Abstract
In the phenomenon of repetition suppression (RS), when a person views a stimulus, the neural activity involved in processing that item is relatively diminished if that stimulus had been previously viewed. Previous noninvasive imaging studies mapped the prevalence of RS for different stimulus types to identify brain regions involved in representing a range of cognitive information. However, these noninvasive findings are challenging to interpret because they do not provide information on how RS relates to the brain's electrophysiological activity. We examined the electrophysiological basis of RS directly using brain recordings from implanted electrocorticographic (ECoG) electrodes in neurosurgical patients. Patients performed a memory task during ECoG recording and we identified high-gamma signals (65–128 Hz) that distinguished the neuronal representation of specific memory items. We then compared the neural representation of each item between novel and repeated viewings. This revealed the presence of RS, in which the neuronal representation of a repeated item had a significantly decreased amplitude and duration compared with novel stimuli. Furthermore, the magnitude of RS was greatest for the stimuli that initially elicited the largest activation at each site. These results have implications for understanding the neural basis of RS and human memory by showing that individual cortical sites exhibit the largest RS for the stimuli that they most actively represent.
Keywords: electrocorticography, gamma band, repetition suppression
1 Introduction
The phenomenon of repetition suppression (RS) is a powerful technique for mapping the functional roles of neurons across different brain areas. In RS, the brain areas that activate when a person views an item generally show a diminished response when a person later sees an identical or similar stimulus. By identifying the areas that exhibit RS across different types of stimuli, researchers have obtained rich insights into the neural basis of various human neuronal processes, including perception, memory, and reasoning (Grill-Spector et al., 2006). Research has used RS to reveal detailed information regarding the types of neuronal processes that occur in different brain areas, such as the findings that perceptual memory information is represented in sensory regions (Tootell et al., 1998) and that abstract stimulus properties are coded by neurons in temporal and frontal cortices (Henson et al., 2004). Further, the magnitude of RS predicts the strength of a person's memory on a trial-by-trial basis (Maccotta & Buckner, 2004) and shows the involvement of different brain regions in distinct memory processes (Gonsalves et al., 2005). Although RS is not a perfect measure of neuronal coding (Sawamura et al., 2006), obtaining a more detailed understanding of RS is likely to shed light on the fundamental nature of human memory and cognition and is considered a key goal of cognitive neuroscience (Weiner & Grill-Spector, 2012).
The phenomenon of RS has been studied with various methods, including scalp electroencephalography (McDonald et al., 2010; Gruber et al., 2006; Gruber & Matthias, 2005; Conrad et al., 2007; Sambeth et al., 2004; Van Strien et al., 2007), magnetoencephalography (McDonald et al., 2010; Friese et al., 2012; Noguchi et al., 2004; Dale et al., 2000; Vidyasagar et al., 2010; Gonsalves et al., 2005), electrocorticography (McDonald et al., 2010; Puce et al., 1999; Hermes et al., 2012), and single-cell recordings (De Baene & Vogels, 2010; Kaliukhovich & Vogels, 2011; Sobotka & Ringo, 1996; Sawamura et al., 2006). Nevertheless, the vast majority of research on RS in humans uses fMRI (Larsson & Smith, 2012; Henson, Shallice, & Dolan, 2000; Sayres & Grill-Spector, 2006; Henson et al., 2004; James & Gauthier, 2006; Maccotta & Buckner, 2004; Harris & Aguirre, 2010; Malach, 2012). Various neural models have been proposed to explain how the RS observed with fMRI is related to the brain's electrical activity (Grill-Spector et al., 2006). These models differ in terms of how they attribute RS to changes in the amplitude, timing, and identities of the neurons that are active when viewing a repeated item. Distinguishing between these theories is further complicated by uncertainty regarding the relation between the fMRI blood-oxygenation signal and underlying neuronal activity (Logothetis et al., 2001; Ekstrom, 2010). Thus, researchers suggested that direct electrophysiological recordings could help to explain RS more fully (Gotts et al., 2012).
We studied RS using direct electrocorticographic (ECoG) brain recordings from neurosurgical patients performing a working-memory task. The high-frequency component of these ECoG signals correlates with neuronal spiking (Manning et al., 2009; K. Miller et al., 2009). These high-frequency signals have revealed neural assemblies that distinguish particular stimuli during cognitive tasks (Jacobs & Kahana, 2009; Blakely et al., 2008; Pei et al., 2011; Pasley et al., 2012). We thus used ECoG to examine RS in detail in humans by comparing the neural representations of individual stimuli between the viewing of novel and repeated items. With this stimulus-based approach, our findings demonstrate that RS is specific to the high-gamma band (65–128 Hz) of ECoG signals and, further, that the neuronal assemblies with the largest initial activations are the ones that exhibit the most RS.
2 Methods
2.1 Patients
We analyzed data from 25 patients who were undergoing invasive seizure monitoring for drug-resistant epilepsy (Jacobs & Kahana, 2010). Throughout ECoG monitoring, patients volunteered to participate in our memory task in free time between clinical procedures on a bedside laptop computer. Each patient participated in between one and five testing sessions. The research protocol was approved by Institutional Review Boards at the Hospital at the University of Pennsylvania (Philadelphia, PA) and the Thomas Jefferson University Hospital (Philadelphia, PA). Informed consent was obtained from each patient or their legal guardians.
2.2 Task
Patients performed the Sternberg working-memory task (Sternberg, 1966); each session lasted about 45 minutes and contained multiple trials. This is a new dataset that is distinct from the one reported by Jacobs and Kahana (2009). Each trial consisted of three phases: encoding, maintenance and response (Fig. 1A). In the encoding phase, patients were first presented a fixation cross and then a list of three uppercase letters were displayed sequentially on a computer screen. Each single letter stimulus remained on the screen for 700 ms and was followed by a blank screen for 275–350 ms (uniformly distributed). Each character had a visual field size of ∼10°, although this varied according to where the subject positioned the laptop on their hospital tray. Patients were instructed to closely attend to each stimulus presentation and to silently hold the identity of each item in memory. After all three list items were presented, the patient attempted to remember all the presented items during a maintenance period. Last, in the response phase, a cue item appeared on the screen, and patients pressed a key to indicate whether the cue item was present or absent in the just-seen list (a target or lure, respectively). Exactly half of the cue items were targets and half were lures, with the order randomized. After the response, a feedback message appeared on the screen, indicating whether the response was correct. Individual patients participated in different numbers of task sessions according to their time and interest.
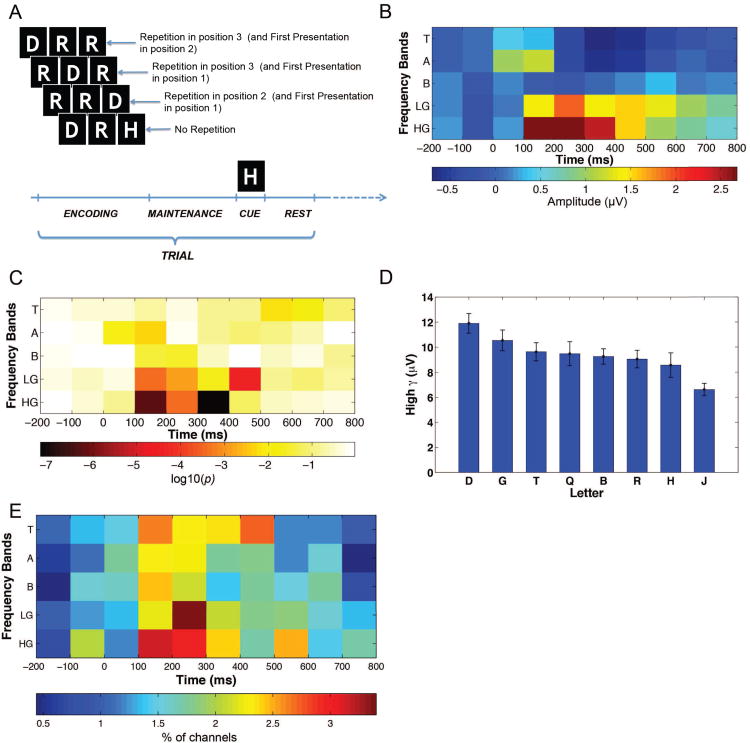
Figure 1.
(A): Schematic representation of Sternberg task with repetitions. Each trial consisted of an encoding period, during which a list of three letters was presented, and a maintenance period, followed by a cue. The participant's task was to identify whether the cue appeared in the just-presented list. (B): The amplitude of neuronal activity at one electrode following letter onset. The amplitude of activity at each timepoint and frequency is normalized related to letter onset (sample electrode from patient 15's left Brodmann area 19). (C): Analysis of letter-related ECoG activity at this same electrode; color indicates log10(p) from an ANOVA. (D): Detail of letter-related activity at this same electrode. The bars show the mean unnormalized high-gamma amplitude for each letter at 100–400 ms. Error bars represent 95% confidence intervals. (E): Time–frequency distribution of the percentage of electrodes exhibiting letter-related activity across the entire dataset.
On average, each patient performed 335 trials across all sessions (∼167 repeats), for a total of 1005 letter presentations. The letters used in this task were one of 8 consonants; vowels were excluded to prevent patients from using mnemonic strategies to remember each list (e.g., remembering the entire list as an easily pronounceable word-like sound). Half the trials had three different list items and half the trials had a repeat. In lists with repeats, the position of the non-repeat item was uniformly distributed across the three list positions.
2.3 Data Analysis
We analyzed brain signals related to viewing each stimulus by measuring the amplitude of ECoG activity in the 800 ms after each item onset. These measurements included all oscillatory activity after item onset, ignoring the signal's phase, in contrast to some previous studies that measured RS with ERP techniques (Anderson et al., 2008; Gilbert et al., 2010) that measure only the portion of the signal that is phase- and time-locked to each stimulus appearance (Fell et al., 2004; Yeung et al., 2004; Jacobs et al., 2006; Hanslmayr et al., 2007). For each electrode, we filtered ECoG activity in five frequency bands: theta (4–8 Hz), alpha (8–16 Hz), beta (16–30 Hz), low gamma (30–65 Hz) and high gamma (65–128 Hz). We then computed the ECoG amplitude in each band with the Hilbert transform (Bruns, 2004; Freeman, 2007) and smoothed it with a 50-ms boxcar filter. We calculated the mean amplitude for each band in each of 8 consecutive 100-ms time intervals after each letter appearance (Fig. 1B).
Our next goal was to identify electrodes that recorded ECoG activity related to processing the identity of each viewed letter (Jacobs & Kahana, 2009). To do this, we used a one-way ANOVA to test whether the amplitude of ECoG activity at each electrode, time bin, and frequency band significantly varied (p < 0.01) between presentations of each individual letter (Fig. 1C). For each electrode measuring letter-related ECoG activity, we then separately ranked the individual letters according to the mean response magnitude at 100-400 ms (Fig. 1D), with rank 1 corresponding to the largest response at that electrode and rank 8 corresponding to the smallest. We also identified the electrodes that activated generally during memory encoding without exhibiting letter-related activity, by comparing the amplitude of ECoG activity after stimulus onset with the activity in the 200-ms prestimulus baseline (t test, p < 0.05).
Next we were interested in identifying ECoG activity related to stimulus repetition. We labeled each stimulus presentation according to whether that item was a repeat or new item within that list. To test for effects of repetition, we employed a four-way ANOVA at each frequency band. The ANOVA factors were the following: Repeated item (whether the stimulus was a novel or repeat presentation) Rank (the rank of the viewed letter), List position (the serial position of the item in the presented list), and Electrode (individual ECoG electrodes). List position and Electrode were random factors and others were fixed. In our analysis of RS that ignored stimulus-related activity, we used a three-way ANOVA that omitted the factor Rank. In addition to the ANOVA, we conducted post-hoc tests to identify individual electrodes exhibiting RS by using paired t tests to compare the mean responses across the first two ranks between novel and repeated presentations (α = 0.05). Repeats appear only in the second or third list positions, unlike novel items, which can also appear in the beginning of each list. Thus, a potential issue is that a neural signal that varies with list position (e.g., Sederberg et al., 2006; Azizian & Polich, 2007; Serruya et al., in press) could incorrectly appear as a correlate of repetition. We corrected for this potential issue in our statistics with the factor List position and in our plots by normalizing each ECoG response relative to the mean response from that same list position for non-repeat items. However, there was no significant effect of List position in the high gamma band (p = 0.9), which suggests that any relevant position effects were minimal in this dataset.
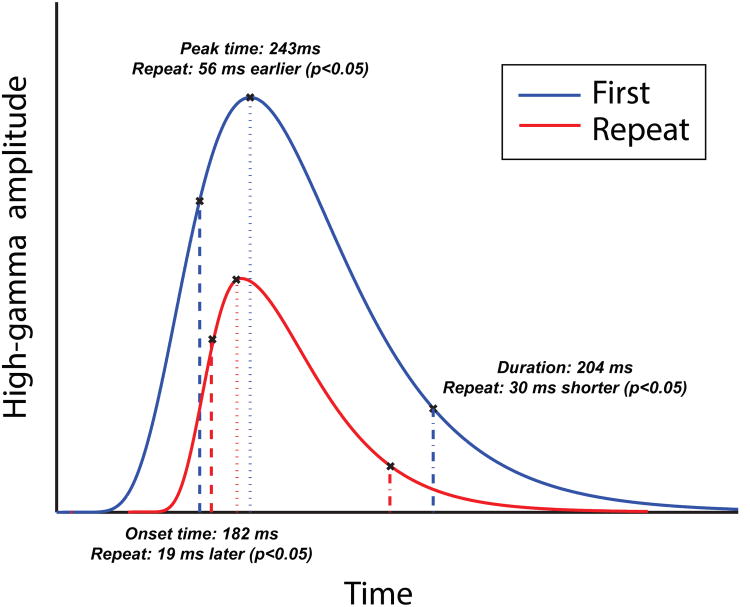
To assess the timecourse of RS, we computed the amplitude timecourse of each electrode's responses to repeat and novel items and measured several temporal features of its shape (Figure 3A). The measurements are Onset time, which is the latency from stimulus onset until the response reaches 75% of its peak increase; Peak time, the latency (in ms) from stimulus onset until the peak ECoG response; and Duration, the length of time from response onset until its subsequent drop 75% of the way back from its peak to its baseline. All response shape measurements were computed separately for novel and repeat items.
Figure 3.
Representation of our analyses examining repetition-related changes in the shape of the high-gamma responses to initial and repeat stimulus presentations.
We also examined repetition-related changes in event-related potentials (ERPs) by computing the time-locked ECoG voltage signal at each electrode and using t tests to compare this signal between repeats and novel items. ECoG signals were low-pass filtered below 30 Hz and then ERPs were computed at the sites that actively exhibited elevated high-gamma activity during the task.
3 Results
We recorded activity from a total of 2262 electrodes implanted in 25 epilepsy patients who performed a working-memory task in free time between clinical procedures. In each trial of the task, the patient memorized a list of three letters, each of which either contained three different items or had one item repeated. Our data analyses sought to characterize neural patterns that differentiated between the processing of novel and repeat items.
3.1 Behavioral data
First, we assessed patients' task performance by measuring their reaction times responding to the memory probes after each list. Overall, patients responded more rapidly to lists containing a repeated item (1393 ms) compared to those that contained three different items (1589 ms; p < 0.05, t test).
3.2 Stimulus-based analysis of repetition suppression
Earlier work showed that a prominent feature of human ECoG recordings was the presence of neuronal patterns that distinguished individual items that were encoded into memory (Jacobs & Kahana, 2009). Here utilized these ECoG measurements of individual stimulus representations to characterize the neuronal basis of repetition suppression (RS). Our strategy was to first identify the ECoG representations of particular stimuli and then to measure changes in these signals when stimuli were repeated.
To identify the ECoG signals that encoded stimulus-related information, at each electrode we performed a one-way ANOVA comparing neuronal responses after the patient viewed different letters in the task's encoding phase. As seen previously, many individual electrodes exhibited increased high-gamma amplitude after each letter appeared on the screen (Fig. 1B; Crone et al., 1998). For each electrode, we used a separate series of ANOVAs to test whether the amplitude of this activity significantly varied according to the identity of the letter that the person viewed (Jacobs & Kahana, 2009). Figure 1C illustrates the results of the ANOVA at an example electrode that exhibited significant letter-related activity in the high-gamma band at 100– 400 ms. This site exhibited the largest amplitude of high-gamma activity when the person viewed the letter “D”, and had a smaller response for other letters, like “J” (Fig. 1D).
Across the entire dataset, significant letter-related ECoG activity (ANOVA p's< 0.01) was most prevalent in the high-gamma band 100–400 ms after stimulus onset (Fig. 1E), where 50 electrodes exhibited activity that differed significantly between individual letters, consistent with previous work (Jacobs & Kahana, 2009). Our next analyses focused on high-gamma activity in this frequency band and time interval (see below for analyses of other frequencies). For each electrode that exhibited significant stimulus-related high-gamma activity, we separately ranked each individual letter according to the magnitude of its response. For example, at the electrode in Figure 1D the letter “D” was ranked first because it elicited the strongest high-gamma amplitude (11.9 μV) and letter “J” was ranked last (6.6 μV).
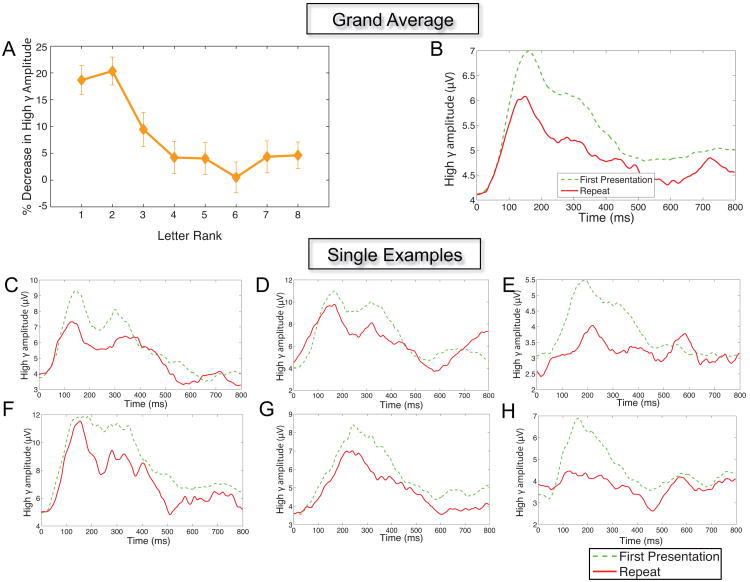
Next, we tested whether the high-gamma representations of individual items changed with repetition, by aggregating across all electrodes that exhibited significant letter-related activity (see Methods). For this purpose, the most critical features of the ANOVA were the factors Repetition and Rank, and their interaction. We did not observe a significant main effect of Repetition (p > 0.7). However, we did observe a significant Repetition × Rank interaction (p < 0.001). This significant interaction showed that high-gamma ECoG activity exhibited RS, but that the magnitude of this effect varied according to the letter's rank. To illustrate this pattern, Figure 2A shows the mean difference in high-gamma amplitude between novel and repeated items, computed seperately for each letter rank. RS is strongest and statistically robust for stimuli with ranks 1 and 2, and this phenomenon diminished for letters with lower ranks.
Figure 2. Examples of repetition suppression in human electrocorticographic data.
Grand Average —(A): rrr Mean difference for high-gamma amplitude between first and repeat presentations for each letter rank, averaged across the dataset. (B): Comparison of average unnormalized high-gamma amplitude time courses between first and repeat presentations. The averages were obtained including all electrodes exhibiting significant repetition suppression and considering only the two highest ranked letters. Single Electrode Examples — Comparison of time courses between first and repeat presentation in single electrodes exhibiting significant repetition suppression at 100–400 ms window. Only the two highest ranked letters were included. (C): Sample electrode from patient 4's right Brodmann area 18. (D): Sample electrode from patient 4's right Brodmann area 19. (E): Sample electrode from patient 11's right Brodmann area 19. (F): Sample electrode from patient 15's left Brodmann area 19. (G-H): Sample electrodes from patient 18's left Brodmann area 19
This phenomenon of RS in high-gamma ECoG activity was also clearly visible at individual electrodes (Figure 2C–H). Of the 50 electrodes that exhibited significant high-gamma stimulus-related activity, 17 exhibited significant RS and only 1 exhibited response enhancement (see Table 1).
Table 1. Summary dataset and high-gamma ECoG repetition effects.
Each row describes for one patient, indicating the testing location (TJ, Thomas Jefferson University Hospital, Philadelphia, USA; UP, University of Pennsylvania Hospital, Philadelphia, USA), handedness, and the counts of electrodes with letter-specific activity, repetition-related suppression and enhancement. Electrode counts are reported overall, as well as separately for temporal and occipital cortices.
| Temporal | Occipital | Overall | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Patient# | Location | Handedness | Total | Letter-Specific | Repetition Suppression | Repetition Enhancement | Total | Letter-Specific | Repetition Suppression | Repetition Enhancement | Total | Letter-Specific | Repetition Suppression | Repetition Enhancement |
| 1 | TJ | R | 40 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 78 | 0 | 0 | 0 |
| 2 | TJ | R | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 63 | 0 | 0 | 0 |
| 3 | TJ | R | 43 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 160 | 0 | 0 | 0 |
| 4 | TJ | R | 57 | 1 | 0 | 0 | 6 | 6 | 3 | 0 | 98 | 7 | 3 | 0 |
| 5 | TJ | R | 60 | 0 | 0 | 0 | 6 | 4 | 0 | 0 | 90 | 4 | 0 | 0 |
| 6 | TJ | R | 44 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 90 | 2 | 0 | 0 |
| 7 | TJ | R | 39 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 106 | 0 | 0 | 0 |
| 8 | TJ | R | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 138 | 0 | 0 | 0 |
| 9 | TJ | R | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 62 | 1 | 0 | 0 |
| 10 | TJ | R | 43 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 120 | 1 | 0 | 0 |
| 11 | TJ | R | 67 | 2 | 2 | 0 | 15 | 6 | 2 | 0 | 110 | 8 | 4 | 0 |
| 12 | TJ | R | 29 | 0 | 0 | 0 | 4 | 3 | 0 | 0 | 96 | 3 | 0 | 0 |
| 13 | TJ | R | 53 | 1 | 0 | 0 | 8 | 1 | 0 | 0 | 99 | 2 | 0 | 0 |
| 14 | TJ | R | 20 | 0 | 0 | 0 | 14 | 6 | 1 | 1 | 48 | 6 | 1 | 1 |
| 15 | TJ | R | 57 | 2 | 1 | 0 | 12 | 3 | 3 | 0 | 96 | 5 | 4 | 0 |
| 16 | TJ | R | 43 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 72 | 1 | 0 | 0 |
| 17 | TJ | R | 54 | 0 | 0 | 0 | 14 | 6 | 2 | 0 | 150 | 8 | 3 | 0 |
| 18 | UP | R | 42 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 80 | 2 | 2 | 0 |
| 19 | UP | L | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 0 | 0 | 0 |
| 20 | UP | R | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 |
| 21 | UP | R | 28 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 36 | 0 | 0 | 0 |
| 22 | UP | L | 28 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 86 | 0 | 0 | 0 |
| 23 | UP | L | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 74 | 0 | 0 | 0 |
| 24 | UP | R | 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 |
| 25 | UP | R | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 68 | 0 | 0 | 0 |
|
| ||||||||||||||
| All patients | 909 | 7 | 3 | 0 | 104 | 38 | 13 | 1 | 2262 | 50 | 17 | 1 | ||
3.3 Timing analysis
ECoG recordings measure aggregate neuronal activity with a high temporal resolution (K. Miller et al., 2012). This allowed us to measure the detailed timecourse of human RS. For each electrode, we computed the detailed timecourse of the mean high-gamma response amplitude to first and repeated stimuli. We then measured the shape of each electrode's response by measuring each response's Onset time, Peak Time, and Duration (see Figure 3).
We compared these measurements between first and repeat presentations of each item. Overall, the initial response times to first presentations were significantly shorter than responses to repeated items (Onset time: p < 0.05, paired t test) and had longer Durations (p < 0.05). The latency of the peak response was also significantly faster for first presentations than repeats (Time to Peak: p < 0.05, t test).
3.4 Analysis of repetition effects at other frequencies
We tested for RS at other frequencies in addition to the high-gamma band (van Gerven et al., in press). As for high gamma, for each other frequency band we identified the electrodes that exhibited stimulus-related ECoG activity. We then used the same ANOVA framework to test for RS at the population level by testing for a interaction between factors Repetition and Rank at each band. Besides high-gamma, the only band where significant RS appeared was the 4–8-Hz theta frequency range (p = 0.001; all other bands: p's> 0.5). However, when we analyzed each electrode individually, we found that theta stimulus-related RS was less robust compared with the high-gamma band. Only 3 electrodes exhibited theta RS total, which is significantly less than the 17 that exhibited high-gamma RS (p < 0.002, χ2 test).
We also tested for broader patterns of RS beyond the electrodes that exhibited stimulus-related activity. This approach is common in fMRI and EEG studies, where individual stimulus representations are not generally observed (Grill-Spector et al., 2006). We identified all electrodes that exhibited increased high-gamma activity during stimulus viewing. Then we performed an ANOVA at each frequency band to identify significant repetition-related changes in ECoG amplitude. This analysis did not reveal significant RS at any band (all p's> 0.9 for factor Repetition, uncorrected). In addition to this population analysis, we tested for RS at the level of individual electrodes, by comparing the counts of electrodes at each band that exhibited RS compared with enhancement. This electrode-count analysis also did not identify significant RS at any frequency (p's> 0.1, uncorrected binomial tests).
3.5 ERP analysis of repetition suppression
In addition to measuring the amplitude of ECoG signals, a different literature has probed human brain signals by assessing event-related potentials (ERPs), which are the subsets of brain signals that are phase-locked to external events (Yeung et al., 2004). We computed ERPs following the onset of each stimulus and compared these ERPs between the viewing of novel and repeated items.
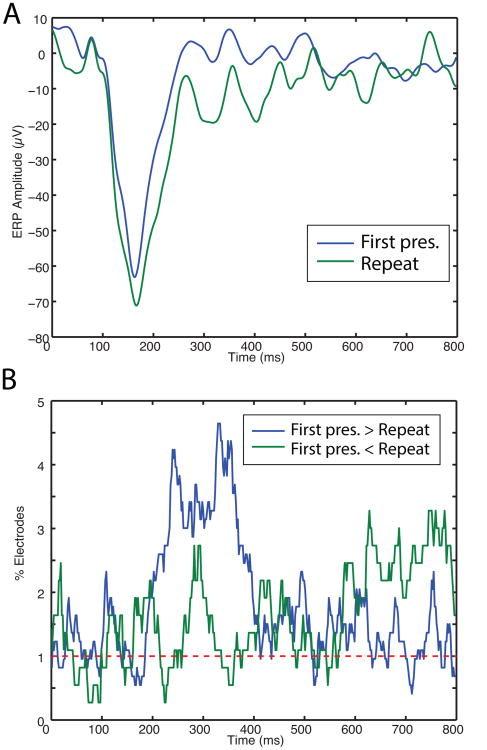
Many individual electrodes exhibited significant ERP changes for viewing repeat items. Figure 4A shows one electrode that exhibits this effect, by displaying a larger negative ERP fluctuation at ∼200 ms for repetitions compared with viewing novel items. A similar pattern was also evident across the population of electrodes (Figure 4B), whereby many individual electrodes had significantly more negative voltage for viewing repetitions compared with novel items. Together these findings are consistent with prior work suggesting increased phase synchrony for repetitions (Gotts et al., 2012), as phase synchrony often manifests as ERP fluctuations (Yeung et al., 2004).
Figure 4. Event-related potential (ERP) analysis of repetition suppression.
A. Example electrode showing a large negative ERP component after stimulus onset. This component was significantly larger for viewing repeated items. B. Population analysis of repetition-related changes in ERPs. Plot indicates the percentage of electrodes that exhibited significant differences in ERPs between the initial viewing of stimuli compared with repetitions. Blue line indicates electrodes with more positive voltage for initial viewings, Green indicates electrodes with more positive voltage for repetitions. Red dashed line indicates the chance level of significant RS patterns.
4 Discussion
We conducted a large-scale analysis of RS using human ECoG data and identified novel neural changes related to viewing repeated stimuli, including stimulus specificity and timing changes. Seventeen ECoG electrodes (out of 50) exhibited high-gamma RS. Only one electrode showed enhancement (p < 0.001, binomial test). This pattern is generally consistent with the findings from previous studies that reliably demonstrate suppressed neural activity with repeated activations via fMRI and other techniques (Buckner et al., 1998; Grill-Spector & Malach, 2001; Henson, Shallice, & Dolan, 2000; Wiggs & Martin, 1998; Puce et al., 1999; Hermes et al., 2012; Dale et al., 2000; Sambeth et al., 2004; E. K. Miller & Desimone, 1994; Swick & Knight, 1997). Although these studies used a variety of experimental paradigms and their methodological details differ greatly, the consistent pattern of RS is one that we replicate.
ECoG data measure human brain activity with a higher spatial and temporal resolution than other human neuroimaging techniques. Here, we found that RS was most apparent in the high-gamma band of these signals. Previous research showed that ECoG high-gamma activity is caused by a broadband signal (K. Miller et al., 2007) that correlates with neuronal spiking (Manning et al., 2009; K. Miller et al., 2009). This link between high-gamma activity and neuronal spiking, in conjunction with our finding of high-gamma RS, suggests that RS manifests neurally as decreased neuronal activity. By showing that RS also appears with high-gamma ECoG, it strengthens the evidence that fMRI can be used to noninvasively estimate neuronal activity and is informative for understanding the complex interrelation between spiking, the fMRI BOLD signal, and oscillatory activity (Logothetis et al., 2001; Ekstrom, 2010). However, to the extent that high-gamma ECoG activity may additionally relate to neuronal synchrony (Ray et al., 2008), an important area of future work is to assess repetition-related changes in synchronous spike timing.
The most novel feature of our study was that we were able to separately measure RS for the neural representations of individual stimuli. Many prior studies of RS aggregated across large cortical regions that showed category-level neuronal responses. This category-wide approach is logical in fMRI and scalp EEG data where large-scale category responses are the dominant cognitive pattern. The spatial blurring inherent in noninvasive brain data largely precludes differentiating between local neuronal populations. Here we were motivated by our prior finding that ECoG signals reveal stimulus-related neural patterns (Jacobs & Kahana, 2009) and this allowed us to study human RS for individual exemplars within a category. This stimulus-based approach was vital for our finding that RS is largest for the stimuli that caused the largest activations at each electrode.
Although RS is most often observed with noninvasive brain measurements, researchers continue to discuss the implications of these measurements for the firing of individual neurons (for reviews, see Grill-Spector et al., 2006; Gotts et al., 2012). We did not directly record individual action potentials, but our ECoG findings nonetheless shed some light on these issues. We observed repetition-related decreases in the duration of high-gamma ECoG responses (Fig. 3). This duration chagne is consistent with one prediction of the Facilitation model of RS (Sobotka & Ringo, 1996; Henson & Rugg, 2003; James & Gauthier, 2006; Grill-Spector et al., 2006), which proposed that repetition caused a decrease in the latency or duration of the neural response. We also observed a significantly later onset time for repeats compared with novel stimuli, which appears to contradict a different prediction of the facilitation model. The Facilitation model was created to explain peceptual-priming data, which is very different from our memory task. However, this onset time difference is nonetheless very relevant for its predictions because the effect happens at such an early latency that is unlikely to be caused by top-down memory processes.
A recent model proposed that the RS observed with fMRI is fundamentally caused by increased low-frequency oscillatory synchronization for repeat items (Gotts et al., 2012). This theory suggests that low-frequency activity improves neuronal efficiency by enhancing the precision of spike timing, thus reducing the total number of action potentials required to perform the task. We tested for changes in low-frequency amplitude for repeated items, but did not observe repetition-related enhancement, both across the entire population of electrodes and for the subset of electrodes that exhibited stimulus-related activity. However, we did identify significant changes in evoked ECoG signals for repetitions, consistent with prior work (Anderson et al., 2008; Gilbert et al., 2010). Evoked ERP patterns can result from either amplitude or phase changes (Fell et al., 2004; Yeung et al., 2004; Hanslmayr et al., 2007). Thus, together these findings generally support the view that there is increased phase synchrony for repetitions but no low-frequency amplitude changes (Gotts et al., 2012).
Across the brain, the overall prevalence of RS in our study is lower than found in some prior fMRI studies. This may be a result of the letter stimuli we used. Stimulus-related ECoG activity for letters is most often observed in visual regions and the patients in our study had electrodes placed in these areas fairly infrequently (Jacobs & Kahana, 2009). Because our analysis of RS is predicated on first observing stimulus-related activity at a particular site, it is likely that a future study could identify a greater prevalence of RS in ECoG signals by using a more diverse stimulus set and having more comprehensive electrode coverage. It is also possible that the prevalence of RS we observed was impacted by the fact that our dataset only included a single stimulus category (letters), and, thus, we could only characterize stimulus-level RS, as in primate single-neuron recordings (E. K. Miller & Desimone, 1994), rather than also measuring RS at the category level.
The phenomenon of RS has become a core neuroimaging method that is used in many studies to compare the nature of neuronal representations and computational schemes across widespread brain areas and behaviors. The vast majority of these studies are conducted with fMRI. Our findings help provide an electrophysiological basis for RS by showing that this activity is prominent in the high-gamma band of ECoG and is thus likely correlated with mean neuronal spiking rates (Manning et al., 2009). An important task going forward is to test for electrophysiological differences in RS across stimuli, brain areas, and tasks, as the detailed properties of RS are likely to vary as a function of the type of item being viewed and how it is processed behaviorally (Henson, Rugg, et al., 2000; Epstein et al., 2008). Our observation that RS is largest for stimuli that elicit the largest neuronal activations has implications for our fundamental understanding of the neural basis of memory by suggesting that individual cortical sites participate in representing only a subset of stimuli. This implies that we are likely to create increasingly detailed insights into the neural basis of human memory with high-resolution recording methods that can measure precise stimulus-related neural patterns, such as high-field fMRI (Yacoub et al., 2008), fMRI with custom pulse sequences or coils (Grill-Spector et al., 2006), and direct brain recordings like ECoG or single-neuron recordings (Jacobs & Kahana, 2010; Engel et al., 2005).
Highlights.
Repetition suppression appears in high-gamma electrocorticographic recordings.
Diminished response amplitude and duration for repetitions.
Repetition suppression was more marked for stimuli eliciting the largest responses at each location.
Acknowledgments
We acknowledge the support of Drexel University's Human Cognition Enhancement Program, the Brain and Behavior Research Fund, and the National Institutes of Health (R01MH55687 and R21NS067316). We thank Michael Kahana for help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson B, Mruczek R, Kawasaki K, Sheinberg D. Effects of familiarity on neural activity in monkey inferior temporal lobe. Cerebral Cortex. 2008;18(11):2540–2552. doi: 10.1093/cercor/bhn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from event-related potentials. Journal of cognitive neuroscience. 2007;19(12):2071–2081. doi: 10.1162/jocn.2007.19.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely T, Miller K, Rao R, Holmes M, Ojemann J. Localization and classification of phonemes using high spatial resolution electrocorticography (ecog) grids. Engineering in medicine and biology society, 2008 embs 2008; 30th annual international conference of the ieee; 2008. pp. 4964–4967. [DOI] [PubMed] [Google Scholar]
- Bruns A. Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? Journal of Neuroscience Methods. 2004;137:321–332. doi: 10.1016/j.jneumeth.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI. I. Retrieval effort versus retrieval success. Neuroimage. 1998 Apr;7(3):151–162. doi: 10.1006/nimg.1998.0327. Retrieved from http://dx.doi.org/10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Conrad N, Giabbiconi CM, Müller MM, Gruber T. Neuronal correlates of repetition priming of frequently presented objects: insights from induced gamma band responses. Neurosci Lett. 2007 Dec;429(2-3):126–30. doi: 10.1016/j.neulet.2007.09.065. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(12):2301. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fmri and meg for high-resolution imaging of cortical activity. Neuron. 2000 Apr;26(1):55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cereb Cortex. 2010 Sep;20(9):2145–65. doi: 10.1093/cercor/bhp277. [DOI] [PubMed] [Google Scholar]
- Ekstrom A. How and when the fmri bold signal relates to underlying neural activity: the danger in dissociation. Brain research reviews. 2010;62(2):233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Moll CKE, Fried I, Ojemann GA. Invasive recordings from the human brain–clinical insights and beyond. Nature Reviews Neuroscience. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- Epstein R, Parker W, Feiler A. Two kinds of fMRI repetition suppression? Evidence for dissociable neural mechanisms. Journal of Neurophysiology. 2008;99(6):2877. doi: 10.1152/jn.90376.2008. [DOI] [PubMed] [Google Scholar]
- Fell J, Dietl T, Grunwald T, Kurthen M, Klaver P, Truatner P, Fernandez G. Neural bases of cognitive ERPs: More than phase reset. Journal of Cognitive Neuroscience. 2004;16:1595–1604. doi: 10.1162/0898929042568514. [DOI] [PubMed] [Google Scholar]
- Freeman W. Hilbert transform for brain waves. Scholarpedia. 2007;2(1):1338. [Google Scholar]
- Friese U, Rahm B, Hassler U, Kaiser J, Gruber T. Repetition suppression and effects of familiarity on blood oxygenation level dependent signal and gamma-band activity. Neuroreport. 2012 Sep;23(13):757–61. doi: 10.1097/WNR.0b013e328356b173. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Gotts S, Carver F, Martin A. Object repetition leads to local increases in the temporal coordination of neural responses. Frontiers in human neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005 Sep;47(5):751–61. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gotts S, Chow C, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cognitive Neuroscience. 2012;3(3-4):227–237. doi: 10.1080/17588928.2012.670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fmr-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001 Apr;107(1-3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nature neuroscience. 2006;9(9):1177. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- Gruber T, Giabbiconi CM, Trujillo-Barreto NJ, Müller MM. Repetition suppression of induced gamma band responses is eliminated by task switching. Eur J Neurosci. 2006 Nov;24(9):2654–60. doi: 10.1111/j.1460-9568.2006.05130.x. [DOI] [PubMed] [Google Scholar]
- Gruber T, Matthias M. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cerebral Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, Birbaumer N. Alpha phase reset contributes to the generation of erps. Cerebral Cortex. 2007;17(1):1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Harris A, Aguirre GK. Neural tuning for face wholes and part in human fusiform gyrus revealed by fMRI adaptation. Journal of Neurophsyiology. 2010;104(1):336–345. doi: 10.1152/jn.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41(3):263–70. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal rolesin episodic retrieval. Journal of Cognitive Neuroscience. 2000;12(6):913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Henson R, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004 Apr;21(4):1674–89. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287(5456):1269. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Hermes D, Siero J, Aarnoutse E, Leijten F, Petridou N, Ramsey N. Dissociation between neuronal activity in sensorimotor cortex and hand movement revealed as a function of movement rate. The Journal of Neuroscience. 2012;32(28):9736–9744. doi: 10.1523/JNEUROSCI.0357-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. NeuroImage. 2006;15(2):978–87. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band ECoG activity. Journal of Neuroscience. 2009;29(33):10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends in Cognitive Sciences. 2010;14(4):162–171. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in bold response reflect accumulation of neural activity. Hum Brain Mapp. 2006 Jan;27(1):37–46. doi: 10.1002/hbm.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition probability does not affect repetition suppression in macaque inferior temporal cortex. Cereb Cortex. 2011 Jul;21(7):1547–58. doi: 10.1093/cercor/bhq207. [DOI] [PubMed] [Google Scholar]
- Larsson J, Smith AT. Fmri repetition suppression: neuronal adaptation or stimulus expectation? Cereb Cortex. 2012 Mar;22(3):567–76. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004 Nov;16(9):1625–32. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Malach R. Targeting the functional properties of cortical neurons using fmr-adaptation. Neuroimage. 2012 Aug;62(2):1163–9. doi: 10.1016/j.neuroimage.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in LFP power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience. 2009 Oct;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, Halgren E. Multimodal imaging of repetition priming: Using fmri, meg, and intracranial eeg to reveal spatiotemporal profiles of word processing. Neuroimage. 2010 Nov;53(2):707–17. doi: 10.1016/j.neuroimage.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994 Jan;263(5146):520–2. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Miller K, Hermes D, Honey C, Hebb A, Ramsey N, Knight R, Fetz E. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Computational Biology. 2012;8(9):e1002655. doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Leuthardt EC, Schalk G, Rao RPN, Anderson NR, Moran DW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. Journal of Neuroscience. 2007;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Zanos S, Fetz EE, den Nijs M, Ojemann J. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. Journal of Neu-roscience. 2009;29(10):3132–3137. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y, Inui K, Kakigi R. Temporal dynamics of neural adaptation effect in the human visual ventral stream. J Neurosci. 2004 Jul;24(28):6283–90. doi: 10.1523/JNEUROSCI.0655-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, David SV, Mesgarani N, Flinker A, Shamma SA, Crone NE, Chang EF. Reconstructing speech from human auditory cortex. PLoS Biology. 2012;10(1):e1001251. doi: 10.1371/journal.pbio.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X, Barbour D, Leuthardt E, Schalk G. Decoding vowels and consonants in spoken and imagined words using electrocorticographic signals in humans. Journal of neural engineering. 2011;8(4):046028. doi: 10.1088/1741-2560/8/4/046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. iii: Effects of top-down processing on face-specific potentials. Cerebral Cortex. 1999;9(5):445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Ray MK, Mackay CE, Harmer CJ, Crow TJ. Bilateral generic working memory circuit requires left-lateralized addition for verbal processing. Cereberal Cortex. 2008;18:1421–1428. doi: 10.1093/cercor/bhm175. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Maes JHR, Quian Quiroga R, Coenen AML. Effects of stimulus repetitions on the event-related potential of humans and rats. Int J Psychophysiol. 2004 Aug;53(3):197–205. doi: 10.1016/j.ijpsycho.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban G, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: A single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49(2):307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J Neurophysiol. 2006 Feb;95(2):995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. NeuroImage. 2006;32(3):1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- Serruya MD, Sederberg PB, Kahana MJ. Power shifts track serial position and modulate encoding in human episodic memory. Cerebral Cortex. doi: 10.1093/cercor/bhs318. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka S, Ringo JL. Mnemonic responses of single units recorded from monkey inferotemporal cortex, accessed via transcommissural versus direct pathways: a dissociation between unit activity and behavior. J Neurosci. 1996 Jul;16(13):4222–30. doi: 10.1523/JNEUROSCI.16-13-04222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight R. Event-related potentials differentiate the effects of aging on word and non-word repetition in explicit and implicit memory tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition; Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23(1):123. doi: 10.1037//0278-7393.23.1.123. [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (v1) inhumans. Proceedings of the National Academy of Sciences. 1998;95(3):811–817. doi: 10.1073/pnas.95.3.811. Retrieved from http://www.pnas.org/content/95/3/811.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerven M, Maris E, Sperling M, Sharan A, Litt B, Anderson C, Jacobs J. Multivariate encoding of letter representations from ECoG signals. NeuroImage in press. [Google Scholar]
- Van Strien JW, Verkoeijen PPJL, Van der Meer N, Franken IHA. Electrophysio-logical correlates of word repetition spacing: Erp and induced band power old/new effects with massed and spaced repetitions. Int J Psychophysiol. 2007 Dec;66(3):205–14. doi: 10.1016/j.ijpsycho.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Vidyasagar R, Stancak A, Parkes LM. A multimodal brain imaging study of repetition suppression in the human visual cortex. Neuroimage. 2010 Jan;49(2):1612–21. doi: 10.1016/j.neuroimage.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Weiner K, Grill-Spector K. Synchrony upon repetition: One or multiple neural mechanisms? Cognitive Neuroscience. 2012;3(3-4):243–244. doi: 10.1080/17588928.2012.689973. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998 Apr;8(2):227–33. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Uğurbil K. High-field fmri unveils orientation columns in humans. Proceedings of the National Academy of Sciences. 2008;105(30):10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the encephalogram: an evaluation of methods. Psychophysiology. 2004;41(6):822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]