Abstract
The peptide repertoire (peptidome) associated with MHC class II molecules (MHCIIs) is influenced by the polymorphic nature of the peptide binding groove but also by cell-intrinsic factors. The invariant chain (Ii) chaperones MHCIIs, affecting their folding and trafficking. Recent discoveries relating to Ii functions have provided insights as to how it edits the MHCII peptidome. In humans, the Ii gene encodes four different isoforms for which structure-function analyses have highlighted common properties but also some non-redundant roles. Another layer of complexity arises from the fact that Ii heterotrimerizes, a characteristic that has the potential to affect the maturation of associated MHCIIs in many different ways, depending on the isoform combinations. Here, we emphasize the peptide editing properties of Ii and discuss the impact of the various isoforms on the MHCII peptidome.
Keywords: invariant chain, p35, di-leucine motif, di-arginine motif, MHCII trafficking, antigen presentation, MHCII
The invariant chain (Ii; CD74) has multiple functions but is best characterized as the main MHC class II (MHCII) chaperone. Ii is a type II protein consisting of a short cytoplasmic tail, a transmembrane region and a luminal domain that can be further partitioned into a membrane-proximal disordered region, the main MHCII-interacting sequence (CLIP), and a C-terminal trimerization domain (1, 2). Mice express two Ii isoforms, p31 and p41, the latter resulting from alternative splicing (3). In humans, the corresponding isoforms are known as p33 and p41. Additionally, around 20% of the Ii mRNAs are translated from an upstream start codon that generates the p35 and p43 isoforms. These bear a 16-amino acid cytoplasmic extension including a strong di-arginine (RxR) ER retention motif (4–6).
Synthesized alongside MHCIIs, Ii can be viewed as: (i) a GUARDIAN that controls access to the MHCII groove; (ii) a SCAFFOLD that assists folding and pairing of α and β MHCII chains; and (iii) a LEADER that directs MHCIIs to the endosomal pathway. It is well established that these Ii functions depend primarily on the ability of its CLIP region to occupy the peptide groove of MHCIIs. Numerous reports showed that Ii proteolysis in endosomes allows HLA-DM to free the groove of CLIP and to catalyze the binding of nominal antigenic peptides [reviewed in Ref. (7)]. Herein, we describe the main chaperone functions of Ii and discuss how the various isoform-specific features can modulate its peptidome-editing properties (Figures 1A–D).
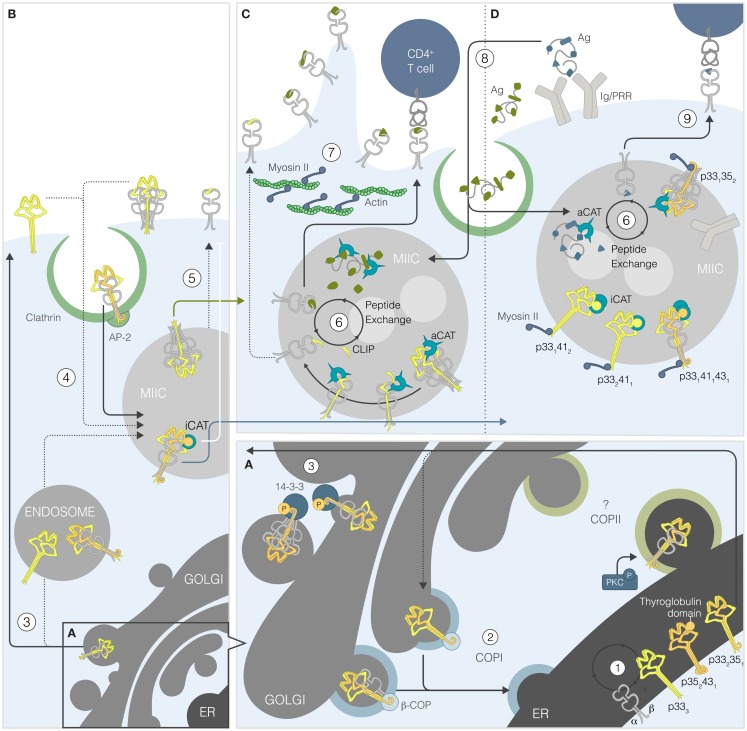
Figure 1.
Portrait of the role of the human invariant chain in MHCII presentation. (A) MHCII α and β chains assemble with Ii in the ER [1]. The four Ii isoforms randomly associate into trimers, some of which bear ER retention motif(s) and/or thyroglobulin domain(s). Unphosphorylated Iip35/p43-containing trimers, associated with MHCIIs or not, exit the ER but are recognized by β-COP and undergo retrograde transport [2]. The MHCII-bound Ii that gets phosphorylated by PKC binds 14-3-3β, thereby preventing β-COP binding and allowing anterograde transport [3]. (B) From the Golgi, the different complexes gain access to the plasma membrane or early endosomes [3]. The complexes at the plasma membrane reach the MHCII-rich compartment (MIIC) after being internalized into clathrin-coated pits [4]. In endosomes, presence of p41/43 will reduce processing by inhibiting cathepsin S (iCAT) and slowing-down Ii processing and/or transport to the cell surface [5]. (C) In multivesicular MIICs, the carboxy-terminal trimerization domain of Ii is cleaved by non-cysteine proteases, generating the p22 fragment. Then, cysteine proteases remove the glycosylated portion to form the p10 fragment before active cathepsin S (aCAT) cuts the anchored portion, leaving CLIP in the MHCII groove. CLIP is then exchanged for an antigenic peptide spontaneously or by DM [6]. Ii degradation frees myosin II, which can restore the cell motility and remodeling of endosomes [7]. (D) Antigens are internalized by pinocytosis or receptor-mediated endocytosis and degraded by proteases, including cathepsins [8]. In the presence of p41/43, processing is more focused given the inactivation of cathepsins. Thus, the MHCIIs that gain access to the plasma membrane present peptides derived from receptor-mediated Ag internalization to CD4+ T cells [9].
Guardian
Early results on the role of Ii have revealed its importance in the presentation of intact Ags (8). By guiding MHCIIs to endosomes while blocking their Ag-binding groove, Ii allows MHCIIs to gain access to peptides from processed Ags and thus influences the pool of associated-peptides (9). This is best exemplified by the differential reactivity of a panel of autoreactive T cell clones co-cultured with Ii+ or Ii− APCs (10). Indeed, the content of the MHCII groove differs in transfected cell lines whether or not Ii is expressed. In a mass spectrometry analysis of DR4-eluted peptides, lack of Ii biased the peptide origin toward cytoplasmic proteins, whereas Ii allowed the binding of peptides derived from exogenous and endocytic proteins (11). Also, the repertoire is strongly skewed in Ii KO mice, as demonstrated by mixed lymphocyte reactions and aberrant CD4+ T cell selection (12–18). In humans however, little is known on the impact of Ii deficiency in different cell types. The effect of Ii on the peptidome varies and following its degradation, the residual CLIP peptide also affects the peptide’s assortment to be presented to T lymphocytes. Indeed, a series of articles from Mellins and collaborators describing the MHCII-CLIP affinity relationship suggest that poor Ii and CLIP chaperoning leads to Ag processing defects with the potential to instigate MHCII-associated autoimmunity (19–23).
In the absence of Ii, both mouse and human MHCIIs bind a collection of long polypeptides, most likely originating from misfolded ER proteins (24–26). Interestingly, even in the presence of Ii, it was recently reported that MHCIIs displayed some ER polypeptides at the plasma membrane, the latter competing with Ii for the class II binding groove (27). Despite the fact that MHCIIs can associate with ER polypeptides, there are numerous functional examples of endogenous and exogenous CD4 T cell epitopes that are presented in the absence of Ii expression [reviewed in Ref. (28)]. While presentation of some of these peptides was negatively affected by the presence of Ii, others were Ii-independent and loaded on recycling MHCIIs (29, 30). Ii is usually produced in vast excess and most if not all MHCIIs mature in association with Ii (31). Still, it is tempting to speculate that under some physiological conditions, the Ii/MHCII protein ratio may decrease sufficiently to impact the peptidome. Accordingly, knocking down Ii in cancers represents a possible therapeutic avenue, allowing these cells to present new antigens to tumor infiltrating T cells (32, 33).
Beside the gross peptidome alterations noted in the non-physiological absence of Ii, subtle variations have been observed when MHCIIs were expressed in the context of specific Ii isoforms. The GUARDIAN role of Ii is not exclusively CLIP-centered but also shaped by the p41/43 thyroglobulin domain, which regulates the proteolytic activity of numerous cathepsins (18, 34–37). Among them, CatS breaks down large polypeptides and provokes MHC/Ii complex dissociation by cleaving Ii between the transmembrane and CLIP regions in APCs (38). In thymic epithelial cells, CatV (CatL in mice) occupies this role although redundancy between cathepsin family members is observed (39, 40). As a result, MHCIIs are freed from Ii cytoplasmic targeting and may egress to the plasma membrane. It has also been suggested that the effect of p41/43 is echoed to surrounding Ii (p33/35), limiting overall processing, and that the thyroglobulin domain chaperones cathepsins, increasing half-life and maintaining a pool of mature enzymes in the MIICs (41, 42). The proportion of p41/43 isoforms varies from 10 to 40% in professional APCs and this provides a mechanism to modulate cathepsin activity (43). Lastly, it has been shown that Ii luminal domain is involved in increasing the half-life of MHCII by delaying endosomal maturation (44). As a result, the pool of antigenic peptide could be skewed toward receptor-mediated protein intake taking place in the highly specialized MIICs (Figure 1D). To efficiently protect the cell from aberrant Ag presentation, the Ii isoforms must work as a team. As seen in Tg mice expressing exclusively one mouse or human Ii isoform, whether p31, p35 or p41, Ag presentation, and CD4 T cell selection can be restored (18, 45–48). Still, Ag presentation by alternatively spliced isoforms was not equivalent, suggesting the existence of divergent pathways (18, 34). Also, the differential outcomes of experimental allergic encephalomyelitis (EAE) or asthma in p31- vs p41-expressing mice point to distinct class II peptide repertoire (45, 49). Along the same lines, it was reported that NOD mice devoid of Ii are protected from developing type 1 diabetes (50). The isoform balance is put into perspective by Baugh et al., which demonstrated that the onset of experimental EAE and rheumatoid arthritis are delayed when cathepsin S inhibitors are administered to mice (51). Further study of the distinct roles of Ii is much needed to understand the implication of the various isoforms in immunity. Altogether, these observations clearly demonstrate the general impact of Ii on the MHCII peptidome and the subtle editing role of the exon 6b-encoded domain.
Scaffold
While transfected MHCIIs could egress from the ER in cell lines, Ii was found to favor the pairing and trafficking of haplotype-matched and -mismatched α and β chains (52, 53). Some key properties of Ii became apparent with the characterization of Ii-deficient mice, although cell-type- and haplotype-dependent differences were reported (54). A detailed sequence of events leading to formation of the MHCII/Ii complex was first described by Cresswell and collaborators (Figure 1A) (55). According to the model proposed by Roche et al. three assembled MHCII αβ heterodimers associate sequentially with a preformed Ii trimer, generating a pentamer, then a heptamer, and ultimately a nonamer with the ability to egress from the ER (56). This dogma was later refined to include intermediate steps such as the initial binding of Ii to an MHCII α chain prior to the pairing of an isotype-matched β chain (57, 58). Although a variety of chaperones such as BIP and calnexin have been shown to interact with the MHCII Ag presentation machinery, their exact role in the final assembly and ER egress of the MHCII/Ii complex is not well defined (55, 59). Furthermore, the interaction between calnexin and MHCIIs until the final nonamer formation suggested that egress is tightly restricted (60). However, given the existence of transport-competent heptamers and pentamers, it does not appear that universal quality control mechanisms are in place to prevent egress of non-stoichiometric complexes (60–62). As MHCII-free Ii trimers can egress from the ER, the relative abundance of MHCIIs and Ii likely influences the complex stoichiometry. Cell-type-specific differences and the affinity of CLIP for the MHCII groove may come into play as well.
In humans, although p33 is the most abundant isoform and generates some homotrimers, it is mostly part of heterotrimers that have also incorporated an RxR-containing moiety (63–65). By analogy to other multi-protein complexes such as the KATP channel (66), the di-arginine motif would prevent premature ER egress of MHCII-unsaturated Ii trimers (i.e., pentamers and heptamers). Indeed, p35/43 require binding of the MHCIIβ chain for anterograde trafficking (67–69). Although less abundant than their respective Iip33/p41 counterparts, p35/43 are dominant as the stochastic incorporation of a single RxR-bearing Ii moiety will prevent ER egress of an heterotrimer (70, 71). Thus, p35/p43 will favor the formation of high-order MHCII/Ii oligomers. Indeed, as p35/43 both need to be phosphorylated by PKC and be associated with MHCIIs to become transport competent, they form larger complexes and egress less efficiently than homotrimers devoid of RxR-containing subunits (Figure 1A) (65, 72, 73). A MHCII molecule binding a p332p351 heterotrimer would have only one chance out of three to egress the ER as a pentamer. This of course is assuming that the MHCII cannot mask the RxR motif in trans. This is an important issue as it was recently suggested that steric hindrance caused by the plasma membrane and the bending of the MHCII/Ii complex only allows formation of pentamers (61). If this holds true, we have to assume that a cis interaction between the MHCII and p35/43 is not required to overcome the retention motif, otherwise many doomed complexes would be formed.
The advantage, if any, conferred by the presence of an ER retention motif in p35/43 remains obscure. A variable Ii/MHCII stoichiometry may modulate the MHCIIs turnaround and thus, the peptidome that is displayed to T cells. One can imagine that although Ii is in excess, its retention of Ii increases the available ER pool and ensures that the ratio of free over Ii-bound MHCIIs is as low as possible. This way, most MHCIIs would acquire their final cargo in the endocytic pathway instead of the ER. Whether or not the cell can modulate its physiology to favor the binding of endogenous ER peptides remains to be seen.
Leader
The fundamental functional distinction between MHCI and MHCII molecules comes from the fact that they acquire peptides in different locations (74). The seminal studies of Ziegler and Unanue demonstrated that the presentation of CD4 T cell epitopes by MHCIIs was inhibited by chloroquine, highlighting the importance of low pH compartments (75, 76). Evidence for a role of Ii in the trafficking of MHCIIs to endosomes has been described in numerous reviews (55, 77, 78). In the absence of Ii, MHCIIs are not transported to endocytic compartments as efficiently and accumulate at the plasma membrane (4, 5). Confocal and electron microscopy experiments using transfected cell lines revealed that a clear colocalization of MHCIIs with endosomal markers or internalized antigens required co-expression of Ii (70, 79). Deletion and site-directed mutagenesis experiments established the importance of the cytoplasmic domain for intracellular trafficking and allowed the mapping of two classical leucine-based endosomal sorting signals in all Ii isoforms (79).
In line with the role of the leucine-based motifs in Ii degradation and CLIP removal, it was shown that deletion of the Ii cytoplasmic tail resulted in the cell-surface display of Ii/MHCII complexes being unable to acquire antigenic peptides (80). In contrast, one can wonder if the specific characteristics of p35/43 affect transport of the complex and, ultimately, the peptidome. Many studies using various Ii+ cell types and transfected cell lines have reported that even in the absence of MHCIIs, some p33/p41 homotrimers gain access to post-Golgi compartments and acquire complex N- and O-linked oligosaccharides (4, 5, 68, 81). However, as mentioned above, p35/p43-containing trimers are retained in the ER (63). The general model stipulates that an unphosphorylated p35 moiety binds β-COP upon arrival at the cis-Golgi sorting station, causing the retrograde transport of the complex in COPI-coated vesicles and the apparent steady-state ER retention (82) (Figure 1A). However, when phosphorylated by PKC on serine 8, Ii recruits 14-3-3β to prevent the binding of β-COP on the RxR motif (47, 56–58, 73). Still, it remains to be determined how the complex is transported from the ER to the Golgi. While largely undefined, export signals have been described in some cargo proteins, allowing their incorporation in COPII-coated transport vesicles originating at ER exit sites (83). Other transmembrane proteins exit through the default pathway (84). Whether the 16-amino acid extension of p35/43 confers specific sorting properties to MHCIIs in such early step as ER egress has yet to be addressed. Another important question that remains is, if the RxR motif is masked by 14-3-3β, why can’t a phosphorylated Ii trimer be released from the Golgi in MHCII-negative cells? Although there is compelling evidence for competition between 14-3-3β and β-COP, the need for MHCIIs in the transport of p35/p43-containing complexes beyond the Golgi apparatus was overlooked in previous studies and remains unexplained.
The stringent quality control mechanism operating at the level of the Golgi suggests that p35/p43-including complexes do not simply reach the plasma membrane through the default pathway. Many groups have studied the route taken by the MHCII/Ii complex to reach the late endosomes/lysosomes [reviewed in Ref. (85)] (Figure 1B). It is now recognized that AP-2 adaptors, which connect cargo and plasma membrane clathrin-coated pits, are important in the sorting of MHCII/Ii complexes to the endocytic pathway. The actual model proposes that the bulk of MHCII/Ii complexes exit the Golgi by a clathrin-independent mechanism en route to the plasma membrane where they are internalized in association with AP-2 [Ref. (86, 87) and references therein). However, one must bear in mind that there could be important cell-type differences in the transport of MHCIIs. Also, in some of the studies looking at the trafficking of Ii, it is not entirely clear which Ii isoform(s) was (were) expressed and in what proportions. Thus, a thorough comparison of p35 and p33 trafficking is much needed.
One clear difference in the trafficking of p33 and p35 is that the latter is not detected at the plasma membrane (71, 72). Kuwana et al. have shown in transfected cells that a dominant-negative form of dynamin caused the cell-surface display of p35, suggesting that in fact, p33 and p35 follow the same path to endosomes (72). The reason why the internalization kinetics of phosphorylated p35 is increased as compared to p33 is not known but may relate to its affinity for AP-2 (88).
Many groups have documented the impact of Ii on the endocytic pathway [see Ref. (78)] (Figure 1C). Ii-expressing cells accumulate large endosomes, in which Ag and MHCIIs degradation is slowed (89–93). Such effects are dependent on the cytoplasmic tail and the luminal trimerization domain common to all Ii isoforms. However, based on studies using cathepsin KO mice, it became clear that the Ii thyroglobulin domain exerts further pressure on MHCII trafficking and maturation by limiting cathepsin-mediated degradation while preventing cathepsin-sensitive epitopes from proteolysis (94–96). Surprisingly, p41/43 and cathepsins were shown to colocalize in compartments not implicated in Ag presentation, a finding suggesting a role in phagocytosis rather than Ag processing (97). In light of the recent results by Faure-André et al. describing Ii-myosin II interactions, it seems that Ii is also involved in cell motility/remodeling (98) (Figures 1C,D). Reduced Ii processing caused by p41/43 would increase the MIIC’s interaction with the myosin II motor, providing necessary extraction force to internalize membrane Ag in these compartments (99). The endocytic pathway is a complex system made of different tubular/vesicular entities and the exact location where MHCIIs acquire peptides is still debated (100, 101). It is unknown if p35/p43 have additional modulatory properties that could translate into a change in the peptidome and the contribution of each isoform to the endocytic landscape covered by Ii remains to be evaluated (102, 103).
Concluding Remarks
The list of functions ascribed to Ii is continuously growing. Beside its many roles in MHCII Ag presentation, Ii was shown to chaperone other presentation molecules, such as CD1d. As this class 1b molecule acquires its ligands in the endocytic pathway, the role of Ii in the selection of lipid Ags is of great interest (104). Recently, Ii was shown to have a key impact in cross-presentation, suggesting that its isoforms may fine tune the peptide repertoire associated with MHCI molecules in DCs (105).
The Ii pool is highly heterogeneous and an important question that remains is the potential isoform-specific influence of post-translational modifications such as the addition of a glycosaminoglycan (chondroitin sulfate, CS) side chain in the ER/Golgi (106). Ii-CS binds CD44 and can enhance T cell responses (107). Given that p35/p43 regulate surface display of Ii trimers (71), it would be interesting to determine in humans the contribution of these isoforms on the chaperone-independent functions of Ii, such as being a cell-surface receptor for the macrophage migration inhibition factor (MIF) and Helicobacter pylori (108). Whether alternative splicing affects the affinity of these ligands for the Ii receptor remains to be measured. Interestingly, the cytoplasmic tail is pivotal in the capacity of Ii to transduce signals in response to MIF or after endosomal cleavage of its transmembrane region by Sppl2a (109–111). The potency of p35/p43 in this context should be tested.
The existence of Ii isoforms offers multiple layers of control over Ag presentation. Major transformations occur during the activation of APCs following, for example, microbial activation of pattern recognition receptors (PRRs) signaling pathways. If mediators of the inflammatory response (IFN-γ, TNF-α, IL-10 etc) can modulate the behavior of Ii isoforms and if the ensuing changes in the expression levels of MHCIIs or Ii can affect the peptidome should be systematically addressed. Evidence that the relative proportions of these isoforms can somehow be regulated comes from the study of chronic lymphocytic leukemia in which overexpression of p35 has been reported (112, 113). Whether p35 plays a role in tumor escape from the immune system by modulating the peptidome remains to be determined. Also, differential p35 expression between B cells from monozygotic twins discordant for type 1 diabetes was shown to affect Ag presentation and could potentially contribute to the development of the disease (114). On a final note, expression of Ii-Ag fusion proteins in APCs represents a potential immunization strategy that targets Ags directly to endosomes and skews the peptidome (115, 116). Alternatively, recombinant proteins have been engineered by replacing CLIP with the sequence of a T cell epitope (117). The efficacy of these promising vaccine approaches may benefit from the study of the biology of the various Ii isoforms.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Canadian Institutes for Health Research (#93592), the Cancer Research Society and the National Science and Engineering Research Council of Canada (#298537) to Jacques Thibodeau.
References
- 1.Singer PA, Lauer W, Dembicl Z, Mayert WE, Lipp J, Koch N, et al. Structure of the murine Ia-associated invariant (Ii) chain as deduced from a cDNA clone. EMBO J (1984) 3(4):873–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cresswell P. Chemistry and functional role of the invariant chain. Curr Opin Immunol (1992) 4:87–92 10.1016/0952-7915(92)90131-W [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto K, Floyd-Smith G, Francke U, Koch N, Lauer W, Dobberstein B, et al. The gene encoding the Ia-associated invariant chain is located on chromosome 18 in the mouse. Immunogenetics (1985) 21(1):83–90 10.1007/BF00372244 [DOI] [PubMed] [Google Scholar]
- 4.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell (1990) 63:707–16 10.1016/0092-8674(90)90137-4 [DOI] [PubMed] [Google Scholar]
- 5.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature (1990) 348:600–5 10.1038/348600a0 [DOI] [PubMed] [Google Scholar]
- 6.Strubin M, Berte C, Mach B. Alternative splicing and alternative initiation of translation explain the four forms of the Ia antigen-associated invariant chain. EMBO J (1986) 5:3485–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol (1995) 11:267–306 10.1146/annurev.cb.11.110195.001411 [DOI] [PubMed] [Google Scholar]
- 8.Stockinger B, Pessara U, Lin RH, Habicht J, Grez M, Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell (1989) 56(4):683–9 10.1016/0092-8674(89)90590-4 [DOI] [PubMed] [Google Scholar]
- 9.Busch R, Rinderknecht CH, Roh S, Lee AW, Harding JJ, Burster T, et al. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol Rev (2005) 207:242–60 10.1111/j.0105-2896.2005.00306.x [DOI] [PubMed] [Google Scholar]
- 10.Lightstone L, Hargreaves R, Bobek G, Peterson M, Aichinger G, Lombardi G, et al. In the absence of the invariant chain, HLA-DR molecules display a distinct array of peptides which is influenced by the presence or absence of HLA-DM. Proc Natl Acad Sci U S A (1997) 94:5772–7 10.1073/pnas.94.11.5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muntasell A, Carrascal M, Alvarez I, Serradell L, van Veelen P, Verreck FA, et al. Dissection of the HLA-DR4 peptide repertoire in endocrine epithelial cells: strong influence of invariant chain and HLA-DM expression on the nature of ligands. J Immunol (2004) 173:1085–93 [DOI] [PubMed] [Google Scholar]
- 12.Tourne S, Nakano N, Viville S, Benoist C, Mathis D. The influence of invariant chain on the positive selection of single T cell receptor specificities. Eur J Immunol (1995) 25:1851–6 10.1002/eji.1830250709 [DOI] [PubMed] [Google Scholar]
- 13.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, et al. Mice lacking the MHC class II-associated invariant chain. Cell (1993) 72:635–48 10.1016/0092-8674(93)90081-Z [DOI] [PubMed] [Google Scholar]
- 14.Grubin CE, Kovats S, DeRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity (1997) 7:197–208 10.1016/S1074-7613(00)80523-3 [DOI] [PubMed] [Google Scholar]
- 15.Wong P, Rudensky AY. Phenotype and function of CD4+ T cells in mice lacking invariant chain. J Immunol (1996) 156:2133–42 [PubMed] [Google Scholar]
- 16.Bodmer H, Viville S, Benoist C, Mathis D. Diversity of endogenous epitopes bound to MHC class II molecules limited by invariant chain. Science (1994) 263:1284–6 10.1126/science.7510069 [DOI] [PubMed] [Google Scholar]
- 17.Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med (1993) 177:1699–712 10.1084/jem.177.6.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikoff EK, Kenty G, Van Kaer L. Distinct peptide loading pathways for MHC class II molecules associated with alternative Ii chain isoforms. J Immunol (1998) 160:3101–10 [PubMed] [Google Scholar]
- 19.Hou T, Macmillan H, Chen Z, Keech CL, Jin X, Sidney J, et al. An insertion mutant in DQA1*0501 restores susceptibility to HLA-DM: implications for disease associations. J Immunol (2011) 187:2442–52 10.4049/jimmunol.1100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallang L, Roh S, Holm A, Bergseng E, Yoon T, Fleckenstein B, et al. Complexes of two cohorts of CLIP peptides and HLA-DQ2 of the autoimmune DR3-DQ2 haplotype are poor substrates for HLA-DM. J Immunol (2008) 181:5451–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doebele RC, Pashine A, Liu W, Zaller DM, Belmares M, Busch R, et al. Point mutations in or near the antigen-binding groove of HLA-DR3 implicate class II-associated invariant chain peptide affinity as a constraint on MHC class II polymorphism. J Immunol (2003) 170(9):4683–92 [DOI] [PubMed] [Google Scholar]
- 22.Rinderknecht CH, Roh S, Pashine A, Belmares MP, Patil NS, Lu N, et al. DM influences the abundance of major histocompatibility complex class II alleles with low affinity for class II-associated invariant chain peptides via multiple mechanisms. Immunology (2010) 131(1):18–32 10.1111/j.1365-2567.2010.03282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinderknecht CH, Belmares MP, Catanzarite TLW, Bankovich AJ, Holmes TH, Garcia KC, et al. Posttranslational regulation of I-Ed by affinity for CLIP. J Immunol (2007) 179:5907–15 [DOI] [PubMed] [Google Scholar]
- 24.Busch R, Vturina IY, Drexler J, Momburg F, Hammerling GJ. Poor loading of major histocompatibility complex class II molecules with endogenously synthesized short peptides in the absence of invariant chain. Eur J Immunol (1995) 25:48–53 10.1002/eji.1830250110 [DOI] [PubMed] [Google Scholar]
- 25.Busch R, Cloutier I, Sekaly RP, Hammerling GJ. Invariant chain protects class II histocompatibility antigens from binding intact polypeptides in the endoplasmic reticulum. EMBO J (1996) 15:418–28 [PMC free article] [PubMed] [Google Scholar]
- 26.Rovere P, Forquet F, Zimmermann VS, Trucy J, Ricciardi-Castagnoli P, Davoust J. Dendritic cells from mice lacking the invariant chain express high levels of membrane MHC class II molecules in vivo. Adv Exp Med Biol (1997) 417:195–201 10.1007/978-1-4757-9966-8_33 [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Arase N, Kohyama M, Hirayasu K, Suenaga T, Jin H, et al. Transport of misfolded endoplasmic reticulum proteins to the cell surface by MHC class II molecules. Int Immunol (2013) 25:235–46 10.1093/intimm/dxs155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceman S, Sant AJ. The function of invariant chain in class II-restricted antigen presentation. Semin Immunol (1995) 7:373–87 10.1006/smim.1995.0042 [DOI] [PubMed] [Google Scholar]
- 29.Long EO, LaVaute T, Pinet V, Jaraquemada D. Invariant chain prevents the HLA-DR-restricted presentation of a cytosolic peptide. J Immunol (1994) 153:1487–94 [PubMed] [Google Scholar]
- 30.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature (1995) 375:603–6 10.1038/375603a0 [DOI] [PubMed] [Google Scholar]
- 31.Marks MS, Blum JS, Cresswell P. Invariant chain trimers are sequestered in the rough endoplasmic reticulum in the absence of association with HLA class II antigens. J Cell Biol (1990) 111:839–55 10.1083/jcb.111.3.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chornoguz O, Gapeev A, O’Neill MC, Ostrand-Rosenberg S. Major histocompatibility complex class II+ invariant chain negative breast cancer cells present unique peptides that activate tumor-specific T cells from breast cancer patients. Mol Cell Proteomics (2012) 11:1457–67 10.1074/mcp.M112.019232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibodeau J, Bourgeois-Daigneault M-C, Lapointe R. Targeting the MHC class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology (2012) 1:908–16 10.4161/onci.21205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson M, Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature (1992) 357:596–8 10.1038/357596a0 [DOI] [PubMed] [Google Scholar]
- 35.Jasanoff A, Song S, Dinner AR, Wagner G, Wiley DC. One of two unstructured domains of Ii becomes ordered in complexes with MHC class II molecules. Immunity (1999) 10:761–8 10.1016/S1074-7613(00)80075-8 [DOI] [PubMed] [Google Scholar]
- 36.Mihelic M, Dobersek A, Guncar G, Turk D. Inhibitory fragment from the p41 form of invariant chain can regulate activity of cysteine cathepsins in antigen presentation. J Biol Chem (2008) 283:14453–60 10.1074/jbc.M801283200 [DOI] [PubMed] [Google Scholar]
- 37.Pierre P, Shachar I, Matza D, Gatti E, Flavell RA, Mellman I. Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells. J Exp Med (2000) 191:1057–62 10.1084/jem.191.6.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med (1995) 181:1729–41 10.1084/jem.181.5.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa T. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science (1998) 280:450–3 10.1126/science.280.5362.450 [DOI] [PubMed] [Google Scholar]
- 40.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev (2005) 207:229–41 10.1111/j.0105-2896.2005.00310.x [DOI] [PubMed] [Google Scholar]
- 41.Fineschi B, Arneson LS, Naujokas MF, Miller J. Proteolysis of major histocompatibility complex class II-associated invariant chain is regulated by the alternatively spliced gene product, p41. Proc Natl Acad Sci U S A (1995) 92:10257–61 10.1073/pnas.92.22.10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lennon-Duménil AM, Roberts RA, Valentijn K, Driessen C, Overkleeft HS, Erickson A, et al. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J (2001) 20:4055–64 10.1093/emboj/20.15.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kämpgen E, Koch N, Koch F, Stöger P, Heufler C, Schuler G, et al. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc Natl Acad Sci U S A (1991) 88:3014–8 10.1073/pnas.88.8.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landsverk OJB, Barois N, Gregers TF, Bakke O. Invariant chain increases the half-life of MHC II by delaying endosomal maturation. Immunol Cell Biol (2011) 89:619–29 10.1038/icb.2010.143 [DOI] [PubMed] [Google Scholar]
- 45.Slavin AJ, Soos JM, Stuve O, Patarroyo JC, Weiner HL, Fontana A, et al. Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J Clin Invest (2001) 108:1133–9 10.1172/JCI200113360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genève L, Chemali M, Desjardins M, Labrecque N, Thibodeau J. Human invariant chain isoform p35 restores thymic selection and antigen presentation in CD74-deficient mice. Immunol Cell Biol (2012) 90:896–902 10.1038/icb.2012.27 [DOI] [PubMed] [Google Scholar]
- 47.Naujokas MF, Arneson LS, Fineschi B, Peterson ME, Sitterding S, Hammond AT, et al. Potent effects of low levels of MHC class II-associated invariant chain on CD4+ T cell development. Immunity (1995) 3:359–72 10.1016/1074-7613(95)90120-5 [DOI] [PubMed] [Google Scholar]
- 48.Shachar I, Elliott EA, Chasnoff B, Grewal IS, Flavell RA. Reconstitution of invariant chain function in transgenic mice in vivo by individual p31 and p41 isoforms. Immunity (1995) 3:373–83 10.1016/1074-7613(95)90121-3 [DOI] [PubMed] [Google Scholar]
- 49.Ye Q, Finn PW, Sweeney R, Bikoff EK, Riese RJ. MHC class II-associated invariant chain isoforms regulate pulmonary immune responses. J Immunol (2003) 170:1473–80 [DOI] [PubMed] [Google Scholar]
- 50.Mellanby RJ, Koonce CH, Monti A, Phillips JM, Cooke A, Bikoff EK. Loss of invariant chain protects nonobese diabetic mice against type 1 diabetes. J Immunol (2006) 177:7588–98 [DOI] [PubMed] [Google Scholar]
- 51.Baugh M, Black D, Westwood P, Kinghorn E, McGregor K, Bruin J, et al. Therapeutic dosing of an orally active, selective cathepsin S inhibitor suppresses disease in models of autoimmunity. J Autoimmun (2011) 36:201–9 10.1016/j.jaut.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 52.Layet C, Germain RN. Invariant chain promotes egress of poorly expressed, haplotype-mismatched class II major histocompatibility complex A alpha B beta dimers from the endoplasmic reticulum/cis-Golgi compartment. Proc Natl Acad Sci U S A (1991) 88:2350–64 10.1073/pnas.88.6.2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekaly RP, Tonnelle C, Strubin M, Mach B, Long EO. Cell surface expression of class II histocompatibility antigens occurs in the absence of the invariant chain. J Exp Med (1986) 164:1490–504 10.1084/jem.164.5.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bikoff EK, Germain RN, Robertson EJ. Allelic differences affecting invariant chain dependency of MHC class II subunit assembly. Immunity (1995) 2:301–10 10.1016/1074-7613(95)90054-3 [DOI] [PubMed] [Google Scholar]
- 55.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol (1994) 12:259–93 10.1146/annurev.iy.12.040194.001355 [DOI] [PubMed] [Google Scholar]
- 56.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature (1991) 354:392–4 10.1038/354392a0 [DOI] [PubMed] [Google Scholar]
- 57.Kvist S, Wiman K, Claesson L, Peterson PA, Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell (1982) 29:61–9 10.1016/0092-8674(82)90090-3 [DOI] [PubMed] [Google Scholar]
- 58.Koch N, McLellan AD, Neumann J. A revised model for invariant chain-mediated assembly of MHC class II peptide receptors. Trends Biochem Sci (2007) 32:532–7 10.1016/j.tibs.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 59.Williams DB, Watts TH. Molecular chaperones in antigen presentation. Curr Opin Immunol (1995) 7:77–84 10.1016/0952-7915(95)80032-8 [DOI] [PubMed] [Google Scholar]
- 60.Anderson KS, Cresswell P. A role for calnexin (IP90) in the assembly of class II MHC molecules. EMBO J (1994) 13:675–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch N, Zacharias M, Ko A, Temme S, Neumann J, Springer S. Stoichiometry of HLA class II-invariant chain oligomers. PLoS One (2011) 6:e17257. 10.1371/journal.pone.0017257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majera D, Kristan KC, Neefjes J, Turk D, Mihelic M. Expression, purification and assembly of soluble multimeric MHC class II-invariant chain complexes. FEBS Lett (2012) 586:1318–24 10.1016/j.febslet.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 63.Lamb CA, Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J Immunol (1992) 148:3478–82 [PubMed] [Google Scholar]
- 64.Arunachalam B, Pan M, Cresswell P. Intracellular formation and cell surface expression of a complex of an intact lysosomal protein and MHC class II molecules. J Immunol (1998) 160:5797–806 [PubMed] [Google Scholar]
- 65.Anderson HA, Roche PA. Phosphorylation regulates the delivery of MHC class II invariant chain complexes to antigen processing compartments. J Immunol (1998) 160:4850–8 [PubMed] [Google Scholar]
- 66.Yuan H, Michelsen K, Schwappach B. 14-3-3 Dimers probe the assembly status of multimeric membrane proteins. Curr Biol (2003) 13:638–46 10.1016/S0960-9822(03)00208-2 [DOI] [PubMed] [Google Scholar]
- 67.Khalil H, Brunet A, Saba I, Terra R, Sekaly RP, Thibodeau J. The MHC class II beta chain cytoplasmic tail overcomes the invariant chain p35-encoded endoplasmic reticulum retention signal. Int Immunol (2003) 15:1249–63 10.1093/intimm/dxg124 [DOI] [PubMed] [Google Scholar]
- 68.Machamer CE, Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol (1982) 129:2564–9 [PubMed] [Google Scholar]
- 69.Khalil H, Brunet A, Thibodeau J. A three-amino-acid-long HLA-DRbeta cytoplasmic tail is sufficient to overcome ER retention of invariant-chain p35. J Cell Sci (2005) 118:4679–87 10.1242/jcs.02592 [DOI] [PubMed] [Google Scholar]
- 70.Lamb CA, Yewdell JW, Bennink JR, Cresswell P. Invariant chain targets HLA class II molecules to acidic endosomes containing internalized influenza virus. Proc Natl Acad Sci U S A (1991) 88:5998–6002 10.1073/pnas.88.14.5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warmerdam PA, Long EO, Roche PA. Isoforms of the invariant chain regulate transport of MHC class II molecules to antigen processing compartments. J Cell Biol (1996) 133:281–91 10.1083/jcb.133.2.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuwana T, Peterson PA, Karlsson L. Exit of major histocompatibility complex class II-invariant chain p35 complexes from the endoplasmic reticulum is modulated by phosphorylation. Proc Natl Acad Sci U S A (1998) 95:1056–61 10.1073/pnas.95.3.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson HA, Bergstralh DT, Kawamura T, Blauvelt A, Roche PA. Phosphorylation of the invariant chain by protein kinase C regulates MHC class II trafficking to antigen-processing compartments. J Immunol (1999) 163:5435–43 [PubMed] [Google Scholar]
- 74.Germain RN. The ins and outs of antigen processing and presentation. Nature (1986) 322:687–98 10.1038/322687a0 [DOI] [PubMed] [Google Scholar]
- 75.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A (1982) 79:175–8 10.1073/pnas.79.1.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziegler K, Unanue ER. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol (1981) 127:1869–75 [PubMed] [Google Scholar]
- 77.Stumptner-Cuvelette P, Benaroch P. Multiple roles of the invariant chain in MHC class II function. Biochim Biophys Acta (2002) 1542:1–13 10.1016/S0167-4889(01)00166-5 [DOI] [PubMed] [Google Scholar]
- 78.Landsverk OJB, Bakke O, Gregers TF. MHC II and the endocytic pathway: regulation by invariant chain. Scand J Immunol (2009) 70:184–93 10.1111/j.1365-3083.2009.02301.x [DOI] [PubMed] [Google Scholar]
- 79.Pieters J, Horstmann H, Bakke O, Griffiths G, Lipp J. Intracellular transport and localization of major histocompatibility complex class II molecules and associated invariant chain. J Cell Biol (1991) 115:1213–23 10.1083/jcb.115.5.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roche PA, Teletski CL, Karp DR, Pinet V, Bakke O, Long EO. Stable surface expression of invariant chain prevents peptide presentation by HLA-DR. EMBO J (1992) 11:2841–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arunachalam B, Lamb CA, Cresswell P. Transport properties of free and MHC class II-associated oligomers containing different isoforms of human invariant chain. Int Immunol (1993) 6:439–51 10.1093/intimm/6.3.439 [DOI] [PubMed] [Google Scholar]
- 82.O’Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell (2002) 111:577–88 10.1016/S0092-8674(02)01040-1 [DOI] [PubMed] [Google Scholar]
- 83.Sato K. COPII coat assembly and selective export from the endoplasmic reticulum. J Biochem (2004) 136:755–60 10.1093/jb/mvh184 [DOI] [PubMed] [Google Scholar]
- 84.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol (2012) 14:20–8 10.1038/ncb2390 [DOI] [PubMed] [Google Scholar]
- 85.Hiltbold EM, Roche PA. Trafficking of MHC class II molecules in the late secretory pathway. Curr Opin Immunol (2002) 14:30–5 10.1016/S0952-7915(01)00295-3 [DOI] [PubMed] [Google Scholar]
- 86.McCormick PJ, Martina JA, Bonifacino JS. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci U S A (2005) 102:7910–5 10.1073/pnas.0502206102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J Biol Chem (2005) 280:19656–64 10.1074/jbc.M501357200 [DOI] [PubMed] [Google Scholar]
- 88.Kongsvik TL, Höning S, Bakke O, Rodionov DG. Mechanism of interaction between leucine-based sorting signals from the invariant chain and clathrin-associated adaptor protein complexes AP1 and AP2. J Biol Chem (2002) 277:16484–8 10.1074/jbc.M201583200 [DOI] [PubMed] [Google Scholar]
- 89.Pieters J, Bakke O, Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci (1993) 106:831–46 [DOI] [PubMed] [Google Scholar]
- 90.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain RN. Relationship between invariant chain expression and major histocompatibility complex class II transport into early and late endocytic compartments. J Exp Med (1993) 177:583–96 10.1084/jem.177.3.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gorvel JP, Escola JM, Bakke O. Invariant chain induces a delayed transport from early to late endosomes. J Biol Chem (1995) 270:2741–6 10.1074/jbc.270.6.2741 [DOI] [PubMed] [Google Scholar]
- 92.Gregers TF, Nordeng TW, Birkeland HC, Sandlie I, Bakke O. The cytoplasmic tail of invariant chain modulates antigen processing and presentation. Eur J Immunol (2003) 33:277–86 10.1002/immu.200310001 [DOI] [PubMed] [Google Scholar]
- 93.Stang E, Bakke O. MHC class II associated invariant chain induced enlarged endosomal structures. A morphological study. Exp Cell Res (1997) 235:79–92 10.1006/excr.1997.3617 [DOI] [PubMed] [Google Scholar]
- 94.Fineschi B, Miller J. Endosomal proteases and antigen processing. Trends Biochem Sci (1997) 22:377–82 10.1016/S0968-0004(97)01116-X [DOI] [PubMed] [Google Scholar]
- 95.Driessen C, Bryant RAR, Lennon-Duménil AM, Villadangos JA, Bryant PW, Shi GP, et al. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol (1999) 147:775–90 10.1083/jcb.147.4.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Villadangos JA, Ploegh HL. Proteolysis in MHC class II antigen presentation: who’s in charge? Immunity (2000) 12:233–9 10.1016/S1074-7613(00)80176-4 [DOI] [PubMed] [Google Scholar]
- 97.Zavasnik-Bergant V, Schweiger A, Bevec T, Golouh R, Turk V, Kos J. Inhibitory p41 isoform of invariant chain and its potential target enzymes cathepsins L and H in distinct populations of macrophages in human lymph nodes. Immunology (2004) 112:378–85 10.1111/j.1365-2567.2004.01879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Faure-André G, Vargas P, Yuseff M, Heuzé M, Vascotto F, Boulanger J, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science (2008) 322:1705–10 10.1126/science.1159894 [DOI] [PubMed] [Google Scholar]
- 99.Yuseff M-I, Pierobon P, Reversat A, Lennon-Duménil A-M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol (2013) 13:475–86 10.1038/nri3469 [DOI] [PubMed] [Google Scholar]
- 100.Castellino F, Germain RN. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity (1995) 2:73–88 10.1016/1074-7613(95)90080-2 [DOI] [PubMed] [Google Scholar]
- 101.Bosch B, Berger AC, Khandelwal S, Heipertz EL, Scharf B, Santambrogio L, et al. Disruption of multivesicular body vesicles does not affect MHC class II-peptide complex formation and antigen presentation by dendritic cells. J Biol Chem (2013) 288(34):24286–92 10.1074/jbc.M113.461996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nordeng TW, Gregers TF, Kongsvik TL, Meresse S, Gorvel JP, Jourdan F, et al. The cytoplasmic tail of invariant chain regulates endosome fusion and morphology. Mol Biol Cell (2002) 13:1846–56 10.1091/mbc.01-10-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gedde-Dahl M, Freisewinkel I, Staschewski M, Schenck K, Koch N, Bakke O. Exon 6 is essential for invariant chain trimerization and induction of large endosomal structures. J Biol Chem (1997) 272:8281–7 10.1074/jbc.272.13.8281 [DOI] [PubMed] [Google Scholar]
- 104.Gelin C, Sloma I, Charron D, Mooney N. Regulation of MHC II and CD1 antigen presentation: from ubiquity to security. J Leukoc Biol (2009) 85:215–24 10.1189/jlb.0308206 [DOI] [PubMed] [Google Scholar]
- 105.Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, Choi KB, et al. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol (2012) 13:237–45 10.1038/ni.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arneson LS, Miller J. The chondroitin sulfate form of invariant chain trimerizes with conventional invariant chain and these complexes are rapidly transported from the trans-Golgi network to the cell surface. Biochem J (2007) 406:97–103 10.1042/BJ20070446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell (1993) 74:257–68 10.1016/0092-8674(93)90417-O [DOI] [PubMed] [Google Scholar]
- 108.Beswick EJ. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol (2009) 15:2855. 10.3748/wjg.15.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matza D, Kerem A, Shachar I. Invariant chain, a chain of command. Trends Immunol (2003) 24:264–8 10.1016/S1471-4906(03)00073-5 [DOI] [PubMed] [Google Scholar]
- 110.Beisner DR, Langerak P, Parker AE, Dahlberg C, Otero FJ, Sutton SE, et al. The intramembrane protease Sppl2a is required for B cell and DC development and survival via cleavage of the invariant chain. J Exp Med (2013) 210:23–30 10.1084/jem.20121072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schneppenheim J, Dressel R, Hüttl S, Lüllmann-Rauch R, Engelke M, Dittmann K, et al. The intramembrane protease SPPL2a promotes B cell development and controls endosomal traffic by cleavage of the invariant chain. J Exp Med (2013) 210:41–58 10.1084/jem.20121069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veenstra H, Jacobs P, Dowdle EB. Processing of HLA-class II invariant chain and expression of the p35 form is different in malignant and transformed cells. Blood (1993) 82:2494–500 [PubMed] [Google Scholar]
- 113.Veenstra H, Jacobs P, Dowdle EB. Abnormal association between invariant chain and HLA class II alpha and beta chains in chronic lymphocytic leukemia. Cell Immunol (1996) 171:68–73 10.1006/cimm.1996.0174 [DOI] [PubMed] [Google Scholar]
- 114.Yan G, Shi L, Penfornis A, Faustman DL. Impaired processing and presentation by MHC class II proteins in human diabetic cells. J Immunol (2003) 170:620–7 [DOI] [PubMed] [Google Scholar]
- 115.Sanderson S, Frauwirth K, Shastri N. Expression of endogenous peptide-major histocompatibility complex class II complexes derived from invariant chain-antigen fusion proteins. Proc Natl Acad Sci U S A (1995) 92:7217–21 10.1073/pnas.92.16.7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holst PJ, Sorensen MR, Mandrup Jensen CM, Orskov C, Thomsen AR, Christensen JP. MHC class II-associated invariant chain linkage of antigen dramatically improves cell-mediated immunity induced by adenovirus vaccines. J Immunol (2008) 180:3339–46 [DOI] [PubMed] [Google Scholar]
- 117.Malcherek G, Wirblich C, Willcox N, Rammensee HG, Trowsdale J, Melms A. MHC class II-associated invariant chain peptide replacement by T cell epitopes: engineered invariant chain as a vehicle for directed and enhanced MHC class II antigen processing and presentation. Eur J Immunol (1998) 28:1524–33 [DOI] [PubMed] [Google Scholar]