Abstract
This review examines the involvement of the motor cortex in Parkinson’s disease (PD), a debilitating movement disorder typified by degeneration of dopamine cells of the substantia nigra. While much of PD research has focused on the caudate/putamen, many aspects of motor cortex function are abnormal in PD patients and in animal models of PD, implicating motor cortex involvement in disease symptoms and their treatment. Herein, we discuss several lines of evidence to support this hypothesis. Dopamine depletion alters regional metabolism in the motor cortex and also reduces interneuron activity, causing a breakdown in intracortical inhibition. This leads to functional reorganization of motor maps and excessive corticostriatal synchrony when movement is initiated. Recent work suggests that electrical stimulation of the motor cortex provides a clinical benefit for PD patients. Based on extant research, we identify a number of unanswered questions regarding the motor cortex in PD and argue that a better understanding of the contribution of the motor cortex to PD symptoms will facilitate the development of novel therapeutic approaches.
Keywords: Parkinson’s Disease, Motor Cortex, Supplementary Motor Area, Premotor Cortex, Dopamine, Functional Imaging, Transcranial Magnetic Stimulation, Deep Brain Stimulation, Plasticity
1. Introduction
The pathological hallmark of Parkinson’s disease (PD) is the death of dopamine (DA) cells in the substantia nigra pars compacta (SNc), which causes bradykinesia, akinesia, resting tremor, rigidity and postural instability (Dauer and Przedborski, 2003; Jankovic, 2008). Treatment with L-DOPA or synthetic DA receptor agonists relieves PD symptoms, but often causes drug-induced dyskinesias (Ahlskog & Muenter, 2001; Stowe et al., 2009). Likewise, deep brain stimulation (DBS) with the electrode placed in either the subthalamic nucleus (STN) or the globus pallidus pars interna (GPi) improves movement in PD patients, but concerns about surgical complications such as hemorrhages or infection can limit their widespread use (Bronstein et al., 2011; Follett et al., 2010).
In devising new therapeutic approaches to PD, the motor cortex has been gaining momentum as a potential target. Evidence for motor cortex involvement in PD pathophysiology and treatment is strong as dynamic changes in motor cortex function are seen in PD patients and in animal models of PD (Lefaucheur, 2005; Ceballos-Baumann, 2003; Goldberg et al., 2004). According to accepted cannon, PD symptoms are thought to result from degeneration of DA-secreting SNc neurons, which synapse in the striatum to facilitate controlled movement (Dauer and Przedborski, 2003). However, this nigrostriatal pathology causes functional alterations in a variety of structures connected to the striatum including the motor cortex (Obeso et al., 2008). At the same time, DA projections from the midbrain directly to the motor cortex are reduced in PD patients, providing a second source of dysfunction (Gaspar et al., 1991). Convergent evidence suggests that the motor cortex is a therapeutic target in PD: direct motor cortex stimulation can reduce the symptoms of PD and L-DOPA-induced dyskinesia (LID; Elahi et al., 2009) while antiparkinsonian therapy modulates the activity of the motor cortex (Lefaucheur, 2005).
Given increasing evidence that abnormal motor cortex function is an important component of PD pathophysiology, this review outlines critical findings while identifying key unanswered questions for the research field. This review will first highlight the intrinsic connectivity of the motor cortex and the basal ganglia before turning to motor cortex pathology in PD. Functional changes in the motor cortex of PD patients before and after treatment will be covered from a “top-down” perspective by examining, in order: regional blood flow and metabolism, gross excitability, plasticity, motor maps, oscillations and synchrony, and lastly, individual cellular activity. For the purposes of this review, the term “motor cortex” is defined as including the primary motor cortex (M1), the supplementary motor area (SMA), and the premotor cortex (PMC).
2. Motor Cortex – Basal Ganglia Connectivity
2.1. Motor Cortex Afferents
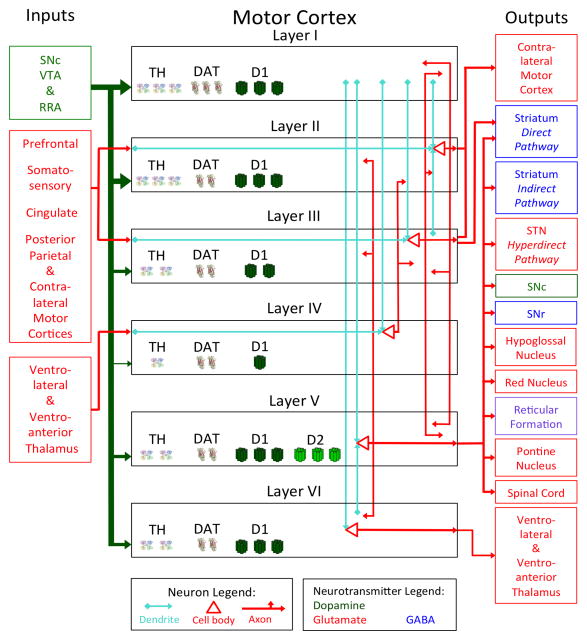
The ventrolateral nucleus of thalamus constitutes most thalamocortical input to the motor cortex, innervating M1, the posterior SMA (SMA proper), the ventral PMC (PMCv) and parts of the dorsal PMC (PMCd) (Geyer et al., 2000; see Figure 1). The ventroanterior thalamic nucleus projects to the anterior SMA (pre-SMA) and parts of the PMCd (Geyer et al., 2000; Martin, 2003). In parts of the anterior motor cortex, these thalamocortical connections synapse in layer IV, following the general pattern for neocortex (Martin, 2003). However, much of the posterior motor cortex (including all of M1) has no anatomically distinct layer IV and thalamocortical connections synapse in layers III and V (Geyer et al., 2000; Keller, 1993). The cerebellum provides inputs to the PMC via a polysynaptic route that relays at the ventrolateral thalamus (Martin, 2003). High order control of movement relies on intracortical connections feeding into the motor cortex from sites including the prefrontal, somatosensory and posterior parietal cortices (Geyer et al., 2000). The motor cortex is also innervated by serotonin (5-HT) from the raphe nuclei (Tork, 1990), norepinephrine (NE) from the locus coeruleus (Lindvall and Bjorklund, 1974) and acetylcholine from the nucleus basalis of meynert (Mesulam et al., 1983).
Figure 1.
Layer-specific input and output model of the primate motor cortex. The neurotransmitter released by a given nuclei is indicated by the color of the text box and the color of the line emanating from it, with DA in green, glutamate in red an GABA in blue (Note: the reticular formation uses acetylcholine, norepinephrine and serotonin and is in purple). For DA projections from the midbrain, the thickness of the line indicates the relative density of the DA fibers to each cortical layer. Arrows indicate the direction of information flow. Within each layer, the location of pyramidal cells and the connections made by their dendrites and axons are schematically represented. Dendrites are depicted in teal while the cell bodies and axons are in red. Only the major synaptic connections are depicted in order to facilitate clarity. Much of the posterior motor cortex does not have a layer IV and thalamocortical axons synapse instead within layers III and V. Within each layer, the relative amount of TH, DAT and D1 receptors in each layer is represented by one, two or three symbols (Note: D2 receptors exclusively localize to layer V).
Abbreviations used: DA = Dopamine; DAT = Dopamine transporter; RRA = Retrorubral area; SNc = Substantia nigra pars compacta; SNr = Substantia nigra pars reticulata; STN = Subthalamic nucleus; TH = Tyrosine hydroxylase; VTA = Ventral tegmental area.
In primates, the supply of DA to the motor cortex is more dense than in any other area of the cortical mantle, with innervation distributed among three midbrain DA nuclei: the SNc, ventral tegmental area (VTA), and retrorubral area (RRA; Gaspar et al., 1992; Williams and Goldman-Rakic, 1998). All cortical layers receive DA although layers I and II are the most densely suppliedwhile layer IV receives the least DA innervation (Goldman-Rakic et al., 1989). D1 receptors are distributed across the cortical layers and found on asymmetrical (excitatory) synapses, while D2 receptors are specifically localized to layer V and form symmetrical (inhibitory) synapses (Lidow and Goldman-Rakic, 1994; Smiley et al., 1994). In contrast to primates, rodents have relatively less DA in the motor cortex than they do in the prefrontal cortex, the DA innervation is preferentially in the deep cortical layers and the fibers originate mostly from the VTA (Berger et al., 1991). Further, a proportion of the SNc neurons that supply the striatum with DA also extend projections to the motor cortex (Debier et al., 2005).
2.2. Motor Cortex Anatomy and Microcircuitry
Layer V of the motor cortex is thicker than in other cortical regions and contains Betz cells, a unique type of pyramidal cell distinguished by their large soma and dendrites that emerge from the soma in all directions (Martin, 2003; Rivara et al., 2003). Betz cells are highest in density in the ventral region of layer V, accounting for about 12% of all pyramidal cells in this area (Rivara et al., 2003). They are most common in the medial region of M1, which is principally responsible for foot and leg movements (Rivara et al., 2003). Betz cells are much less common in the SMA and the PMC, and are primarily observed near the anatomical boundaries of M1 (Geyer et al., 2000).
Within the subregions of the motor cortex, there is elaborate interconnectivity. The pre-SMA, SMA proper, PMCd and PMCv all provide inputs to M1; however, M1 provides reciprocal feedback only to the SMA proper and the PMCd (Geyer et al., 2000). While the SMA proper and pre-SMA have monosynaptic reciprocal connections, the PMCd and PMCv are connected only disynaptically (Geyer et al., 2000).
Within a cortical column, information tends to flow into the superficial layers and propagate ventrally. In cats, the apical dendrites of M1 pyramidal cells in all layers extend dorsally with most reaching layer I (see Figure 1; Ghosh et al., 1988). By contrast, only layers II and layer V have ventrally projecting basal dendrites, each of which travel down one layer (Ghosh et al., 1988). Innervation by intrinsic motor cortex axon collaterals is most extensive in layers II, III and V (Aroniadou & Keller, 1993; Keller, 1993). By stimulating individual M1 neurons in a known layer and measuring the response in other layers, Weiler et al. (2008) showed that the strongest signal propagation occurred in layer V when layers II/III were stimulated. By contrast, the strongest ascending pathway was from layer V into layers II/III. Top-down information flow is also commensurate with the observation that the motor cortex has a large number of output structures despite having comparatively fewer input nuclei.
From a functional perspective, complex movements tend to recruit anterior motor cortex regions first and the chain of activation moves in a posterior direction. The PMCd seems to be important for guiding movements using available sensory information (Abe and Hanakawa, 2009). By contrast, many PMCv neurons fire in response to visual or somatosensory stimuli or when an organism is manipulating an object directly (Luppino and Rizzolatti, 2000). The SMA is considered to be key for movement planning (Goldberg, 1985) and while simple movements require only the SMA proper, more complex motor tasks activate the pre-SMA as well (Luppino and Rizzolatti, 2000). M1 is critical for fine motor control, such as the independent use of digits among primate species (Canedo, 1997; Geyer et al., 2000).
2.3. Motor Cortex Efferents
Motor cortex output is almost exclusively glutamatergic and occurs mainly via the deepest two layers. The bottom layer, layer VI, sends projections back to the ventrolateral and ventroanterior thalamus (Martin, 2003). Layer V projects to a diversity of structures in the brain stem involved in movement execution and postural stability including the red nucleus, reticular formation, SNc, substantia nigra pars reticulata (SNr) and the hypoglossal nucleus (Canedo, 1997; Martin, 2003). Motor cortex outputs bound for the cerebellum first synapse in the pontine nuclei of the pons (Martin, 2003). The corticospinal tract also emanates from layer V and while most of these neurons terminate in the cervical spinal cord, some axons travel as far as the lumbar region (Canedo, 1997).
The efferent connections to the basal ganglia are somatotopically organized and form three distinct pathways, known as the direct, indirect and hyperdirect pathways (Ebrahimi et al., 1992). Motor cortex neurons that connect with the direct and indirect pathways synapse on distinct sets of striatal neurons: striatal output neurons of the direct pathway project to the SNr and GPi while other neurons follow the indirect pathway synapsing at the globus pallidus pars externa (GPe) and STN en route to the GPi (Obeso et al., 2008). Almost all of these corticostriatal neurons emanate from layer V; however, a subset of direct pathway neurons have cell bodies in the deep part of layer III (Lei et al., 2004). Motor cortex layer V also projects to the STN, comprising what is known as the hyperdirect pathway, so named as these signals have the shortest latency to travel from the cortex to the GPi.
Alterations in corticostriatal signaling may be a primary cause of both PD and LID (Braak and Del Tredici, 2008; Calabresi et al., 1996; Cenci, 2007). These heterosynapses contain axoaxonal connections between corticostriatal and nigrostriatal neurons, which in turn make axodendritic contacts with striatal medium spiny neurons (Wilson, 1987). Nigrostriatal DA release activates D2 receptors at these synapses to reduce corticostriatal glutamate release, thus serving as a feedback loop to filter cortical inputs to the striatum (Bamford et al., 2004).
The GPi is the convergence point for the direct, indirect and hyperdirect pathways. The net result of these initially divergent pathways is that cortical stimulation evokes four temporally distinct GPi responses, with early and late excitation mediated through the STN, punctuated by inhibition from the striatum through the direct and indirect pathways (Kita and Kita, 2011; Ryan and Clark, 1991). GPi neurons project to the ventrolateral thalamus, providing inhibitory GABAergic input to thalamic cells partly responsible for glutamatergic excitation of motor cortex neurons.
3. Motor Cortex Pathology in Parkinson’s Disease
3.1. Brainstem Neurotransmitter Innervation
Generally, PD patients have reduced motor cortex neurotransmitter innervation from brainstem nuclei as a result of disease progression. At autopsy, patients generally have lost more than 75% of locus coeruleus NE neurons and SNc DA neurons (Dauer and Przedborski, 2003; Zarow et al., 2003). At the same time, DA-secreting cells in the VTA and RRA, acetylcholine neurons of the nucleus basalis of meynert and 5-HT cells of raphe nuclei are all pathologically affected to some extent although average cell death is probably less than 50% (Braak et al., 2003; Zarow et al., 2003). Thus, neurochemical signaling in the motor cortex of PD patients is disrupted as a direct result of brainstem nuclei cell death, but few studies have been conducted which quantify the nature and severity of motor cortex pathology. Perhaps the most comprehensive study on this topic was performed by Gaspar and colleagues (1991) who used post-mortem tissue to examine catecholamine loss in PD patients. The researchers found that the number of fibers staining positively for tyrosine hydroxylase (TH) (almost exclusively DA neurons: see Hokfelt et al., 1977) was reduced by around 70% in layers I and III (where DA innervation is relatively high) and reduced by 25–30% in layer VI (where DA density is lower). Relative to DA, NE terminals were more severely affected with reductions of 80–90% with no layer-specific differences. For both DA and NE, the authors did not find differences in deafferentation severity between M1, the SMA and the PMC (Gaspar et al., 1991).
The two most popular animal models of PD recapitulate some level of motor cortex DA pathology. In a widely-used model of PD where the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is delivered systemically, primate studies have shown that the toxin causes long-term reductions in tissue concentrations of DA, 5-HT and NE (Elsworth et al., 1990; Pifl et al., 1991). These studies demonstrate that MPTP causes the most severe reduction in striatal DA supply (>90%), but that motor cortex DA levels are also affected (~50–85%).
The other popular model of PD utilizes the catecholamine neurotoxin 6-hydroxydopamine (6-OHDA), delivered intracerebrally to the SNc, the striatum or the tract that connects the two structures, known as the medial forebrain bundle. The medial forebrain bundle lesion produces the most severe striatal DA lesion (>99%: Francardo et al., 2011) and was shown to reduce M1 TH fibers by 93% (Halje et al., 2012). Even if 6-OHDA is injected directly into the striatum, there is still some reduction in TH-positive fibers innervating the motor cortex (Debier et al., 2005). Thus, each of the two most widely-used toxin-based animal models of PD disturb motor cortex DA supply. While the construct validity for the MPTP and 6-OHDA models is therefore high, it may be difficult to dissociate the contribution of striatal versus motor cortex DA loss to the symptoms of PD.
3.2. Lewy Bodies
The histological hallmark of PD is the presence of Lewy bodies, which are cytosolic protein inclusions dense in E3 ligases and alpha-synuclein and are toxic to cells in high concentrations (Moore et al., 2005). By analyzing post-mortem tissue from PD patients at various disease stages, Braak et al. (2003) determined that frontal cortex Lewy body inclusions are first evident in prefrontal areas; as the disease progresses, Lewy bodies appear in the PMC, then in the SMA and finally in M1. Abnormal aggregation of alpha-synuclein in the motor cortex without classical Lewy bodies has also been observed, suggesting that this may occur during earlier disease stages (Caviness et al., 2011). At this point, how motor cortex Lewy bodies influence cortical function remains unknown.
3.3. Gray and White Matter Abnormalities
When the motor cortex of PD patients and healthy controls are examined with structural imaging, there are minor differences between PD patients and controls. In M1, reduced gray matter has been correlated with increased bradykinesia (Lyoo et al., 2011). In the SMA, PD patients showed gray matter reductions compared to controls though cortical thinning was not significantly correlated with PD symptom severity (Jubault et al., 2011). This cell loss is not neccesarily caused by Lewy bodies as one study found that intra-cortically projecting pyramidal cells in the anterior SMA were reduced by 45% relative to controls without the presence of cortical Lewy bodies or the loss of other types of neurons (MacDonald and Halliday, 2002). A decrease in fractional anisotropy (a marker for white matter patency) around the SMA has been reported, indicative of reduced coherence of white matter tracts entering or exiting the structure (Karagulle Kendi et al., 2008). At the same time, this study and others failed to find any changes in overall gray and white matter density in any motor cortex region of PD patients compared to age-matched controls (Karagulle Kendi et al., 2008; Cerasa et al., 2011). The fact that many of the aforementioned studies observed minimal effects with large samples (up to 142 individuals) suggests that differences in gray and white matter between PD patients and controls are not major contributors to PD symptoms.
4. Functional Changes in the Motor Cortex in Parkinson’s Disease
4.1. Regional Blood Flow and Metabolism
Advances in medical imaging have allowed for the non-invasive visualization of blood flow changes in the motor cortex of PD patients, first with positron emission tomography (PET) or single photon emission computed tomography (SPECT) and later with functional magnetic resonance imaging (fMRI). The use of fMRI provides high spatial resolution, but safety concerns often preclude scanning PD patients with implanted DBS electrodes (Ceballos-Baumann, 2003). Importantly, most imaging studies have been conducted on patients with early to moderate PD who respond well to therapeutic intervention with DBS or DA replacement.
During resting conditions, PD patients show activation patterns that are equivalent to healthy controls in all regions of the motor cortex (Berding et al., 2001; Hilker et al., 2004; Playford et al., 1992; Rascol et al., 1992; Samuel et al., 1997). When participants are asked to move, differences emerge between age-matched controls and PD patients who are OFF treatment (Table 1A). Evidence suggests that, during movement, M1 activity is increased in PD patients OFF medication. Relative to healthy controls, PD patients show increased M1 blood oxygen level-dependent (BOLD) fMRI signal during the performance of motor tasks based on timing (Haslinger et al., 2001; Yu et al., 2007) or sequence repetition (Sabatini et al., 2000). This has been interpreted to suggest that M1 hyperactivity represents an attempt to compensate for striatal pathology. In contrast to fMRI data, two studies using PET/SPECT scans on PD patients performing hand movements have shown reductions in primary sensorimotor cortex blood flow compared to age-matched controls (Catalan et al., 1999; Rascol et al., 1992). The lower spatial resolution of PET scans necessitates grouping larger cortical areas together, so it is possible that overall decreases in primary sensory cortex blood flow masked increased M1 blood flow, leading different researchers to draw opposing conclusions.
Table 1.
Compilation of studies using PD patients to examine changes in regional blood flow or metabolism within subregions of the motor cortex
| A) | |||
|---|---|---|---|
| Primary PD | PD OFF Relative to Healthy Controls | ||
| M1 Activity | SMA Activity | PMC Activity | |
| Rest |
No Change Berding et al. 2001 |
No Change Berding et al. 2001 Hilker et al. 2004 Playford et al. 1992 Rascol et al. 1992 |
No Change Berding et al. 2001 Samuel et al. 1997 |
| Movement |
Increased Yu et al. 2007 Haslinger et al. 2001 Sabatini et al. 2000 |
Decreased overall Playford et al. 1992 Rascol et al. 1992 Decreased in pre-SMA Haslinger et al. 2001 Sabatini et al. 2000 Yu et al. 2007 Increased in SMA proper Sabatini et al. 2000 |
Increased Catalan et al. 1999 Sabatini et al. 2000 Samuel et al. 1997 Mixed Haslinger et al. 2001 |
| B) | ||||
|---|---|---|---|---|
| Use of DA Therapy | PD ON Relative to PD OFF | |||
| M1 Activity | SMA Activity | PMC Activity | ||
| L-DOPA | Rest |
Decreased Asanuma et al. 2006 |
No Change Berding et al. 2001 |
No Change Berding et al. 2001 |
| Movement |
Decreased Haslinger et al. 2001 |
Increased Haslinger et al. 2001 Martinu et al. 2012 |
Increased Martinu et al. 2012 Mixed Haslinger et al. 2001 |
|
| Apomorphine | Rest |
No Change (Sensorimotor) Rascol et al. 1992 |
No Change Jenkins et al. 1992 Rascol et al. 1992 |
No Change Jenkins et al. 1992 |
| Movement |
Increased (Sensorimotor) Rascol et al. 1992 |
Increased Jenkins et al. 1992 Rascol et al. 1992 |
No Change Jenkins et al. 1992 |
|
| C) | ||||
|---|---|---|---|---|
| Use of Stimulations | PD ON Relative to PD OFF | |||
| M1 Activity | SMA Activity | PMC Activity | ||
| STN DBS | Rest |
Decreased Asanuma et al. 2006 Haslinger et al. 2005 Limousin et al. 1997 |
Decreased Hershey et al. 2003 Haslinger et al. 2005 |
Decreased Haslinger et al. 2005 Limousin et al. 1997 |
| Movement |
Decreased Ceballos-Baumann et al. 1999 |
Increased Limousin et al. 1997 Increased in pre-SMA and Decreased in SMA proper Ceballos-Baumann et al. 1999 |
Increased Ceballos-Baumann et al. 1999 |
|
| Gpi DBS | Rest |
No Change Limousin et al. 1997 |
Increased Davis et al. 1997 |
No data available |
| Movement |
No Change Limousin et al. 1997 |
Increased Valalik et al. 2009 |
Increased Valalik et al. 2009 |
|
Note. Participants were studied at rest and while performing hand movements using functional magnetic resonance imaging, positron emission tomography or single photon emission computed tomography. These results are listed as revealing changes in regional “activity” although different methods (and tracers) measure distinct aspects of motor cortex physiology. Null results are only listed if no other studies have shown significant changes in that region or experimental condition. Studies where the experimenters could not distinguish between the sensory and motor cortices are excluded unless noted as sensorimotor cortex.(A) PD patients studied OFF medication. (B) Effects of L-DOPA and apomorphine administration on PD patients. (C) Changes in activation as a result of DBS in the STN or the GPi. Abbreviations used: DBS = Deep brain stimulation; GPi = Globus pallidus pars interna; M1 = Primary motor cortex; PD = Parkinson’s disease; PMC = Premotor cortex; SMA = Supplementary motor area; STN = Subthalamic nucleus.
The SMA displays reduced activity in most untreated PD patients. This change may relate directly to the available supply of DA since, among healthy humans, a diet deficient in DA precursors reduced the BOLD signal from the SMA and impaired the ability to judge stimulus timing (Coull et al., 2012). Early studies using radioisotope scans showed an overall decrease in SMA blood flow during movement compared to age-matched controls (Playford et al., 1992; Rascol et al., 1992). Subsequent studies utilizing fMRI more conclusively distinguished that it is the pre-SMA that displays a reduction in cerebral blood flow (Haslinger et al., 2001; Yu et al., 2007). One study even found that while the pre-SMA had a reduced BOLD signal, the BOLD for the SMA proper was increased relative to controls (Sabatini et al., 2000). Although a direct link between behavioral phenotype and SMA hypoactivity remains speculative, it is interesting to note that the SMA is responsible for internally-generated movements (Goldberg, 1985) and this type of movement is particularly difficult for PD patients to perform (Jankovic, 2008).
During movement, the PMC has increased blood flow among PD patients relative to healthy adults, as assessed using PET for detection of H215O as well as fMRI (Catalan et al., 1999; Sabatini et al., 2000; Samuel et al., 1997). This region is responsible for integrating sensory information and for performing externally-guided movements (Abe and Hanakawa, 2009). The fact that PD patients have relatively preserved motor responses when cued by their environment (Rubinstein et al., 2002) suggests that PMC function may be relatively spared in PD.
Pharmacotherapy with L-DOPA or the non-specific DA agonist apomorphine tends to reverse metabolic deficits seen in primary PD (Table 1B). PD patients who respond well to L-DOPA show decreased M1 metabolic rate (assessed by 18F-fluorodeoxyglucose uptake) and reduced BOLD activation in the ON state relative to the OFF state regardless of whether or not they are moving (Asanuma et al., 2006; Haslinger et al., 2001). Interestingly, in a study where PD patients OFF medication showed decreased sensorimotor cortex blood flow during movement, administration of apomorphine still led to a reversal of the observed deficit by increasing sensorimotor cortex activity (Rascol et al., 1992). Regional cerebral blood flow in the SMA is increased by both L-DOPA and apomorphine during movement as assessed with PET, SPECT and fMRI (Haslinger et al., 2001; Jenkins et al., 1992; Martinu et al., 2012; Rascol et al., 1992). DA-replacement therapy does not seem to change PMC metabolic rate or blood flow when the patient is at rest (Berding et al., 2001; Jenkins et al., 1992). Data on activity changes caused by L-DOPA during movement are somewhat inconsistent: one study reported a global increase in PMC BOLD response (Martinu et al., 2012) while another found that specific voxel clusters within the PMC showed increased or decreased BOLD response as a result of L-DOPA treatment (Haslinger et al., 2001).
Unfortunately, there are few studies that investigate drug-induced dyskinesias using neuroimaging techniques since peripheral body movements can disrupt brain scans or confound interpretations of blood flow changes in the motor cortex. When imaging is used to study patients with LID, they are usually given a subclinical threshold dose of L-DOPA to avoid provoking involuntary movements. Using PET for H215O, it was shown that patients with LID had reduced M1 blood flow after L-DOPA, just as is seen in patients without LID (Hershey et al., 1998). However, another PET study using a tracer that binds to activated NMDA receptors found that giving L-DOPA to patients with LID caused an increase in NMDA receptor activation in M1 compared to patients without LID (Ahmed et al., 2011). Thus, abnormal NMDA signaling in M1 may predict the manifestation of LID in PD patients.
When PD patients are moving, the effects of STN DBS on motor cortex activation are similar to those seen with L-DOPA (Table 1C). Thus, in moving patients, turning ON an STN stimulator decreases M1 blood flow (Ceballos-Baumann et al., 1999) while increasing blood flow in the SMA (Limousin et al., 1997) and the PMC (Ceballos-Baumann et al., 1999). A region-specific increase in the pre-SMA and a decrease in the SMA proper has been reported, which is noteworthy since this is the precise opposite of what is observed in PD patients OFF treatment (Ceballos-Baumann et al., 1999). Curiously, when a patient is at rest, STN DBS decreases regional blood flow in the SMA and the PMC (Haslinger et al., 2005; Hershey et al., 2003; Limousin et al., 1997). The fact that STN DBS always reduces motor cortex activity when patients are at rest provides evidence for the emerging hypothesis that the therapeutic mechanism of action is an antidromic modulation of the motor cortex via axonal feedback in the corticosubthalamic pathway (Gradinaru et al., 2009; Li et al., 2012).
There are fewer studies examining GPi DBS, but these stimulators appear to increase anterior motor cortex activity (Table 1C). A reduction in sensorimotor blood flow (using PET detection of 15O-butanol) with GPi DBS has been reported during movement (Valalik et al., 2009), but was not found during rest or movement in M1 by itself (Limousin et al., 1997). An increase in SMA blood flow is seen when the GPi electrode is turned ON and this increase is observed whether or not the patient is moving (Davis et al., 1997; Valalik et al., 2009). Along the same lines, turning ON a GPi electrode increases PMC blood flow during movement (Valalik et al., 2009).
When the neuroimaging studies are examined as whole, several patterns emerge (see Table 1). First, increases in M1 blood flow are generally associated with an akinetic PD state, and a subsequent decrease in blood flow after intervention coincides with a decrease in PD symptoms. The SMA displays the opposite pattern: it is hypoactive when PD symptoms are present and shows increased activity when treatment is applied. Changes in the PMC are a mix of both patterns. PD patients OFF-treatment show greater blood flow in the PMC relative to healthy controls (similar to M1) while antiparkinsonian interventions lead to further increases in regional blood flow (similar to the SMA). The fact that region-specific increases and decreases in blood flow and metabolic rate are seen within the motor cortex of PD patients may suggest that some of these changes are compensatory for subcortical DA depletion.
A second possibility is that PD causes reduced motor cortex firing among neurons that are targets of the ventroanterior nucleus (pre-SMA and parts of the PMCd), which relays information from the caudate while increasing the firing of neurons that are innervated by the ventrolateral thalamus (M1, SMA proper and the rest of the PMC), which relays information from the putamen and pallidum (Geyer et al., 2000). Still a third possibility is that DA terminal loss in PD alters the balance of glutamatergic and GABAergic signaling. Since DA receptors are found at both excitatory and inhibitory synapses in the motor cortex (Goldman-Rakic et al., 1989; Smiley et al., 1994), a preferential reduction in activity of either synapse type could cause region-specific increases or decreases in neuron firing rates.
4.2. Gross Excitability
Individuals with PD show abnormal electrophysiological responses to motor cortex stimulation. In a typical experiment testing motor cortex excitability, transcranial magnetic stimulation (TMS) is applied to M1 in order to stimulate a motor-evoked potential (MEP) in a particular hand muscle. In “paired-pulse” TMS, two electrical pulses are applied in sequence (separated by 1–200 ms) and the magnitude of the second MEP is inhibited or facilitated by the presence of first pulse (presumably through activation of GABA or glutamate currents). Differences in inhibition/excitation seen in PD patients versus healthy controls are assumed to reflect global changes in the balance of GABA and glutamate signaling within M1 of PD patients (Lefaucheur, 2005).
Among PD patients, paired-pulse TMS research indicates that GABAergic tone in M1 is reduced. Short-interval intracortical inhibition (SICI) refers to a reduction in the amplitude of the second MEP when two pulses are separated by 1–6 ms (Chen, 2004). SICI is GABAA receptor-mediated and since PD patients typically display a smaller SICI than controls, this suggests that their GABAA currents are diminished (Ridding et al., 1995). Alleviation of the behavioral symptoms of PD using treatment with L-DOPA, direct DA receptor agonists or DBS all normalize SICI relative to controls (Cunic et al., 2002; Pierantozzi et al., 2001, 2002; Strafella et al., 2000; Ziemann et al., 1996). These convergent findings suggest that restoration of inhibition in the motor cortex is critical to alleviation of PD symptoms. Indeed, DA has been shown to be critical for activating cortical interneurons and increasing the signal-to-noise ratio in other cortical regions (Kroener et al., 2009). Thus, enhancement of DA signaling in the motor cortex may facilitate movement execution in PD patients.
Further evidence for reduced tonic inhibition in PD is shown by a reduction in the cortical silent period (CSP). The CSP appears dependent on GABAB receptor activation and refers to a period of time after muscle contraction during which M1 stimulation cannot produce a MEP in the contracted muscle (Bagnato et al., 2006; Cantello et al., 1991). L-DOPA tends to normalize (prolong) the CSP duration (Lefaucheur et al., 2004; Ridding et al., 1995) but not among patients with LID (Morgante et al., 2006). STN DBS alone has been shown to increase the CSP among most, but not all, PD patients (Baumer et al., 2009; Fraix et al., 2008). In one study, GPi DBS at a therapeutic intensity both reduced dyskinesia and normalized the CSP relative to healthy controls, suggesting that this GABAB receptor population in M1 may be involved in dyskinesia expression (Chen et al., 2001a).
The body of electrophysiological data indicate that GABAergic inhibition is impaired in the motor cortex, which may prevent proper selection of desired motor programs. Conventional models of PD pathophysiology predict a reduction in motor cortex glutamatergic excitation, but make no predictions about changes in GABA signaling (e.g. Obeso et al., 2000; Wichmann and DeLong, 1996). If this classical model is correct, the reduced GABAergic inhibition seen in PD patients may reflect a compensatory adjustment based on a lack of glutamatergic drive. Alternatively, the increase in motor cortex excitability could be due to a reduction in DA innervation: TH-positive synapses with pyramidal cells tend to be symmetric (inhibitory) and the application of DA reduces the firing rates of motor cortex cells (Awenowicz and Porter, 2002; Bernardi et al., 1982; Goldman-Rakic et al., 1989). Further investigations are required to determine if the increase in M1 excitability seen in PD patients is compensatory for striatal DA loss or if it is directly related to DA deafferentation.
4.3. Plasticity
A key role for DA in the motor cortex is to facilitate motor learning; thus it is not surprising that the ability to learn new motor tasks is impaired in PD patients (Doyon, 2008; Floel et al., 2005). In humans, motor cortex plasticity can be experimentally induced and studied using the paired associative stimulation (PAS) protocol (Stefan et al., 2000). In this paradigm, baseline cortical excitability is established by quantifying the magnitude of a MEP evoked by TMS at a site in M1 (typically a hand muscle is used). Subsequently, electrical stimulation of a sensory nerve is continuously paired with M1 stimulation using TMS. This pairing causes a transient increase in the MEP amplitude evoked by a single pulse of M1 TMS. The magnitude of the increase is then used as a marker of M1 plasticity (Stefan et al., 2000). Using the PAS paradigm, mid-stage PD patients did not show any MEP facilitation as a result of conditioning, indicating a lack of cortical plasticity (Ueki et al., 2006). By contrast, early-stage PD patients showed no plasticity-like effects on their more affected bodily hemisphere, but showed relatively normal plasticity on their less affected side (Kojovic et al., 2012). Importantly, L-DOPA was shown to restore PAS-induced plasticity in patients without LID, but not among patients with LID (Morgante et al., 2006), indicating that dysfunctional M1 plasticity is a persistent and disruptive factor in dyskinesia.
High frequency theta burst stimulation (TBS) can also be used to measure cortical plasticity. The TBS paradigm requires only M1 stimulation and can produce potentiation or attenuation of MEP amplitude, which are used as proxy markers of long-term potentiation (LTP) or long-term depression (LTD), respectively (Huang et al., 2005). Curiously, standard TBS parameters that produce motor learning in healthy controls have failed to find any such effects in PD patients either ON and OFF antiparkinsonian medication whether or not they manifest LID (Eggers et al., 2010; Suppa et al., 2011). The fact that PD patients show measureable plasticity with some protocols but not others indicates that specific types of motor learning are impaired in PD and that only certain types of learning can be restored by drug therapy. Determining the physiological correlates of each type of learning paradigm will shed light on the specific impairments in PD.
A modified TBS protocol that produces plasticity-like effects in PD patients was recently devised and this novel method provides support for the hypothesis that LID is associated with a specific lack of synaptic depotentiation in M1. Using this new TBS paradigm, Huang et al. (2011) demonstrated normal potentiation and depotentiation in M1 of patients without LID who were given their full dose of L-DOPA. Importantly, neither plasticity-like effect was seen if patients were given only half of their normal dose of L-DOPA. Among patients with LID, half of their usual dose of L-DOPA restored LTP-like effects, but not LTD-like effects. These findings suggest that an inability to initiate M1 LTD is implicated in LID expression, paralleling previous findings in the striatum (Picconi et al., 2003).
Data showing impaired motor cortex plasticity in PD may explain the widely-observed finding that PD patients have a reduced ability to learn motor sequences, whether the learning is implicit or explicit (Doyon, 2008). Moreover, the deficits in sequence learning are accentuated in later disease states (Muslimovic et al., 2007). PD patients show reduced activity in the pre-SMA during the acquisition of motor sequences and reduced SMA proper activity during sequence retrieval, suggesting that deficient SMA processing contributes directly to deficits in motor learning among PD patients (Nakamura et al., 2001).
In preclinipcal models, selective loss of motor cortex DA impairs motor cortex LTP and skills learning, but doesn’t appear to contribute to PD symptoms. In rats, injection of 6-OHDA into M1 produced an impairment in learning a skilled reach task; later, local delivery of L-DOPA into M1 restored the ability to learn the task (Molina-Luna et al., 2009). The same study showed impaired LTP in M1 slice preparations treated with either a D1 or D2 receptor antagonist, suggesting activation of both receptors in M1 is necessary for new motor learning. Taken together, the available evidence suggests that motor cortex DA is critical for normal motor learning and plasticity, but not necessary for normal motor performance.
4.4. Motor Maps
In addition to blunting motor cortex plasticity, a reduction in the supply of DA causes a reorganization of motor maps that blurs the normally discrete somatotopic organization of M1 and the SMA. Mapping topographical motor representations through non-invasive stimulation is possible in humans by using TMS. One such study investigated unilaterally impaired PD patients without medication and found that imagining a finger movement elicited an increase in the number of excitable cortical sites within the unaffected motor cortex, but had no effect on the parkinsonian side (Filippi et al., 2001). Performing an actual muscle contraction did elicit an increase in M1 excitability, but this increase was attenuated in the affected hemisphere when compared to both the intact hemisphere and healthy control participants.
In animal models, intracortical microstimulation has been used to map the size and location of motor cortex representations by systematically stimulating regions of M1/SMA with an intracortical electrode while observing the location of the motor response (Donoghue and Wise, 1982; Neafsey et al., 1986). In macaques with 93% bilateral SNc DA cell loss from MPTP treatment, microstimulation mapping revealed a decrease in the total size of the motor map and an increase in the average current required to evoke a movement (Escola et al., 2003). The finding that movements were more difficult to evoke with M1 stimulation implicates this deficit as a physiological correlate of PD-induced akinesia.
Functional reorganization of the motor cortex likely depends on several features of the DA lesion. A severe unilateral striatal DA depletion (99%) caused a bilateral reduction in the number of motor cortex sites where electrical stimulation could evoke a motor movement (Viaro et al., 2011). Interestingly, stimulation of the lesioned motor cortex often produced ipsilateral motor movements, an effect which was rarely seen in the intact hemisphere or amongst sham-lesioned animals. These ipsilateral movements signal a degree of interhemispheric compensation, which may also be occurring in early-stage PD patients with a unilateral motor impairment. The size of the motor map was smallest 15 days after DA lesion, but then increased over the next 4 months (Viaro et al., 2011). Since cell death from 6-OHDA is complete three weeks post-injection (Jeon et al., 1995), these protracted changes in motor map size indicate that plasticity in the motor cortex is ongoing when DA depletion is stable.
When using striatal 6-OHDA infusions that achieved approximately 60% SNc DA cell loss, it was found that bilateral but not unilateral lesions reduced the size of cortical forelimb representations (Brown et al., 2009). Striatal 6-OHDA injections may be a useful model of early-stage PD since the magnitude of DA loss is less than with SNc or medial forebrain bundle lesions (Francardo et al., 2011). Thus, the animal research supports clinical evidence (e.g. Filippi et al., 2001) suggesting that motor maps are altered across disease stages in PD patients. What has not been determined are the individual contributions of striatal and M1 DA depletion to cortical motor map alterations.
Antiparkinsonian therapy leads to a partial restoration of motor maps. Following 6-OHDA lesions in rats, a single de novo dose of L-DOPA (6 mg/kg) increased the area of excitable cortex close to the level of controls (Viaro et al., 2011). STN DBS has also been investigated using microstimulation mapping and the results are similar to L-DOPA: 60 seconds of DBS lead to a 12% increase in forelimb representation size among DA-lesioned rats, while increasing the forelimb map of sham-lesioned animals by only 1.5% (Brown et al., 2011). Therefore, at least functional restoration of motor maps in M1 and the SMA seems to be an essential step towards relief of PD symptoms.
4.5. Oscillations and Synchrony
In healthy brains, at rest, local field potential recordings of M1 have revealed prominent oscillations at beta frequency ranges (roughly 10–30 Hz; Schnitzler and Gross, 2005). During movement, the power of the M1 beta band falls and is replaced by gamma range oscillations (roughly 30–80 Hz), which are coherent with the basal ganglia and spinal cord (Schoffelen et al., 2005). This smooth transition is thought to underlie controlled movement. PD patients seem to have difficulty transitioning between the two cortical states as beta oscillations actually increase in power during movement (Crowell et al., 2012; Engel and Fries, 2010).
Some have argued that the transmission of these beta oscillations to the basal ganglia promotes akinesia (Hutchison et al., 2004). There is a strong correlation between prominent beta oscillations in the motor cortex and the symptoms of PD in both humans and animal models (Fogelson et al., 2006; Mallet et al., 2008; Sharott et al., 2005; Silberstein et al., 2005). Even early-stage PD patients (with a Unified Parkinson’s Disease Rating Scale [UPDRS] score <10) who displayed only a unilateral impairment, showed increased beta band activity in M1 compared to age-matched controls (Pollok et al., 2012). Since abnormal M1 activity is present at the earliest disease states and is easily detectable with non-invasive methods such as electroencephalographs, this raises the possibility that analysis of local field potentials could be useful in diagnosing PD.
The cause of powerful beta oscillations in PD patients is not clear, but they may be driven by deficits in synaptic DA or activated DA receptors. In an attempt to answer this question, Costa and colleagues (2006) used a procedure to rapidly deplete synaptic DA: mice with a genetic knockout of the dopamine transporter (DAT) gene were treated with alpha-methyl-p-tyrosine to pharmacologically inhibit TH and thus prevent new DA synthesis. Within 15 min of TH inhibitor injection, the power of M1 and striatal beta oscillations rose among DAT knockout mice but not among controls. Critically, this effect was reversed by L-DOPA, which can be converted to DA without TH availability. This finding demonstrated that rapid DA depletion could produce PD-like field potential changes in M1 and the striatum, arguing against the idea that beta oscillations emerge as a result of long-term plasticity. Along these lines, acute pharmacological blockade of D1/D2 receptors that impaired movement in healthy rats produced beta band oscillations in one study (Dejean et al., 2009) but not in another (Mallet et al., 2008). Although these authors used the same drugs at the same doses, Dejean et al. (2009) examined beta band activity on the rat’s second exposure to DA antagonists while Mallet et al. (2008) tested rats on their first drug injection; therefore, it is possible that multiple exposures to DA antagonists are required for beta band M1 oscillations to emerge.
Despite the findings of rapid changes in beta oscillations, the time course of the emergence of beta oscillations after DA lesion suggests that they are, in part, compensatory in nature. Using the 6-OHDA rat model, the power of the beta band (and it’s coherence with the beta band in the SNr) was increased one week after lesion (Brazhnik et al., 2012). At three weeks post-lesion, the frequency with the greatest power in (and coherence between) the SNr and M1 increased (from 31 to 34 Hz) suggesting that protracted changes occur in M1 as a result of an acute DA lesion. Not only do beta band oscillations correlate with PD symptoms, but artificially enhancing the power of beta oscillations in the motor cortex (by stimulating the STN at 20 Hz) was capable of enhancing bradykinesia among PD patients (Chen et al., 2007), suggesting that beta oscillations causally contribute to PD symptoms. This argument is bolstered by the findings that antiparkinsonian treatment with L-DOPA or STN DBS reduces the power of the beta band in the motor cortex of humans and in animal models of PD (Brazhnik et al., 2012; Silberstein et al., 2005).
While M1 beta oscillations may be a biological signature of PD symptoms, recent evidence suggests that abnormal high gamma band oscillations (~80 Hz) in M1 are implicated in LID. Using the unilateral 6-OHDA rat model of PD, administration of L-DOPA caused an increase in 80 Hz M1 oscillations that were temporally contiguous with the behavioral manifestation of LID (Halje et al., 2012). Under normal circumstances, a rise in M1 gamma band field potentials portends movement initiation (Schoffelen et al., 2005), but excessively powerful 80 Hz oscillations may cause an inability to terminate movement. The motor circuits of PD patients seem to have difficulty switching between akinetic (beta band) and prokinetic (gamma band) oscillations (Schnitzler and Gross, 2005). Among PD patients who manifest LID, L-DOPA may restore the ability to generate basal ganglia gamma range oscillations without restoring the ability to transition smoothly between beta and gamma oscillations, causing such patients to manifest persistent, stereotypic involuntary movements.
While many argue that the frequency of the field potentials in the motor cortex and basal ganglia leads to the symptoms of PD (Hutchison et al., 2004; Schnitzler and Gross, 2005), others take the position that synchrony of neurons between the motor cortex and basal ganglia is the essential feature that portends the symptoms of PD (Brown, 2007; Hammond et al., 2007). Within a largely self-contained system such as the basal ganglia, neurons will trend towards correlated firing patterns unless there is some mechanism that is designed to block this type of emergent synchrony (Bergman et al., 1998). Computational modeling suggests that increased synchrony within a neuronal network reduces the efficiency of information processing by creating redundant circuits (Schneidman et al., 2003). In healthy brains, the motor cortex and basal ganglia exhibit coherent oscillations while an animal is at rest, but striatal DA breaks this synchronous activity while simultaneously facilitating movement (Hammond et al., 2007). It is believed that DA regulates cortical inputs via activation of D2 receptors on corticostriatal axons, which selectively inhibits certain glutamatergic axons in order to focus excitatory inputs on a subset of striatal medium spiny neurons (Bamford et al., 2004). In patients with PD, corticostriatal coherence at beta frequencies is associated with PD symptoms; L-DOPA desynchronizes activity in this frequency band and increases cortex-basal ganglia synchrony in the gamma band, which coincides with symptom relief (Williams et al., 2002). Without DA to uncouple activity between the cortex and striatum, the motor circuit begins to oscillate at the same fundamental synchronous frequency, the net behavioral results of which are akinesia, bradykinesia and potentially tremor.
It has been challenging to determine the source of the pathological beta oscillations seen in PD because many structures exhibit this phenotype following DA depletion including the striatum, STN, GPi, SNr and motor cortex (Brazhnik et al., 2012; Brown et al., 2001; Goldberg et al., 2004; Sharott et al., 2005; Williams et al., 2002). However, mounting evidence suggests that the motor cortex generates these beta oscillations by slowly entraining the basal ganglia in the absence of striatal DA, the presence of which normally prevents excessive corticostriatal synchronization. Cortical neurons, even those outside the motor cortex, often oscillate in the beta frequency range when the organism is engaged in certain tasks and synchronized activity between cortical columns is considered to be essential for organized behavioral outputs (Singer, 1999). Indeed, the level of M1 cortico-cortical synchrony is increased after DA lesion (Goldberg et al., 2002). When corticostriatal slices from a healthy rat brain were bathed in the glutamate agonist kainic acid and the muscarinic agonist carbachol, M1 neurons showed oscillations in the high beta range, around 28Hz (Yamawaki et al., 2008). Notably, this beta rhythm was most apparent among layer V cortical neurons, which project to the basal ganglia, providing in vitro proof of concept that M1 can generate beta band local field potentials.
A more recent in vivo study employed spike timing to show that M1 was driving beta oscillations in the SNr. The experiment used unilaterally 6-OHDA-lesioned rats and showed that coherent oscillations between M1 and the SNr were prominent only after DA depletion (Brazhnik et al., 2012). Critically, analyses of the waveforms of the coherent local field potentials between the cortex and SNr showed that the cortical peaks led by an average of 17 ms, consistent with the idea that the cortex is controlling SNr field potentials through the direct pathway in the striatum from cortex→striatum→SNr. Furthermore, cortical ablation almost completely abolishes STN oscillations in both intact and DA-lesioned animals (Magill et al., 2001) providing additional evidence for the cortex as the rhythm generator for the internally-projecting basal ganglia structures.
4.6. Cellular Activity
In the period before and after the onset of movement, PD patients show irregular electrical activity in M1 (Chen et al., 2001b; Lefaucheur, 2005). Among healthy primates, in the 100–200 ms before and after movement began, there was a sharp rise and fall in the number of M1 action potentials with the peak occurring at movement onset (Watts and Mandir, 1992). By contrast, when primates were rendered parkinsonian with the neurotoxin MPTP, the rise and fall of M1 action potentials before and after movement onset was more gradual and the peak number of neurons firing was reduced (Doudet et al., 1990; Watts and Mandir, 1992). When primates were studied at rest, MPTP treatment still increased the number of bursting cells in M1 without leading to an overall change in firing rate (Goldberg et al., 2002). Increases in burst firing amongst motor cortex neurons in a PD state may be caused by a reduction in the activity of GABAergic interneurons (as suggested by Lefaucheur, 2005), which renders the motor cortex unable to select the appropriate motor circuit for movement execution. Indeed, 6-OHDA lesions altered the activity of rat M1 interneurons, increasing the number that were firing in-phase with a beta band local field potential (Brazhnik et al., 2012). This aberrant activity is also detectable in muscles, as PD patients and MPTP monkeys show irregular bursting in electromyograph recordings made during hand movements (Doudet et al., 1990; Watts and Mandir, 1992).
Alterations in SMA single cell activity are particularly evident during movement preparation. Under normal circumstances, SMA neurons often display peak firing rates just prior to movement onset (Escola et al., 2003; Watts and Mandir, 1992). When primates were treated with MPTP and trained to move a joystick in response to a cue stimulus, their SMA cells showed reduced firing rates after the cue but prior to movement (Escola et al., 2003; Watts and Mandir, 1992). When a healthy primate’s limbs were moved by experimenters, most SMA cells responded to the movement of only one joint; after MPTP administration, more cells began to respond to multiple joint movements (Escola et al., 2002). Irregular activity of SMA neurons may reflect difficulty in performing internally-guided movement and thus contribute to akinesia.
The changes seen in motor cortex cellular activity after DA lesion may also depend on which cell type is being recorded. Pasquereau and Turner (2011) recorded from M1 and compared activity changes in corticostriatal and pyramidal tract neurons before and after MPTP lesion in primates. Pyramidal neurons showed a decrease in basal firing rate and increases in the time spent burst firing, the number of spikes per burst and the average burst duration. MPTP lesion also increased the number pyramidal cells firing in the beta frequency band. In contrast, corticostriatal neurons showed no significant lesion-induced changes in activity state, which is surprising given the presumed importance of corticostriatal connections in the pathophysiology of beta oscillations specifically and PD generally.
Modulation of cellular activity in the motor cortex by pharmacotherapies that relieve PD symptoms or provoke LID has not been sufficiently investigated up to this point. However, administration of L-DOPA has been shown to increase c-fos expression within M1 of DA-lesioned dyskinetic rats, but not in a normal intact M1 (Halje et al., 2012; Ostock et al., 2011), suggesting that M1 hyperactivity is associated with LID. In these studies, dyskinesia evoked by systemic L-DOPA was reduced by local M1 infusion of a 5-HT1A receptor agonist (Ostock et al., 2011) or a D1 receptor antagonist (Halje et al., 2012). These results indicate that LID may be exacerbated by hyperactivity of M1 and highlight the potential usefulness of anti-dyskinetic compounds that target the motor cortex.
5. The Motor Cortex as a Therapeutic Target in Parkinson’s Disease
5.1. Transcranial Magnetic Stimulation
Given the evidence for pathological motor cortex activity in PD, there has been considerable interest in electrophysiological therapies targeting this region. Bidirectional effects on cortical activity can be produced in PD patients depending on the frequency of TMS stimulation: cortical stimulation at 5 Hz or greater has a net excitatory effect while a frequency of 1 Hz or lower reduces cortical excitability (Fitzgerald et al., 2006). Measureable changes in cortical excitability generally subside within 30 min, but clinical improvements from repeated stimulation sessions can be detected for several months after stimulation, indicating a role for TMS in inducing therapeutic neural plasticity (Wu et al., 2008).
Several dozen small clinical studies of TMS have been performed, and most have shown improvements in PD symptoms, as assessed by changes in UPDRS motor scores. Two meta-analyses have been conducted on the clinical TMS PD literature, finding an average effect size (using Cohen’s d) of about 0.6, and a reduction in UPDRS motor scores by about 20% (Elahi et al., 2009; Fregni et al., 2005). These analyses demonstrate that the most effective antiparkinsonian effects from TMS are conferred by stimulation of M1 at frequencies between 5 and 25 Hz. Lower frequency stimulation has been used, but UPDRS changes are smaller and less consistent between studies (e.g. Filipovic et al., 2010; Lefaucheur et al., 2004). If multiple sessions of TMS are performed, reductions in UPDRS motor scores can last for over a month after the final session (Khedr et al., 2003).
While excitatory M1 TMS is most useful for PD symptoms, LID has been successfully reduced by inhibitory TMS over M1 or the SMA. Application of 1 Hz TMS to M1 can reduce LID but clinical improvement has only been shown at 24 h post-stimulation and was not seen when patients were tested two weeks later (Filipovic et al., 2009; Wagle-Shukla et al., 2007). Likewise, 1 Hz stimulation of the SMA was capable of reducing apomorphine-induced dyskinesia when assessed up to 30 min later (Koch et al., 2005). These data are consistent with circuit models of LID that predict excessive glutamate release in the motor cortex during LID as a result of thalamic disinhibition (e.g. Cenci, 2007; Obeso et al., 2008). To date, there is no research showing a lasting reduction (>1 day) in dyskinesia symptoms using TMS.
In order to provide more consistent motor cortex stimulation than is practical using TMS, researchers have developed epidural and subdural electrodes that are implanted over the motor cortex. The potential efficacy of this approach is bolstered by pre-clinical research with MPTP-treated primates: 130 Hz epidural stimulation of M1 reduced behavioral akinesia and bradykinesia, reduced hyperactivity in the STN and GPi, and increased metabolism in the SMA (Druout et al., 2004). Most clinical studies using this approach have shown some improvements in cardinal PD symptoms (Fasano et al., 2008; Gutierrez et al., 2009), although some have only shown improvements in tremor (Moro et al., 2011). To the authors’ knowledge, no studies have been conducted which compare the degree of clinical improvement with motor cortex electrode stimulation versus that provided by DBS or TMS.
5.2. Therapeutic Mechanism of Cortical Stimulation
The therapeutic mechanism(s) of excitatory TMS applied to M1 may stem, in part, from an enhancement of striatal DA signaling. In healthy rats, just 2 sec of 25 Hz frontal cortex stimulation caused an increase in striatal DA concentration and a reduction in DA metabolism as assessed by whole-tissue chromatography 10 sec later (Ben-Shachar et al., 1997). Likewise, 20 min of intermittent 25 Hz frontal cortex stimulation enhanced striatal DA release for the next three hours as measured by in vivo microdialysis (Kanno et al., 2004). TMS-induced striatal DA release has also been shown in healthy humans using PET to analyze radioligand displacement (Strafella et al., 2003). In human PD patients, it appears that with increasing DA cell loss, the actual amount of TMS-evoked DA release is reduced while the percent increase in DA release relative to basal conditions is increased (Strafella et al., 2005).
Immediate DA signaling facilitation may account for the transient effects of single-session TMS, but the fact that multiple sessions have greater antiparkinsonian effects over a longer duration of time indicate that long-term changes in cortical electrophysiology are occurring. It is likely that the protracted effects are due to an increase in basal excitability of certain groups of cells. The inhibitory effects of low frequency TMS may result from activation of low-threshold inhibitory interneurons, while the excitatory effects of high frequency TMS may come from activation of large diameter axons of Betz cells in cortical layer V (Lefaucheur, 2009).
5.3. Pharmacological Modulation of the Motor Cortex
A variety of compounds affecting the DA and glutamate systems are currently used to treat PD and LID symptoms (see Cenci et al., 2011), but it remains an open question as to whether any of these drugs have therapeutic or deleterious effects through their actions on the motor cortex. At present, it is known that 5-HT1A receptor activation or D1 receptor blockade within M1 reduces LID in animal models (Halje et al., 2012; Ostock et al., 2011). When L-DOPA or a synthetic DA receptor agonist is given, it is unclear what effect activation of motor cortex D1 or D2 receptors have on PD or LID symptoms. The NMDA antagonist amantadine is the only drug with established anti-LID efficacy (Wolf et al., 2010) and M1 NMDA receptor activation is increased in LID (Ahmed et al., 2011). Thus, it is possible that amantadine exhibits part of its therapeutic action by blockading receptors in M1. In general, determining the contribution of motor cortex neurotransmitter signaling to the pathogenesis of PD and LID will aid in the development of future therapeutics.
5.4. Deep Brain Stimulation and the Motor Cortex
The therapeutic benefit of STN DBS has been recognized since the 1990s (Limousin et al., 1998). However, it was long speculated that it’s efficacy was due to a disruption of intrinsic STN activity or orthodromic effects on the GPe (e.g. Garcia et al., 2005). Using optogenetics, Gradinaru and colleagues (2009) showed that selective inhibition or excitation of STN cells was not capable of modifying motor performance in 6-OHDA-lesioned rodents. Instead, they demonstrated that high frequency stimulation (120 Hz) of STN afferents had antiparkinsonian effects while low frequency stimulation (20 Hz) exacerbated PD symptoms. These effects precisely parallel the clinical STN DBS literature which shows that 120 Hz stimulation is antiparkinsonian (Follett et al., 2010) while 20 Hz worsens the symptoms of PD (Chen et al., 2007). Gradinaru et al. (2009) also showed that 120 Hz stimulation of M1 cells in layer V attenuated PD symptoms, which may be the mechanism by which motor cortex electrodes are antiparkinsonian at 120 Hz (Gutierrez et al., 2009).
The importance of antidromic activity in the therapeutic effect of STN DBS was demonstrated by Li and colleagues (2012) who implanted bilateral STN DBS electrodes in a unilaterally 6-OHDA-lesioned rat. The authors stimulated the STN at a number of frequencies and found that the frequency with the greatest antiparkinsonian properties (125 Hz) also caused the greatest number of antidromic spikes per second in M1 corticosubthalamic neurons. The firing rate of corticosubthalamic neurons was reduced by 6-OHDA lesion and 125 Hz STN DBS elevated the firing rate to the level of unlesioned controls. Importantly, in the hemisphere contralateral to lesion, STN DBS did not affect M1 neuron firing rate and caused few antidromic spikes in M1, suggesting that STN DBS is alleviating a pathological M1 state that is specific to PD rather than generally modulating cortical activity. Emerging research thus supports the idea that modulation of M1 corticosubthalamic neuron activity is an important therapeutic target for the treatment of PD.
6. Conclusions and Future Directions
In summary, compelling evidence for pathological motor cortex activity in PD continues to accrue. Despite such findings, several essential questions remain unanswered. For example, it is not clear whether abnormal cortical activities are primarily attributable to striatal DA depletion or whether motor cortex DA depletion also contributes. This remains an open question since (at least in a rat) the same SNc cells which project to the striatum also project to the motor cortex (Debier et al., 2005). Presumably, the three midbrain nuclei which supply the motor cortex with DA (the SNc, VTA and RRA) have different functional roles in modulating M1 physiology, but the unique contribution of each has yet to be dissociated.
In addition, accumulating research suggests that GABAergic interneurons of the motor cortex are hypoactive in PD, perhaps as a result of altered frontal cortex DA supply (Gaspar et al., 1991; Kroener et al., 2009). This is also demonstrated in paired-pulse TMS studies demonstrating reduced GABAA and GABAB receptor mediated inhibition within M1 (Lefaucheur, 2005). Single cell recordings have also confirmed dysfunctional M1 interneuron activity in a PD model (Brazhnik et al., 2012). Thus, identifying methods that enhance cortico-cortical inhibition may be constitute novel therapeutic approaches to PD.
From a treatment standpoint, divergent methods of antiparkinsonian therapy (e.g. DBS and DA replacement) cause similar changes in motor cortex function, hinting that they work through convergent, but under-explored mechanisms. Finally, electrical modulation of motor cortex activity using TMS, subdural electrodes and STN DBS has provided preliminary evidence that the motor cortex represents a valid target for PD therapy. As yet, little is known about the potential mechanisms through which these stimulators work, but basic research may soon discover candidate pathways. Unearthing these mechanisms may shed further light on the changes in the motor cortex that are desired to achieve a positive therapeutic outcome.
Highlights.
Parkinson’s patients have reduced monoamine innervation of the motor cortex
Intracortical inhibition by motor cortex interneurons is reduced in Parkinson’s
Impaired cortical plasticity leads to deficits in motor learning in Parkinson’s
The Parkinsonian motor cortex is abnormally synchronized with the basal ganglia
Motor cortex stimulation is a potential therapeutic approach to Parkinson’s disease
Acknowledgments
The authors wish to thank Dr. Patricia M. Di Lorenzo, Dr. Lisa M. Savage, Corinne Ostock, Melissa Conti, Adam Goldenberg and Jennifer Gold for reading drafts of this manuscript.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke Grant R01-NS059600 (C.B.) and the Center for Development and Behavioral Neuroscience at Binghamton University (C.B.). The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Lindenbach, Email: dlinden1@binghamton.edu.
Christopher Bishop, Email: cbishop@binghamton.edu.
References
- Abe M, Hanakawa T. Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behavioral Brain Research. 2009;198:13–23. doi: 10.1016/j.bbr.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Movement Disorders. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain. 2011;134:979–986. doi: 10.1093/brain/awr028. [DOI] [PubMed] [Google Scholar]
- Aroniadou VA, Keller A. The patterns and synaptic properties of horizontal intracortical connections in the rat motor cortex. Journal of Neurophysiology. 1993;70:1553–1569. doi: 10.1152/jn.1993.70.4.1553. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson’s disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awenowicz PW, Porter LL. Local application of dopamine inhibits pyramidal tract neuron activity in the rodent motor cortex. Journal of Neurophysiology. 2002;88:3439–3451. doi: 10.1152/jn.00078.2002. [DOI] [PubMed] [Google Scholar]
- Bagnato S, Agostino R, Modugno N, Quartarone A, Berardelli A. Plasticity of the motor cortex in Parkinson’s disease patients on and off therapy. Movement Disorders. 2006;21:639–645. doi: 10.1002/mds.20778. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Baumer T, Hidding U, Hamel W, Buhmann C, Moll CKE, Gerloff C, Orth M, Siebner HR, Munchau A. Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson’s disease. Movement Disorders. 2009;24:672–676. doi: 10.1002/mds.22417. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Belmaker RH, Grisaru N, Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. Journal of Neural Transmission. 1997;104:191–197. doi: 10.1007/BF01273180. [DOI] [PubMed] [Google Scholar]
- Berding G, Odin P, Brooks DJ, Nikkhah G, Matthies C, Peschel T, Shing M, Kolbe H, van Den Hoff J, Fricke H, Dengler R, Samii M, Knapp WH. Resting regional cerebral glucose metabolism in advanced Parkinson’s disease studied in the off and on conditions with [(18)F]FDG-PET. Movement Disorders. 2001;16:1014–1022. doi: 10.1002/mds.1212. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends in Neurosciences. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P. Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Research. 1982;245:267–274. doi: 10.1016/0006-8993(82)90809-5. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Experimental Neurology. 2008;212:226–229. doi: 10.1016/j.expneurol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. Journal of Neuroscience. 2012;32:7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR. Deep Brain Stimulation for Parkinson Disease: An Expert Consensus and Review of Key Issues. Archives of Neurology. 2011;68:165–165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AR, Antle MC, Hu B, Teskey GC. High frequency stimulation of the subthalamic nucleus acutely rescues motor deficits and neocortical movement representations following 6-hydroxydopamine administration in rats. Experimental Neurology. 2011;231:82–90. doi: 10.1016/j.expneurol.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Brown AR, Hu B, Antle MC, Teskey GC. Neocortical movement representations are reduced and reorganized following bilateral intrastriatal 6-hydroxydopamine infusion and dopamine type-2 receptor antagonism. Experimental Neurology. 2009;220:162–170. doi: 10.1016/j.expneurol.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Current Opinion in Neurobiology. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. Journal of Neuroscience. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends in Neurosciences. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Canedo A. Primary motor cortex influences on the descending and ascending systems. Progress in Neurobiology. 1997;51:287–335. doi: 10.1016/s0301-0082(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson’s disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41:1449–1456. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain. 1999;122:483–495. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Lue LF, Beach TG, Hentz JG, Adler CH, Sue L, Sadeghi R, Driver-Dunckley E, Evidente VG, Sabbagh MN, Shill HA, Walker DG. Parkinson’s disease, cortical dysfunction, and alpha-synuclein. Movement Disorders. 2011;26:1436–1442. doi: 10.1002/mds.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO. Functional imaging in Parkinson’s disease: activation studies with PET, fMRI and SPECT. Journal of Neurology. 2003;250:I15–23. doi: 10.1007/s00415-003-1103-1. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Archives of Neurology. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in l-DOPA-induced dyskinesia. Trends in Neurosciences. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE, Odin P. Current options and future possibilities for the treatment of dyskinesia and motor fluctuations in Parkinson’s disease. CNS & Neurological Disorders Drug Targets. 2011;10:670–684. doi: 10.2174/187152711797247885. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Messina D, Pugliese P, Morelli M, Lanza P, Salsone M, Novellino F, Nicoletti G, Arabia G, Quattrone A. Increased prefrontal volume in PD with levodopa-induced dyskinesias: A voxel-based morphometry study. Movement Disorders. 2011;26:807–812. doi: 10.1002/mds.23660. [DOI] [PubMed] [Google Scholar]
- Chen C, Litvak V, Gilbertson T, Kuhn A, Lu C, Lee S, Tsai C, Tisch S, Limousin P, Hariz M. Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Experimental Neurology. 2007;205:214–221. doi: 10.1016/j.expneurol.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg RR, Lozano AM, Lang AE. Effects of internal globus pallidus stimulation on motor cortex excitability. Neurology. 2001a;56:716–723. doi: 10.1212/wnl.56.6.716. [DOI] [PubMed] [Google Scholar]
- Chen R, Kumar S, Garg RR, Lang AE. Impairment of motor cortex activation and deactivation in Parkinson’s disease. Clinical Neurophysiology. 2001b;112:600–607. doi: 10.1016/s1388-2457(01)00466-7. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Coull JT, Hwang HJ, Leyton M, Dagher A. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. Journal of Neuroscience. 2012;32:16704–16715. doi: 10.1523/JNEUROSCI.1258-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, Starr PA. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135:615–630. doi: 10.1093/brain/awr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology. 2002;58:1665–1672. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taub E, Houle S, Lang AE, Dostrovsky JO, Tasker RR, Lozano AM. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nature Medicine. 1997;3:671–674. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- Debeir T, Ginestet L, Francois C, Laurens S, Martel JC, Chopin P, Marien M, Colpaert F, Raisman-Vozari R. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Experimental Neurology. 2005;193:444–454. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dejean C, Hyland B, Arbuthnott G. Cortical Effects of Subthalamic Stimulation Correlate with Behavioral Recovery from Dopamine Antagonist Induced Akinesia. Cerebral Cortex. 2009;19:1055–1063. doi: 10.1093/cercor/bhn149. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. Journal of Comparative Neurology. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Doudet DJ, Gross C, Arluison M, Bioulac B. Modifications of precentral cortex discharge and EMG activity in monkeys with MPTP-induced lesions of DA nigral neurons. Experimental Brain Research. 1990;80:177–188. doi: 10.1007/BF00228859. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Current Opinion in Neurology. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, Dauguet J, Lefaucheur JP, Dolle F, Conde F, Bottlaender M, Peschanski M, Keravel Y, Hantraye P, Palfi S. Functional Recovery in a Primate Model of Parkinson’s Disease following Motor Cortex Stimulation. Neuron. 2004;44:769–778. doi: 10.1016/j.neuron.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Ebrahimi A, Pochet R, Roger M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neuroscience Research. 1992;14:39–60. doi: 10.1016/s0168-0102(05)80005-7. [DOI] [PubMed] [Google Scholar]
- Eggers C, Fink GR, Nowak DA. Theta burst stimulation over the primary motor cortex does not induce cortical plasticity in Parkinson’s disease. Journal of Neurology. 2010;257:1669–1674. doi: 10.1007/s00415-010-5597-1. [DOI] [PubMed] [Google Scholar]
- Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function-Systematic review of controlled clinical trials. Movement Disorders. 2009;24:357–363. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Deutch AY, Redmond DE, Jr, Sladek JR, Jr, Roth RH. MPTP reduces dopamine and norepinephrine concentrations in the supplementary motor area and cingulate cortex of the primate. Neuroscience Letters. 1990;114:316–322. doi: 10.1016/0304-3940(90)90583-u. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Current Opinion in Neurobiology. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Escola L, Michelet T, Douillard G, Guehl D, Bioulac B, Burbaud P. Disruption of the proprioceptive mapping in the medial wall of parkinsonian monkeys. Annals of Neurology. 2002;52:581–587. doi: 10.1002/ana.10337. [DOI] [PubMed] [Google Scholar]
- Escola L, Michelet T, Macia F, Guehl D, Bioulac B, Burbaud P. Disruption of information processing in the supplementary motor area of the MPTP-treated monkey: a clue to the pathophysiology of akinesia? Brain. 2003;126:95–114. doi: 10.1093/brain/awg004. [DOI] [PubMed] [Google Scholar]
- Fasano A, Piano C, De Simone C, Cioni B, Di Giuda D, Zinno M, Daniele A, Meglio M, Giordano A, Bentivoglio AR. High frequency extradural motor cortex stimulation transiently improves axial symptoms in a patient with Parkinson’s disease. Movement Disorders. 2008;23:1916–1919. doi: 10.1002/mds.21977. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson’s disease. Movement Disorders. 2009;24:246–253. doi: 10.1002/mds.22348. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Rothwell JC, Bhatia K. Low-frequency repetitive transcranial magnetic stimulation and off-phase motor symptoms in Parkinson’s disease. Journal of the Neurological Sciences. 2010;291:1–4. doi: 10.1016/j.jns.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Filippi MM, Oliveri M, Pasqualetti P, Cicinelli P, Traversa R, Vernieri F, Palmieri MG, Rossini PM. Effects of motor imagery on motor cortical output topography in Parkinson’s disease. Neurology. 2001;57:55–61. doi: 10.1212/wnl.57.1.55. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Annals of Neurology. 2005;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson’s disease. Cerebral Cortex. 2006;16:64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. New England Journal of Medicine. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Fraix V, Pollak P, Vercueil L, Benabid AL, Mauguiere F. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Clinical Neurophysiology. 2008;119:2513–2518. doi: 10.1016/j.clinph.2008.07.217. [DOI] [PubMed] [Google Scholar]
- Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiology of Disease. 2011;42:327–340. doi: 10.1016/j.nbd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L, D’Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson’s disease: more or less? Trends in Neurosciences. 2005;28:209–216. doi: 10.1016/j.tins.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in Parkinson’s disease. Annals of Neurology. 1991;30:365–374. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Stepniewska I, Kaas JH. Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. Journal of Comparative Neurology. 1992;325:1–21. doi: 10.1002/cne.903250102. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anatomy and Embryology. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Fyffe RE, Porter R. Morphology of neurons in area 4 gamma of the cat’s cortex studied with intracellular injection of HRP. Journal of Comparative Neurology. 1988;277:290–312. doi: 10.1002/cne.902690212. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary Motor Area Structure and Function - Review and Hypotheses. Behavioral and Brain Sciences. 1985;8:567–588. [Google Scholar]
- Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike Synchronization in the Cortex-Basal Ganglia Networks of Parkinsonian Primates Reflects Global Dynamics of the Local Field Potentials. Journal of Neuroscience. 2004;24:6003–6010. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. Journal of Neuroscience. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Science. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JC, Seijo FJ, Vega MAÁ, González FF, Aragoneses BL, Blázquez M. Therapeutic extradural cortical stimulation for Parkinson’s Disease: Report of six cases and review of the literature. Clinical Neurology and Neurosurgery. 2009;111:703–707. doi: 10.1016/j.clineuro.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Halje P, Tamte M, Richter U, Mohammed M, Cenci MA, Petersson P. Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. Journal of Neuroscience. 2012;32:16541–16551. doi: 10.1523/JNEUROSCI.3047-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends in Neurosciences. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson’s disease. Neuroimage. 2005;28:598–606. doi: 10.1016/j.neuroimage.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Stambuk MK, Carl JL, McGee-Minnich LA, Perlmutter JS. Altered thalamic response to levodopa in Parkinson’s patients with dopa-induced dyskinesias. Proceedings of the National Academy of Science. 1998;95:12016–12021. doi: 10.1073/pnas.95.20.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Weisenbach S, Kalbe E, Burghaus L, Ghaemi M, Lehrke R, Koulousakis A, Herholz K, Sturm V, Heiss WD. Subthalamic Nucleus Stimulation Restores Glucose Metabolism in Associative and Limbic Cortices and in Cerebellum: Evidence from a FDG-PET Study in Advanced Parkinson’s Disease. Journal of Cerebral Blood Flow & Metabolism. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Medical Biology. 1977;55:21–40. [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Lu CS, Chuang WL, Chen RS. Abnormal bidirectional plasticity-like effects in Parkinson’s disease. Brain. 2011;134:2312–2320. doi: 10.1093/brain/awr158. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, Brown P. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings. Journal of Neuroscience. 2004;24:9240–9243. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ. Impaired activation of the supplementary motor area in Parkinson’s disease is reversed when akinesia is treated with apomorphine. Annals of Neurology. 1992;32:749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O. Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage. 2011;55:462–467. doi: 10.1016/j.neuroimage.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. Journal of the Neurological Sciences. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Karagulle Kendi AT, Lehericy S, Luciana M, Ugurbil K, Tuite P. Altered diffusion in the frontal lobe in Parkinson disease. AJNR American Journal of Neuroradiology. 2008;29:501–505. doi: 10.3174/ajnr.A0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. Intrinsic synaptic organization of the motor cortex. Cerebral Cortex. 1993;3:430–441. doi: 10.1093/cercor/3.5.430. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. European Journal of Neurology. 2003;10:567–572. doi: 10.1046/j.1468-1331.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- Kita H, Kita T. Cortical Stimulation Evokes Abnormal Responses in the Dopamine-Depleted Rat Basal Ganglia. Journal of Neuroscience. 2011;31:10311–10322. doi: 10.1523/JNEUROSCI.0915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Brusa L, Caltagirone C, Peppe A, Oliveri M, Stanzione P, Centonze D. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology. 2005;65:623–625. doi: 10.1212/01.wnl.0000172861.36430.95. [DOI] [PubMed] [Google Scholar]
- Kojovic M, Bologna M, Kassavetis P, Murase N, Palomar FJ, Berardelli A, Rothwell JC, Edwards MJ, Bhatia KP. Functional reorganization of sensorimotor cortex in early Parkinson disease. Neurology. 2012;78:1441–1448. doi: 10.1212/WNL.0b013e318253d5dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 2009;4:6507. doi: 10.1371/journal.pone.0006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clinical Neurophysiology. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: influence of antiparkinsonian treatment and cortical stimulation. Clinical Neurophysiology. 2005;116:244–253. doi: 10.1016/j.clinph.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Treatment of Parkinson’s disease by cortical stimulation. Expert Review of Neurotherapeutics. 2009;9:1755–1771. doi: 10.1586/ern.09.132. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. Journal of Neuroscience. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, Arbuthnott GW, Yung WH. Therapeutic deep brain stimulation in parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proceedings of the National Academy of Science. 1994;91:4353–4356. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Annals of Neurology. 1997;42:283–291. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. New England Journal of Medicine. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiologica Scandinavica Supplementum. 1974;412:1–48. [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The organization of the frontal motor cortex. Physiology. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Ryu YH, Lee MS. Cerebral cortical areas in which thickness correlates with severity of motor deficits of Parkinson’s disease. Journal of Neurology. 2011;258:1871–1876. doi: 10.1007/s00415-011-6045-6. [DOI] [PubMed] [Google Scholar]
- MacDonald V, Halliday GM. Selective loss of pyramidal neurons in the pre-supplementary motor cortex in Parkinson’s disease. Movement Disorders. 2002;17:1166–1173. doi: 10.1002/mds.10258. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted Dopamine Transmission and the Emergence of Exaggerated Beta Oscillations in Subthalamic Nucleus and Cerebral Cortex. Journal of Neuroscience. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Neuroanatomy text and atlas. 3. McGraw-Hill; New York: 2003. [Google Scholar]
- Martinu K, Degroot C, Madjar C, Strafella AP, Monchi O. Levodopa influences striatal activity but does not affect cortical hyper-activity in Parkinson’s disease. European Journal of Neuroscience. 2012;35:572–583. doi: 10.1111/j.1460-9568.2011.07979.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, Luft AR. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE. 2009;4:e7082. doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annual Review of Neuroscience. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Moro E, Schwalb JM, Piboolnurak P, Poon YYW, Hamani C, Hung SW, Arenovich T, Lang AE, Chen R, Lozano AM. Unilateral subdural motor cortex stimulation improves essential tremor but not Parkinson’s disease. Brain. 2011;134:2096–2105. doi: 10.1093/brain/awr072. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130:2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, Hacking A, Moeller JR, Ghez C, Eidelberg D. Functional networks in motor sequence learning: abnormal topographies in Parkinson’s disease. Human Brain Mapping. 2001;12:42–60. doi: 10.1002/1097-0193(200101)12:1<42::AID-HBM40>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Research. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Annals of Neurology. 2008;64:S30–46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends in Neurosciences. 2000;23:S8–19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Ostock CY, Dupre KB, Eskow Jaunarajs KL, Walters H, George J, Krolewski D, Walker PD, Bishop C. Role of the primary motor cortex in l-DOPA-induced dyskinesia and its modulation by 5-HT1A receptor stimulation. Neuropharmacology. 2011;61:753–760. doi: 10.1016/j.neuropharm.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary Motor Cortex of the Parkinsonian Monkey: Differential Effects on the Spontaneous Activity of Pyramidal Tract-Type Neurons. Cerebral Cortex. 2011;21:1362–1378. doi: 10.1093/cercor/bhq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA–induced dyskinesia. Nature Neuroscience. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Marciani MG, Bernardi G, Giacomini P, Stanzione P. Effect of apomorphine on cortical inhibition in Parkinson’s disease patients: a transcranial magnetic stimulation study. Experimental Brain Research. 2001;141:52–62. doi: 10.1007/s002210100839. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Mazzone P, Marciani MG, Rossini PM, Stefani A, Giacomini P, Peppe A, Stanzione P. Deep brain stimulation of both subthalamic nucleus and internal globus pallidus restores intracortical inhibition in Parkinson’s disease paralleling apomorphine effects: a paired magnetic stimulation study. Clinical Neurophysiology. 2002;113:108–113. doi: 10.1016/s1388-2457(01)00694-0. [DOI] [PubMed] [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Annals of Neurology. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Sudmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. Journal of Physiology. 2012;590:3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A. Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Archives of Neurology. 1992;49:144–148. doi: 10.1001/archneur.1992.00530260044017. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Annals of Neurology. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Rivara CB, Sherwood CC, Bouras C, Hof PR. Stereologic characterization and spatial distribution patterns of Betz cells in the human primary motor cortex. The Anatomical Record. 2003;270:137–151. doi: 10.1002/ar.a.10015. [DOI] [PubMed] [Google Scholar]
- Rubinstein TC, Giladi N, Hausdorff JM. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Movement Disorders. 2002;17:1148–1160. doi: 10.1002/mds.10259. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Clark KB. The role of the subthalamic nucleus in the response of globus pallidus neurons to stimulation of the prelimbic and agranular frontal cortices in rats. Experimental Brain Research. 1991;86:641–651. doi: 10.1007/BF00230538. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain. 1997;120:963–976. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Schneidman E, Bialek W, Berry MJ., 2nd Synergy, redundancy, and independence in population codes. Journal of Neuroscience. 2003;23:11539–11553. doi: 10.1523/JNEUROSCI.23-37-11539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nature Reviews Neuroscience. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of β-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. European Journal of Neuroscience. 2005;21:1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Pogosyan A, Kuhn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain. 2005;128:1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proceedings of the National Academy of Science. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database of Systematic Reviews. 2009:CD006564. doi: 10.1002/14651858.CD006564.pub2. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. European Journal of Neuroscience. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Valzania F, Nassetti SA, Tropeani A, Bisulli A, Santangelo M, Tassinari CA. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson’s disease: a transcranial magnetic stimulation study. Clinical Neurophysiology. 2000;111:1198–1202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Belvisi D, Conte A, Iezzi E, Modugno N, Fabbrini G, Berardelli A. Lack of LTP-like plasticity in primary motor cortex in Parkinson’s disease. Experimental Neurology. 2011;227:296–301. doi: 10.1016/j.expneurol.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Annals of the New York Academy of Sciences. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Ali Kotb M, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H. Altered plasticity of the human motor cortex in Parkinson’s disease. Annals of Neurology. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- Valalik I, Emri M, Lengyel Z, Mikecz P, Tron L, Csokay A, Marian T. Pallidal Deep Brain Stimulation and L-Dopa Effect on PET Motor Activation in Advanced Parkinson’s Disease. Journal of Neuroimaging. 2009;19:253–258. doi: 10.1111/j.1552-6569.2008.00304.x. [DOI] [PubMed] [Google Scholar]
- Viaro R, Morari M, Franchi G. Progressive Motor Cortex Functional Reorganization Following 6-Hydroxydopamine Lesioning in Rats. Journal of Neuroscience. 2011;31:4544–4554. doi: 10.1523/JNEUROSCI.5394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle-Shukla A, Angel MJ, Zadikoff C, Enjati M, Gunraj C, Lang AE, Chen R. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology. 2007;68:704–705. doi: 10.1212/01.wnl.0000256036.20927.a5. [DOI] [PubMed] [Google Scholar]
- Watts RL, Mandir AS. The role of motor cortex in the pathophysiology of voluntary movement deficits associated with parkinsonism. Neurology Clinics. 1992;10:451–469. [PubMed] [Google Scholar]
- Weiler N, Wood L, Yu J, Solla SA, Shepherd GM. Top-down laminar organization of the excitatory network in motor cortex. Nature Neuroscience. 2008;11:360–366. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Current Opinion in Neurobiology. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. Journal of Comparative Neurology. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, Ott E, Kloiber I, Haubenberger D, Auff E, Poewe W. Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Movement Disorders. 2010;25:1357–1363. doi: 10.1002/mds.23034. [DOI] [PubMed] [Google Scholar]
- Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A. Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics. 2008;5:345–361. doi: 10.1016/j.nurt.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Stanford IM, Hall SD, Woodhall GL. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35:222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Archives of Neurology. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Bruns D, Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neuroscience Letters. 1996;208:187–190. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]