Key Points
In contrast to c-Src and Yes, Lyn stabilizes endothelial junctions through interaction and phosphorylation of FAK.
Abstract
The Src family kinases (SFKs) c-Src and Yes mediate vascular leakage in response to various stimuli including lipopolysaccharide (LPS) and vascular endothelial growth factor (VEGF). Here, we define an opposing function of another SFK, Lyn, which in contrast to other SFKs, strengthens endothelial junctions and thereby restrains the increase in vascular permeability. Mice lacking Lyn displayed increased mortality in LPS-induced endotoxemia and increased vascular permeability in response to LPS or VEGF challenge compared with wild-type littermates. Lyn knockout mice repopulated with wild-type bone marrow–derived cells have higher vascular permeability than wild-type mice, suggesting a role of endothelial Lyn in the maintenance of the vascular barrier. Small interfering RNA–mediated down-regulation of Lyn disrupted endothelial barrier integrity, whereas expression of a constitutively active mutant of Lyn enhanced the barrier. However, down-regulation of Lyn did not affect LPS-induced endothelial permeability. We demonstrate that Lyn association with focal adhesion kinase (FAK) and phosphorylation of FAK at tyrosine residues 576/577 and 925 were required for Lyn-dependent stabilization of endothelial adherens junctions. Thus, in contrast to c-Src and Yes, which increase vascular permeability in response to stimuli, Lyn stabilizes endothelial junctions through phosphorylation of FAK. Therefore, therapeutics activating Lyn kinase may strengthen the endothelial barrier junction and hence have anti-inflammatory potential.
Introduction
The vascular endothelium controls the transendothelial exchange of solutes, fluid, and proteins, as well as the migration of cells from blood to extravascular tissues. Endothelial disruption can lead to extravasation of blood components, metastasis, and accumulation of fluid, resulting in tissue edema, inflammation, and cancer.1,2 Endothelial barrier hyperpermeability is characterized by the formation of intercellular gaps between adjacent endothelial cells, the predominant mechanism of vascular leakage and transmigration. Multiple signaling pathways regulate endothelial permeability, but ultimately they all induce the opening of endothelial adherens junctions (AJs).3
The Src family nonreceptor tyrosine kinases (SFKs) has been well recognized as signaling enzymes that regulate cell growth, differentiation, cell adhesion, carcinogesesis, and immune cell function.4 Mounting evidence has demonstrated that the SFKs, such as c-Src and Yes, play an essential role in promoting endothelial permeability in response to inflammatory mediators.5-9 Vascular endothelial growth factor (VEGF) increases vascular permeability through activation of c-Src and Yes.5-7 SFKs also contribute to increases in endothelial permeability in response to thrombin10 and superoxide anion.8,9 SFKs regulate endothelial barrier integrity through phosphorylation and activation of the proteins involved in paracellular and junctional transport. c-Src associates with AJs through its interaction with vascular-endothelial cadherin (VE-cadherin),11 where it phosphorylates VE-cadherin and induces disruption of AJs, leading to the formation of intercellular gaps.7,12,13
In addition to the effect of SFKs on intercellular junctions, they can affect permeability through the regulation of focal adhesions,14,15 the specialized subcellular structures that mediate endothelial cell attachment to the extracellular matrix via transmembrane and cytoskeletal linker proteins. Focal adhesion kinase (FAK), which regulates focal adhesion formation and turnover, is a major determinant of vessel wall permeability.16 Interestingly, FAK appears to be involved in both increasing or decreasing in endothelial permeability elicited by stimulation with the protease activated receptor 1 or the sphingosine-1-phosphate receptor 1, respectively.16 FAK can be phosphorylated by SFKs at multiple sites, including tyrosine 576/577 and 925.17,18 It is not clear whether the distinct roles of FAK in the regulation of endothelial barrier are due to differential FAK phosphorylation by different kinases. In this regard, it has been shown that thrombin induces robust phosphorylation of FAK at tyrosine 397, 576, and 925. In contrast, S1P induces more robust phosphorylation at tyrosine 576 of FAK.14 In this present study, we describe a new role for the SFK member, Lyn, in regulating endothelial barrier function through phosphorylating FAK. In contrast to c-Src and Yes, Lyn did not increase endothelial permeability in response to inflammatory stimuli but stabilized endothelial barrier and prevented the increase in permeability in response to inflammatory mediators such as lipopolysaccharide (LPS). These data reveal a novel Lyn-dependent modulation of endothelial permeability and show that different SFK members have distinct functions in either disrupting or preserving the endothelial barrier. Hence, approaches increasing Lyn activity, as it is distinct from other SFKs, may be useful in preventing inflammation and inappropriate transmigration of blood and cancer cells.
Methods
Materials
LPS 0111:B4 was purchased from Sigma (St Louis, MO). Lyn, FAK, and control small interfering RNA (siRNA) oligonucleotides, and mouse monoclonal antibodies against Fyn (FYN-59), c-Src (B-12) and rabbit polyclonal antibodies against Lyn, FAK, or phosphorylated FAK at residue Tyr861 were from Santa Cruz Biotechnology (Santa Cruz, CA). A rabbit monoclonal antibody against Lyn (C13F9) and rabbit polyclonal antibodies against phosphorylated FAK at residues Tyr397, 576/577, or 925 were from Cell Signaling Technology (Beverly, MA). A mouse anti-phosphotyrosine monoclonal antibody 4G10 was from EMD Millipore Corp. (Billerica, MA). Human umbilical vein endothelial cells (HUVECs) and human pulmonary artery endothelial cells (HPAECs) were from Lonza (Conshohocken, PA). Lyn-deficient mice were generated as described previously19 and were backcrossed to C57BL/6J background. Wild-type littermates from Lyn heterozygote breeding were used as controls. Mice were bred and maintained in the University of Illinois Animal Care Facility and the University of Kentucky Animal Care Facility following institutional and National Institutes of Health guidelines after approval by the Institutional Animal Use and Care Committee.
Cell culture and transfection
HUVECs and HPAECs were cultured with endothelial growth medium-2 (Lonza) and supplemented with 10% fetal bovine serum (FBS). siRNA transfection reagent was used for Lyn and FAK siRNA transfection, following the manufacturer’s instruction. siGLO RISC-Free control siRNA labeled with DY-547 (Thermo Fisher Scientific, Waltham, MA) was cotransfected with Lyn or FAK siRNA to indicate the siRNA-transfected cells without interfering with target gene silencing.
Adenoviral infection of HUVECs
Recombinant adenovirus containing a mutant of human Lyn cDNA (LynY508F) under the control of a cytomegalovirus promoter was custom generated by Vector Biolabs. A cytomegalovirus-null virus was used as a control. The adenovirus containing LynY508F or the null virus was added to (final concentration 3 × 107 pfu/mL) wells or dishes with serum-free medium containing 5 μg/mL polybrene. Equal volume of fresh medium containing 10% FBS was added after 4 hours.
Immunofluorescence staining and confocal microscopy
HUVECs cultured onto 0.2% gelatin-coated coverslips were fixed with 4% paraformaldehyde for 20 minutes, followed by permeabilization with 0.1% Triton X-100 for 5 minutes. Thereafter, cells were incubated with primary antibody against endogenous VE-cadherin for 1 hour, followed by incubation with Alexa-488–conjugated anti-mouse secondary antibody, using Hanks balanced salt solution containing 1% bovine serum albumin as a blocking buffer. Images were acquired using an LSM 510 confocal microscope (Zeiss), equipped with a ×63 water immersion objective with appropriate filter sets.
Determination of pulmonary microvascular permeability
Lung microvessel filtration coefficient (Kf,c) in isolated lung preparation was measured to determine pulmonary microvascular permeability to liquid as described previously.20 In brief, after the standard 20-minute equilibration perfusion to establish an isogravimetric condition, the outflow pressure was rapidly elevated by 10 cm of H2O for 20 minutes. Lung preparations gained weight in response to the pressure increase, reflecting the net liquid accumulation. Lungs were dissected free of nonpulmonary tissue, and lung dry weight was determined. The Kf,c was calculated from the slope of the recorded weight change normalized to the pressure change and to lung dry weight.
Irradiation and bone marrow–derived cell repopulation
Male Lyn+/+ mice and Lyn−/− mice (6-8 weeks old) were lethally irradiated with a total of 900 rad divided into 2 doses (450 rad/dose, 3 hours apart) from a cesium γ source. Bone marrow–derived cells were harvested from Lyn−/− or Lyn+/+ male mice (6-8 weeks old) and injected into irradiated recipient Lyn+/+ mice or Lyn−/− mice (1 × 107 donor cells per animal). The mice were used for experiments at 6 weeks after irradiation. Lyn expression in bone marrow cells of recipient mice was detected by immunoblotting assay.
LPS-induced mortality
Lyn+/+ and Lyn−/− mice (8-10 weeks old) were injected intraperitoneally with 12 mg/kg LPS in a volume of 200 µL saline. Mortality was assessed at 24, 48, 72, 96, and 120 hours. Statistical analysis was performed using the Fisher exact test for the evaluation of differences in survival rate.
Isolation of mouse lung microvascular endothelial cells
Lyn+/+ and Lyn−/− mice (3-4 weeks old) were killed by CO2 inhalation. The lungs were taken out and transferred into a 15-mL tube containing Dulbecco’s modified Eagle medium + 20% FBS + pen/strep (isolation medium). Lung lobes were minced finely with scissors. Minced lung was incubated with prewarmed collagenase type II (2 mg/mL) at 37°C with gentle agitation for 45 minutes. The digested tissues were passed through a 70-μm cell strainer. After washed once with the isolation medium, cells were incubated with rat anti-mouse CD31 coated Dynabeads for 10 minutes at room temperature with gentle agitation, and the cells pulled down by the beads were cultured and confirmed by VE-cadherin immunostaining (supplemental Figure 1 on the Blood Web site).
Measurement of transendothelial electrical resistance
Endothelial barrier function was assessed through measuring real-time changes in electrical resistance across endothelial monolayers as described.21 Briefly, HUVECs were seeded onto gold microelectrode arrays (Applied Biophysics) and cultured to confluence. Impedance across the cells was measured at 4 kHz, and the resistive portion of the impedance was normalized to its initial value.
Immunoprecipitation and western blotting
Endothelial cells or bone marrow cells were solubilized in sodium dodecyl sulfate (SDS) sample buffer and analyzed by western blotting. To determine the phosphorylation levels of FAK, the cells were solubilized in radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM sodium ortho-vanadate, and protease inhibitors. The cleared lysates were incubated with 5 μg of rabbit anti-FAK antibody and 30 μL of protein A/G agarose beads for 4 hours at 4°C. After washing, precipitated proteins were analyzed by immunoblotting with the anti-phosphotyrosine monoclonal antibody 4G10.22
Statistical analyses
The survival assay was analyzed by the Fisher exact test. Significance in experiments comparing 2 groups was examined by a 2-tailed Student's t test.
Results
Increased lung microvascular permeability and mortality in Lyn−/− mice
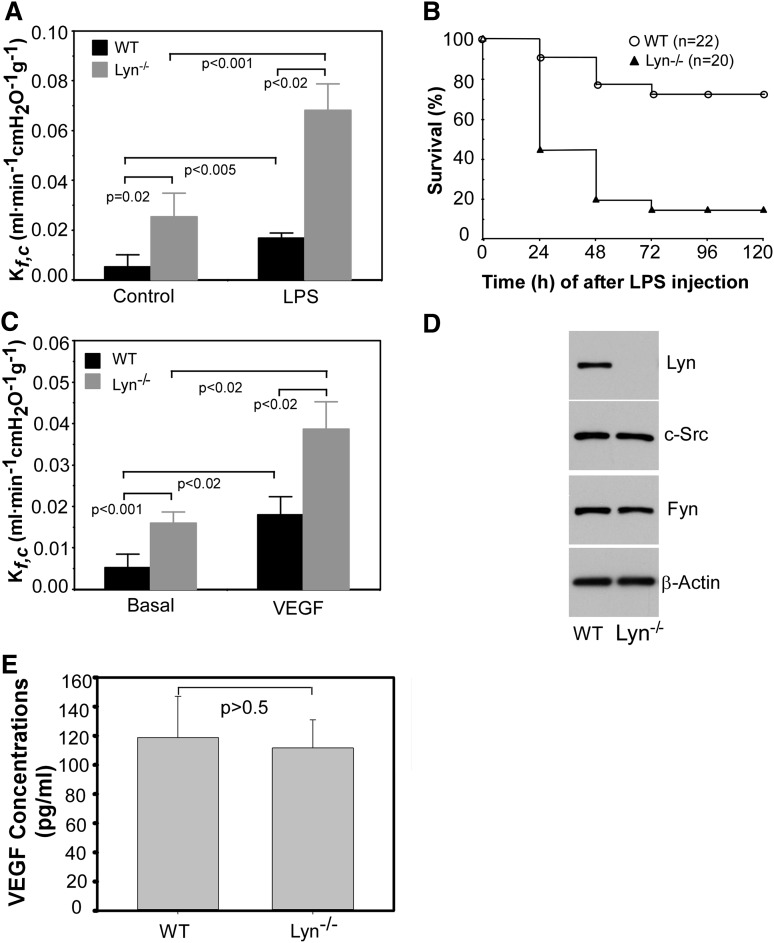
Sepsis and its accompanying crisis of transudation of fluid and proteins into tissue remains a major cause of death in intensive care units. A classical example of sepsis-induced vascular leakage syndrome is the development of acute lung injury, which is associated with the development of protein-rich pulmonary edema.23,24 Gram-negative bacterial LPS or endotoxin, a component of the outer membrane of Gram-negative bacteria, plays a critical role in the pathogenesis of sepsis. A mouse model of endotoxemia induced by LPS infusion/injection stimulates vessel wall leakiness.25 SFKs play critical roles in the molecular mechanism underlying increased vascular permeability. Inhibition of c-Src and Yes protects against LPS-induced increases in vascular permeability.26-28 As LPS can also activate Lyn,29 we investigated the role of Lyn in the regulation of vascular permeability by determining mouse lung capillary filtration coefficient (Kf,c).30,31 Injection of LPS (intraperitoneally) significantly increased the Kf,c compared with basal conditions in wild-type mice (Figure 1A). The increase in Kf,c in Lyn-deficient mice challenged with LPS was significantly greater than that of wild-type mice. Surprisingly, basal Kf,c of Lyn-deficient mice was also significantly greater than that of wild-type mice, suggesting a role of Lyn kinase in stabilizing endothelial barrier. Mortality studies in mice challenged with intraperitoneal administration of LPS showed that 80% of Lyn-deficient mice died within 96 hours after LPS challenge, whereas only 20% of wild-type littermates died during this time period (P < .001; Figure 1B). These results demonstrate a novel protective role of Lyn kinase in septic shock in contrast to the other Src kinases c-Src and Yes, which mediate increased vascular permeability in response to LPS.28
Figure 1.
Lyn knockout mice have increased lung microvascular endothelial permeability and higher mortality rate in response to LPS challenge. (A) The lung capillary filtration coefficient (Kf,c) was determined in Lyn+/+ and Lyn−/− mice before or at 6 hours after administration of LPS (5 mg/kg body weight). Bars indicate means ± standard error (n = 5). P < .005 and .001 between wild-type littermates and Lyn−/− mice under control or LPS administration, respectively, by unpaired 2-tailed Student t test. P < .05 by Bonferroni correction analysis of multiple comparisons. (B) Kaplan-Meier survival plots for Lyn+/+ (WT) and Lyn−/− (Lyn−/−) mice. Mice were challenged with LPS (12 mg/kg) via intraperitoneal injection. The percentage of surviving animals was observed at 24, 48, 72, 96, and 120 hours after LPS injection. The difference in survival between WT and Lyn−/− mice was significant (P < .001). (C) Lung microvascular permeability in Lyn+/+ and Lyn−/− mice before or at 6 hours after challenging with VEGF (3 μg/kg body weight). Bars indicate means ± standard deviation (SD; n = 6). P values indicated in the figure were analyzed by unpaired 2-tailed Student t test. P < .05 by Bonferroni correction analysis of multiple comparisons. (D) Lung microvascular endothelial cells isolated from Lyn−/− and wild-type littermates were cultured and solubilized, and Lyn, c-Src, and Fyn in cell lysates were detected by western blot. β-actin was detected by western blot with mouse monoclonal antibody (Sigma) to verify equal loading. (E) VEGF in plasma from Lyn−/− and wild-type littermates was measured by enzyme-linked immunosorbent assay (ELISA) assay with a VEGF ELISA kit (Sigma). Difference was assessed using unpaired 2-tailed Student t test, P > .5. Values are means ± SD (n = 3).
As VEGF increases vascular permeability in a c-Src– and Yes-dependent manner,5 we studied VEGF-induced vascular permeability in Lyn-deficient mice. As expected, injection of VEGF increased the Kf,c in wild-type mice. The Kf,c in Lyn-deficient mice in response to VEGF treatment was significantly greater compared with that of wild-type littermates (Figure 1C). Increased lung permeability in Lyn−/− mice is unlikely due to compensatory increases in the expression of other isoforms of SFKs, because lung microvascular endothelial cells from the Lyn knockout mice express similar levels of c-Src and Fyn as wild-type mice (Figure 1D). In addition, plasma VEGF levels of Lyn knockout mice were similar to that of wild-type mice (Figure 1E).
Lyn−/− mice repopulated with wild-type bone marrow–derived cells have higher basal Kf,c than wild-type mice
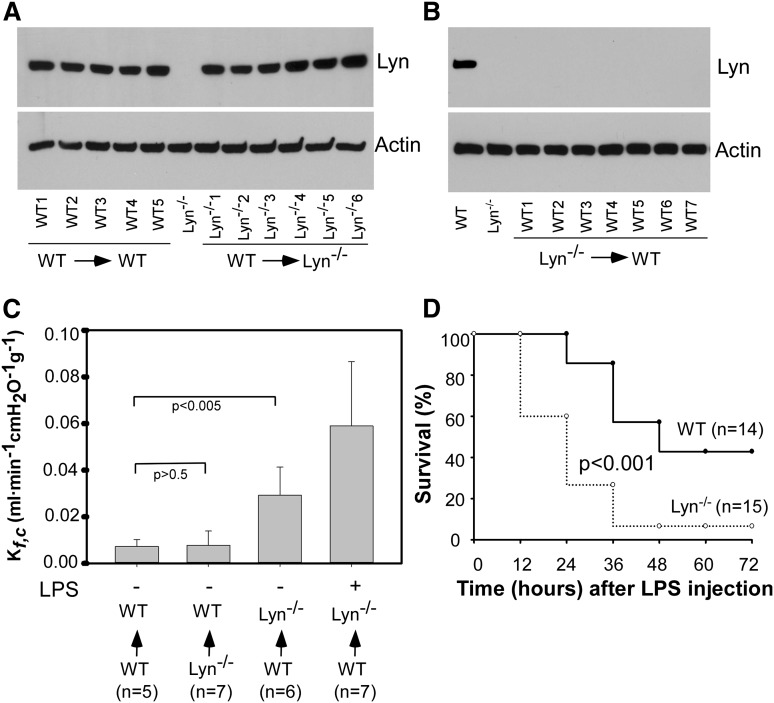
Lyn kinase plays a key role in white blood cell function,32-34 an important mechanism contributing to pathogenesis of increased vascular permeability.35 To exclude the possibility that the increase in the basal Kf,c in Lyn-deficient mice stems from an alteration in white blood cell function, Lyn knockout mice were repopulated with wild-type mouse bone marrow–derived cells by bone marrow transplantation. Wild-type mice repopulated with wild-type mouse bone marrow–derived cells were used as controls. Wild-type mice repopulated with Lyn−/− bone marrow–derived cells by bone marrow transplantation were used as an additional control. Chimerism of Lyn−/− mice repopulated with wild-type donor cells and chimerism of wild-type mice repopulated with Lyn−/− donor cells was confirmed by western blot detecting Lyn expression in bone marrow cells. The expression levels of Lyn in bone marrow cells from Lyn knockout mice repopulated with wild-type mouse bone marrow–derived cells was similar to that of wild-type mice repopulated with wild-type mouse bone marrow–derived cells (Figure 2A). Lyn expression in bone marrow cells from wild-type mice repopulated with Lyn−/− bone marrow–derived cells was abolished (Figure 2B). The basal Kf,c of Lyn knockout mice repopulated with wild-type bone marrows was much higher than that of wild-type mice repopulated with wild-type bone marrows (Figure 2C). In response to LPS challenge, Kf,c of these mice (Figure 2C) reached to a level similar to Lyn−/− mice (Figure 1A). In contrast, basal Kf,c of wild-type mice repopulated with Lyn−/− bone marrows was similar to that of wild-type mice repopulated with wild-type bone marrows. These data imply that lack of Lyn in endothelial cells, but not in white blood cells, is responsible for increased vascular permeability in Lyn-deficient mice.
Figure 2.
Endothelial Lyn contributes to increased lung permeability and higher mortality rate of Lyn−/− mice in response to LPS challenge. (A) Expression of Lyn in bone marrow cells from Lyn+/+ (WT→WT, n = 5) and Lyn−/− (WT→ Lyn−/−, n = 6) mice repopulated with Lyn+/+ donor bone marrow cells was detected by western blot with a rabbit polyclonal antibody against Lyn. Bone marrow cells from a Lyn−/− mouse were used as a control. β-actin was detected by western blot with mouse monoclonal antibody (Sigma) to verify equal loading. (B) Expression of Lyn in bone marrow cells from wild-type mice repopulated with Lyn−/− donor bone marrow cells was detected by western blot with a rabbit polyclonal antibody against Lyn. Bone marrow cells from a wild-type and Lyn−/− mouse were used as controls. β-actin was detected by western blot with mouse monoclonal antibody to verify equal loading. (C) Lung microvascular permeability in Lyn+/+ and Lyn−/− mice repopulated with either Lyn+/+ or Lyn−/− donor bone marrow cells was measured. In some experiments, Lyn−/− mice repopulated with Lyn+/+ donor bone marrow cells were injected with LPS. Six hours after LPS injection, lung microvascular permeability was measured. Bars indicate means ± SD (n = 5∼7). (D) Wild-type and Lyn−/− mice repopulated with wild-type donor bone marrow cells were challenged with LPS (8 mg/kg) via intraperitoneal injection. Survival rates were observed at 24, 48, 72, 96, and 120 hours after LPS injection. The difference in survival between WT and Lyn−/− mice was significant (P < .001).
Accordingly, mortality rate in the Lyn knockout mice repopulated with wild-type bone marrows was much higher than that of wild-type mice repopulated with wild-type bone marrows (P < .001; Figure 2D). These results confirmed a protective role of endothelial Lyn in septic shock. Mice subjected to bone marrow transplantation were more sensitive to LPS challenge. Therefore, a low dose of LPS was applied.
Lyn kinase is the predominant SFK maintaining basal endothelial barrier function
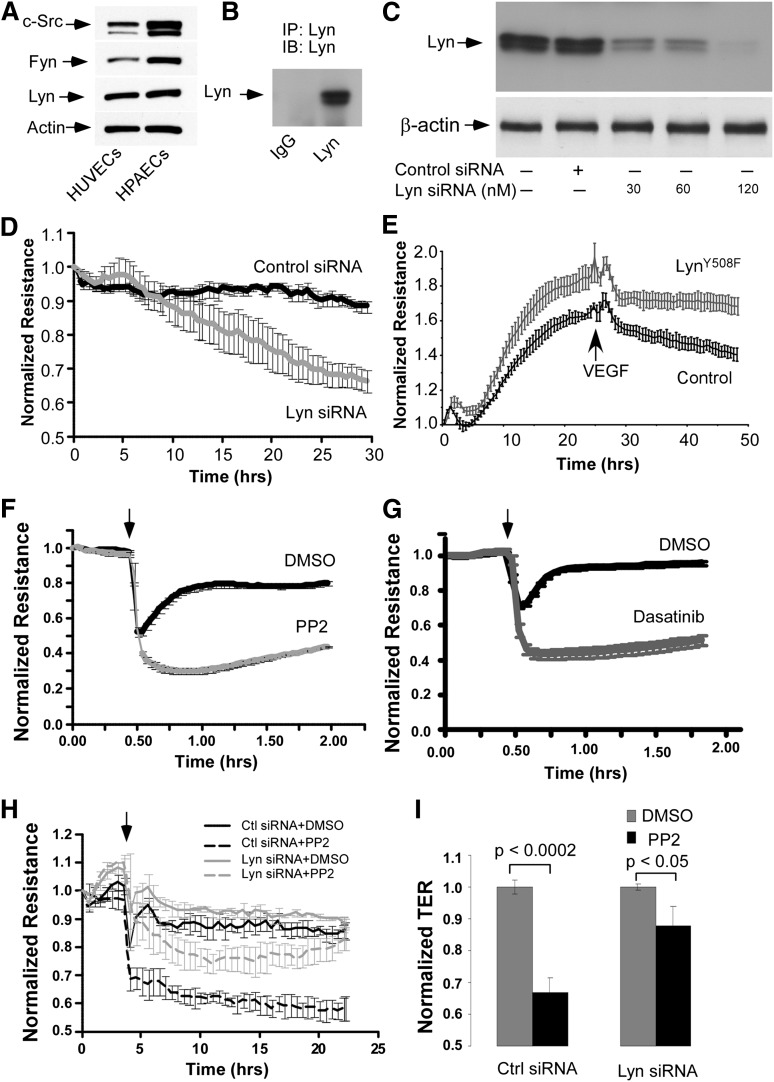
Lyn kinase is preferentially expressed in hematopoietic cells such as macrophages, monocytes, and platelets.36 Lyn kinase was first reported to be present in developing blood vessels in embryonic and early postnatal mouse brain, but not in endothelium outside the brain.37 Other studies reported that Lyn kinase is expressed in adult endothelial cells.28,38 Here, we confirmed the expression of Lyn in HPAECs and HUVECs (Figure 3A-B). To explore the basis of Lyn regulation of endothelial barrier function, Lyn expression in HUVECs was down-regulated by transfection of HUVECs with Lyn siRNA (Figure 3C). As shown in Figure 3D, down-regulation of Lyn markedly reduced basal transendothelial electrical resistance (TER), a measure of junctional integrity, indicating that Lyn serves to homeostatically regulate endothelial integrity. To further establish a role of Lyn in stabilizing endothelial barrier function, TER was measured in HUVEC monolayers infected with null adenovirus or a virus containing a cDNA of the constitutively active Lyn kinase mutant, LynY508F.39 HUVEC monolayers infected with LynY508F had higher TER vs HUVEC monolayers infected with a null virus (Figure 3E). Overexpression of the constitutively active Lyn kinase mutant also blunted endothelial disruption in response to VEGF stimulation (Figure 3E). These results indicate that the Lyn-mediating stabilization of endothelial barrier restrains the increase in endothelial permeability in response to the prototypic permeability-increasing mediator VEGF.
Figure 3.
Expression of Lyn kinase in endothelial cells and the role of Lyn in regulating TER. (A) HUVECs or HPAECs were lysed with radioimmunoprecipitation assay buffer. Total cell lysate proteins were analyzed by SDS-polyacrylamide gel electrophoresis. Lyn, c-Src, and Fyn in cell lysates were detected by western blot with a rabbit monoclonal anti-Lyn antibody (Cell Signaling) or mouse monoclonal antibodies against c-Src or Fyn (Santa Cruz). A mouse monoclonal antibody against β-actin was used to verify equal loading. (B) Lyn in HUVEC lysates was immunoprecipitated by a monoclonal anti-Lyn antibody and detected by western blot with a polyclonal antibody against Lyn (Santa Cruz). (C) HUVECs grown on 6-well plates were transiently transfected with increasing doses of control or Lyn siRNA for 48 hours. Lyn kinase was visualized by western blot analysis with a polyclonal antibody against Lyn (upper). The membrane was probed with anti–β-actin antibody (lower) to verify equal loading. (D) HUVECs were grown to confluence on gold microelectrodes and then transfected with control or Lyn siRNA oligonucleotide (60 nM). TER across the HUVEC monolayers was measured. (E) HUVECs were infected with null adenovirus (Control) or a virus containing a cDNA of the constitutively active Lyn kinase mutant (Lyn), LynY508F. VEGF (200 ng/mL) was added 24 hours after infection with the viruses, and TER was monitored. (F and G) HUVECs were grown to confluence on gold microelectrodes and then added with vehicle (dimethylsulfoxide [DMSO]), (F) PP2 (10 μM), or (G) Dasatinib (100 nM). TER across the HUVEC monolayers was measured. (H) Forty-eight hours after transfection of control or Lyn siRNA oligonucleotide (60 nM), HUVECs seeded on gold microelectrodes were used for TER measurement. At the time indicated by the arrows, cells were incubated with vehicle (DMSO) or PP2 (10 μM). (I) Histogram of normalized TER obtained from H at the time point of 15 hours. Data represent means ± SD of changes in TER from 4 independent experiments.
We observed that treatment of HUVECs with a pan-SFK inhibitor, PP2, markedly reduced basal TER (Figure 3F). Another SFK inhibitor, dasatinib, also decreased basal TER in HUVECs (Figure 3G). These results demonstrate a previously unappreciated role of SFKs in maintaining basal endothelial barrier function. To address the question whether Lyn is responsible for this effect of SFKs on endothelial cells, we examined the effect of PP2 on endothelial permeability of HUVEC monolayer in which Lyn expression was suppressed by siRNA prior to PP2 treatment. As shown in Figure 3, the effect of PP2 on basal endothelial barrier function was significantly diminished in the HUVECs lacking Lyn (Figure 3H-I), indicating that impairment of basal barrier function by PP2 treatment is mainly attributed to inhibition of Lyn. For comparison of the effect of PP2 on endothelial permeability of Lyn down-regulated and control endothelial cells, at 48 hours after transfection of HUVECs with Lyn or control siRNA, TER values of both groups were normalized prior to PP2 treatment, although down-regulation of Lyn produced a sustained decrease in TER (Figure 3D).
Knockdown of Lyn kinase results in gap formation between endothelial cells
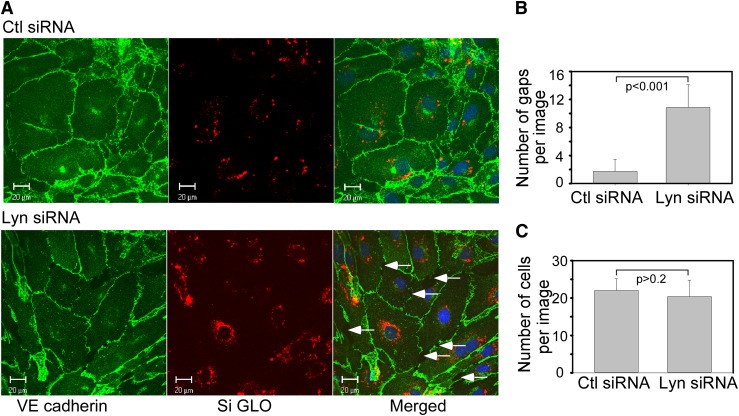
To visualize the effect of Lyn on the integrity of endothelial junctions, we examined subcellular distribution of VE-cadherin, a molecular marker of cell-cell AJs in HUVEC monolayers transfected with Lyn siRNA or control siRNA. Down-regulation of Lyn kinase resulted in distinct discontinuities in VE-cadherin distribution and formation of numerous interendothelial junctional gaps consistent with a key role of Lyn in promoting AJ integrity (Figure 4A-B). Cell numbers per image was not affected by down-regulation of Lyn (Figure 4C), suggesting that Lyn is not required for cell growth.
Figure 4.
Down-regulation of Lyn kinase in confluent endothelial cells disrupts AJ. (A) HUVECs were transfected with control or Lyn siRNA together with DY-547–labeled siGLO RISC-free control siRNA to allow visualization of siRNA transfected cells. Forty-eight hours after transfection, cells were fixed and immunostained for VE-cadherin. In control siRNA transfected HUVECs, VE-cadherin staining was continuous along cell borders indicative of its presence at the AJs. In Lyn siRNA-transfected cells, VE-cadherin was lost from AJs, resulting in formation of interendothelial gaps, as pointed by the arrows. (B) Quantification of gaps and (C) cell numbers in 4 random images (mean ± SD).
Lyn kinase is not required for LPS-induced increases in endothelial permeability
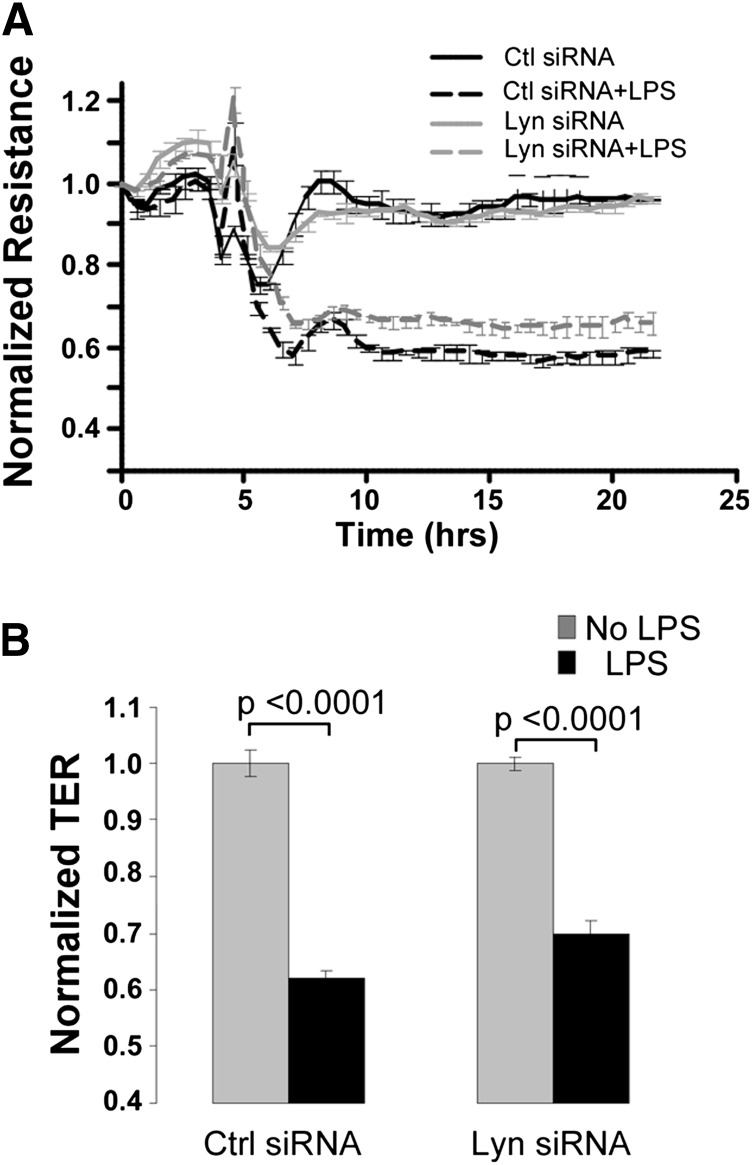
To determine whether Lyn contributes to the increased endothelial permeability in response to LPS, HUVECs were transfected with Lyn siRNA or control siRNA. At 48 hours after transfection, HUVEC monolayers were stimulated with LPS. The TER values were normalized to 1 prior to LPS treatment. Down-regulation of Lyn did not attenuate an LPS-induced decrease in TER (Figure 5A-B), suggesting that, unlike other SFKs, Lyn is not required for LPS-induced increased endothelial permeability.
Figure 5.
Down-regulation of Lyn kinase does not inhibit the LPS-induced increase in endothelial permeability. (A-B) Transfection of Lyn siRNA did not inhibit the LPS-induced TER decline. Confluent HUVECs grown on gold microelectrodes were transfected with control or Lyn siRNA for 48 hours and used for TER measurements. (A) Cells were stimulated with LPS (1 mg/mL) in the presence of FBS (2.5%). (B) Bar graph indicated normalized TER relative to non–LPS-treated groups at 15 hours after LPS stimulation.
Lyn binds and phosphorylates FAK in endothelial cells
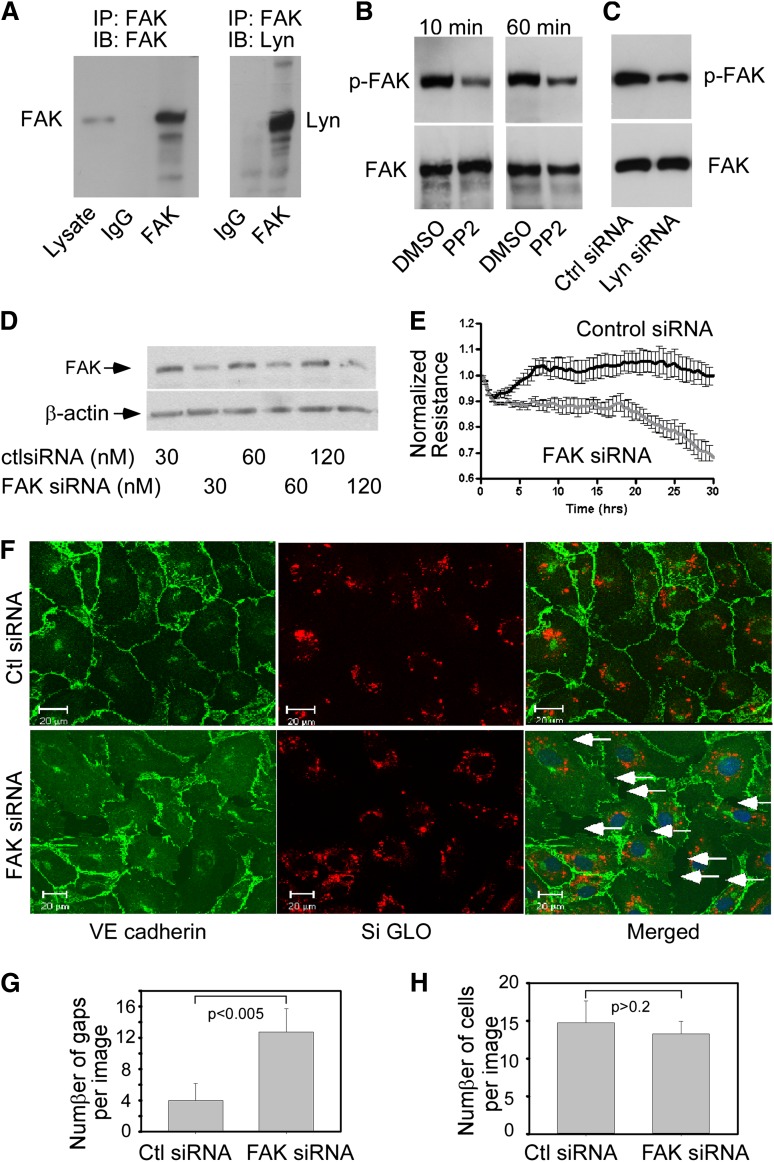
FAK is a critical regulator of endothelial barrier function.3,40 c-Src kinase is a known binding partner of FAK that regulates FAK activity in many cell types.41 The high sequence homology between c-Src and Lyn led us to address the concept that Lyn could also interact with FAK and that Lyn-induced endothelial barrier enhancement might be mediated by FAK. To test this hypothesis, we first performed coimmunoprecipitation experiments to determine whether Lyn interacts with FAK. A mouse monoclonal antibody against FAK, but not control mouse IgG, coimmunoprecipitated Lyn kinase (Figure 6A). As FAK is a protein tyrosine kinase whose activity is regulated by phosphorylation of tyrosine residues, we examined the effects of PP2 or Lyn down-regulation on FAK phosphorylation to determine whether Lyn kinase regulates FAK activity. FAK was immunoprecipitated from HUVEC lysates, and tyrosine phosphorylation of FAK was detected using the anti–phospho-tyrosine monoclonal antibody 4G10 (Figure 6B). Treatment of HUVECs with PP2 significantly reduced phosphorylation of FAK. Transfection of HUVECs with Lyn siRNA, but not control siRNA, also reduced FAK phosphorylation (Figure 6C), indicating that Lyn kinase regulates FAK activity in endothelial cells.
Figure 6.
Lyn kinase regulates FAK phosphorylation and the role of FAK in regulating endothelial integrity. (A) Lyn kinase was co-immunoprecipitated with FAK. HUVECs grown in 60-mm culture dishes were lysed, and the cell extracts were incubated with anti-FAK antibody followed by incubation of protein A/G beads. Immunoprecipitates and total cell lysates were analyzed by western blotting with anti-FAK and anti-Lyn antibodies as indicated. (B) FAK phosphorylation was inhibited by PP2. HUVECs were incubated with either PP2 (10 μM) or vehicle (DMSO) for 10 and 60 min, and cell lysates were immunoprecipitated with anti-FAK antibody. Phosphorylated FAK was detected by western blotting using 4G10, an anti-phosphotyrosine antibody. The same membrane was reprobed for total FAK with an anti-FAK antibody. (C) Effect of Lyn silencing on the phosphorylation of FAK. HUVECs were transfected with control or Lyn siRNA. Forty-eight hours after transfection, cells were harvested to determine the level of phosphorylated FAK as described in B. Similar results were obtained in 3 independent experiments. (D) Knockdown of FAK by transfection of specific siRNA in HUVECs. Cells were transiently transfected with indicated concentrations of control or FAK siRNA. Forty-eight hours after transfection, cells were harvested and lysed for western blot analysis. (E) Effect of FAK silencing on TER measurement. Confluent HUVECs seeded onto microelectrodes were transfected with control or FAK siRNA as indicated. Three hours after transfection, the changes in TER across HUVEC monolayers were continuously recorded for 30 hours. Recorded values are plotted as the mean from 4 independent experiments as means ± SD. (F) Disruption of AJ in FAK siRNA transfected confluent HUVEC monolayers. HUVECs were cotransfected with control or FAK siRNA together with siGLO control siRNA as indicated. Forty-eight hours after transfection, cells were fixed and immunostained for VE-cadherin. Knockdown of FAK resulted in formation of interendothelial gaps, as pointed by the arrows. (G) Quantification of gaps and (H) cell numbers in 4 random images (mean ± SD).
Role of FAK in regulating endothelial barrier function
If Lyn functions to preserve endothelial barrier function through FAK-mediated signaling, FAK should be important for maintaining endothelial barrier integrity. Therefore, we examined whether down-regulation of FAK itself can impair endothelial barrier function. Transfection of HUVECs with siRNA targeted to FAK reduced the amount of endogenous FAK by 70% compared with control nonsilencing siRNA (Figure 6D). Similar to down-regulation of Lyn, knockdown of FAK significantly reduced TER values in HUVECs (Figure 6E). As with Lyn knock-down, down-regulation of FAK produced discontinuities in VE-cadherin distribution accompanied by the formation of intercellular gaps (Figure 6F-G). The number of cells per image was not affected by down-regulation of FAK (Figure 6H), suggesting that FAK is not required for cell growth.
Identification of Lyn-dependent phosphorylation sites in FAK
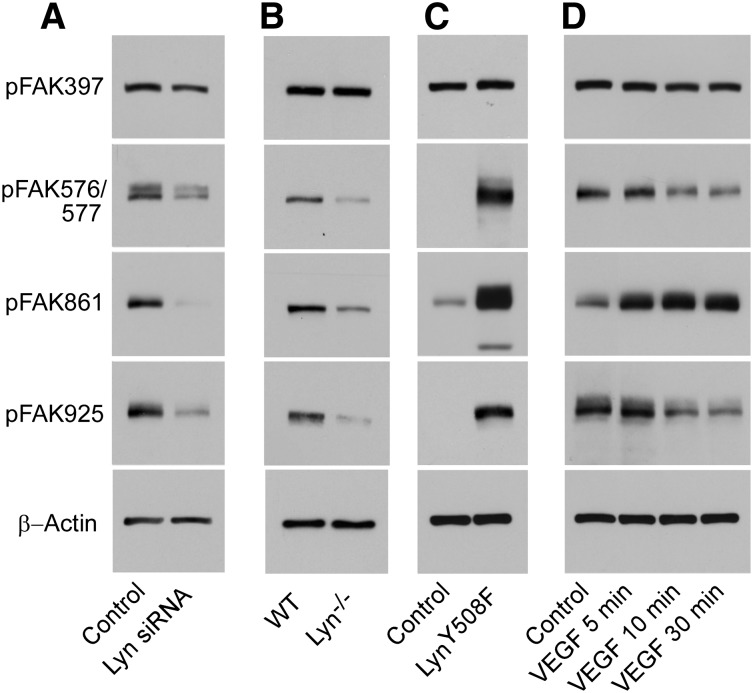
To identify Lyn kinase–dependent phosphorylation sites in FAK, HUVECs were transfected with Lyn siRNA and FAK phosphorylation was analyzed by western blot using rabbit polyclonal phospho-specific antibodies against FAK. Phosphorylation of FAK at residues Tyr576/577, Tyr861, and Tyr925 was reduced by down-regulation of Lyn kinase (Figure 7A). Phosphorylation of FAK at residue Tyr397 was not affected. Consistently, phosphorylation of FAK at residues Tyr576/577, Tyr861, and Tyr925 was reduced in mouse lung microvascular endothelial cells from Lyn−/− mice compared with that of wild-type mice (Figure 7B). Furthermore, phosphorylation of FAK at residues Tyr576/577, Tyr861, and Tyr925, but not Tyr397, in HUVECs infected with a virus containing the constitutively active mutant of Lyn kinase, LynY508F, was increased vs HUVECs infected with a null virus (Figure 7C). To avoid overexposure of the bands from the cells expressing LynY508F, figures were selected with a short exposure time, under which condition the basal FAK phosphorylation was not obvious. In contrast, stimulation of HUVECs with VEGF increased phosphorylation of FAK only at residue Tyr861, in agreement with previous reports.42 FAK phosphorylation at residues Tyr576/577 and Tyr925 was even decreased by VEGF stimulation (Figure 7D). Thus, Lyn-dependent phosphorylation of FAK at residues Tyr576/577 and Tyr925 may be critical for stabilizing the endothelial barrier function.
Figure 7.
Lyn kinase regulates FAK phosphorylation. (A) Effect of Lyn silencing on FAK phosphorylation. HUVECs were transfected with control siRNA or Lyn siRNA. Forty-eight hour after transfection, cells were solubilized, and phosphorylation of FAK was detected by western blotting with rabbit polyclonal antibodies specifically recognizing the phosphorylated FAK residues Tyr397, Tyr576/577, Tyr861, or Tyr925. A mouse monoclonal antibody against β-actin was used to verify equal loading. Images are representative of 3 experiments. (B) Lung microvascular endothelial cells isolated from Lyn−/− and wild-type littermates were cultured and solubilized in SDS sample buffer. Phosphorylation of FAK was detected by western blotting as described above. (C) Effect of expression of constitutively active mutant of Lyn on the phosphorylation of FAK. HUVECs were transfected with a recombinant adenovirus encoding a constitutively active mutant of Lyn kinase (LynY508F) or null adenovirus (Control) and FAK phosphorylation was analyzed. Twenty-four hours after infection, cells were solubilized, and phosphorylation of FAK was detected by western blotting. (D) Stimulation of FAK phosphorylation by VEGF. HUVECs were incubated with VEGF (200 ng/mL) for various lengths of time. Cells were then solubilized and phosphorylation of FAK was detected by western blotting.
Discussion
Mounting evidence indicates that SFKs are key players in regulating endothelial barrier function and promoting pathologic increases in vascular permeability as observed in acute lung injury.5-7,28 Therefore, pharmacologic inactivation of SFKs becomes a potential therapeutic target for preserving the integrity of endothelial barrier, thereby alleviating vascular dysfunction and edema formation.3 The present study demonstrated for the first time to our knowledge an essential and potentially important role of Lyn in strengthening barrier function, thus opposing the permeability-increasing effects of other SFK members. An interesting study reported that expression of constitutively active c-Src mutant resulted in increased endothelial permeability, whereas activation of SFKs by knockdown of Csk (a negative regulator of SFKs) or expression of dominant negative Csk failed to decrease barrier function.43 As Csk regulates all SFK members including Lyn, the discrepant effects of c-Src and DN-Csk on endothelial permeability are explained by our findings that increased endothelial permeability by activation of Src is counteracted by activation of Lyn in the Csk down-regulated cells. Taken together, our results suggest that SFKs have distinct even opposing actions in regulating endothelial permeability even though they share high structural homology.
As Src signaling is critical for endothelial permeability regulation, oncogenic, and invasive processes, efforts have been devoted to translating Src inhibitors into therapy for systemic inflammation conditions.3 Dasatinib, the most clinically studied Src inhibitor in this regard, potently inhibits Src and other SFKs, such as Lyn, Yes, and Fyn. Peripheral edema is a common adverse event associated with the use of dasatinib.44,45 Pleural effusion, the most important and limiting toxicity during dasatinib treatment, often requires treatment discontinuation that impairs the otherwise high efficacy of the drug.44-47 A very recent study reported that 52 of 172 patients with chronic myeloid leukemia developed pleural effusion during treatment with dasatinib.48 However, the molecular mechanism by which administration of dasatinib causes edema and pleural effusion is unknown. Our results showing that dasatinib treatment impaired endothelial barrier integrity due to Lyn inhibition may provide a possible explanation behind dasatinib-related edema and pleural effusion. Thus, development of isoform-specific Src inhibitors should take into account distinct and varied effects of different Src kinases on endothelial permeability.
Our data indicate that Lyn−/− mice have greater increases in lung endothelial permeability in response to LPS or VEGF challenge, which is consistent with the effect of Lyn in maintaining basal barrier integrity. Increased lung endothelial permeability in Lyn−/− mice is not due to increases in endothelial apoptosis or necrosis, because lung microvascular endothelial cells from Lyn−/− mice have similar rates of the apoptosis and necrosis as the lung microvascular endothelial cells from wild-type mice (supplemental Figure 2). We show that Lyn−/− mice repopulated with wild-type mouse bone marrow–derived cells have higher lung permeability than wild-type mice, further demonstrating the important role of endothelial Lyn in the maintenance of basal barrier integrity. Our results indicate that knockdown of Lyn in HUVECs had no effect on LPS-induced TER decline (Figure 5). Consistently, it has been recently reported that, distinct from c-Src, Fyn, and Yes, Lyn is neither activated nor contributes to disrupted barrier integrity by LPS stimulation.28 These data together suggest that Lyn is not required for LPS-mediated dysfunction of endothelial barrier and that Lyn protects from endothelial barrier function is due to its effect in maintaining basal barrier integrity. We further demonstrated that expression of a constitutively active Lyn mutant promoted basal barrier function and counteracted VEGF-induced endothelial hyperpermeability (Figure 3E). Thus, activation of Lyn in endothelial cells may represent an important means of strengthening the endothelial junction, thereby having potential anti-inflammatory effects.
The present study identifies a possible mechanism whereby Lyn strengthens the endothelial barrier. FAK, a well-known SFK substrate, is critical for recovery of endothelial barrier function and in promoting barrier function.49,50 Our data indicate that FAK forms a complex with Lyn in ECs, and down-regulation of FAK causes VE-cadherin discontinuities at endothelial borders resulting in formation of intercellular gaps, demonstrating a critical role of FAK in maintaining basal endothelial barrier integrity. These findings are consistent with a recent report showing that the kinase activity of FAK was required for endothelial barrier maintenance.50 As a tyrosine kinase, FAK activity is dependent on tyrosine phosphorylation sites.18 With the exception of Tyr397 that is a major autophosphorylation site, FAK phosphorylation at Tyr576, 577, 861, and 925 is mediated by SFKs.18,51 We are the first to demonstrate that FAK phosphorylation at tyrosine residues 576/577 and 925 is facilitated by Lyn (Figure 7). In contrast, FAK phosphorylation at tyrosine residues 576/577 and 925 was inhibited by VEGF stimulation. These results suggest that the role of Lyn in strengthening barrier function might be mediated by phosphorylating FAK tyrosine residues 576/577 and 925. In addition, Lyn stimulated FAK phosphorylation on tyrosine 861, which was known to be a major phosphorylation site for SFKs and involved in Src signaling pathways mediating VEGF-induced endothelial migration and antiapoptosis.28,51,52 Whether Tyr861 phosphorylation of FAK by SFKs is associated with endothelial permeability remains unknown.
In summary, we uncovered a novel role of Lyn in enhancing endothelial barrier function. Unlike other members of SFKs in endothelial cells, Lyn plays a critical role in maintenance of endothelial integrity and strengthening endothelial barrier function. This specialized function of Lyn is mediated through phosphorylation of FAK at tyrosine residues 576/577 and 925. Lyn therefore represents an attractive target for the development of novel therapeutics for inflammatory diseases.
Supplementary Material
Acknowledgments
The authors thank Drs Alan Daugherty and Susan S. Smyth for critical reading of the manuscript and Dr Richard Charnigo for assistance with statistical analysis.
This work was supported by American Heart Association Midwest Affiliate grant-in-aid 0855698G (to Z.L.) and in part by the American Society of Hematology bridge grant award (to Z.L.) and the Center of Biomedical Research Excellence in Obesity and Cardiovascular Disease grant P20RR021954 from the National Institutes of Health/National Center for Research Resources.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.H. designed research, performed experiments, analyzed data, and wrote the paper; G.Z. performed important experiments and analyzed data; E.J.W., Y.L., and J.F. performed important experiments; S.M.V. was involved in mouse lung perfusion assays; C.A.L. provided Lyn knockout mice; X.D. and D.A.C. were involved in analysis of results; A.B.M. was involved in research design, analyzed data and wrote the paper; and Z.L. designed research, performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.H. is Vascular Biology Section, Department of Medicine, Boston University, Boston, MA.
Correspondence: Zhenyu Li, Division of Cardiovascular Medicine, Saha Cardiovascular Center, University of Kentucky, 741 South Limestone St, Lexington, KY 40536-0200; e-mail: zhenyuli08@uky.edu.
References
- 1.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87(2):300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 2.Kim MP, Park SI, Kopetz S, Gallick GE. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res. 2009;335(1):249–259. doi: 10.1007/s00441-008-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2(6):467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 5.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 6.Paul R, Zhang ZG, Eliceiri BP, et al. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7(2):222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 7.Weis S, Shintani S, Weber A, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113(6):885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src). Am J Physiol. 1999;276(6 Pt 1):L989–L998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- 9.Shi S, Garcia JG, Roy S, Parinandi NL, Natarajan V. Involvement of c-Src in diperoxovanadate-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L441–L451. doi: 10.1152/ajplung.2000.279.3.L441. [DOI] [PubMed] [Google Scholar]
- 10.Tiruppathi C, Naqvi T, Sandoval R, Mehta D, Malik AB. Synergistic effects of tumor necrosis factor-alpha and thrombin in increasing endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2001;281(4):L958–L968. doi: 10.1152/ajplung.2001.281.4.L958. [DOI] [PubMed] [Google Scholar]
- 11.Lambeng N, Wallez Y, Rampon C, et al. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 2005;96(3):384–391. doi: 10.1161/01.RES.0000156652.99586.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery. 2002;132(2):180–185. doi: 10.1067/msy.2002.125305. [DOI] [PubMed] [Google Scholar]
- 13.Wallez Y, Cand F, Cruzalegui F, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26(7):1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 14.Shikata Y, Birukov KG, Birukova AA, Verin A, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17(15):2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Kayyali US, Sousa AM, Rajan T, Lechleider RJ, Day RM. Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src. Am J Respir Cell Mol Biol. 2007;37(4):485–493. doi: 10.1165/rcmb.2006-0439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res. 2012;83(1):31–44. doi: 10.1016/j.mvr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16(10):5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15(2):954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7(1):69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 20.Vogel SM, Gao X, Mehta D, et al. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics. 2000;4(2):137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Liu G, Profirovic J, Niu J, Voyno-Yasenetskaya T. Zyxin is involved in thrombin signaling via interaction with PAR-1 receptor. FASEB J. 2009;23(12):4193–4206. doi: 10.1096/fj.09-131862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang B, Zhang G, Stefanini L, et al. The Src family kinases and protein kinase C synergize to mediate Gq-dependent platelet activation. J Biol Chem. 2012;287(49):41277–41287. doi: 10.1074/jbc.M112.393124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 24.Tumurkhuu G, Koide N, Dagvadorj J, et al. The mechanism of development of acute lung injury in lethal endotoxic shock using alpha-galactosylceramide sensitization. Clin Exp Immunol. 2008;152(1):182–191. doi: 10.1111/j.1365-2249.2008.03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119(10):2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severgnini M, Takahashi S, Tu P, et al. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am J Respir Crit Care Med. 2005;171(8):858–867. doi: 10.1164/rccm.200407-981OC. [DOI] [PubMed] [Google Scholar]
- 27.Lee HS, Moon C, Lee HW, Park EM, Cho MS, Kang JL. Src tyrosine kinases mediate activations of NF-kappaB and integrin signal during lipopolysaccharide-induced acute lung injury. J Immunol. 2007;179(10):7001–7011. doi: 10.4049/jimmunol.179.10.7001. [DOI] [PubMed] [Google Scholar]
- 28.Gong P, Angelini DJ, Yang S, et al. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283(19):13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanová I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268(28):20725–20728. [PubMed] [Google Scholar]
- 30.Gao X, Kouklis P, Xu N, et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1218–L1225. doi: 10.1152/ajplung.2000.279.6.L1218. [DOI] [PubMed] [Google Scholar]
- 31.Zhao YY, Gao XP, Zhao YD, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116(9):2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira S, Lowell C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J Immunol. 2003;171(3):1319–1327. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 33.Beavitt SJ, Harder KW, Kemp JM, et al. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J Immunol. 2005;175(3):1867–1875. doi: 10.4049/jimmunol.175.3.1867. [DOI] [PubMed] [Google Scholar]
- 34.Charles N, Watford WT, Ramos HL, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30(4):533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindbom L. Regulation of vascular permeability by neutrophils in acute inflammation. Chem Immunol Allergy. 2003;83:146–166. doi: 10.1159/000071559. [DOI] [PubMed] [Google Scholar]
- 36.Yamanashi Y, Mori S, Yoshida M, et al. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotropic virus type I. Proc Natl Acad Sci USA. 1989;86(17):6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achen MG, Clauss M, Schnürch H, Risau W. The non-receptor tyrosine kinase Lyn is localised in the developing murine blood-brain barrier. Differentiation. 1995;59(1):15–24. doi: 10.1046/j.1432-0436.1995.5910015.x. [DOI] [PubMed] [Google Scholar]
- 38.Chang R, Chicoine LG, Cui H, et al. Cytokine-induced arginase activity in pulmonary endothelial cells is dependent on Src family tyrosine kinase activity. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L688–L697. doi: 10.1152/ajplung.00504.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harder KW, Parsons LM, Armes J, et al. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15(4):603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 40.Grinnell KL, Harrington EO. Interplay between FAK, PKCδ, and p190RhoGAP in the regulation of endothelial barrier function. Microvasc Res. 2012;83(1):12–21. doi: 10.1016/j.mvr.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5(4):413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliceiri BP, Puente XS, Hood JD, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem. 2010;285(10):7045–7055. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109(12):5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 45.Gnoni A, Marech I, Silvestris N, Vacca A, Lorusso V. Dasatinib: an anti-tumour agent via Src inhibition. Curr Drug Targets. 2011;12(4):563–578. doi: 10.2174/138945011794751591. [DOI] [PubMed] [Google Scholar]
- 46.Masiello D, Gorospe G, III, Yang AS. The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on dasatinib. J Hematol Oncol. 2009;2:46. doi: 10.1186/1756-8722-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE. Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer. 2010;116(2):377–386. doi: 10.1002/cncr.24734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latagliata R, Breccia M, Fava C, et al. Incidence, risk factors and management of pleural effusions during dasatinib treatment in unselected elderly patients with chronic myelogenous leukaemia. Hematol Oncol. 2013;31(2):363–369. doi: 10.1002/hon.2020. [DOI] [PubMed] [Google Scholar]
- 49.Knezevic N, Tauseef M, Thennes T, Mehta D. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med. 2009;206(12):2761–2777. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Peng X, Sun S, Park AY, Guan JL. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 2010;189(6):955–965. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228(3):662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360(Pt 1):255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.