dmca1DAR66 indicate similar physiological phenotypes. Thus, results from the different alleles are combined in analysis to increase statistical power. All these stocks were raised in the presence of conventional fly medium and maintained at room temperature.
Preparations and Electrophysiology
Preparation of wandering third instar larvae and intracellular recordings of excitatory junctional potentials (EJPs) and miniature EJPs (mEJPs) were performed as described previously (Lee et al., 2008). Briefly, evoked EJPs upon segmental nerve stimulation were recorded from muscle 6 or 7 in abdominal segments 3 through 6, mostly from 4th and 5th segments, in HL3.1 saline (Feng et al., 2004) containing Ca2+ at specified concentrations. The correction procedure for nonlinear summation of synaptic potential (Martin, 1955; Lee et al., 2008) was applied for EJP amplitude comparison. Miniature EJPs (mEJPs) at the resting membrane potential more negative than −60 mV were analyzed for their frequency and amplitude (MiniAnalysis, Synaptosoft Inc., Fort Lee, NJ, USA). Quantal content was calculated by dividing the mean EJP amplitude (corrected for nonlinear summation) by the mean mEJP amplitude at the same NMJs. All EJP data were collected using CLAMPEX and FETCHEX (version 5.5, Molecular Devices Corporation, Sunnyvale, CA, USA) and analyzed using CLAMPFIT (version 6.0, Molecular Devices Corporation) and Origin software (version 6.0, OriginLab Corporation, Northampton, MA, USA). Selective blockade of Sh K+ channel function was achieved by a bath application of 4-AP (0.2 mM, Sigma-Aldrich Corp., St. Louis, MO, USA) at least for 2 min before data collection.
Direct electrotonic stimulation of the nerve terminal
In order to examine the Ca2+ influx-dependent excitability in the nerve terminal, transmitter release was evoked by direct electrotonic stimulation of the nerve terminals after nerve action potentials were blocked with 3 μM TTX (Ganetzky and Wu, 1982). Depolarization (1 ms) was applied through the suction electrode near the nerve entry point in the proximity of motor terminals to increase the effectiveness of electrotonic stimulation. With increasing stimulus intensity, electrotonically evoked EJCs gradually increased in amplitude until a saturation level was reached. Under this condition, further amplification of the Ca2+-supported excitability in presynaptic axon terminals can be achieved by removing the repolarization force using K+ channel blockers 4-AP (4-Aminopyridine, 200 μM) and TEA (Tetraethylammonium, 20 mM). As a result, sustained presynaptic Ca2+ action potentials leads to strikingly prolonged EJPs, with durations up to a few seconds (Ganetzky and Wu, 1982, 1983; Ueda and Wu, 2009). In order to obtain consistent results, we adjusted the stimulus voltage to the level where these plateau EJPs were triggered most frequently.
Muscle current injection
Muscle action potentials were triggered by depolarizing current injection into muscle cells bathed in the HL3.1 saline containing 2 mM Sr2+, 0 mM Ca2+, and 16 mM Mg2+. Drosophila larval muscles normally do not display overshooting Ca2+ spikes. However, depolarizing current injection with saline containing Sr2+ can readily trigger muscle action potentials since Sr2+ is a more effective charge carrier through the Ca2+ channels, suppressing Ca2+ channel inactivation and partially blocking K+ channels (Hille, 2001). The Ca2+-free saline (nominally 0 mM) also helped to suppress muscle contraction. The high Mg2+ concentration (16 mM) compensates for the decreased surface charge screening effect due to the absence of Ca2+.
Pharmacological agents
Tetrodotoxin (TTX) was obtained from Sigma (St. Louis, MO, USA). Charybdotoxin (ChTX) was purchased from Alomone Labs (Jerusalem, Israel).
Immunohistochemistry and Image analysis
Wandering larvae at the third instar stage were fixed and prepared as described (Lee et al., 2008). Briefly, dissected larvae were fixed with either 100% ice-cold methanol for 5 min for immunostaining with both anti-DGluRIIA and -DGluRIIB antibodies, otherwise with 3.7% formaldehyde in phosphate buffer saline for 20 min. The primary antibodies used include: monoclonal Brp (NC82, 1:50, Developmental Studies Hybridoma Bank (DSHB), Univ. of Iowa) and DGluRIIA antibody (8B4D2, 1:50, DSHB, Univ. of Iowa), and rabbit polyclonal DGluRIIB (1:500) antibody (a generous gift from Dr. A. DiAntonio at Washington Univ., St. Louis). The fluorescent secondary antibodies at 1:250 were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The overall structure of NMJs was visualized with FITC-conjugated anti-HRP antibody. For direct comparison of DGluRIIA and DGluRIIB density among different genotypes, all larvae were processed together, treated with the same antibody and washout solutions within individual tubes and washout chambers throughout the procedure.
Images of NMJs on muscles 6/7 from abdominal segments 3 through 4 were collected using Zeiss LSM5 and LSM700 confocal microscopes (Carl Zeiss Microscopy, LLC, One Zeiss Drive, Thornwood, NY, USA) with an oil-immersed 40X objective and analyzed as previously described (Lee et al., 2008). For direct comparison of DGluRII subunit density measurements across genotypes, all larvae in the same batch were subjected to the same settings for exposure and signal detection. The average pixel intensity readings for DGluRIIA and DGluRIIB signals and their ratio (IIA/IIB) from the collapsed Z-stacked images for each genotype were then normalized to the average value from the WT NMJs to facilitate comparisons across different genotypes.
Statistical Analyses
For comparison between two genotypes, a Chi-Square test was carried out as specified in figure legends. For multiple comparison, we used One-way ANOVA followed by multiple comparisons using Fisher’s LSD test. P-values less than 0.05 was considered to be significantly different. All statistical analyses were performed using Origin software (version 6.0, OriginLab Corporation).
RESULTS
Effects of cacophony and Dmca1D mutations on Ca2+ dependency of synaptic transmission
As described above, it is well established that cac-encoded Ca2+ channels play an important role in regulating nerve-evoked synaptic responses. However, the role of Dmca1D-encoded L-type Ca2+ channels in synaptic transmission remains to be documented. Thus, we first compared how cac and Dmca1D mutations affect synaptic transmission in HL3.1 recording saline. Since the known null alleles of either cac or Dmca1D mutations are embryonic lethal (Smith et al., 1996; Eberl et al., 1998), the viable alleles of cac and Dmca1D mutations were used here (cacS and Dmca1DAR66). At 0.2 mM [Ca2+]o, we were able to detect reliable EJP responses upon nerve stimulation in both WT and Dmca1D larvae. At lower Ca2+ levels, the response amplitude of these EJPs was more variable, reflecting fluctuation in the number of released quanta. However, there were no significant differences in EJP amplitude between WT and Dmca1D [Fig. 1]. In contrast, a drastic reduction in transmitter release was found in all cacS larvae examined [Fig. 1], with some larvae (2/14) displaying complete response failures, i.e. no quanta released [Data not shown], consistent with the previous reports on a different synaptic preparation (Kawasaki et al., 2000; Kawasaki et al., 2002). As [Ca2+]o increased, EJP amplitude in both WT and Dmca1D larvae was drastically enhanced and reached saturated levels around 1.0 mM of [Ca2+]o. However, EJP amplitude in cacS mutants remained significantly smaller at physiological [Ca2+]o [0.5 mM or higher, Fig. 1(B)]. The above observations support the notion that synaptic vesicle release is regulated by Ca2+ influx mainly through cac-encoded Ca2+ channels in the presynaptic terminal.
Figure 1.
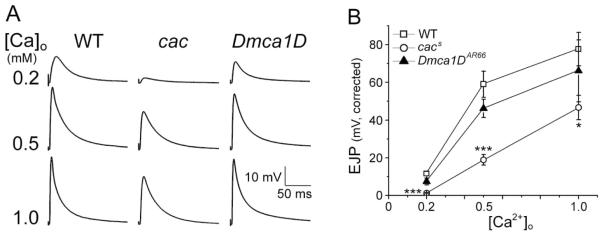
Altered Ca2+ dependency of EJP responses in cac mutants. (A) Representative traces of EJPs at different [Ca2+]o for WT, cac, and Dmca1D larvae. Note smaller EJPs in cac mutants than WT and Dmca1D at all [Ca2+]o. (B) Pooled EJP data at different [Ca2+]o for WT, cacS, and Dmca1DAR66 larvae. EJP amplitude is corrected for nonlinear summation (see Methods). The number of larvae for WT, cacS, and Dmca1DAR66 was 50, 14, and 9 at 0.2 mM Ca2+; 13, 13, and 8 at 0.5 mM Ca2+; 6, 6, and 4 at 1.0 mM Ca2+. Mean ± SEM are indicated. *, P<0.05 and ***, P<0.001, One-way ANOVA with Fisher’s LSD test for multiple comparisons.
Effects of cacophony and Dmca1D mutations on pre and postsynaptic membrane excitability
We examined the effects of cac and Dmca1D mutations on Ca2+-supported excitability in presynaptic axon terminals, using a previously established protocol of direct electrotonic stimulation of the terminal (Ganetzky and Wu, 1982, 1983; Ueda and Wu, 2009). After silencing Na+ spikes with TTX, the nerve terminal was depolarized by the electrotonic spread of a stimulus of increased duration, which lead to EJPs of graded amplitudes in response to varying stimulus voltage at WT NMJs (Wu et al., 1978). After removal of the repolarization force by K+ channel blockers 4-AP and TEA, the EJP response was no longer graded and displayed explosive all-or-none plateau potentials [Fig. 2(A)] due to sustained presynaptic Ca2+ spikes (Ganetzky and Wu, 1982; 1983), as first described in the squid giant synapse and the frog neuromuscular junction under similar conditions (Katz and Miledi, 1969a,b). As shown in Figure 2, such prolonged plateau EJPs were intact in the majority of Dmca1D muscle fibers tested (4 out of 6 muscles, 3 larvae), but were observed in cac larvae only in rare occasions [2 out of 8 muscles, 3 larvae; Fig. 2(A)]. Our result from the hypomorphic alleles (cacs, see Methods) is consistent with the previous imaging study on the same cac mutants, showing a decrease in Ca2+ influx-induced fluorescence signal (Macleod et al., 2006).
Figure 2.
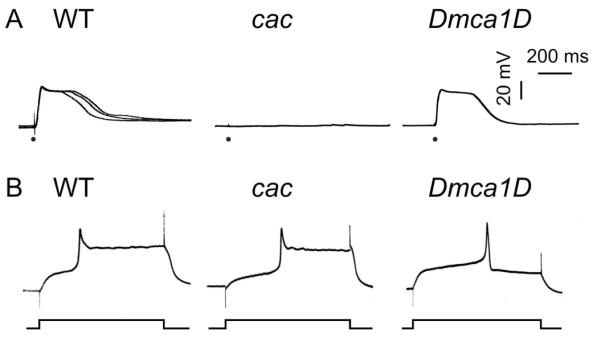
Preferential functional expression of cac and Dmca1D Ca2+ channels in pre- and post-synaptic sites. (A) Representative traces of EJPs triggered electrotonically in the presence of blockers of Na+ (TTX, 3 μM) and K+ (4-AP, 200 μM; TEA, 20 mM) channels at a low concentration of external Ca2+ (0.1 mM). ● indicates electrotonic stimulation of the nerve terminal (1 ms duration, see Methods). Rare occurrences of plateau EJPs in cac indicated weakened Ca2+ influx in the presynaptic terminals (see text). WT: 11 out of 11 NMJs (2 larvae of CS and 3 larvae of OR); cacs: 2 out of 8 NMJs (p < 0.01; 3 larvae); Dmca1DAR66: 4 out of 6 muscles (p > 0.05; 3 larvae). Chi-square test with sequential Bonferroni adjustment for multiple comparisons. (B) Representative traces of muscle action potentials triggered by current injection in saline containing 2 mM Sr2+, which partially blocks K+ channels and is a more effective charge carrier through muscle Ca2+ channels. Shortened muscle action potentials in Dmca1DAR66 (5 out of 5 muscles, 2 larvae; p < 0.001) in contrast to prolonged Sr2+ spikes in WT (10 out of 10 muscles, 8 larvae of CS) indicated weakened Ca2+ channel function in the muscle. Prolonged Sr2+ spikes were observed in all cacS muscle fibers (9 out of 9 muscles, 3 larvae; p > 0.05). Chi-square test with sequential Bonferroni adjustment for multiple comparisons.
We also examined the Ca2+-supported excitability in postsynaptic muscle membrane using the current injection technique (see Methods). As previously shown, Drosophila larval muscles normally do not display overshooting Ca2+ spikes unless the repolarizing K+ currents are suppressed by K+ channel blockers, such as TEA, or by using Sr2+ as the charge carrier, which also blocks K+ channels (Singh and Wu, 1990; Ueda and Wu, 2006). We found that by replacing Ca2+ with Sr2+, depolarizing current pulses (700 ms) evoked an overshooting spike potential followed by a sustained plateau lasting hundreds of milliseconds in both WT and cac larval muscles [Fig. 2(B)]. In contrast, we did not observe sustained plateau potentials in Dmca1DAR66, a hypomorph (Eberl et al., 1998), which was able to produce only brief overshooting spikes (10 ms). This is consistent with the previous finding that postsynaptic muscle Ca2+ currents are predominantly mediated by dihydropyridine-sensitive Ca2+ channels (Gielow et al., 1995).
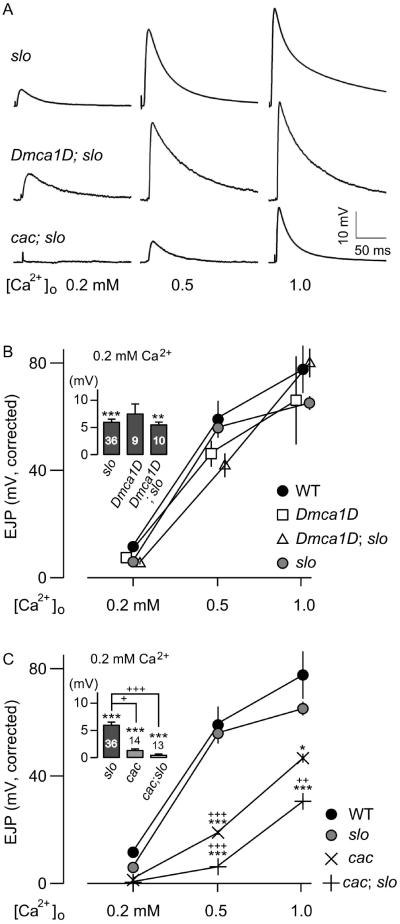
Effects of cac and Dmca1D on slo-induced synaptic homeostasis in double mutants
As described above, the known null alleles of either cac or Dmca1D mutations are embryonic lethal (Smith et al., 1996; Eberl et al., 1998), therefore the viable alleles of cac and Dmca1D mutations were used here (cacS and Dmca1DAR66). Despite an undetermined amount of residual currents that could remain in these hypomorphic mutant alleles, their profound functional consequences are clearly indicated by the severe EJP reduction in cacS [Fig. 1] and in the striking genetic interactions of both cacS and Dmca1DAR66 with slo in synaptic morphology previously described (Lee and Wu, 2010). These more commonly used cac and Dmca1D mutant alleles were thus employed in double mutant experiments described below. Another viable cac allele, cacNT27, displayed similar phenotypes of decreased EJP responses [Data not shown].
In Dmca1D;slo double mutants, there were only a few viable Dmca1D;slo third-instar larvae, few of which succeeded in eclosion, as expected from the severe lethality of Dmca1D mutants (Eberl et al., 1998). Surprisingly, the EJP amplitude in viable Dmca1D;slo escapees was comparable to that of slo single mutants, regardless of [Ca2+]o levels [Fig. 3(B); see also Fig. 1]. In other words, Ca2+-dependent homeostatic adjustment of EJP amplitude in slo mutants was apparently undisturbed in Dmca1D;slo double mutants. Thus, functional interaction between Dmca1D-encoded Ca2+ and slo BK channels in other cellular compartments or cell types may be responsible for the lethality of the double mutants.
Figure 3.
Effects of cac and Dmca1D mutations on slo EJP amplitude in double-mutant combinations. (A) Representative traces of EJPs at different [Ca2+]o for slo, cacs;;slo, and Dmca1DAR66;slo larvae. Note similar response amplitudes between slo and Dmca1DAR66;slo in contrast to smaller EJPs in cacs;;slo. (B) Pooled EJP data for Dmca1DAR66, slo, and the double mutants. (C) Pooled EJP data for cacs, slo, and the double mutants. Note further reduction in EJP amplitudes at different Ca2+ levels in cacs;;slo double mutants compared to cac single mutants. EJP amplitudes are corrected for nonlinear summation (see Methods). The insets show at a higher resolution the differences in EJP amplitudes at 0.2 mM [Ca2+]o. Mean ± SEM are indicated. * or +, P<0.05, ** or ++, P<0.01 and *** or +++, P<0.001, One-way ANOVA with Fisher’s LSD test for multiple comparisons, WT (*) or slo (+) vs. genotypes indicated.
In contrast to Dmca1D, striking interactions between cac and slo led to severely impaired synaptic transmission and an apparent suppression of slo-induced homeostatic regulation of EJP size [Fig. 3(C)]. Similar to Dmca1D;slo double mutants, several cac;;slo combinations of hypomorphic alleles are largely pupal lethal, while double mutants with the slo4 null allele were nearly larval lethal. Viable hypomorphic double-mutant larvae were smaller in size, and the adult escapees exhibited an uncoordinated walking behavior, abnormal wing posture, poor flight ability, and short lifespan [Data not shown].
In addition to these developmental and behavioral abnormalities, we found a drastically reduced EJP amplitude in cac;;slo double mutants. At lower external Ca2+ concentrations (0.2 mM of [Ca2+]o), complete synaptic failures (no detectable synaptic responses upon nerve stimulation) occurred in the majority of preparations [8/13; Fig. 3(A,C)], with only a few larvae displayed occasional EJP responses upon repetitive nerve stimulation [Data not shown]. Furthermore, there was no indication of slo-induced homeostatic responses in cac;;slo, i.e. restoration of EJP amplitude to the WT level at physiological [Ca2+]o, but rather, a further decrement of EJP amplitudes compared to either single mutant [Fig. 3(A,C)]. These findings illustrate the important functional coupling between cac-encoded N-type Ca2+ and slo-encoded BK channels in maintaining synaptic function and homeostasis. It is surprising that weakening of repolarization due to slo BK channel disruption could actually result in further EJP amplitude reduction in cac;;slo double mutants as compared to cac single mutants, indicating altered developmental regulation of the transmitter release mechanism. To test this notion, we employed a highy specific antagonists of BK channels, charybdotoxin (ChTx, 50 nM, Elkins et al., 1986, Singh and Wu, 1990) to eliminate acutely Slo BK K+ currents in the cac mutant background. Consistently, reduction in EJP amplitude in cac;;slo could not be mimicked by the acute blockade of Slo BK current by ChTx in cac mutants, confirming a long-term regulatory effect conferred by cac-slo interaction [Table 1].
Table 1.
Effects of charybdotoxin (ChTx) on evoked EJPs. EJPs were recorded from the same muscles before and after ChTx (50 nM) treatment. Note that the amplitude and failure rate of EJPs were not significantly affected by ChTx. Data for cac is pooled from cacS (n = 2) and cacNT27 (n = 3). Mean±SEM are indicated.
| Genotype | Before ChTx | After ChTx | n | ||
|---|---|---|---|---|---|
|
| |||||
| ejp (mV) | Failure (%) | ejp (mV) | Failure (%) | ||
| WT | 12 ± 1.2 | 0 ± 0 | 13 ± 2.7 | 0 ± 0 | 5 |
| cac | 2.7 ± 0.78 | 22 ± 5.6 | 2.4 ± 1.1 | 36 ± 16 | 5 |
Effects of cac and Dmca1D on slo-induced homeostatic regulation of postsynaptic quantal response
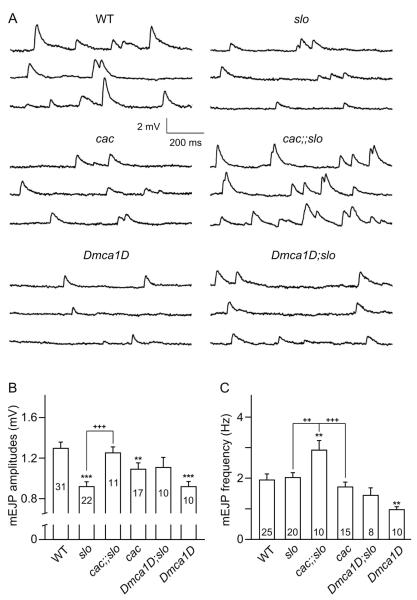
Spontaneous miniature EJPs (mEJPs) represent the postsynaptic quantal response to the randomly occurring unitary fusion event of presynaptic transmitter vesicles. The amplitude of these events in part reflects postsynaptic receptor properties or density. We have previously demonstrated that homeostatic adjustment of synaptic strength in slo mutants is partially achieved via a reduction in quantal size (Lee et al., 2008). We thus examined the consequences of interaction between Slo and pre- vs. post-synaptic Ca2+ channels in single and double mutants by monitoring these quantal events.
We found that although cac mutations caused a moderate, but significant, reduction in mEJP amplitude, intriguingly, cac;;slo double mutants displayed significantly greater mEJP amplitudes compared to the reduced mEJP size in slo single mutants, essentially restored to the WT-level quantal size [Fig. 4(A,B)]. This observation again supports a major role of cac in slo-induced homeostatic reduction in postsynaptic quantal response. We found that a similar trend of quantal size increase might also be present in Dmca1D;slo double mutants although it did not reach a statistically significant level [Fig. 4(A,B)].
Figure 4.
Effects of cac and Dmca1D mutations on spontaneous quantal release. (A) Examples of mEJP traces for WT, cacS, Dmca1DAR66, slo, cacS;;slo, and Dmca1DAR66;slo larvae. (B, C) Pooled data for mEJP amplitude (B) and frequency (C). Note fewer mEJPs in Dmca1DAR66 mutants in contrast to more frequent occurrence in cacS;;slo, and greater mEJP amplitude in WT and cacS;;slo compared to slo. The number of larvae is indicated for each genotype. Mean ± SEM. *, P<0.05, ** or ++, P<0.01 and *** or +++, P<0.001, One-way ANOVA with Fisher’s LSD test for multiple comparisons, WT (*) vs. other genotypes, or each pair indicated (+).
We further examined the rate of spontaneous vesicle fusion events in presynaptic terminals and discovered unexpected alterations associated with Dmca1D, cac, and slo interactions in different manners. Interestingly, among the three single mutants, mEJP frequency was significantly reduced only in Dmca1D mutants compared to WT [Fig. 4(C), P<0.01, WT vs. Dmca1D; see also examples of mEJPs in Fig. 4(A)]. Given its postsynaptic localization at the NMJ, a transsynaptic signaling mechanism may be involved to exert the Dmca1D mutational effects on the presynaptic release process. When Dmca1D was combined with slo in double mutants, the frequency of mEJPs was not different from that of slo or WT larvae [Fig. 4(C)]. Unexpectedly, a stronger genetic interaction between cac and slo was indicated by a drastic increase in spontaneous mEJP frequency in cac;;slo double mutants, clearly surpassing that in WT and either single mutant [Fig. 4(C); see also examples of mEJP traces in Fig. 4(A)].
Together with the restoration of quantal size, the contrasting effects of combining Dmca1D and cac with slo on the mEJP frequency suggest different modes of regulation. Apparently, a trans-synaptic molecular mechanism exists to confer the Dmca1D interaction with slo vs. a presynaptic mechanism for cac interaction with slo to produce the observed regulations on the presynaptic release efficacy.
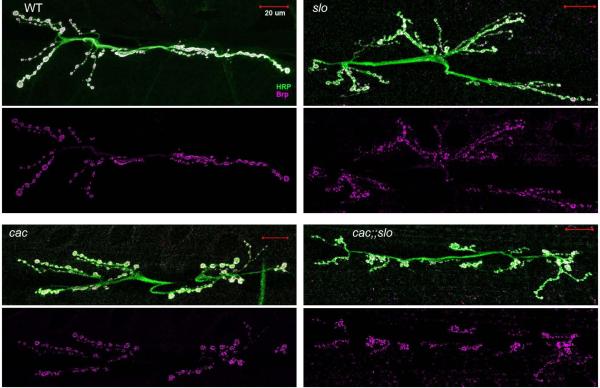
Effects of cac mutations on presynaptic active zone distributions and postsynaptic glutamate receptor properties
As described above, our data demonstrate alterations in the size and frequency of quantal events induced by cac mutations in combinations with slo [Fig.4]. Thus, we performed immunostaining of pre- and post-synaptic molecular markers to probe the synaptic components responsible for quantal size determinations and transmitter release regulations. We employed the antibodies specific for the presynapyic active zone protein Bruchpilot and the postsynaptic receptor subunits GluRIIA and B. Our results from NC82 staining [Fig. 5] clearly demonstrated that Bruchpilot proteins in the active zones remained abundant in cac;;slo double mutants. The NMJ structure visualized with anti-HRP immunoreactivity (see Methods) also indicated that the general morphology of the nerve-terminal arbors in double mutants was not drastically altered, with nearly normal numbers of synaptic boutons and Bruchpilot reactive puncta, although there were still abundant satellite boutons (cf. Lee & Wu, 2010). However, the modification in satellite bouton numbers could not be the cause of enhanced spontaneous mEJP frequencies in cac;;slo because a similar level of satellite bouton overgrowth occurred in slo without a corresponding increase in spontaneous mEJPs [Fig. 4]. The resolution of our NC82 immunostaining images does not allow firm quantitative conclusions on potentially altered number or intensity of active zone immuno reactivity. Future ultrastructural analysis is required to examine whether alterations of the active zone are responsible for the drastically decreased release efficacy (or low quantal content) indicated by the diminished cac;;slo EJPs.
Figure 5.
Gross morphology of NMJs and active zone distribution in slo, cac, and cac;slo mutations. Boutons in slo and cacS;slo exhibited frequent satellite boutons (anti-HRP antibody staining; Lee & Wu, 2010) with active zones (anti-BRP antibody staining). However, no significant alterations in the intensity of anti-BRP staining was observed in slo, cacS, and cacS;slo NMJs compared to WT (cacNT27: 5 NMJs from 2 larvae; cacs: 5 NMJs from 2 larvae; cacs;slo1: 6 NMJs from 2 larvae; slo1: 4 NMJs from 2 larvae; WT: 5 NMJs from 3 larvae).
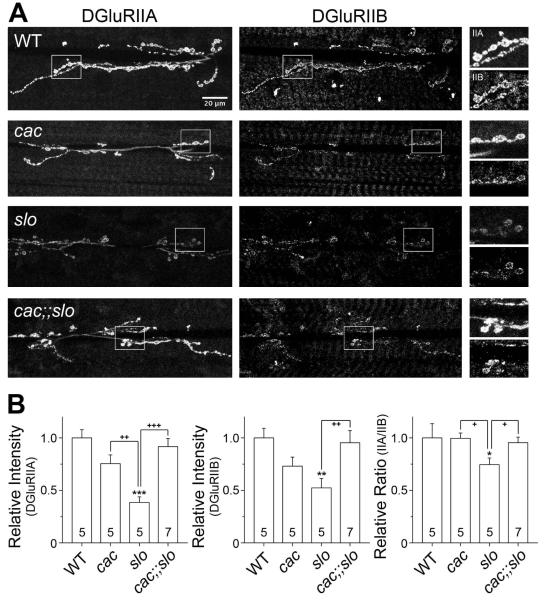
We have previously reported that the regulation of quantal event amplitude, i.e. mEJPs, may in part involve modification in postsynaptic receptor composition or density, which contributes to homeostatic regulation of synaptic strength in the absence of Slo BK channel activities (Lee et al. 2008). Importantly, our data indicate clear restoration of quantal size in cac;;slo mutants, in contrast to reduced mEJP amplitude in slo single mutants. Thus, we performed immunohistochemical analysis of postsynaptic glutamate receptor properties in WT as well as slo, cac, and cac;;slo mutants. For direct comparisons of receptor density, we took precautions to obtain more quantitative results from simultaneous GluRIIA and GluRIIB immunostaining. The larval preparations from different genotypes were processed together and went through the staining procedure in the same chamber. Figure 6 shows a clear decrease in GluRIIA and GluRIIB immunoreactivity in slo, which may underlie the reduced mEJP size in slo. Furthermore, there was a reduced GluRIIA/GluRIIB (IIA/IIB) ratio because of the more drastic reduction in IIA than IIB [Fig. 6], in line with our observation that also demonstrates a positive correlation between the receptor subtype ratio (IIA/IIB) and quantal size (Lee et al., 2008). Significantly, the postsynaptic receptor density and IIA/IIB ratio were restored to nearly-WT levels in cac;;slo double mutants [Fig. 6], consistent with the restoration of quantal size to the WT mEJP level in the electrophysiological recordings [Fig. 4(B)]. Taken together, these observations further confirm a major role of cac in slo-induced homeostatic reduction.
Figure 6.
Effects of slo, cac, and cac;;slo mutations on postsynaptic glutamate receptor density and composition. (A) Relative distributions of postsynaptic DGluRII subunits are shown for each genotype indicated. Note that samples of all genotypes were treated simultaneously throughout the entire immunohistochemical and image analysis procedures (see Methods). Boxed regions in the DGluRIIA and DGluRIIB staining images (two left panels) are magnified twice and presented on the right. Scale bar, 20 μm. (B) Data for the pixel intensity of each DGluRII subunit and their relative ratio are pooled and compared among different genotypes. Note that all values were normalized to that of WT. Mean ± SEM are indicated. * or +, P<0.05, ** or ++, P<0.01 and *** or +++, P<0.001, One-way ANOVA with Fisher’s LSD test for multiple comparisons, WT (*) vs. other genotypes, or slo (+) vs. paired genotypes.
Effects of acute pharmacological blockade of slo BK channels on quantal size
To gain insight into the time scale of the potential homeostatic mechanisms, it is important to establish whether such phenotype could be mimicked by acute blockade of functional interaction between these two channel types. We applied ChTx (50 nM) to eliminate acutely Slo BK K+ currents in the cac mutant background. As shown in Figure 7, the ChTx treatment in two independent alleles of cac mutants failed to reproduce the phenotypes of cac;;slo in the upregulation of the frequency of mEJPs [Fig. 7, cacS;cacNT27, Data not shown]. Furthermore, there was no significant change in either mEJP or EJP amplitude following an application of ChTx in cac mutants [Fig. 7(C) and Table 1]. Taken together, these results indicate that disrupted coupling between presynaptic Ca2+ and BK channels can lead to an altered development of synaptic vesicle pool distribution or release mechanism, leading to the striking increase in spontaneous fusion events.
Figure 7.
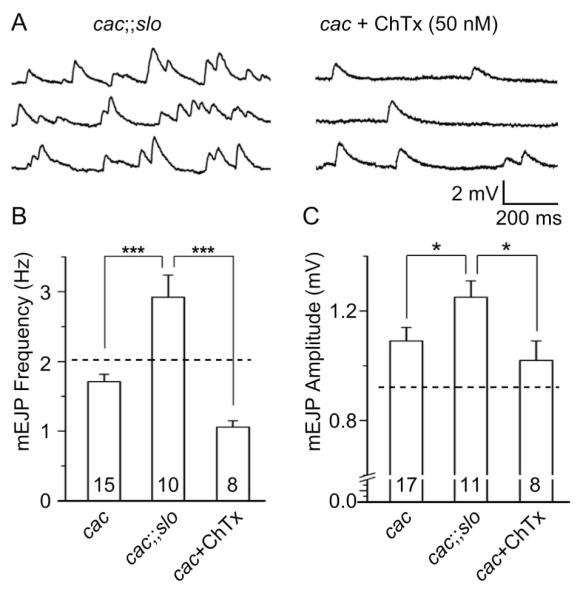
Lack of acute effects of BK current blockade by ChTx on spontaneous release in cac mutants in contrast to striking chronic effects of of BK channel disruption in cac;;slo double mutants. (A) Examples of mEJP traces for cacS;;slo double mutants (left) and cacS mutants treated with ChTx (50 nM). (B, C) Pooled data for mEJP frequency and amplitude. Dashed lines indicate the values for slo mutants (cf. Fig. 4). The results of ChTx treatment on cacS mEJPs are compared with corresponding cacS and cacS;;slo data (cf. Fig. 4). Note that the phenotype of larger and higher frequency of mEJPs in cacS;;slo could not be reproduced by acute blockade of slo BK channels in cacS mutants. Mean ± SEM are indicated. *, P<0.05 and ***, P<0.001, One-way ANOVA with Fisher’s LSD test for multiple comparisons between genotypes paired.
Effects of cac and Dmca1D on slo-induced homeostatic upregulation of presynaptic 4-AP-sensitive Sh IA
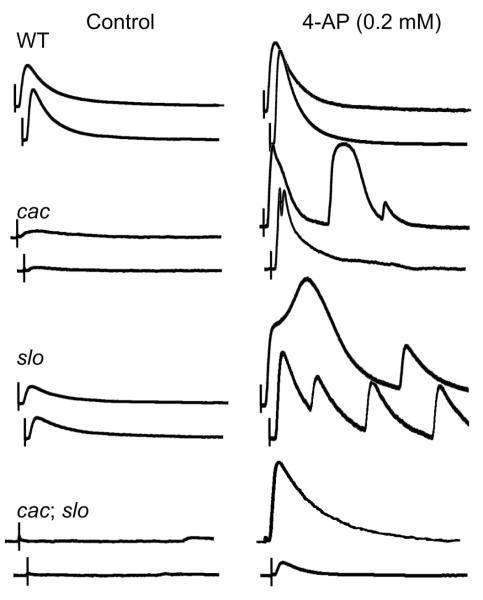
The strong synergistic effects of cac and slo mutations on mEJP and EJP properties prompted us to seek indications for a role of cac Ca2+ channels in another prominent feature of slo-induced synaptic homeostatic responses, i.e. upregulation of presynaptic Shaker (Sh) IA in compensation for the loss of repolarizing Slo BK current. As previously demonstrated, 4-AP treatment produces a striking effect on slo NMJs, in which supernumerary or prolonged EJPs of increased amplitudes can be evoked by a single nerve stimulus (Lee et al., 2008). Such multiple EJPs suggested presynaptic hyperexcitability upon 4-AP blockade of upregulated Sh IA, a consequence of eliminating both voltage-activated IA and Ca2+-activated BK, causing motor axon terminal repetitive firing that was not observed in WT larvae under the same treatment [Fig. 8]. We examined how this feature is further modified by cac and Dmca1D mutations in double mutants at a low Ca2+ level (0.2 mM, well below saturation levels of EJPs in order to optimize the sensitivity of detection).
Figure 8.
Altered 4-AP sensitivity of slo, cac, and cac;;slo double mutants. Representative traces of EJP responses before and after a 4-AP treatment (0.2 mM) in WT, slo, cacS, and cacS;;slo larvae. Unlike in WT, selective blockade of Sh IA by 4-AP in slo and cacS mutants induced prolonged and supernumerary EJPs, indicative of presynaptic hyperexcitability (cf. Lee et al., 2008). In cacS;;slo double mutant larvae, EJPs increased in size significantly but supernumerary EJPs rarely occurred at 0.2 mM 4-AP. Saline contained 0.2 mM Ca2+. Scale bar, 10 mV and 100 ms.
Importantly, despite drastically decreased transmission compared to slo, cac single mutants displayed certain aspects similar to slo larvae following 4-AP treatment, i.e., a tendency of producing broadened and supernumerary EJPs not found in WT larvae [Fig. 8]. It is likely that reduced Ca2+ current in cac mutants would result in weakened activation of Slo (BK) K+ current, thus indirectly trigger slo-like upregulation of Sh IA. Our previous voltage-clamp studies show that reduction in Slo current in cultured cac neurons is compensated for by a Sh IA increase to maintain a nearly constant total K+ conductance (Peng and Wu, 2007). Thus, a similar compensatory mechanism may be activated in cac NMJs. Along with reduced Slo current, 4-AP blockade of upregulated Sh IA in cac could lead to the phenotype observed here [Fig. 8].
The increment of EJP amplitude in cac;;slo after 0.2 mM 4-AP treatment was clearly to a lesser extent as compared to that in either single mutants [Fig. 8]. However, the proportional increase in quantal content could be in fact higher than WT because of the much suppressed release in cac;;slo before 4-AP treatment [Fig. 3 and Fig. 8; compare cac;;slo with cac or slo]. Notably, cac;;slo mutants, in some rare cases, were capable of generating supernumerary EJPs with further increment of 4-AP [from 0.2 to 2 mM; Data not shown]. These results suggest that slo-induced homeostatic upregulation of presynaptic Sh IA might still be present in cac;;slo, albeit to a lesser extent.
In contrast to cac;;slo, 4-AP-treated Dmca1D;slo double mutants displayed supernumerary EJPs similar to slo [Data not shown], indicating that slo-induced enhancement of 4-AP sensitivity could occur with disrupted Dmca1D-encoded postsynaptic Ca2+ channels.
The highly nonlinear nature of supernumerary and prolonged EJPs caused by 4-AP treatment precludes quantitative estimates of Sh IA upregulation from dosage-dependent measurements. However, qualitative assessments of the increase of EJP sizes after 4-AP treatment clearly indicate that the 4-AP effects were greatest on slo and cac, less on cac;;slo, and least on WT.
DISCUSSION
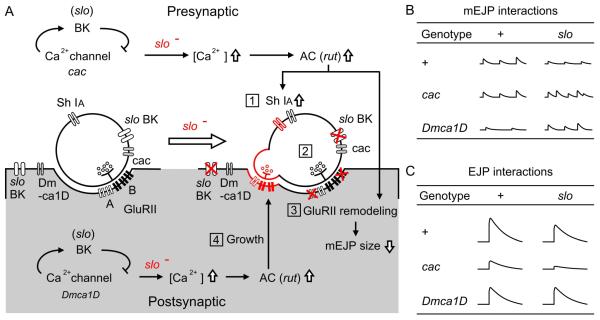
In this study, we extended our previous findings to investigate the roles of two identified Ca2+ channels in slo mutation-induced homeostatic regulation of presynaptic transmitter release and postsynaptic quantal response. We first characterized the functional roles of the cac- and Dmca1D-encoded Ca2+ channels in membrane excitability and transmitter release control at the larval NMJ [Fig. 1 and Fig. 2]. In different double mutant combinations with slo, we further determined the effects of cac and Dmca1D mutations on slo-induced homeostatic regulation in the pre- and post-synaptic compartments. Figure 9 summarizes several observed features of synaptic homeostasis that have been found in mutants of slo BK channels in the previous and present studies, i.e. cAMP-dependent upregulation of presynaptic Sh IA, postsynaptic modification of glutamate receptor composition, and synaptic bouton growth (Lee et al., 2008; Lee and Wu, 2010), as well as further modifications of these phenotypes by cac and Dmca1D in cac;;slo and Dmca1D;slo double mutants. Our results indicate separate roles of two distinct Ca2+ channels in slo-induced homeostatic regulatory processes, with cac and Dmca1D preferentially affecting synaptic function and structure, respectively [Fig. 9]. Our work also revealed several unexpected chronic effects of cac and Dmca1D mutations on both quantal size and spontaneous mEJP discharge frequencies [Fig. 4 and Fig. 7].
Figure 9.
Summary diagrams for slo mutation-induced homeostatic adjustments in synaptic transmission and growth and the potential roles of cac and Dmca1D in pre- and post-synaptic compartments. (A) The homeostatic adjustments are high-lighted in red and numbered: (1) up regulation of presynaptic Sh channel, (2) unaltered mEJP frequency, (3) decreased mEJP amplitude due to modified postsynaptic DGluRII subunit density and composition, and (4) increased number of satellite bouton growth. The potential roles of cac and Dmca1D Ca2+ channels and their site of action, as well as the rut adenylyl cyclase (AC)-mediated adjustments are depicted. (B and C) Modifications in mEJP (B) and EJP (C) amplitudes are summarized for cac, Dmca1D, slo, and their double-mutant combinations.
Types of voltage-gated Ca2+ channels in Drosophila
The influx of Ca2+ via voltage-activated Ca2+ channels influences a diversity of cellular function and growth processes. In vertebrates, subtypes of Ca2+ channels, e.g. L, N, P, Q, and R types, vary in threshold, inactivation, and pharmacological properties, and are identified with distinct functional domains of the α1 pore-forming subunit (Tsien et al., 1988; Catterall, 2000; Ertel et al., 2000). In Drosophila, the existence of distinct Ca2+ channel types in neurons and muscles has been suggested by differences in sensitivity to the various Ca2+ channel blockers (Pauron et al., 1987; Greenberg et al., 1989; Leung et al., 1989; Pelzer et al., 1989; Leung and Byerly, 1991; Singh and Wu 1999). Subsequent genetic and molecular studies have identified two genes encoding distinct Ca2+ channel α1 subunits; cac encodes a subunit that shares sequence similarities to vertebrate N-type Ca2+ or CaV2 channels (von Schilcher 1976; Smith et al., 1996; Rieckhof et al., 2003), and Dmca1D encodes a subunit homologous to rabbit brain L-type or CaV1 channels (Zheng et al., 1995). Our report here further extends the functional characterization of these two channels in larval nerve and muscle. A third class of Ca2+ channel α1 subunits of low voltage-activated (LVA) CaV3 channels has been inferred from sequence homology of a gene, Dmca1G (Littleton and Ganetzky, 2000). Further, patch-clamp studies have demonstrated the existence of a LVA current in adult thoracic motor neuron somata (Worrell and Levine, 2008; Ryglewski et al., 2012) and in cultured embryonic neuroblasts (Peng and Wu, 2007). However, it remains to be determined whether the Dmca1G-encoded channel also plays a role in regulating NMJ function or structure. Another gene, straightjacket, is known to encode an auxiliary subunit for the cac Ca2+ channel (Dickman et al., 2008; Ly et al., 2008). This α2-delta subunit is important for localization of Cac channels to trigger synaptic transmission (Dickman et al., 2008; Ly et al., 2008) and for regulating synaptic vesicle recycling (Kuromi et al., 2010). The α2-delta subunit can conceivably modify slo-induced synaptic homeostasis as well.
Importance of intact cac Ca2+ channels in slo-induced regulation of mEJP and EJP amplitudes
One important feature of slo-induced synaptic homeostasis is to reduce the quantal size. We found normal density and subunit composition of postsynaptic DGluRII [Fig. 6] in correlation with normal size of mEJPs [Fig. 4] in cac;;slo double mutants. This result demonstrates that Cac channel function is required for the slo -induced down regulation of mEJP amplitude [Fig. 9]. In fact, a central role of Cac channels has been established in a different form of synaptic homeostatic regulation upon perturbations of postsynaptic glutamate receptor activities (Petersen et al., 1997; Frank et al., 2006; Frank et al., 2009). In these studies, reducing quantal size by philanthotoxin (PhTx) or dGluRIIA mutations leads to homeostatic increase in presynaptic neurotransmitter release, to restore synaptic strength. Such homeostatic responses are suppressed by cac mutations affecting CaV2.1 (Frank et al., 2006; Frank et al., 2009).
Although the exact mechanisms remain unknown, the slo-induced quantal size adjustment may involve trans-synaptic signaling to allow presynaptically localized Cac channels to confer modification of slo-induced adjustment of postsynaptic receptor density and composition [Fig. 7]. One possibility is that the severe reduction in the EJP size at cac;;slo NMJs reflect drastically decreased vesicle release, which may limit the discharge of unknown factors stored along with the excitatory neurotransmitter glutamate. For example, neuropeptides may be co-released with transmitter during exocytosis. Recent studies have implicated spontaneous vesicle release as a part of molecular mechanisms to regulate synaptic strength during activity-dependent synaptic plasticity in Aplysia cultures (Jin et al., 2012a; Jin et al., 2012b).
Nerve-evoked and spontaneous vesicle release in single and double mutants
One interesting and unexpected finding is a clear effect of interaction between cac and slo on the spontaneous discharge of synaptic vesicles. Although neither cac nor slo single mutants showed altered mEJP frequencies, the double mutants displayed a striking increase in the rate of spontaneous mEJPs [Fig. 4]. This interaction is likely to be localized to the presynaptic site to influence spontaneous vesicle release. Our attempt of immuno-chemical staining against the active zone protein Bruchpilot [Brp; recognized by NC82 antibodies, Fig. 5] failed to detect obvious changes in numbers of active zones. Future electron microscopy observations will be required to examine whether more abundant synaptic vesicles or altered proportion of vesicle pools exist near release sites and other subcellular locations (Sudhof, 2004) at cac;;slo larval NMJs.
Another notable non-additive interaction between cac and slo mutations is indicated by the unusually small EJP size in the double mutants. Quantal content deduced from mean EJP and mEJP amplitudes was decreased in cac larvae, but was even more drastically reduced in cac;;slo double mutants (nearly to the level of complete transmission failure at the lower Ca2+ levels, 0.2 mM), indicating further suppression of presynaptic neurotransmitter release [Fig. 3; 9.57±1.72 mV in WT vs. 0.16±0.16 mV in cac;;slo]. This severely suppressed vesicle release could in principle be caused by either a further decrease in Ca2+ influx through Cac channels or a further increase in slo-induced Sh IA upregulation. However, our 4-AP treatment experiments do not support a striking upregulation of Sh IA. Whether Ca2+ influx might be further reduced by the loss of both Cac Ca2+ and Slo BK channel activities in mutants remains to be addressed.
Importance of intact Dmca1D Ca2+ channels in slo-induced morphological modifications
Our data indicate that unlike cac mutations, altered Dmca1D function did not exhibit clear modifications of physiological aspects of slo-induced synaptic homeostasis, including quantal size reduction and ShIA upregulation. This is in contrast to the previous report that a slo-induced, cAMP-dependent aberrant growth of satellite boutons is suppressed in Dmca1D;slo double mutants, but not in cac;;slo double mutants (Lee and Wu, 2010). This interaction leads to abundant satellite boutons and likely involves postsynaptic functional coupling of Dmca1D Ca2+ and slo BK channels to achieve this trans-synaptic effect on bouton growth regulation [Fig. 9]. As previously shown, Ca2+/calmodulin-activated rut adenylyl cyclase (AC) is required for Sh IA upregulation and satellite bouton overgrowth in slo (Lee et al., 2008; Lee and Wu, 2010). Conceivably, presynaptic and postsynaptic Ca2+ influx can contribute to the activation of rut AC and the initiation of cAMP cascade [Fig. 9].
Recently, synpatotagmin IV (Syt4), a potential Ca2+ sensor for postsynaptic release of retrograde signaling molecule, has been implicated in an increase in presynaptic spontaneous vesicle release following a tetanic nerve stimulation at embryonic NMJs in Drosophila (Yoshihara et al., 2005; Barber et al., 2009). It will be interesting to determine whether Dmca1D channels provide Ca2+ sources for activating Syt4-dependent retrograde signaling involved in trans-synaptic homeostasis regulations.
It should be noted that Dmca1D is also implicated in voltage-clamp studies of the motoneuron soma (Worrell and Levine, 2008). Its role in neuronal homeostatic regulations, especially the slo-induced modifications, remains to be investigated. Clearly, the exact mechanisms and additional interacting players in the slo-induced synaptic homeostasis require further investigations in the future. At the present time, it is technically challenging to directly determine the properties of normal and altered Ca2+ currents at the neuromuscular junction of the mutants in this study. Patch-clamp whole-cell recording from neuronal somata may not obtain the correct picture of the nerve terminals because expression of specific types of Ca2+ channels may depend on the cell type and the cellular compartments of the neuron (Peng and Wu, 2007; Worrell and Levine, 2008).
In summary, together with their preferential localization, the distinct interactions between slo-encoded BK and two types of Ca2+ channels imply their differential roles in synaptic homeostasis. Our results demonstrate the predominant contributions of cac- and Dmca1D-encoded Ca2+ channels to the functional and structural aspects, respectively, of the slo-induced synaptic modifications.
ACKNOWLEDGEMENTS
We thank Dr. T. Littleton for providing fly stocks and Dr. A. DiAntonio for a generous gift of DGluRIIB antibody. This work was supported by NIH GM 088804 to CFW.
REFERENCES
- Barber CF, Jorquera RA, Melom JE, Littleton JT. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J Cell Biol. 2009;187:295–310. doi: 10.1083/jcb.200903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila α2δ voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl DF, Ren D, Feng G, Lorenz LJ, Van Vactor D, Hall LM. Genetic and developmental characterization of Dmca1D, a calcium channel alpha1 subunit gene in Drosophila melanogaster. Genetics. 1998;148:1159–1169. doi: 10.1093/genetics/148.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci USA. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Drosophila mutants with opposing effects on nerve excitability: Genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982;47:501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: Synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Gielow ML, Gu GG, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15:6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R, Streissnig J, Koza A, Devay P, Glossman H, Hall L. Native and detergent-solubilized membrane extracts from Drosophila heads contain binding sites for the phenylalkylamine calcium channel blockers. Insect Biochem. 1989;19:309–322. [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sinauer; Sunderland: 2001. [Google Scholar]
- Jin I, Puthanveettil S, Udo H, Karl K, Kandel ER, Hawkins RD. Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci USA. 2012a;109:9131–9136. doi: 10.1073/pnas.1206914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin I, Udo H, Rayman JB, Puthanveettil S, Kandel ER, Hawkins RD. Spontaneous transmitter release recruits postsynaptic mechanisms of long-term and intermediate-term facilitation in Aplysia. Proc Natl Acad Sci USA. 2012b;109:9137–9142. doi: 10.1073/pnas.1206846109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969a;203:459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium ringer. J Physiol. 1969b;203:689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Ueno K, Kidokoro Y. Two types of Ca2+ channel linked to two endocytic pathways coordinately maintain synaptic transmission at the Drosophila synapse. Eur J Neurosci. 2010;32:335–346. doi: 10.1111/j.1460-9568.2010.07300.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Ueda A, Wu CF. Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by Slowpoke and Shaker K+ channel mutations in Drosophila. Neuroscience. 2008;154:1283–1296. doi: 10.1016/j.neuroscience.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wu CF. Orchestration of stepwise synaptic growth by K+ and Ca2+ channels in Drosophila. J Neurosci. 2010;30:15821–15833. doi: 10.1523/JNEUROSCI.3448-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Branton WD, Phillips HS, Jan L, Byerly L. Spider toxins selectively block calcium currents in Drosophila. Neuron. 1989;3:767–772. doi: 10.1016/0896-6273(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Leung HT, Byerly L. Characterization of single calcium channels in Drosophila embryonic nerve and muscle cells. J Neurosci. 1991;11:3047–3059. doi: 10.1523/JNEUROSCI.11-10-03047.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ. Straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel α 1 subunit. J Cell Biol. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod GT, Chen L, Karunanithi S, Peloquin JB, Atwood HL, McRory JE, Zamponi GW, Charlton MP. The Drosophila cacts2 mutation reduces presynaptic Ca2+ entry and defines an important element in Ca(v)2.1 channel inactivation. Eur J Neurosci. 2006;23:3230–3244. doi: 10.1111/j.1460-9568.2006.04873.x. [DOI] [PubMed] [Google Scholar]
- Marder E, Abbott LF, Turrigiano GG, Liu Z, Golowasch J. Memory from the dynamics of intrinsic membrane currents. Proc Natl Acad Sci USA. 1996;93:13481–13486. doi: 10.1073/pnas.93.24.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition on the end-plate potential. J Physiol. 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Pym ECG, Tong A, Davis GW. Rab3-GAP Controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron. 2012;69:749–762. doi: 10.1016/j.neuron.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauron D, Qar J, Barhanin J, Fournier D, Cuany A, Pralavorio M, Berge JB, Lazdunski M. Identification and affinity labeling of very high affinity binding sites for the phenylalkylamine series of Ca+ channel blockers in the Drosophila nervous system. Biochemistry. 1987;26:6311–6315. doi: 10.1021/bi00394a003. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Pelzer S, Barhanin J, Pauron D, Trautwein W, Lazdunski M, Pelzer D. Diversity and novel pharmacological properties of Ca2+ channels in Drosophila brain membranes. Embo J. 1989;8:2365–2371. doi: 10.1002/j.1460-2075.1989.tb08365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci. 2007;27:1072–1081. doi: 10.1523/JNEUROSCI.4746-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Van Kempen GThH, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in α-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine RB, Duch C. Ca(v)2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J Physiol. 2012;590:809–825. doi: 10.1113/jphysiol.2011.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Wu CF. Properties of potassium currents and their role in membrane excitability in Dorosphila larval muscle-fibers. J Exp Biol. 1990;152:59–76. doi: 10.1242/jeb.152.1.59. [DOI] [PubMed] [Google Scholar]
- Singh S, Wu CF. Ionic currents in larval muscles of Drosophila. NEUROMUSCULAR JUNCTIONS IN DROSOPHILA Book Series: INTERNATIONAL REVIEW OF NEUROBIOLOGY. 1999;Volume: 43:191–220. doi: 10.1016/s0074-7742(08)60546-2. Published: 1999. [DOI] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Schuster CM, Goodman CS, Atwood HL. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci. 1996;16:3877–3886. doi: 10.1523/JNEUROSCI.16-12-03877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, LeMasson G, Marder E. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci. 2006;26:6238–6248. doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Role of rut adenylyl cyclase in the ensemble regulation of presynaptic terminal excitability: reduced synaptic strength and precision in a Drosophila memory mutant. J Neurogenet. 2009;23:185–199. doi: 10.1080/01677060802471726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schilcher F. The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Bio. 1976;17:187–196. doi: 10.1016/s0091-6773(76)90444-2. [DOI] [PubMed] [Google Scholar]
- Worrell JW, Levine RB. Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J Neurophysiol. 2008;100:868–878. doi: 10.1152/jn.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Zheng W, Feng G, Ren D, Eberl DF, Hannan F, Dubald M, Hall LM. Cloning and characterization of a calcium channel alpha 1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J Neurosci. 1995;15:1132–1143. doi: 10.1523/JNEUROSCI.15-02-01132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Jan LY, Jan YN, Benzer S. Drosophila mutant with a temperature-sensitive block in nerve-conduction. Proc Natl Acad Sci USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]