Abstract
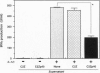
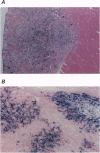
The p40 subunit of interleukin 12 (IL-12p40) has been known to act as an IL-12 antagonist in vitro. We here describe the immunosuppressive effect of IL-12p40 in vivo. A murine myoblast cell line, C2C12, was transduced with retro-virus vectors carrying the lacZ gene as a marker and the IL-12p40 gene. IL-12p40 secreted from the transfectant inhibited the IL-12-induced interferon gamma (IFN-gamma) production by splenocytes in vitro. Survival of C2C12 transplanted into allogeneic recipients was substantially prolonged when transduced with IL-12p40. Cytokine (IL-2 and IFN-gamma) production and cytotoxic T lymphocyte induction against allogeneic C2C12 were impaired in the recipients transplanted with the IL-12p40 transfectant. Delayed-type hypersensitivity response against C2C12 was also diminished in the IL-12p40 recipients. Furthermore, serum antibodies against beta-galactosidase of the T-helper 1-dependent isotypes (IgG2 and IgG3) were decreased in the IL-12p40 recipients. These results indicate that locally produced IL-12p40 exerts a potent immunosuppressive effect on T-helper 1-mediated immune responses that lead to allograft rejection. Therefore, IL-12p40 gene transduction would be useful for preventing the rejection of allografts and genetically modified own cells that are transduced with potentially antigenic molecules in gene therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bette M., Jin S. C., Germann T., Schäfer M. K., Weihe E., Rüde E., Fleischer B. Differential expression of mRNA encoding interleukin-12 p35 and p40 subunits in situ. Eur J Immunol. 1994 Oct;24(10):2435–2440. doi: 10.1002/eji.1830241026. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Podlaski F. J., Wolitzky A. G., Quinn P. M., Nunes P., Stern A. S., Gately M. K. IL-12: monoclonal antibodies specific for the 40-kDa subunit block receptor binding and biologic activity on activated human lymphoblasts. J Immunol. 1991 Sep 1;147(5):1548–1556. [PubMed] [Google Scholar]
- Cox G. A., Cole N. M., Matsumura K., Phelps S. F., Hauschka S. D., Campbell K. P., Faulkner J. A., Chamberlain J. S. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993 Aug 19;364(6439):725–729. doi: 10.1038/364725a0. [DOI] [PubMed] [Google Scholar]
- Dai Y., Schwarz E. M., Gu D., Zhang W. W., Sarvetnick N., Verma I. M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarki V. J., Belloni P., Nijjar T., Smith J., Couto L., Rabier M., Clift S., Berns A., Cohen L. K. Gene therapy for hemophilia A: production of therapeutic levels of human factor VIII in vivo in mice. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1023–1027. doi: 10.1073/pnas.92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Desai B. B., Wolitzky A. G., Quinn P. M., Dwyer C. M., Podlaski F. J., Familletti P. C., Sinigaglia F., Chizonnite R., Gubler U. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991 Aug 1;147(3):874–882. [PubMed] [Google Scholar]
- Germann T., Bongartz M., Dlugonska H., Hess H., Schmitt E., Kolbe L., Kölsch E., Podlaski F. J., Gately M. K., Rüde E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995 Mar;25(3):823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- Gillessen S., Carvajal D., Ling P., Podlaski F. J., Stremlo D. L., Familletti P. C., Gubler U., Presky D. H., Stern A. S., Gately M. K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995 Jan;25(1):200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Huard J., Bouchard J. P., Roy R., Malouin F., Dansereau G., Labrecque C., Albert N., Richards C. L., Lemieux B., Tremblay J. P. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve. 1992 May;15(5):550–560. doi: 10.1002/mus.880150504. [DOI] [PubMed] [Google Scholar]
- Karpati G., Acsadi G. The potential for gene therapy in Duchenne muscular dystrophy and other genetic muscle diseases. Muscle Nerve. 1993 Nov;16(11):1141–1153. doi: 10.1002/mus.880161102. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Waldburger K. E., Goldman S. J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995 Jan 1;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau R. S., Singer S. M., McDevitt H. O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995 Jan;16(1):34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Ling P., Gately M. K., Gubler U., Stern A. S., Lin P., Hollfelder K., Su C., Pan Y. C., Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995 Jan 1;154(1):116–127. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F., Fischer S., Guckes S., Jin S., Kaulen H., Schmitt E., Rüde E., Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993 Sep;23(9):2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Zimmer G. J., Fogelman I., Wolf S. F., Abbas A. K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994 Mar 1;152(5):2172–2179. [PubMed] [Google Scholar]
- Nickerson P., Steurer W., Steiger J., Zheng X., Steele A. W., Strom T. B. Cytokines and the Th1/Th2 paradigm in transplantation. Curr Opin Immunol. 1994 Oct;6(5):757–764. doi: 10.1016/0952-7915(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Schoenhaut D. S., Chua A. O., Wolitzky A. G., Quinn P. M., Dwyer C. M., McComas W., Familletti P. C., Gately M. K., Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992 Jun 1;148(11):3433–3440. [PubMed] [Google Scholar]
- Stern A. S., Podlaski F. J., Hulmes J. D., Pan Y. C., Quinn P. M., Wolitzky A. G., Familletti P. C., Stremlo D. L., Truitt T., Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembleau S., Germann T., Gately M. K., Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995 Aug;16(8):383–386. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Wakimoto H., Abe J., Tsunoda R., Aoyagi M., Hirakawa K., Hamada H. Intensified antitumor immunity by a cancer vaccine that produces granulocyte-macrophage colony-stimulating factor plus interleukin 4. Cancer Res. 1996 Apr 15;56(8):1828–1833. [PubMed] [Google Scholar]
- Wolf S. F., Temple P. A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R. M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- Wysocka M., Kubin M., Vieira L. Q., Ozmen L., Garotta G., Scott P., Trinchieri G. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995 Mar;25(3):672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xiang Z., Ertl H. C., Wilson J. M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]