Abstract
Whereas selenium was found to act as an insulin-mimic and to be anti-diabetic in earlier studies, recent animal experiments and human trials have shown unexpected risk of prolonged high Se intake in potentiating insulin resistance and type 2 diabetes. Elevating dietary Se intakes (0.4 to 3.0 mg/kg of diet) above the nutrient requirements, similar to overproduction of selenoproteins, led to insulin resistance and(or) diabetes-like phenotypes in mice, rats, and pigs. Although its diabetogenic mechanism remains unclear, the high Se intake elevated activity or production of selenoproteins including GPx1, MsrB1, SelS, and SelP. This up-regulation diminished intracellular reactive oxygen species (ROS) and then dys-regulated key regulators of β cells and insulin synthesis and secretion, leading to chronic hyperinsulinaemia. Over-scavenging intracellular H2O2 also attenuated oxidative inhibition of protein tyrosine phosphatases and suppressed insulin signaling. High Se intake might affect expression and(or) function of key regulators for glycolysis, gluconeogenesis, and lipogenesis. Future research is needed to find out if certain forms of Se metabolites in addition to selenoproteins and if mechanisms other than intracellular redox control mediate the diabetogenic effect of high Se intakes. Furthermore, a potential interactive role of high Se intakes in the interphase of carcinogenesis and diabetogenesis should be explored to make the optimal use of Se in human nutrition and health.
Keywords: Diabetes, Insulin, Reactive oxygen species, Selenium, Selenoprotein
Introduction
Selenium (Se) was discovered in 1817 and reported in 1818 by Jöns Jacob Berzelius [1]. It was initially found as a toxic element because of Se poisoning in animals and humans [2]. However, Se deficiency was later shown to be more practically problematic and deleterious or fatal in animals [3,4] and humans [5]. In 1957, Se was recognized as an essential nutrient for animals [6] and 15 years later cellular glutathione peroxidase (GPx1) became the first identified Se-dependent enzyme [7,8]. Another landmark of Se biology was seen in 1996 when Clark and colleagues reported a striking effect of Se supranutrition on decreasing mortality of three types of human cancers [9].
Diabetes mellitus is one of the most costly chronic diseases, with an estimated worldwide prevalence of 366 million in 2011 and an expected rise to 552 million by 2030 [10]. In 2007, the prevalence of diabetes in the USA was 7.8% [11]. Meanwhile, China has the largest diabetic population in the world, accounting for 92.4 million adults in 2007–2008 [12]. There are four types of diabetes: Type 1 diabetes, Type 2 diabetes, gestational diabetes, and maturity onset diabetes of the young (MODY). Type 2 diabetes accounts for 90% of all diabetes and is characterized by peripheral insulin resistance, with an insulin-secretory defect that varies in severity. Although mechanisms for insulin resistance and diabetes are not fully understood, a growing body of evidence suggests that oxidative stress plays an important role in both of their onset and progress [13,14]. While there was a high hope for using antioxidants including Se to prevent and treat diabetes and its complications, a number of recent human trials have actually shown an alarming correlation between high Se intake or body Se status and diabetic risks [15–21]. Before this revealing, overexpression of GPx1, the “oldest” and most abundant Se-dependent protein, was shown to induce type 2 diabetes-like phenotypes in mice [22–24]. After this initial linking of selenoprotein to glucose and lipid metabolism, several new animal studies have provided compelling evidence and mechanism for the pro-diabetic potential of prolonged high Se intakes in different species.
Two tales of Se on diabetes
Se as an insulin mimic
Early studies indicated that inorganic Se acted as an insulin-mimic [25]. High doses of sodium selenate (0.1 to10 mM for 10 or 20 min) stimulated glucose uptake in isolated rat adipocytes through enhancing translocation of glucose transporters to plasma membrane, and activating serine/threonine kinases including p70 S6 kinase [26, 27]. Moreover, sodium selenate also produced dose-dependent stimulation of glucose uptake in dissected skeletal muscle of rats with the maximal response reached at 100 mM (60 min) [28]. Intraperitoneal injection or oral administration of sodium selenate improved glucose homeostasis in Type 1 and Type 2 diabetic animals [29–33]. Similarly, the insulin-like and anti-diabetic effects of sodium selenite and selenomethionine were also observed in diabetic animals [34–39], although their effects were shown to be weaker than sodium selenate. Mechanisms underlying differentiated effects of various selenium compounds have been reviewed [40]. However, all these insulin-like effects were mainly observed at high Se doses (0.9–4.5 mg/kg body weight) [29–39].
Deficiencies of Se and selenoprotein on diabetes
Data from earlier epidemiologic investigations showed correlations between abnormal glucose or lipid metabolism and decreased plasma Se concentrations or selenoperoxidase activity in diabetic subjects [41–48]. Likewise, there were also similar correlations or associations in animals. Thompson et al [49] reported that feeding chicks with Se-deficient diet (< 0.02 mg Se/kg of diet) for 3–5 weeks resulted in poor growth, poor feathering, atrophy of the pancreas, and impaired lipid absorption, compared with Se-supplemented controls. Souness et al [50] showed that rats fed a Se-deficient diet (Se content was too low to be detected) for 7–8 weeks had lower insulin-stimulated glucose oxidation in adipocytes compared with that of control rats fed the same diet supplemented with 0.5 mg Se/kg of diet as sodium selenite. Asayama et al [51] reported that Se deficiency impaired islet function and free radical scavenging systems in rats, resulting in decreased insulin secretory reserve. Furthermore, feeding both normal and diabetic rats [52] with a Se-deficient diet (< 0.025 mg Se/kg of diet) for 10 weeks elevated their plasma glucose concentrations and induced albuminuria and glomerular sclerosis, compared with those fed 0.27 or 0.78 mg Se/kg of diet. While Se deficiency caused renal oxidative stress, Se supplementation to diabetic rats prevented not only oxidative stress but also renal structural injury. Thus, supplementing Se was perceived as an effective strategy to prevent and treat diabetes.
Likewise, two groups have recently suggested that selenoprotein deficiency in mice was closely associated with diabetes or metabolic syndrome. Labunskyy et al [53] reported that reducing selenoprotein synthesis by overexpressing an i(6)A(−) mutant selenocysteine tRNA promoted glucose intolerance and led to a type 2 diabetes-like phenotype in mice. Seale et al [54] showed that knockout (KO) of selenocysteine lyase (Scly) in mice affected hepatic glucose and lipid homeostasis. Mice lacking Scly and raised on a Se-adequate diet exhibited hyperinsulinemia, hyperleptinemia, glucose intolerance, hepatic steatosis, and increased hepatic oxidative stress, but maintained selenoprotein levels and circulating Se status. Upon dietary Se deficiency, Scly KO animals developed several characteristics of metabolic syndrome, such as obesity, fatty liver, and hypercholesterolemia, with aggravated hyperleptinemia, hyperinsulinemia, and glucose intolerance. Altogether, these findings suggest a dependence of glucose and lipid homeostasis on Scly activity.
Elevations of Se intake and selenoprotein expression on diabetes
As mentioned above, a number of animal studies have been conducted to determine impacts of high Se intakes or overexpression of selenoprotein on glucose and lipid metabolism in mice, rats, and pigs. The main findings are summarized in Table 1.
Table 1.
Diabetogenic potential of elevated Se intakes or selenoprotein expression in animals
| Treatment | Finding | Reference |
|---|---|---|
| The GPX1-overexpressing (OE) and wild type (WT) male mice (n = 80) were fed a Se-adequate diet (0.4 mg/kg) from 8 to 24 weeks of age | Compared with the WT, the OE mice developed hyperglycemia, hyperinsulinemia, increased β-cell mass, hyper-secretion of insulin, insulin resistance, and obesity | 22, 55 |
| C57BL/6J mice (n = 6–7 per group) were fed either Se-deficient Torula yeast-based diet or diets supplemented with Se at 0.1 and 0.4 mg (sodium selenite)/kg of diet for 3 months | Mice in the 0.4 mg Se/kg group showed decreased insulin sensitivity and hyperinsulinemia compared to those fed the Se-deficient diet and 0.1 mg of Se/kg of diet | 53 |
| Three groups of rats (n = 10) were fed either a Se-deficient diet or diets supplemented with Se at 75 or 150 μg/kg of diet for 8 weeks | Rats in groups of 75 and 150 μg Se/kg diet had greater body weight, liver PTP1b activity, and liver triglyceride concentrations than the control group fed the Se-deficient diet | 58 |
| Seven groups of rats (n = 7) were fed either a Se-deficient diet or diets contained Se (as selenite or selenite) at 0.20, 1.0, and 2.0 mg/kg of diet for 8 weeks | All Se-supplemented animals featured a higher body weight, elevated liver GPx1 expression and activity, increased liver PTP1b activity, and reduced PTP1b glutathionylation, compared to their Se-deficient controls | 59 |
| Female Wistar rats were fed a Se-deficient (0.01 mg/kg of diet) corn–soy basal diet (BD) or BD+Se (as Se-yeast) at 0.3 or 3.0 mg/kg of diet from 5 weeks before breeding to day 14 postpartum. Offspring (n = 8/diet) born to dams fed 0.3 and 3.0 mg of Se/kg were fed with the same respective diet until age 112 days | Compared with the 0.3 mg of Se/kg of diet, the 3.0 mg of Se/kg of diet induced hyperinsulinemia, insulin resistance, and glucose intolerance in the dams at late gestation and/or day 14 postpartum and in the offspring at age 112 days | 60 |
| Weanling male pigs (n = 24) were fed a Se-deficient (< 0.02 mg of Se/kg of diet) corn-soy basal diet supplemented with 0, 0.3, or 3.0 mg of Se/kg of diet as Se-enriched yeast for 16 weeks | Compared with those fed 0.3 mg of Se/kg of diet, pigs fed 3.0 mg of Se/kg of diet became hyperinsulinemic and had lower tissue levels of serine/threonine protein kinase (Akt) | 62 |
| Male pigs were fed either a Se-adequate (0.17 mg of Se/kg of diet) or a high Se (0.50 mg of Se/kg of diet) diet for 16 weeks | The fasting plasma insulin and cholesterol levels were non-significantly increased in the pigs fed the high-Se diet, whereas fasting glucose concentrations did not differ between the two groups. Dietary Se oversupply affected expression and activity of proteins involved in energy metabolism in major was probably not sufficient to induce diabetes | 63 |
| Rats were intraperitoneally injected with saline (control) or 1.3, 1.6 and 3.8 mg of Se/kg of body weight as sodium selenite | Sodium selenite administration caused hyperglycemia in rats and elevated plasma corticosterone levels, but did not change plasma insulin levels | 57 |
Mice
The GPx1 overexpressing (OE) mice became obese at 6 months of age, and developed hyperglycemia, hyperinsulinemia, hyperlipidemia, and insulin resistance, along with elevated pancreatic β cell mass, islet insulin secretion, plasma leptin concentration, and hepatic lipogenesis [22–24]. Diet restriction (3 vs. 5 g of feed/day) of OE mice from 2 to 6 months of age [55] prevented all their phenotypes except for fasting hyperinsulinemia and hyper-secretion of insulin after glucose stimulation [55]. While dietary Se deficiency [23] did not rescue these two primary phenotypes of GPx1 overproduction in the feed-restricted OE mice, it exerted a strong effect on mRNA and(or) protein levels of 14 molecules involved in islet insulin synthesis and secretion and hepatic lipogenesis [23]. Dietary Se deficiency exhibited a hypoinsulinemic trend in OE mice and a strong hypolipidemic effect in the liver of WT mice. Because the overwhelming metabolic effect of diet restriction and the relatively short length of Se deficiency might preclude further benefit of dietary Se depletion in this study [23], a consecutive study [56] was conducted by Yan et al to explore whether dietary Se deficiency in the full-fed OE mice could completely rescue their phenotypes. While dietary Se deficiency (<0.02 mg of Se/kg of diet from 1 to 5 months of age) indeed precluded the GPx1 overproduction in the full-fed OE mice, 3 of their phenotypes, including hyperglycemia, insulin resistance, and elevated hepatic lipid profiles [22], were nearly rescued. Meanwhile, their hyperinsulinemia and aggravated glucose stimulated insulin secretion (GSIS) were also improved by dietary Se deficiency [56]. Mechanistically, this alleviation resulted from modulating the expression and/or function of proinsulin genes, lipogenesis rate-limiting enzyme genes, and key glycolysis and gluconeogenesis enzymes in islets, liver, and muscle. Taken together, these findings suggest that GPx1 was an important regulator of energy metabolism and insulin synthesis, secretion, and function. The C57BL/6 J mice fed a Torula yeast-based diet supplemented with Se at 0.4 mg/kg of diet for 3 months developed hyperinsulinemia and had decreased insulin sensitivity, compared with those fed a Se-deficient diet and the diet supplemented with 0.1 mg of Se/kg of diet [53].
Rats
Rasekh et al. [57] showed that acute intraperitoneal administration of sodium selenite to the rats (1.6 mg/kg of body weight or more) caused hyperglycemia in a time- and dose-dependent manner. While sodium selenite did not change plasma insulin levels in either fasted or fed animals, increases in corticosterone levels of the rats suggested the involvement of gluconeogenesis in this hyperglycemic response. In contrast to the nearly toxic doses of Se used in the above-mentioned study, Mueller et al [58] reported that rats received diets supplemented with sodium selenate to obtain final Se concentrations of 75 or 150 μg Se/kg of diet for 8 weeks had markedly elevated body weight, higher liver protein tyrosine phosphatase 1b (PTP1b) activity and higher liver triglyceride concentrations than the control group fed a Se-deficient diet. It is now well recognized that protein tyrosine phosphatases (PTPases) counteract insulin signaling and that maintaining the reduced state of PTPases supports this effect. They [59] also found that rats fed diets containing Se as selenite or selenate (final Se concentrations of 0.2, 1 and 2 mg/kg diet) for 8 weeks featured a higher body weight compared to their Se-deficient controls. In another recent study [60], female Wistar rats were fed a Se-deficient (0.01 mg/kg of diet) corn–soy basal diet (BD) or BD+Se (as Se-yeast) at 0.3 or 3.0 mg/kg of diet from 5 weeks before breeding to day 14 postpartum, and offspring of the 0.3 and 3.0 mg Se/kg of diet dams were fed with the same respective diet until age 112 days. Compared with the 0.3 mg Se/kg of diet, the 3.0 mg Se/kg of diet induced hyperinsulinemia, insulin resistance, and glucose intolerance in the dams at late gestation and/or day 14 postpartum and in the offspring at age of 112 days. Furthermore, plasma triglyceride levels in the dams were increased by the high dietary Se intake on day 19 of gestation. This hyperlipidemic effect of the high-Se diet in the gestating dams was also similar to the data from the above studies by Mueller et al [58,59].
Pigs
As a better model than rodents for human nutrition and medicine, pigs share with humans a greater metabolic similarity and disease susceptibility to develop type 2 diabetes or metabolic syndrome [61]. Feeding pigs with 3.0 mg of Se/kg of diet for 16 weeks induced hyperinsulinemia compared with those fed 0.3 mg of Se/kg of diet [62]. More specifically, the Se-overdosed pigs had >50% plasma insulin levels than the Se-adequate pigs to maintain similar plasma glucose concentrations, indicating an early sign of insulin resistance. Unlike rats [58–60], pigs fed the high-Se diet (3 mg of Se/kg of diet) did not develop hyperlipidemia compared with those fed 0.3 mg of Se/kg of diet. Meanwhile, Pinto et al [63] reported that after 16 weeks of intervention, fasting plasma insulin and cholesterol levels were increased in pigs fed 0.50 mg of Se/kg of diet (as Se-yeast) compared with those fed 0.17 mg of Se/kg of diet, although fasting glucose concentrations did not differ between the two groups.
Other models
Past studies using high Se intakes not designed for diabetes research, but for cancer chemoprevention or selenium toxicity research, have often overlooked or ignored glucose homeostasis or energy metabolism. However, there was at least one study illustrating such link. That study was aimed at characterizing Se action on normal rat heart function [64] and showed that sodium selenite administration to the normal rats (5 μmol or 0.86 mg/kg of body weight per day) for 4 weeks caused a slight but significant increase in blood glucose level, and a significant decrease in plasma insulin level.
Putative mechanisms of high Se on diabetes
ROS on islet insulin synthesis and secretion
Compared with liver, islets contain only 1% catalase, 2% GPx1, and 29% SOD1 activities [65–67]. Accordingly, β cells are considered to be low in antioxidant defense and susceptible to oxidative stress. In diabetic subjects, the β cell apoptosis seems to be a more deciding factor than replication in controlling the cell mass compared with control subjects [68]. Thus, maintaining pancreatic islet β cell mass is recognized as a pivotal prevention from pathogenesis of both types 1 and 2 diabetes [69]. The β cell apoptosis can be triggered by high glucose [70] and cytokines that elevate ROS production [71]. Furthermore, the key regulators of β cells and insulin synthesis are responsive to ROS, such as mitochondrial uncoupling protein 2 (UCP2), and the transcriptional factors, pancreatic duodenal homebox 1 (PDX1) and forkhead box A2 (FOXA2). In general, elevated ROS contribute to β cell apoptosis and defective insulin synthesis via affecting expression and function of these transcriptional factors. Although being considered to be wasteful and deleterious, ROS, especially H2O2, function as important factors in normal cellular signal transduction [72, 73], although contradictory results have been published regarding their impacts on acute glucose exposure and roles in GSIS. While numerous studies have described the negative effects of ROS generation in β cells including attenuation of GSIS [74], emerging evidence indicates that ROS derived from glucose metabolism, in particular H2O2, serve as additional metabolic signals to elicit GSIS [75–78]. This view has been reviewed elsewhere [79, 80].
ROS on insulin sensitivity
In insulin-responsive tissues, actual roles of ROS in insulin signaling depend on the balance of ROS production and antioxidant defense. Excessive ROS is involved in the multifactorial etiology of insulin resistance, and the subsequent development of type 2 diabetes [13,14]. Meanwhile, elevating ROS may activate a variety of serine/threonine kinases that in turn phosphorylate multiple targets, including the insulin receptor (IR) and the insulin receptor substrate (IRS) proteins [81]. In consequence, increased serine phosphorylation of IRS-1 decreases insulin-stimulated tyrosine phosphorylation of the protein, leading to insulin resistance. On the other hand, H2O2 may prolong phosphorylation of key proteins in the insulin signaling cascade by an oxidative inhibition of PTP1b [82–85].
Selenoproteins and antioxidant enzymes on tissue ROS tone and related signaling
Overexpression or knockout of GPx1 altered intracellular ROS status and subsequent redox regulation of key events in insulin synthesis, secretion, and function, resulting in dys-regulated glucose and lipid metabolism [22, 24]. More specifically, GPx1 overproduction up-regulated PDX1 mRNA and protein levels and attenuated degradation of PDX1 protein in islets [55]. The decrease in phosphorylated PDX1 protein was likely due to a reductive environment in islets, and the decreased phosphorylation of AKT at Thr 308 [86, 87], in line with the idea that phosphorylation of PDX1 is required for degradation by the proteasome machinery. An elevated functional PDX1 protein in islets resulted in hypertrophy of β cell mass, and subsequent increased pancreatic and plasma insulin concentrations [88–90]. In contrast, the reverse was induced by the GPx1 knockout [24]. Furthermore, GPx1 overexpression resulted in hyperacetylation of histone 3 and 4 (H3 and H4) in the PDX1 gene promoter [55] that may help explain in part the increased islet PDX1 mRNA levels. However, GPx1 overproduction had no significant effect on islet FOXA2 mRNA levels. Unlike the GPx1 overproduction, the GPx1 knockout did not affect islet PDX1 mRNA and H3 and H4 acetylation [24]. GPx1 overproduction down-regulated islet UCP2 protein and elevated mitochondrial membrane potential, contributing to the accelerated GSIS and hyperinsulinemia [55]. In contrast, knockout of GPx1 alone or together with SOD1 up-regulated UCP2 protein in pancreas and decreased islet ATP content [24]. Both changes could contribute to the attenuated GSIS in these mice. In the GPx1 overexpressing mice, insulin resistance was associated with an attenuated insulin-stimulated phosphorylation of IR β subunit and AKT at Ser 473 and Thr 308 in liver and muscle [22]. These decreased phosphorylations were presumably caused by the diminished intracellular H2O2, which lifted the oxidative inhibition of protein tyrosine phosphatases. In contrast, knockout of GPx1 resulted in enhanced phosphorylation of AKT in muscle [24], and rendered mice resistant to a high fat diet induced-insulin resistance via an enhanced oxidation of phosphatase with tensin homology (PTEN) selectively in muscle tissue [91].
SelP (in humans encoded by the Sepp1 gene), a secretory protein primarily produced by the liver [92, 93], contains ten selenocysteine residues and functions as a Se transporter [94]. A pioneer study by Walter et al [95] reported the stimulation of the Sepp1 promoter activity by the forkhead box transcriptional factor FoxO1a in hepatoma cells and its attenuation by insulin. Moreover, the production of SelP was regulated similarly to that of the gluconeogenic enzyme glucose-6-phosphatase, by concerted action of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) and the transcriptional factors FoxO1a and hepatocyte nuclear factor-4α (HNF-4α) [96]. They also found that treatment of rat hepatocytes with high glucose resulted in increased Sepp1 mRNA expression and secretion. Furthermore, the treatment with metformin induced dose-dependent down-regulation of Sepp1 mRNA expression and secretion, and suppressed glucocorticoid-stimulated production of SelP [97]. Recently, Misu et al [98] found a positive correlation between hepatic Sepp1 mRNA levels and insulin resistance in humans, along with a positive correlation between serum SelP levels and both fasting plasma glucose and hemoglobin A1c (HbA1c) levels. Administration of purified SelP impaired insulin signaling and dysregulated glucose metabolism both in vitro and in vivo. In contrast, genetic deletion and RNA interference-mediated knockdown of SelP improved systemic insulin sensitivity and glucose tolerance in mice. The metabolic actions of SelP were mediated, at least partly, by inactivation of adenosine monophosphate-activated protein kinase (AMPK). Accordingly, the metabolic effect of SelP on insulin sensitivity was similar to that of GPx1. However, SelP did not show effect on β cell mass or insulin synthesis and secretion.
Walder and colleagues showed that Tanis (in humans encoded by the Sels gene) was regulated by glucose and altered in the diabetic state [99,100]. Furthermore, Tanis overexpression in H4IIE cells reduced glucose uptake, basal and insulin-stimulated glycogen synthesis, and glycogen content, attenuated the suppression of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by insulin, and had no effect on insulin-stimulated IR phosphorylation or triglyceride synthesis [101]. These results suggested that Tanis might be involved in the regulation of glucose metabolism, and increased expression of Tanis could contribute to insulin resistance in the liver. Furthermore, emerging evidences suggest that elevations of Sels [102–105] or Sepp1 [106,107] mRNA and protein expression were observed in type 2 diabetic patients.
It has been widely accepted that catalase, GPx, and SOD represent the three most important intracellular antioxidant enzymes. SOD1 (Cu,Zn-SOD) comprises over 90% of the total cellular SOD activity, and functions upstream of GPx1 in catalyzing dismutation of superoxide ion into H2O2. Catalase shares a common substrate of H2O2 with GPx1, but with a lower affinity for H2O2. Altered expressions of SOD1 and catalase have produced variable metabolic outcomes. Whereas β cell-specific or global overexpression of SOD1 enhanced mouse resistance to alloxan-induced diabetes [108, 109], β cell-specific overexpression of catalase aggravated onset of type 1 diabetes in nonobese diabetic mice [110]. It seems that the role of catalase in glucose metabolism is similar to that of GPx1, but different from that of SOD1. A comprehensive review of this topic can be found elsewhere [48].
High Se intake on tissue redox status and selenoprotein expression
High dietary Se intake may induce the generation of superoxide radicals and/or other ROS [33,111–115]. This type of ROS elevation is implicated in the molecular mechanisms for the insulin-like effects of Se, as elevated H2O2 may activate insulin signaling by an oxidative inhibition of PTP-1b [82–85]. Meanwhile, a high selenite diet (1.0–2.0 mg of Se/kg of diet) [59] resulted in a lower GSSG/GSH ratio in the rat liver, compared with a Se adequate diet (0.2 mg of Se/kg of diet). This antioxidant effect was in accordance with increased plasma GPx3 activity by high Se over adequate Se supplements, although the high selenite diet had no effect on the activities of GPx1 and SOD in the liver, and even decreased catalase activity. Using the Se-enriched yeast [116], Zhou et al. have demonstrated that 3.0 mg of Se/kg of diet enhanced (43–88%) GPx activity among four tissues of pigs (liver, testis, thyroid, and pituitary) compared to those fed 0.3 mg of Se/kg of diet. However, the high Se diet did not affect activities of plasma GPx3 and other three antioxidant enzymes in any of the four tissues. The increased GPx activity was in accordance with data from another pig study [63]. Moreover, similar increases in hepatic or erythrocyte GPx activity by high Se diets over adequate Se supplements have also been seen in mice [117], rats [118], and fish [119]. Thus, the increases in liver and erythrocyte GPx activity seem to be a plausible mediator for the high Se intake to disturb glucose and lipid metabolism.
While Zhou et al [116] found little effect of dietary Se concentrations (0.02, 0.3, and 3.0 mg/kg diet) on mRNA levels of 12 selenoprotein genes in thyroid, pituitary, liver, or muscle, Liu et al [62] reported that mRNA expression of the remaining 13 selenoproteins in 10 tissues of pigs responded to dietary Se in three patterns. But, there was no common regulation for any given gene across all tissues or for any given tissue across all genes [62]. Dietary Se affected 2, 3, 3, 5, 6, 7, 7, and 8 selenoprotein genes in muscle, hypothalamus, liver, kidney, heart, spleen, thyroid, and pituitary, respectively. Protein abundance of GPx1, Sepp1, Selh, and Sels in 6 tissues was also regulated by dietary Se concentrations in three ways. The high Se diet (3.0 mg of Se/kg of diet) resulted in greater protein levels of GPx1 in heart and testis, Sepp1 in thyroid and testis, Selh in liver and kidney, and Sels in thyroid compared with Se adequate diet (0.3 mg of Se/kg of diet). As reported previously, these selenoproteins have the biochemical potential to be involved in glucose metabolism. In the rat study [60], dietary Se produced dose-dependent increases in GPx1 mRNA or GPx activity in pancreas, liver, and erythrocytes of dams. The 3.0 mg of Se/kg of diet decreased Selh, Sepp1, and Sepw1, but increased Sels mRNA levels in the liver of the offspring, compared with the 0.3 mg of Se/kg of diet. Expression of 6 selenoprotein genes, in particular Gpx1, was linked to gestational diabetes and insulin resistance. Likewise, Labunskyy et al showed that high-Se diet (0.4 mg/kg of diet), compared with the diet containing 0.1 mg of Se/kg of diet, resulted in slight elevation of GPx1 and MsrB1 protein levels in mice [53]. In contrast, protein expression of mitochondrial thioredoxin reductase 3 (TrxR3) in livers and kidneys was less responsive to changes in dietary Se levels. Moreover, Pinto et al [63] observed an increase in GPx activity in the skeletal muscle of pigs fed a high Se diet compared with the controls. However, the protein expression of GPx1 and thioredoxin reductase 1 (TrxR1) was not altered by Se supplementation. No significant changes in mRNA levels of any of the selenoproteins assayed in liver, skeletal muscle or visceral adipose tissue (VAT) were found in the pigs fed the high Se diet over the Se-adequate diet.
High Se intake on key regulators of beta cells and insulin
Given the positive effect of GPx1 overproduction on beta cell mass and insulin synthesis and secretion [22–24], the above-mentioned elevated tissue GPx activity by the high Se diets compared with the Se-adequate diets in various species might represent one of the pathways for the high Se intake regulation of insulin levels. While the elevated tissue GPx activity could attenuate insulin sensitivity by diminishing oxidative inhibition of PTP1b, Mueller et al [59] reported a high Se diet (1.0–2.0 mg of Se/kg of diet as selenite or selenate) markedly elevated liver PTP1b activity in rats through reduction of glutathionylation of PTP1b, compared with the Se adequate diet (0.2 mg of Se/kg of diet). Apparently, this elevation of PTP1b activity attenuated insulin-stimulated tyrosine phosphorylation of IRS, resulting in impairment of insulin signaling. Moreover, insulin resistance induced by the high Se diet in the dams and offspring of rats was associated with down-regulation of mRNA levels of hepatic Insr, Irs1, and Akt2 genes and/or hepatic IR and AKT protein levels [60]. The high Se intake also reduced mRNA levels of hepatic Irs2 in the dams as well as those of muscle Irs2 in the offspring. Because these genes code for key insulin signal proteins [120], downregulation of their mRNA expression or protein production may compromise insulin sensitivity. The high Se diet-induced porcine hyperinsulinemia was concurrent with Akt protein decreases in liver and other tissues [62].
High Se intake on key regulators of glucose and lipid metabolism
The altered expression and function of key enzymes and factors for glucose and lipid metabolism is also implicated in the mechanisms for the pro-diabetic potential of high Se intake. This notion is supported by the finding that high Se diets increased gene expression of forkhead box O1 and PGC-1α, and reduced gene expression of the glycolytic enzyme pyruvate kinase in skeletal muscle of pigs [63]. Moreover, high Se diets enhanced mRNA expression of sterol regulatory element-binding transcription factor 1 and lipoprotein lipase (LPL) (1.90 fold, P = 0.17), decreased mRNA levels of PGC-13 (55%, P = 0.27), and affected the phosphorylation of AMPK and mitogen-activated protein kinases in visceral adipose tissue [63]. The elevated expression of SREBP-1c was in accordance with data from a rat study in which high Se diet elevated liver PTP1b activity, possibly by activating lipogenic mechanisms involving the activation of SREBP-1c [59]. Likewise, high Se intake reduced mRNA levels of hepatic FoxO1 and muscle Pgc-1 in the rat offspring [60].
Perspective and conclusion
Feeding mice, rats, and pigs with high Se diets containing 0.4 to 3.0 mg of Se/kg of diet for extended periods of time induced hyperinsulinemia, hyperglycemia, insulin resistance, glucose intolerance, and altered lipid metabolism. This type of effect seems to be independent of different forms of Se sources, compositions of basal diets, and physiological stages. Thus, it is hard to deny a causative relationship between prolonged high Se intakes and pro-diabetic potential.
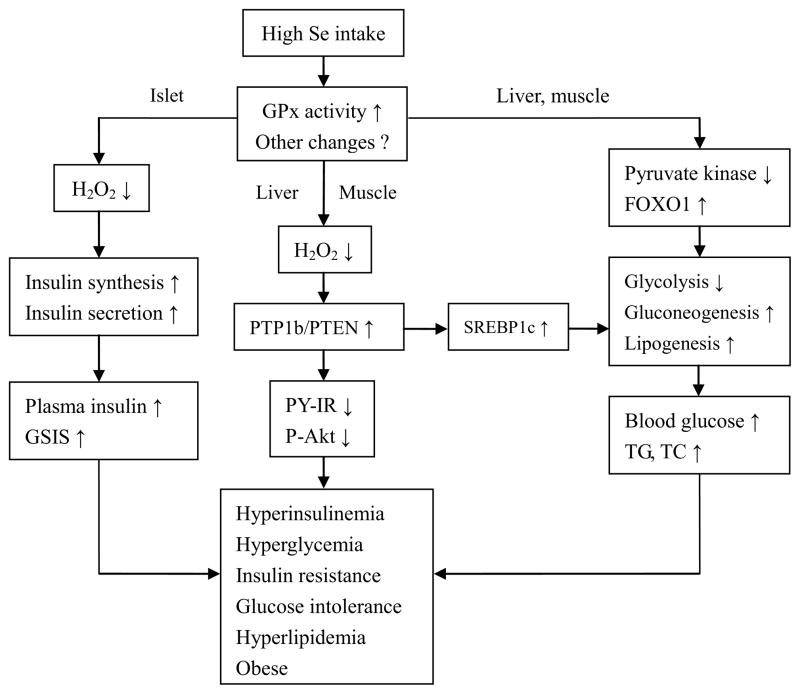
As illustrated in Fig. 1, high Se intake may lead to elevated activity or production of GPx1 and other selenoproteins including MsrB1, SelS, ad SelP. This type of up-regulation diminishes intracellular ROS and dys-regulates key regulators of β cells and insulin synthesis and secretion, leading to chronic hyperinsulinaemia. Over-scavenging intracellular H2O2 also attenuates oxidative inhibition of protein tyrosine phosphatases including PTP1b or PTEN, suppressing insulin-stimulated IR/IRS/PI3-K/Akt signaling. At the same time, un-inhibited PTP1b stimulates the lipogenic pathway [121–124], promoting lipogenesis and further aggravating insulin resistance. High Se intake may affect expression and(or) function of the key regulators for glycolysis, gluconeogenesis, and lipogenesis. These pathways might contribute to the pro-diabetic potential of high Se intake cooperatively or independently [40, 48, 125–127]. Although expression and activity of many selenoproteins are saturated at the adequate Se intake, several selenoproteins or their mRNA levels are affected by higher Se intake [128–130]. Because the low molecular weight Se metabolites require a higher dietary selenium intake for their saturation [131–134], it is tempting to test if those metabolites [135] mediate the pro-diabetic potential of high Se intake.
Figure 1.
Scheme of potential regulatory pathways and mechanisms for the diabetogenic potential of high Se intake. ↑, Activation or increase; ↓, inhibition or decrease; Akt, protein kinase B; FOXO1, forkhead box O1; GPx1, glutathione peroxidase-1; GSIS, glucose-stimulated insulin secretion; H2O2, hydrogen peroxide; IR, insulin receptor; P, phosphorylation; PY, tyrosine phosphorylation; PTEN, phosphatase with tensin homology; PTP1b, protein tyrosine phosphatase 1b; SREBP1c, sterol regulatory element binding protein-1c; TC, total cholesterol; TG, triglyceride.
There are at least three reasons for the discrepancy on dietary Se role in diabetes between the past and present experiments. First, many of the past animal studies used diabetic animals [29–39], but the present studies have been conducted in normal animals [53,57–60, 62–63]. Second, the past experiments used high or nearly toxic doses of Se (0.9–4.5 mg/kg body weight) [29–39], while recent experiments used Se levels not exceeding their maximal tolerable limits (≤33.0 mg Se/kg diet). Finally, many of the past animal studies lasted only 2 to 8 weeks, whereas in most of recent animal studies the duration of Se supplementation has been 12 weeks or longer [53,57–60, 62–63].
The causative relationship between prolonged high Se intake and pro-diabetic potential in different animal species was consistent with findings from several major human trials including the Nutritional Prevention of Cancer (NPC) [15] and the Selenium and Vitamin E Cancer Prevention Trial (SELECT) [16] trials. Most striking, Faghihi et al [136] have recently conducted a randomized, double-blind placebo-controlled trial and assessed the effects of supplemental Se (200 μg/day or placebo was administered orally for 3 months) on blood glucose, lipid profile, and oxidative stress in 60 patients with type 2 diabetes. At endpoint, plasma Se concentration reached to 71.98 (45.08) μg/L in Se recipients compared with 45.38 (46.45) μg/L in placebo recipients (P < 0.01). Between-group comparison showed that fasting plasma glucose, glycosylated hemoglobin A1c, and high-density lipoprotein cholesterol were higher in the Se recipient arm. Apparently, the Se supplementation in patients with type 2 diabetes was associated with adverse effects on blood glucose homeostasis, although plasma Se concentration was raised from deficient status to the optimal concentration of antioxidant activity. They suggested that until results of further studies became available, indiscriminate use of Se supplements in patients with type 2 diabetes should warrant caution. Caution should also be given to relate the animal responses to high Se intake to human cases because many factors such as genetic variation, living style, and environment could affect onset and development of diabetes and the potential role of Se in this regard. At the mechanist level, many challenging questions remain to be answered. It is important to find out if certain forms of Se metabolites or selenoproteins mediate the diabetogenic effect of high Se intake. It is also imperative to reveal if biochemical or molecular mechanisms other than modulating intracellular redox status are also involved in the diabetogenic action of high Se intake. Lastly, it is most important to elucidate the metabolic significance and mechanistic basis for a potential interactive role of high Se intakes in the interphase of carcinogenesis and diabetogenesis. This is because prolonged high Se diet induces hyperinsulinemia and insulin resistance in insulin-responsive tissues, and insulin signaling is recognized for being pro-carcinogenic [137,138]. Thus, it is tempting to speculate that high Se intake may suppress carcinogenesis by inhibiting insulin signaling. However, several human cancer trials including the NPC [9,15,139] and SELECT [16] have yielded inconsistent results in that regard. Seemingly, a U-shaped relation exists between the dietary Se intake/body Se status and cancer risk. If the body Se status reaches or rises above a threshold, higher Se intake may turn into potentiating the cancer risk. Prudently, indiscriminant Se supplementation to healthy subjects with adequate Se intake should not be recommended to avoid the possible double risks of diabetogenic and pro-carcinogenic potential of excessive Se.
Acknowledgments
The research conducted by the authors was supported in part by NIH grant DK53018 (X.G.L.), the National Natural Science Foundation of China (J. Z., No 21001045 and 31270870), and the Fundamental Research Funds for the Central Universities, HUST: No 2012QN145 (J. Z.).
List of Abbreviations
- ACC
Acetyl-CoA carboxylase
- AMPK
adenosine monophosphate-activated protein kinase
- BD
basal diet
- FOXA2
forkhead box A2
- FoxO1
forkhead box O1
- GPx1
glutathione peroxidase 1
- GSIS
glucose stimulated insulin secretion
- HbA1c
hemoglobin A1c
- HNF-4α
hepatocyte nuclear factor-4α
- H2O2
hydrogen peroxide
- H3
histone 3
- H4
histone 4
- Insr
insulin receptor
- IR
insulin receptor
- IRS
insulin receptor substrate
- JNK
c-Jun N-terminal kinase
- KO
knockout
- LPL
lipoprotein lipase
- MAPK
mitogen-activated protein kinase
- MODY
maturity onset diabetes of the young
- MsrB1
methionine-R-sulfoxide reductase 1
- mTOR
mammalian target of rapamycin
- NPC
Nutritional Prevention of Cancer
- OE
overexpressing
- PDX1
pancreatic duodenal homebox 1
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisomal proliferator-activated receptor-γ coactivator 1α
- PI3-K
phosphatidylinositol 3-kinase
- PTEN
phosphatase with tensin homology
- PTPase
phosphotyrosine phosphatase
- PTP1b
protein tyrosine phosphatase 1b
- ROS
reactive oxygen species
- Scly
selenocysteine lyase
- Se
selenium
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- SelP
selenoprotein P
- SelS
selenoprotein S
- SelT
selenoprotein T
- SOD
superoxide dismutase
- SREBP-1c
sterol regulatory element binding protein-1c
- TrxR1
thioredoxin reductase 1
- UCP2
uncoupling protein 2
- VAT
visceral adipose tissue
- WT
wild type
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnér ES. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Whanger P, Vendeland S, Park YC, Xia Y. Metabolism of subtoxic levels of selenium in animals and humans. Ann Clin Lab Sci. 1996;26:99–113. [PubMed] [Google Scholar]
- 3.Moir DC, Masters HG. Hepatosis dietetica, nutritional myopathy, mulberry heart disease and associated hepatic selenium level in pigs. Aust Vet J. 1979;55:360–364. doi: 10.1111/j.1751-0813.1979.tb15889.x. [DOI] [PubMed] [Google Scholar]
- 4.Reilly C, editor. Selenium in food and health. London: Blackie Academic and Professional; 1996. [Google Scholar]
- 5.Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr. 1993;57:259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz K, Foltz CM. J Am Chem Soc. 1957;79:3292–3293. [Google Scholar]
- 7.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 8.Flohe L, Günzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 9.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 10.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 11.National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics. 2007 fact sheet. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2008. [Google Scholar]
- 12.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. China National Diabetes and Metabolic Disorders Study Group. Prevalence of Diabetes among Men and Women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 13.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440: 944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 14.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 15.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 16.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30:829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 18.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117:1409–1413. doi: 10.1289/ehp.0900704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010;10:564. doi: 10.1186/1471-2458-10-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang KC, Lee LT, Lee YS, Huang HY, Chen CY, Huang KC. Serum selenium concentration is associated with metabolic factors in the elderly: a cross-sectional study. Nutr Metab (Lond) 2010;7:38. doi: 10.1186/1743-7075-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obeid O, Elfakhani M, Hlais S, Iskandar M, Batal M, Mouneimne Y, Adra N, Hwalla N. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of lebanese adults. Biol Trace Elem Res. 2008;123:58–65. doi: 10.1007/s12011-008-8112-0. [DOI] [PubMed] [Google Scholar]
- 22.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, Lei XG. Impacts of dietary selenium deficiency on metabolic phenotypes of diet-restricted GPX1-overexpressing mice. Antioxid Redox Signal. 2011;14:383–390. doi: 10.1089/ars.2010.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391–401. doi: 10.1089/ars.2010.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stapleton SR. Selenium: an insulin-mimetic. Cell Mol Life Sci. 2000;57:1874–1879. doi: 10.1007/PL00000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990;265:1124–1128. [PubMed] [Google Scholar]
- 27.Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH. Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem. 1998;178:367–375. doi: 10.1023/a:1006819906820. [DOI] [PubMed] [Google Scholar]
- 28.Fürnsinn C, Englisch R, Ebner K, Nowotny P, Vogl C, Waldhäusl W. Insulin-like vs. non-insulin-like stimulation of glucose metabolism by vanadium, tungsten, and selenium compounds in rat muscle. Life Sci. 1996;59:1989–2000. doi: 10.1016/s0024-3205(96)00550-4. [DOI] [PubMed] [Google Scholar]
- 29.McNeill JH, Delgatty HLM, Battell ML. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991;40:1675–1678. doi: 10.2337/diab.40.12.1675. [DOI] [PubMed] [Google Scholar]
- 30.Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996;39:3–11. doi: 10.1007/BF00400407. [DOI] [PubMed] [Google Scholar]
- 31.Battell ML, Delgatty HLM, McNeill JH. Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats. Mol Cell Biochem. 1998;179:27–34. doi: 10.1023/a:1006819227506. [DOI] [PubMed] [Google Scholar]
- 32.Mueller AS, Pallauf J, Rafael J. The chemical form of selenium affects insulinomimetic properties of the trace element: investigations in type II diabetic dbdb mice. J Nutr Biochem. 2003;14:637–647. doi: 10.1016/j.jnutbio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006;17:548–560. doi: 10.1016/j.jnutbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Sheng XQ, Huang KX, Xu HB. Influence of alloxan-induced diabetes and selenite treatment on blood glucose and glutathione levels in mice. J Trace Elem Med Biol. 2005;18:261–267. doi: 10.1016/j.jtemb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Zeng J, Zhou J, Huang K. Effect of selenium on pancreatic proinflammatory cytokines in streptozotocin-induced diabetic mice. J Nutr Biochem. 2009;20:530–536. doi: 10.1016/j.jnutbio.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Ayaz M, Can B, Ozdemir S, Turan B. Protective effect of selenium treatment on diabetes-induced myocardial structural alterations. Biol Trace Elem Res. 2002;89:215–226. doi: 10.1385/bter:89:3:215. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir S, Ayaz M, Can B, Turan B. Effect of selenite treatment on ultrastructural changes in experimental diabetic rat bones. Biol Trace Elem Res. 2005;107:167–179. doi: 10.1385/BTER:107:2:167. [DOI] [PubMed] [Google Scholar]
- 38.Can B, Ulusu NN, Kilinc K, Leyla Acan N, Saran Y, Turan B. Selenium treatment protects diabetes-induced biochemical and ultrastructural alterations in liver tissue. Biol Trace Elem Res. 2005;105:135–150. doi: 10.1385/bter:105:1-3:135. [DOI] [PubMed] [Google Scholar]
- 39.Erbayraktar Z, Yilmaz O, Artmann AT, Cehreli R, Coker C. Effects of selenium supplementation on antioxidant defense and glucose homeostasis in experimental diabetes mellitus. Biol Trace Elem Res. 2007;118:217–226. doi: 10.1007/s12011-007-0037-5. [DOI] [PubMed] [Google Scholar]
- 40.Mueller AS, Mueller K, Wolf NM, Pallauf J. Selenium and diabetes: an enigma? Free Radic Res. 2009;43:1029–1059. doi: 10.1080/10715760903196925. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-Alarcon M, López-G de la Serrana H, Perez-Valero V, Lopez-Martinez C. Serum and urine selenium concentrations as indicators of body status in patients with diabetes mellitus. Sci Total Environ. 1999;228:79–85. doi: 10.1016/s0048-9697(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 42.Kljai K, Runje R. Selenium and glycogen levels in diabetic patients. Biol Trace Elem Res. 2001;83:223–229. doi: 10.1385/BTER:83:3:223. [DOI] [PubMed] [Google Scholar]
- 43.Hawkes WC, Alkan Z, Lang K, King JC. Plasma selenium decrease during pregnancy is associated with glucose intolerance. Biol Trace Elem Res. 2004;100:19–29. doi: 10.1385/BTER:100:1:019. [DOI] [PubMed] [Google Scholar]
- 44.Faure P, Ramon O, Favier A, Halimi S. Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Invest. 2004;34:475–481. doi: 10.1111/j.1365-2362.2004.01362.x. [DOI] [PubMed] [Google Scholar]
- 45.Whiting PH, Kalansooriya A, Holbrook I, Haddad F, Jennings PE. The relationship between chronic glycaemic control and oxidative stress in type 2 diabetes mellitus. Br J Biomed Sci. 2008;65:71–74. doi: 10.1080/09674845.2008.11732800. [DOI] [PubMed] [Google Scholar]
- 46.Akbaraly TN, Arnaud J, Rayman MP, Hininger-Favier I, Roussel AM, Berr C, Fontbonne A. Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab (Lond) 2010;7: 21–27. doi: 10.1186/1743-7075-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roman M, Lapolla A, Jitaru P, Sechi A, Cosma C, Cozzi G, Cescon P, Barbante C. Plasma selenoproteins concentrations in type 2 diabetes mellitus--a pilot study. Transl Res. 2010;156:242–250. doi: 10.1016/j.trsl.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal. 2011;14:489–503. doi: 10.1089/ars.2010.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson JN, Scott ML. Impaired lipid and vitamin E absorption related to atrophy of the pancreas in selenium-deficient chicks. J Nutr. 1970;100:797–809. doi: 10.1093/jn/100.7.797. [DOI] [PubMed] [Google Scholar]
- 50.Souness JE, Stouffer JE, Chagoya de Sanchez V. The effect of selenium-deficiency on rat fat-cell glucose oxidation. Biochem J. 1983;214:471–477. doi: 10.1042/bj2140471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asayama K, Kooy NW, Burr IM. Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: decrease of islet manganosuperoxide dismutase. J Lab Clin Med. 1986;107:459–464. [PubMed] [Google Scholar]
- 52.Reddi AS, Bollineni JS. Selenium-deficient diet induces renal oxidative stress and injury via TGF-β1 in normal and diabetic rats. Kidney Int. 2001;59:1342–1353. doi: 10.1046/j.1523-1755.2001.0590041342.x. [DOI] [PubMed] [Google Scholar]
- 53.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14: 2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seale LA, Hashimoto AC, Kurokawa S, Gilman CL, Seyedali A, Bellinger FP, Raman AV, Berry MJ. Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol Cell Biol. 2012;32:4141–4154. doi: 10.1128/MCB.00293-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 56.Yan X, Pepper MP, Vatamaniuk MZ, Roneker CA, Li L, Lei XG. Dietary selenium deficiency partially rescues type 2 diabetes-like phenotypes of glutathione peroxidase-1-overexpressing male mice. J Nutr. 2012;142:1975–1982. doi: 10.3945/jn.112.164764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasekh HR, Potmis RA, Nonavinakere VK, Early JL, Iszard MB. Effect of selenium on plasma glucose of rats: role of insulin and glucocorticoids. Toxicol Lett. 1991;58:199–207. doi: 10.1016/0378-4274(91)90174-5. [DOI] [PubMed] [Google Scholar]
- 58.Mueller AS, Klomann SD, Wolf NM, Schneider S, Schmidt R, Spielmann J, Stangl G, Eder K, Pallauf J. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008;138:2328–2336. doi: 10.3945/jn.108.089482. [DOI] [PubMed] [Google Scholar]
- 59.Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J. Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem. 2009;20:235–247. doi: 10.1016/j.jnutbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, Wang KN, Lei XG. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52:1335–1342. doi: 10.1016/j.freeradbiomed.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patterson JK, Lei XG, Miller DD. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med (Maywood) 2008;233:651–664. doi: 10.3181/0709-MR-262. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Zhao H, Zhang Q, Tang J, Li K, Xia XJ, Wang KN, Li K, Lei XG. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr. 2012;142:1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto A, Juniper DT, Sanil M, Morgan L, Clark L, Sies H, Rayman MP, Steinbrenner H. Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J Inorg Biochem. 2012;114:47–54. doi: 10.1016/j.jinorgbio.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Ayaz M, Ozdemir S, Yaras N, Vassort G, Turan B. Selenium-induced alterations in ionic currents of rat cardiomyocytes. Biochem Biophys Res Commun. 2005;327:163–173. doi: 10.1016/j.bbrc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Grankvist K, Marklund S, Täljedal I. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 67.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and anti-oxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 68.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 69.Rhodes C. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 70.Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 71.Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 72.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 73.Rhee SG. Measuring H2O2 produced in response to cell surface receptor activation. Nat Chem Biol. 2007;3:244–246. doi: 10.1038/nchembio0507-244. [DOI] [PubMed] [Google Scholar]
- 74.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7: 38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 78.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 79.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function. Diabetes Obes Metab. 2010;12:141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 80.Collins S, Pi J, Yehuda-Shnaidman E. Uncoupling and reactive oxygen species (ROS) – A double-edged sword for β-cell function? “Moderation in all things”. Best Pract Res Clin Endocrinol Metab. 2012;26:753–758. doi: 10.1016/j.beem.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 82.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 83.Storz G, Tartaglia LA, Ames BN. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 84.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 85.Hayes GR, Lockwood DH. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci USA. 1987;84:8115–8119. doi: 10.1073/pnas.84.22.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 87.Boucher MJ, Selander L, Carlsson L, Edlund H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem. 2006;281:6395–6403. doi: 10.1074/jbc.M511597200. [DOI] [PubMed] [Google Scholar]
- 88.Mosley AL, Corbett JA, Ozcan S. Glucose regulation of insulin gene expression requires the recruitment of p300 by the beta-cell-specific transcription factor Pdx-1. Mol Endocrinol (Baltimore) 2004;18:2279–2290. doi: 10.1210/me.2003-0463. [DOI] [PubMed] [Google Scholar]
- 89.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 90.Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. 2000;20:900–911. doi: 10.1128/mcb.20.3.900-911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in seleniumhomeostasis. Annu Rev Nutr. 2005;25:215–235. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 93.Carlson BA, Novoselov SV, Kumaraswamy E, Lee BJ, Anver MR, Gladyshev VN, Hatfield DL. Specific excision of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver demonstrates an essential role of selenoproteins in liver function. J Biol Chem. 2004;279:8011–8017. doi: 10.1074/jbc.M310470200. [DOI] [PubMed] [Google Scholar]
- 94.Saito Y, Takahashi K. Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem. 2002;269:5746–5751. doi: 10.1046/j.1432-1033.2002.03298.x. [DOI] [PubMed] [Google Scholar]
- 95.Walter P, Steinbrenner H, Barthel A, Klotz LO. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008;365:316–321. doi: 10.1016/j.bbrc.2007.10.171. [DOI] [PubMed] [Google Scholar]
- 96.Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008;48:1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 97.Speckmann B, Sies H, Steinbrenner H. Attenuation of hepatic expression and secretion of selenoprotein P by metformin. Biochem Biophys Res Commun. 2009;387:158–163. doi: 10.1016/j.bbrc.2009.06.143. [DOI] [PubMed] [Google Scholar]
- 98.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto K, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 99.Walder K, Kantham L, McMillan JS, Trevaskis J, Kerr L, De Silva A, Sunderland T, Godde N, Gao Y, Bishara N, Windmill K, Tenne-Brown J, Augert G, Zimmet PZ, Collier GR. Tanis: a link between type 2 diabetes and inflammation? Diabetes. 2002;51:1859–1866. doi: 10.2337/diabetes.51.6.1859. [DOI] [PubMed] [Google Scholar]
- 100.Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress – SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–190. doi: 10.1016/S0014-5793(04)00296-0. [DOI] [PubMed] [Google Scholar]
- 101.Gao Y, Walder K, Sunderland T, Kantham L, Feng HC, Quick M, Bishara N, de Silva A, Augert G, Tenne-Brown J, Collier GR. Elevation in Tanis expression alters glucose metabolism and insulin sensitivity in H4IIE cells. Diabetes. 2003;52: 929–934. doi: 10.2337/diabetes.52.4.929. [DOI] [PubMed] [Google Scholar]
- 102.Karlsson HK, Tsuchida H, Lake S, Koistinen HA, Krook A. Relationship between serum amyloid A level and Tanis/SelS mRNA expression in skeletal muscle and adipose tissue from healthy and type 2 diabetic subjects. Diabetes. 2004;53:1424–1428. doi: 10.2337/diabetes.53.6.1424. [DOI] [PubMed] [Google Scholar]
- 103.Du JL, Sun CK, Lü B, Men LL, Yao JJ, An LJ, Song GR. Association of SelS mRNA expression in omental adipose tissue with Homa-IR and serum amyloid A in patients with type 2 diabetes mellitus. Chin Med J (Engl) 2008;121:1165–1168. [PubMed] [Google Scholar]
- 104.Liu J, Tang H, Niu L, Xu Y. Upregulation of Tanis mRNA expression in the liver is associated with insulin resistance in rats. Tohoku J Exp Med. 2009;219:307–310. doi: 10.1620/tjem.219.307. [DOI] [PubMed] [Google Scholar]
- 105.Olsson M, Olsson B, Jacobson P, Thelle DS, Björkegren J, Walley A, Froguel P, Carlsson LM, Sjöholm K. Expression of the selenoprotein S (SELS) gene in subcutaneous adipose tissue and SELS genotype are associated with metabolic risk factors. Metabolism. 2011;60:114–120. doi: 10.1016/j.metabol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96:E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- 107.Misu H, Ishikura K, Kurita S, Takeshita Y, Ota T, Saito Y, Takahashi K, Kaneko S, Takamura T. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012;7:e34952. doi: 10.1371/journal.pone.0034952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kubisch H, Wang J, Bray T, Phillips J. Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic beta-cells against oxidative stress. Diabetes. 1997;46:1563–1566. doi: 10.2337/diabetes.46.10.1563. [DOI] [PubMed] [Google Scholar]
- 109.Kubisch H, Wang J, Luche R, Carlson E, Bray T, Epstein C, Phillips J. Transgenic copper/zinc superoxide dismutase modulates susceptibility to type I diabetes. Proc Nat Acad Sci USA. 1994;91:9956–9959. doi: 10.1073/pnas.91.21.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: reactive oxygen species may have a protective role in pancreatic beta-cells. Diabetes. 2006;55:1592–1604. doi: 10.2337/db05-1357. [DOI] [PubMed] [Google Scholar]
- 111.Spallholz JE. Free radical generation by selenium compounds and their prooxidant toxicity. Biomed Environ Sci. 1997;10:260–270. [PubMed] [Google Scholar]
- 112.Chen JJ, Boylan LM, Wu CK, Spallholz JE. Oxidation of glutathione and superoxide generation by inorganic and organic selenium compounds. Biofactors. 2007;31:55–66. doi: 10.1002/biof.5520310106. [DOI] [PubMed] [Google Scholar]
- 113.Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–726. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 114.Brigelius-Flohé R. Selenium compounds and selenoproteins in cancer. Chem Biodivers. 2008;5: 389–395. doi: 10.1002/cbdv.200890039. [DOI] [PubMed] [Google Scholar]
- 115.Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–1666. doi: 10.1093/carcin/20.9.1657. [DOI] [PubMed] [Google Scholar]
- 116.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr. 2009;139:1061–1066. doi: 10.3945/jn.109.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng W-H, Combs GF, Jr, Lei XG. Knockouts of cellular glutathione peroxidase affect selenium-dependent parameters similarly in mice fed adequate and excessive dietary selenium. Biofactors. 1998;7:311–421. doi: 10.1002/biof.5520070403. [DOI] [PubMed] [Google Scholar]
- 118.Whanger PD, Butler JA. Effects of various dietary levels of selenium as selenite or selenomethionine on tissue selenium levels and glutathione peroxidase activity in rats. J Nutr. 1988;118:846–852. doi: 10.1093/jn/118.7.846. [DOI] [PubMed] [Google Scholar]
- 119.Lin Y-H, Shiau S-Y. Dietary selenium requirements of juvenile grouper. Epinephelus malabaricus. Aquaculture. 2005;250:356–363. [Google Scholar]
- 120.Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabetes Med. 2005;22:674–682. doi: 10.1111/j.1464-5491.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 121.Shimizu S, Ugi S, Maegawa H, Egawa K, Nishio Y, Yoshizaki T, Shi K, Nagai Y, Morino K, Nemoto K, Nakamura T, Bryer-Ash M, Kashiwagi A. Protein-tyrosine phosphatase 1B as new activator for hepatic lipogenesis via sterol regulatory element-binding protein-1 gene expression. J Biol Chem. 2003;278:43095–43101. doi: 10.1074/jbc.M306880200. [DOI] [PubMed] [Google Scholar]
- 122.Shi K, Ugi S, Shimizu S, Sekine O, Ikeda K, Egawa K, Yoshizaki T, Nagai Y, Nishio Y, Takada T, Torii R, Kimura H, Kashiwagi A, Maegawa H. Membrane localization of protein-tyrosine phosphatase 1B is essential for its activation of sterol regulatory element-binding protein-1 gene expression. Biochem Biophys Res Commun. 2007;363:626–632. doi: 10.1016/j.bbrc.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Ferré P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 124.Sanderson SO, Smyrk TC. The use of protein tyrosine phosphatase 1B and insulin receptor immunostains to differentiate nonalcoholic from alcoholic steatohepatitis in liver biopsy specimens. Am J Clin Pathol. 2005;123:503–509. doi: 10.1309/1PX2-LMPQ-UH1E-E12U. [DOI] [PubMed] [Google Scholar]
- 125.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2011;48:40–45. doi: 10.3164/jcbn.11-002FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Finley JW, Kong AN, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem. 2011;59:6837–6846. doi: 10.1021/jf2013875. [DOI] [PubMed] [Google Scholar]
- 127.Lei XG, Wang X. Glutathione Peroxidase 1 and Diabetes. In: Hatfield DL, Berry MJ, Gladyshev VN, editors. Selenium: Its Molecular Biology and Role in Human Health. NY: Springer; 2012. pp. 261–270. [Google Scholar]
- 128.Yang JG, Hill KE, Burk RF. Dietary selenium intake controls rat plasma selenoprotein P concentration. J Nutr. 1989;119:1010–1012. doi: 10.1093/jn/119.7.1010. [DOI] [PubMed] [Google Scholar]
- 129.Behne D, Kyriakopoulos A, Gessner H, Walzog B, Meinhold H. Type I iodothyronine deiodinase activity after high selenium intake, and relations between selenium and iodine metabolism in rats. J Nutr. 1992;122:1542–1546. doi: 10.1093/jn/122.7.1542. [DOI] [PubMed] [Google Scholar]
- 130.Sunde RA, Evenson JK, Thompson KM, Sachdev SW. Dietary selenium requirements based on glutathione peroxidase-1 activity and mRNA levels and other Se-dependent parameters are not increased by pregnancy and lactation in rats. J Nutr. 2005;135:2144–2150. doi: 10.1093/jn/135.9.2144. [DOI] [PubMed] [Google Scholar]
- 131.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 132.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 133.Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, Broadley MR, Motley AK, Fairweather-Tait SJ. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, Byrne DW, Burk RF. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92:525–531. doi: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pinto A, Speckmann B, Heisler M, Sies H, Steinbrenner H. Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J Inorg Biochem. 2011;105:812–820. doi: 10.1016/j.jinorgbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 136.Faghihi T, Radfar M, Barmal M, Amini P, Qorbani M, Abdollahi M, Larijani B. A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: effects on glucose homeostasis, oxidative stress, and lipid profile. Am J Ther. 2013 Apr 29; doi: 10.1097/MJT.0b013e318269175f. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 137.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 138.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8: 915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 139.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11: 630–639. [PubMed] [Google Scholar]