Abstract
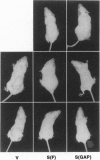
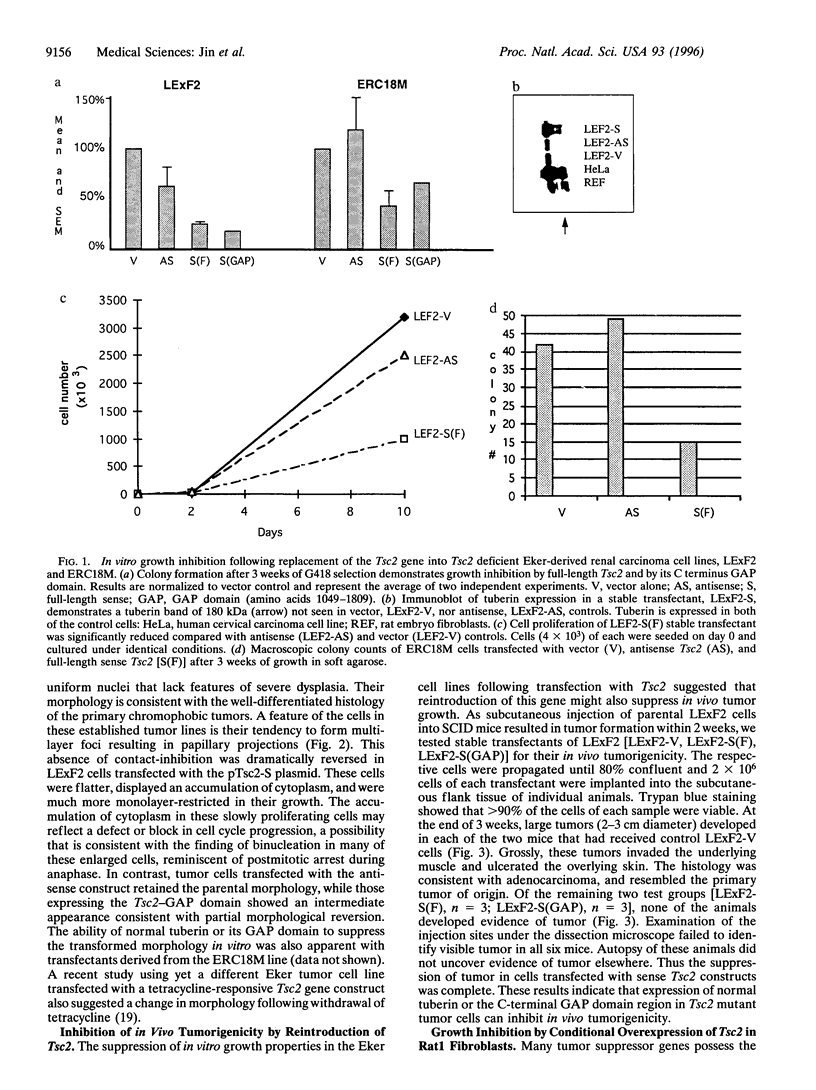
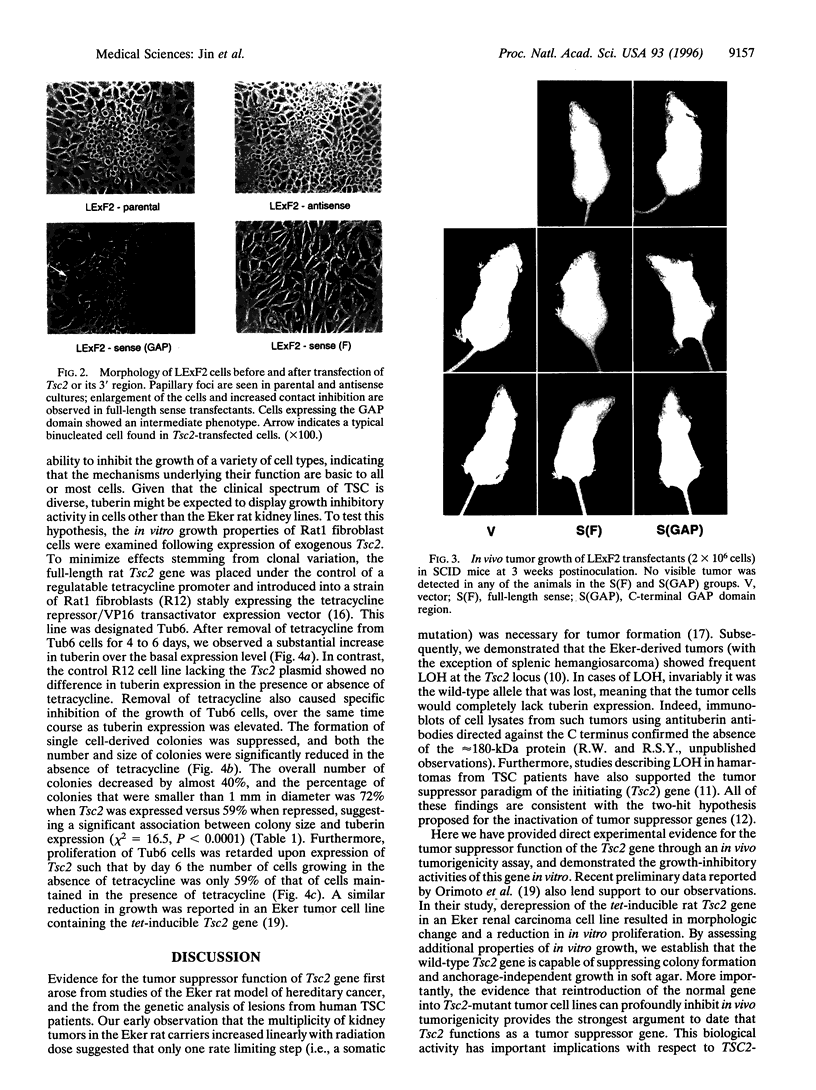
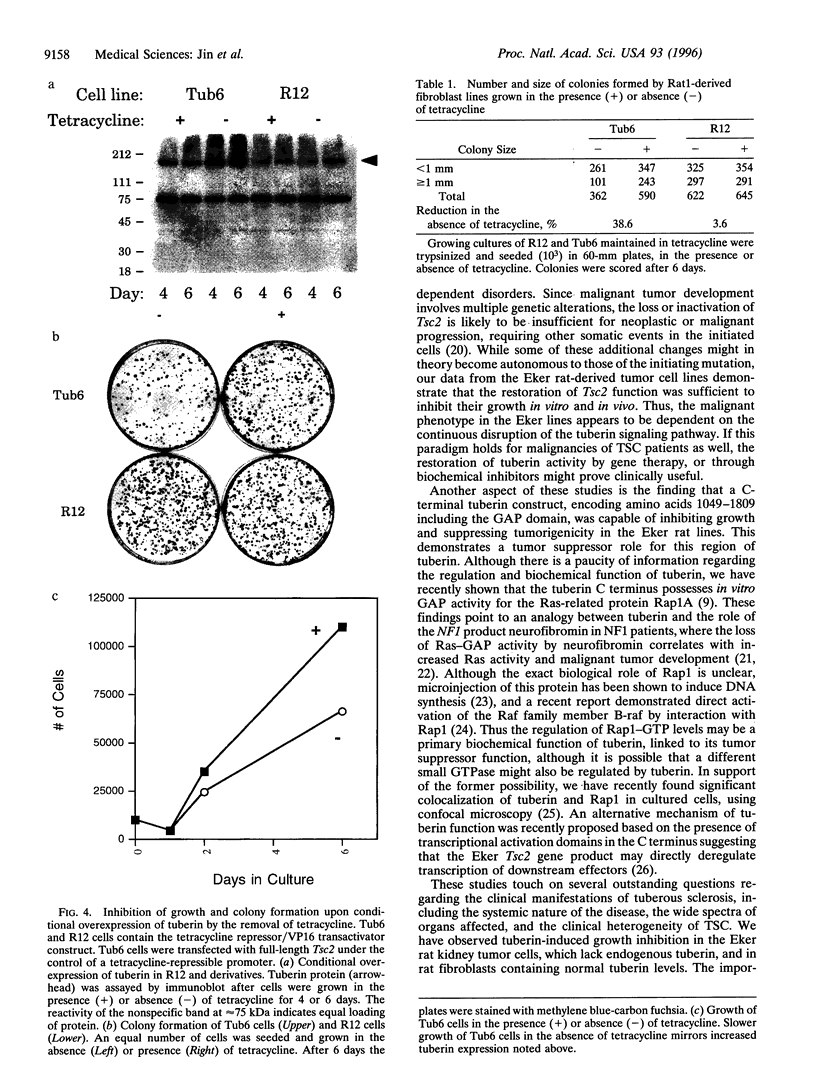
The Tsc2 gene, which is mutationally inactivated in the germ line of some families with tuberous sclerosis, encodes a large, membrane-associated GTPase activating protein (GAP) designated tuberin. Studies of the Eker rat model of hereditary cancer strongly support the role of Tsc2 as a tumor suppressor gene. In this study, the biological activity of tuberin was assessed by expressing the wild-type Tsc2 gene in tumor cell lines lacking functional tuberin and also in rat fibroblasts with normal levels of endogenous tuberin. The colony forming efficiency of Eker rat-derived renal carcinoma cells was significantly reduced following reintroduction of wild-type Tsc2. Tumor cells expressing the transfected Tsc2 gene became more anchorage-dependent and lost their ability to form tumors in severe combined immunodeficient mice. At the cellular level, restoration of tuberin expression caused morphological changes characterized by enlargement of the cells and increased contact inhibition. As with the full-length Tsc2 gene, a clone encoding only the C terminus of tuberin (amino acids 1049-1809, including the GAP domain) was capable of reducing both colony formation and in vivo tumorigenicity when transfected into the Eker rat tumor cells. In normal Rat1 fibroblasts, conditional overexpression of tuberin also suppressed colony formation and cell growth in vitro. These results provide direct experimental evidence for the tumor suppressor function of Tsc2 and suggest that the tuberin C terminus plays an important role in this activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu T. N., Gutmann D. H., Fletcher J. A., Glover T. W., Collins F. S., Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992 Apr 23;356(6371):713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Papageorge A. G., Fletcher J. A., Diehl S. R., Ratner N., Vass W. C., Lowy D. R. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992 Apr 17;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Geist R. T., Gutmann D. H. The tuberous sclerosis 2 gene is expressed at high levels in the cerebellum and developing spinal cord. Cell Growth Differ. 1995 Nov;6(11):1477–1483. [PubMed] [Google Scholar]
- Green A. J., Smith M., Yates J. R. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat Genet. 1994 Feb;6(2):193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- Hino O., Klein-Szanto A. J., Freed J. J., Testa J. R., Brown D. Q., Vilensky M., Yeung R. S., Tartof K. D., Knudson A. G. Spontaneous and radiation-induced renal tumors in the Eker rat model of dominantly inherited cancer. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Johnson M. R., DeClue J. E., Felzmann S., Vass W. C., Xu G., White R., Lowy D. R. Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol Cell Biol. 1994 Jan;14(1):641–645. doi: 10.1128/mcb.14.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Hirayama Y., Kobayashi E., Kubo Y., Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Genet. 1995 Jan;9(1):70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T., Shimizu K., Yamamori B., Kuroda S., Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J Biol Chem. 1996 Jan 19;271(3):1258–1261. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- Orimoto K., Tsuchiya H., Kobayashi T., Matsuda T., Hino O. Suppression of the neoplastic phenotype by replacement of the Tsc2 gene in Eker rat renal carcinoma cells. Biochem Biophys Res Commun. 1996 Feb 6;219(1):70–75. doi: 10.1006/bbrc.1996.0183. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H., Orimoto K., Kobayashi K., Hino O. Presence of potent transcriptional activation domains in the predisposing tuberous sclerosis (Tsc2) gene product of the Eker rat model. Cancer Res. 1996 Feb 1;56(3):429–433. [PubMed] [Google Scholar]
- Wiederholt W. C., Gomez M. R., Kurland L. T. Incidence and prevalence of tuberous sclerosis in Rochester, Minnesota, 1950 through 1982. Neurology. 1985 Apr;35(4):600–603. doi: 10.1212/wnl.35.4.600. [DOI] [PubMed] [Google Scholar]
- Wienecke R., König A., DeClue J. E. Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J Biol Chem. 1995 Jul 7;270(27):16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- Xiao G. H., Jin F., Yeung R. S. Germ-line Tsc2 mutation in a dominantly inherited cancer model defines a novel family of rat intracisternal-A particle elements. Oncogene. 1995 Jul 6;11(1):81–87. [PubMed] [Google Scholar]
- Xiao G. H., Jin F., Yeung R. S. Identification of tuberous sclerosis 2 messenger RNA splice variants that are conserved and differentially expressed in rat and human tissues. Cell Growth Differ. 1995 Sep;6(9):1185–1191. [PubMed] [Google Scholar]
- Yeung R. S., Buetow K. H., Testa J. R., Knudson A. G., Jr Susceptibility to renal carcinoma in the Eker rat involves a tumor suppressor gene on chromosome 10. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8038–8042. doi: 10.1073/pnas.90.17.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Everitt J. I., Jin F., Walker C. L. Allelic loss at the tuberous sclerosis 2 locus in spontaneous tumors in the Eker rat. Mol Carcinog. 1995 Sep;14(1):28–36. doi: 10.1002/mc.2940140107. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Jin F., Lee W. C., Testa J. R., Knudson A. G. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kawata M., Miura Y., Musha T., Sasaki T., Kikuchi A., Takai Y. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol Cell Biol. 1992 Aug;12(8):3407–3414. doi: 10.1128/mcb.12.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]