Abstract
Antimicrobial activities were detected in the haemolymph of the spider Acanthoscurrria rondoniae. A novel antifungal peptide, rondonin, was purified by reverse phase high performance liquid chromatography (RP-HPLC). Rondonin has an amino acid sequence of IIIQYEGHKH and a molecular mass of 1236.776 Da. This peptide has identity to a C-terminal fragment of the “d” subunit of haemocyanin from the spiders Eurypelma californicum and Acanthoscurria gomesiana. A synthetic peptide mimicking rondonin had identical characteristics to those of the isolated material, confirming its sequence. The synthetic peptide was active only against fungus. These data led us to conclude that the antifungal activity detected in the plasma of these spiders is the result of enzymatic processing of a protein that delivers oxygen in the haemolymph of many chelicerate. Several studies have suggested that haemocyanins are involved in the arthropod immune system, and the activity of this haemocyanin fragment reinforces this idea.
Keywords: Antimicrobial peptide, Haemocyanin fragment, Rondonin, Acanthoscurria rondoniae, Spider
1. Introduction
Invertebrate animals can be found in almost every habitat in the world. Because many invertebrates live in environments in which microorganisms thrive, their widespread distribution and survival are primarily due to successful defences that efficiently recognise and combat potentially harmful microorganisms [37]. Invertebrates only possess innate immunity, which is considered to be an ancient defence mechanism [16].
One characteristic of innate immunity is the production of antimicrobial substances, which are often peptides or polypeptides [12]. Several of these antimicrobial peptides (AMPs) have been recognised as important components of the nonspecific host defence or innate immune system in a variety of organisms and have been isolated and characterised from plants and animals, including insects, molluscs, crustaceans, amphibians, birds, fish, mammals, and humans [2,17,3,11]. Several antimicrobial peptides were isolated from the venom and haemolymph of venomous arthropods such as scorpions and spiders [22]. The haemolymph of invertebrates are the main source of antimicrobial peptides [14]. The first biochemical study of an antimicrobial peptide in arachnids demonstrated the presence of an antibacterial peptide in the haemolymph of the scorpion species Leiurus quinquestriatus [5] and Androctonus australis [8]. Gomesin was the first antimicrobial peptide isolated from spider blood cells [34]. Other antimicrobial peptides were found in the plasma of shrimp [6], freshwater crayfish [24], the plasma of the tarantula spider Acanthoscurria gomesiana, named theraphosinin [34], and crude haemolymph of Agelena labyrinthica [46]. These peptides are typically relatively short, positively charged (cationic), and amphiphilic [19,40] and generally interact with the outer membranes of microorganisms due to their negative charge [20]. Compared to cationic AMPs, much less is known about how anionic AMPs work [39,36,13].
While most AMPs are derived from a biologically inactive proprotein that is processed into an active state, some AMPs are derived from larger, functional proteins, such as haemoglobin [10,23,25,41] and haemocyanin [6,24]. Arthropod haemocyanins are composed of heterogeneous subunits in the 75-kDa range that combine to form either a regular cubic single hexamer (1×6) or multiple hexamers (2–8×6) depending upon the species and physiological conditions [29]. The haemocyanin of the North American tarantula Eurypelma californicum is a native 24-mer protein complex consisting of two identical dodecamers with an estimated total molecular mass of approximately 1800 kDa [33,26,27,29]. Formation of the 24-mer complex requires the aggregation of seven different subunits in a constant stoichiometric amount with four copies of each of the subunits a, d, e, f, and g and two copies of subunits b and c [28,29]. Since antimicrobial peptides have been characterised in haemocytes of A. gomesiana and Acanthoscurria rondoniae which belongs to the same genera, we choose this species to look for the presence of these peptides. So, in the present study, we report the first isolation and characterisation of an antifungal fragment of haemocyanin from arachnids.
2. Experimental procedures
2.1. Microorganisms
Fungal and bacterial strains were obtained from various sources. Escherichia coli SBS363 and Micrococcus luteus A270 were from the Pasteur Institut, Paris; Candida albicans (MDM8) was from the Department of Microbiology from the University of São Paulo, Brazil; and E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 (Strain Boston 41501), Staphylococcus aureus ATCC 29213 and S. epidermides ATCC 12228 were from the American Type Culture Collection (ATCC). The following human clinical yeast isolates, which can be agents of candidiasis disease, obtained from the Oswaldo Cruz Institute, Brazil, were also used: Trichosporon sp. IOC 4569, Candida krusei IOC 4559, C. glabrata IOC 4565, C. albicans IOC 4558, C. parapsilosis IOC 4564, C. tropicalis IOC 4560 and C. guilliermondii IOC 4557. The filamentous fungi Aspergilus niger, Cladosporium sp. and Penicilium expansum and Beauveria bassiana, an entomopathogenic fungus, were isolated from a mummified spider.
2.2. Spider blood
The spiders (Acanthoscurria rondoniae, a tarantula of the Theraphosidae family) were kept alive in the biotherium of the Special Laboratory of Toxinology Applied of the Institute Butantan (São Paulo, Brazil) (Fig. 1). These animals were collected under Licence Permanent Zoological Material no. 11024-3-IBAMA and Special Authorisation for Access to Genetic Patrimony no. 001/2008. The haemolymph (approximately 10 mL) from animals of either sex at different stages of development was collected by cardiac puncture with an apyrogenic syringe. To avoid haemocyte degranulation and coagulation, the haemolymph was collected in the presence of sodium citrate buffer (0.14 M NaCl, 0.1 M glucose, 30 mM trisodium citrate, 26 mM citric acid, 10 mM EDTA, pH 4.6 (2:1, v/v)) [38]. The plasma was separated, and the blood cells were removed by centrifugation at 800g for 10 min at 4 °C.
Fig. 1.

Specimen of the spider Acanthoscurria rondoniae (Theraphosidae, Mygalomorphae).
2.3. Antimicrobial activity assays
During the purification procedure, the antimicrobial activities of the samples were monitored by a liquid growth inhibition assay using the Gram-negative bacteria Escherichia coli SBS363, Gram-positive bacteria Micrococcus luteus A270 that were cultured in poor broth nutrient medium (PB: 1.0 g peptone in 100 ml of water containing 86 mM NaCl at pH 7.4; 217 mOsM), whereas yeast strain Candida albicans MDM8 was cultured in poor dextrose broth (1/2 PDB:1.2 g potato dextrose in 100 ml of H2O at pH 5.0; 79 mOsM). Determination of antimicrobial peptide was performed using 5-fold microtiter broth dilution assay in 96-well sterile plates at a final volume of 100 μL. Mid-log phase culture were diluted to a final concentration of 1×105 colony forming units/mL. Dried fractions were dissolved in 200 μL of water ultrapure and 20 μL applied into each well and added to 80 μL of the bacterium/yeast dilution. The fractions were tested in triplicate. The microtiter plates were incubated for 18 h at 30 °C; growth inhibition was determined by measuring absorbance at 595 nm.
2.4. Purification of rondonin from plasma
For purification of antimicrobial peptides, the plasma was homogenised and then trifluoracetic acid was added to a final concentration of 0.05%. The sample was agitated on ice for 30 min and centrifuged at 16,000g at 4 °C. The acidic supernatant was loaded onto classic Sep-Pak C18 cartridges equilibrated in acidified water (TFA 0.05%). After washing with acidified water, three stepwise elutions were successively performed with 5%, 40% and 80% acetonitrile (ACN/TFA 0.05%). The 40% Sep-Pak fraction was concentrated in a vacuum centrifuge and reconstituted in Milli-Q water (Millipore™) and directly subjected to C18 reverse-phase on a semi-preparative Jupiter C18 column equilibrated at room temperature with 0.05% trifluoracetic acid in water. The sample was purified using acetonitrile/water/0.05% trifluoracetic acid gradients of 2–60% acetonitrile in 60 min at a flow rate 1.5 mL/min. Ultraviolet absorbance was monitored at 225 nm. The eluted peaks fractions were collected by hand and were vacuum dried (Speed-Vac Savant) and used for assay of antimicrobial activity and determination of amino acid sequence.
2.5. Rondonin mass analysis by MALDI-TOF and LC/MS
Briefly, 0.35 μL of sample in Milli-Q water was mixed with 0.35 μL of saturated matrix α-cyano-4-hydroxycinnamic acid solution deposited onto the sample slide and dried on the bench. The analysis was performed with the spectrometer operating in positive mode, which detects positively charged ions. To determine the amino acid sequence of rondonin, the doubly charged ions were subjected to “de novo” sequencing in a Q-TOF Ultima API (Micromass) spectrometer operating in positive ionisation mode. The spectrum was analysed, and the “y” and “b” fragments were used to elucidate the primary structure of the molecule.
2.6. Peptide synthesis of rondonin
Synthetic rondonin was synthesised by solid phase peptide synthesis using the Fmoc procedure [1]. The peptide was purified by reverse phase (Shim-pack Prep-ODS, 5 μ, 20 mm×250 mm Shimadzu Co.) semi-preparative HPLC, and the purity and identity of the peptide were confirmed by MALDI-TOF mass spectrometry and analytical HPLC using the conditions described above.
2.7. Minimal inhibitory concentration (MIC) of rondonin
The minimal inhibitory concentration was determined using the synthetic peptide against the Gram-negative bacterial strains, the Gram-positive bacterial strains, the fungal strains and the yeast strains, as described above (experimental procedures 2.1 and 2.3). The peptide was dissolved in sterile Milli-Q water at a final concentration of 670 μM. Determination of minimal inhibitory concentrations (MICs) for rondonin was performed using a 5-fold microtiter broth dilution assay of stock solution (670 μM) and serial dilution in 96-well sterile plates at a final volume of 100 μL where 20 μL of stock solution was applied into each well at serial dilution 2-fold microtiter broth dilution and added to 80 μL of the bacterium/yeast dilution. Microbial growth was measured by monitoring the increase in OD at 595 nm after incubation at 30 °C for 18 h (modified [8]). The rondonin was tested in duplicate. The MIC is defined as the minimal concentration of peptide that caused 100% growth inhibitions [47].
2.8. Antifungal assay of rondonin
The antifungal assay was performed using a 5-fold microtiter broth dilution assay and serial dilution in 96-well sterile plates at a final volume of 100 μL where 20 μL of stock solution (670 μM) was applied into each well at serial dilution 2-fold microtiter broth dilution and added to 80 μL of the yeast dilution. The inhibition growth curve of rondonin was determined by incubating twice the concentration of the MIC (67 μM) of rondonin with C. albicans MDM8 at 30 °C for various amounts of time (0, 10 min, 1 h, 3 h, 5 h, 8 h, 10 h, 12 h, 18 h, and 24 h) and counting the number of conidia present; the viability of the yeast was verified by incubating the colonies on a nutrient agar plate (1.5%). The rondonin was tested in triplicate.
2.9. Haemolytic assay to determine rondonin toxicity
Human erythrocytes from a healthy donor were collected in 0.15 M citrate buffer, pH 7.4, and washed three times by centrifugation (700g, 10 mins, 4 °C) with 0.15 M phosphate-buffered saline (PBS), pH 7.4. After the final centrifugation, the erythrocytes were suspended in PBS, pH 7.4. Aliquots of 50 μL containing rondonin at concentrations ranging from 0.2 to 134 μM were added to 50 μl of a 3% suspension of erythrocytes in the wells of U-shaped bottom plates and incubated for 3 h at 37 °C. The supernatant was first collected and haemolysis was determined by measuring the absorbance at 414 nm of each well in a Victor3 (1420 Multilabel Counter/Victor3, Perkin Elmer). The haemolysis percentage was expressed in relation to a 100% lysis control (erythrocytes incubated with 0.1% triton X-100); PBS was used as a negative control. The rondonin was tested in triplicate.
3. Results
3.1. Antimicrobial activity of rondonin measured by liquid growth inhibition
Antimicrobial activity of synthetic rondonin was screened using 3 species of Gram-positive bacteria, 3 species of Gram-negative bacteria, 7 species of yeast and 2 species of fungi. Rondonin was tested against these organisms at concentrations ranging from 0.1 to 67 μM and demonstrated antimicrobial activity against all 7 species of yeast and one species of filamentous fungus (Table 1).
Table 1.
Antimicrobial activity spectrum of synthetic rondonin compared to gomesin. The minimal inhibitory concentrations (MICs) of the synthetic peptides were determined by liquid growth inhibition. MICs are expressed as the (a) and (b) interval of concentrations where (a) is the highest concentration tested at which the microorganisms are growing and (b) is the lowest concentration that causes 100% growth inhibition.
| Minimal inhibitory concentration (μM) |
||||||
|---|---|---|---|---|---|---|
| Microorganisms | Rondonin | Gomesin | ||||
| Gram-positive bacteria | ||||||
| Micrococcus luteus A270 | ND | 0.4 | – | 0.8a | ||
| Staphylococcus aureus ATCC 29213 | ND | 1.2 | – | 2.5b | ||
| Staphylococcus epidermides ATCC 12228 | ND | NA | ||||
| Gram-negative bacteria | ||||||
| Escherichia coli SBS363 | ND | 0.4 | – | 0.8a | ||
| Escherichia coli ATCC 25922 | ND | 0.2 | – | 0.9b | ||
| Pseudomonas aeruginosa ATCC 27853 (Strain Boston 41501) | ND | NA | ||||
| Fungi | ||||||
| Beauveria bassiana | ND | NA | ||||
| Trichosporon sp IOC 4569 | 1.1 | – | 2.1 | 0.6 | – | 1.2b |
| Yeasts | ||||||
| Candida albicans MDM8 | 16.75 | – | 33.5 | 0.15 | – | 0.3a |
| Candida krusei IOC 4559 | 16.75 | – | 33.5 | 5.0 | – | 10.0b |
| Candida glabrata IOC 4565 | 8.37 | – | 16.5 | 12.5 | – | 25 |
| Candida albicans IOC 4558 | 8.37 | – | 16.5 | 5.0 | – | 10.0b |
| Candida parapsilosis IOC 4564 | 16.75 | – | 33.5 | 2.5 | – | 5.0b |
| Candida tropicalis IOC 4560 | 8.75 | – | 16.5 | 0.3 | – | 0.6b |
| Candida guilliermondii IOC 4557 | 16.75 | – | 33.5 | 2.5 | – | 5.0b |
3.2. Purification of rondonin from plasma
The plasma of the spider A. rondoniae from the Theraphosidae family was collected (10 mL) and dissolved in acidified Milli-Q water as previously described. The supernatant obtained by centrifugation was applied to a Sep-Pak C18 column and subjected to three successive extractions of increasing concentrations of acetonitrile (5%, 40% and 80% ACN) to pre-purify antimicrobial peptides. The material eluted at 40% ACN was subjected to fractionation by RP-HPLC, which resulted in fractions with antimicrobial activity (Fig. 2). All fractions were analysed in the liquid growth inhibition assay using M. luteus, E. coli, and C. albicans. We found six fractions that showed antimicrobial activity only against C. albicans: 2, 3, 7, 11, 12, and 13. Only the fraction 2, named rondonin, was purified to homogeneity.
Fig. 2.
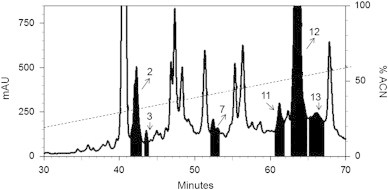
Purification of rondonin from spider plasma by reverse phase HPLC. An acidic extract obtained from A. rondoniae plasma was submitted to solid phase extraction on Sep-Pak C18 cartridges. The fraction that eluted at 40% acetonitrile was analysed on a semi-preparative Jupiter C18 column with a linear gradient from 2% to 60% acetonitrile in acidified water over 60 min (dotted line) at a flow rate of 1.5 mL/min.
3.3. Minimal inhibitory concentration
The MIC of synthetic rondonin was tested against Gram-negative bacterial strains, Gram-positive bacterial strains, fungal strains and yeast strains. Synthetic rondonin showed activity against all yeasts tested and one fungus. However, no activity could be detected against Gram-positive and Gram-negative bacteria and the fungus Aspergilus niger, Cladosporium sp., Penicilium expansum and B. bassiana strains tested in the range of concentration investigated (above 67 μM). MICs are expressed as the (a) and (b) interval of concentrations where (a) is the highest concentration tested at which the microorganisms are growing and (b) is the lowest concentration that causes 100% growth inhibition. We compare our results with the synthetic gomesin performed by Silva et al. [35] and Yamane [44]. As shown in Table 1, rondonin could be a specific antifungal activity against yeasts.
3.4. MALDI-TOF and LC/MS analysis
Analysis by mass spectrometry MALDI-TOF revealed a single molecule with a mass of 1236.776 Da (Fig. 3). Following the methodology of Budnik et al. [4], “de novo” sequencing (Fig. 4) of this molecule revealed a sequence of 10 amino acids, IIIQYEGHKH (Fig. 5), that showed identity to the C-terminus fragment of the subunit “d” of haemocyanin from the tarantula Eurypelma californicum (Theraphosidae) [42]. Furthermore, when compared to a database of partial genomes, rondonin showed identity with the C-terminus fragment of subunit “d” and 90% similarity to a fragment (ILIQYEGHKH) of subunit “f” of haemocyanin from the spider A. gomesiana (http://www.compsysbio.org/partigene/). Therefore, in the present study, we report the first isolation and characterisation of a fragment of haemocyanin with antifungal activity from arachnids.
Fig. 3.
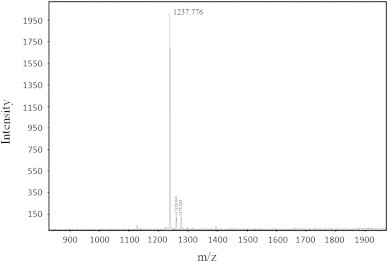
MALDI-TOF spectrum of fraction 2 (rondonin). Analysis of fraction 2 by mass spectrometry showed a single molecule with an m/z of 1237.776.
Fig. 4.
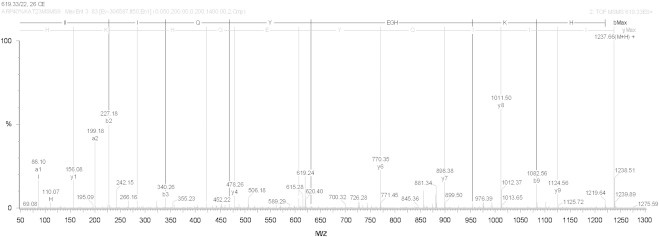
Representative “de novo” sequencing of fraction 2 fromA. rondoniaeplasma in a Q-TOF Micro ™ (Micromass). The fragments shown correspond to the b and y series. The peptide sequence following the b and y series orientation is shown on the top of the graph. Mass spectrometric “de novo” peptide sequencing was performed in positive ionisation mode on a Q-TOF Micro™ fitted with an electrospray ion source.
Fig. 5.

Eurypelma californicum(Theraphosidae) haemocyanin subunits (a–g). The peptide showed identity with the C-terminal fragment from subunit “d” [42].
3.5. Microbicidal activity of rondonin
The microbicidal properties of rondonin were determined by the Neubauer chamber and plate count method. When synthetic rondonin was incubated with C. albicans at a concentration two times higher than the respective MIC, no living yeast were detected after 10 min (data not shown). Fig. 6 shows that rondonin inhibits the growth of Candida albicans MDM8 compared to the control.
Fig. 6.
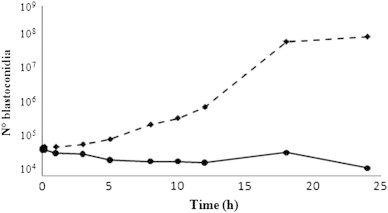
Growth inhibition ofCandida albicansMDM8 by rondonin. Synthetic rondonin (67 μM, solid line) or water (control, dotted line) was added to an exponential phase culture of C. albicans. Aliquots were removed at various times, and the number of blastoconidia were counted in a Newbauer chamber. To test the viability of blastoconidia, 20 μL aliquots for each time point were incubated on Luria Bertani agar plates for 48 h at 30 °C.
3.6. Toxicity to human red blood cells
Haemolytic assays were used to assess the toxicity of peptides towards HRBCs in vitro. Incubating HRBCs (w/v) with various concentrations of rondonin for 3 h at 37 °C did not affect the OD at 414 nm. Triton X-100 was used as a positive control and taken as the 100% haemolysis value. PBS was used as a negative control and taken as the 0% haemolysis value. These results demonstrate that rondonin is not haemolytic (Fig. 7).
Fig. 7.
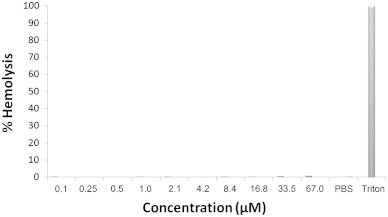
Toxicity of rondonin with HRBCs. Synthetic rondonin was incubated with human erythrocytes ranging from 0.2 to 134 μM for 3 h at 37 °C. The haemolysis percentage was expressed in relation to 0% haemolysis with PBS and 100% lysis control (0.1% triton X-100).
4. Discussion
Considering the studies of the immune system of invertebrates, the knowledge of the mechanisms involved in the immune response of arachnids is less studied than Limulus polyphemus [18]. The life expectancy of many invertebrates is as long as the lifespan of vertebrates, despite the continuous challenge of pathogens. All multicellular animals are subject to frequent microbial challenges and the attack of endo- and ectoparasites. In addition to defences against predators, survival depends on the presence of an efficient immune system that can quickly remove or inactivate pathogenic organisms. The immune system of the tarantula spider is particularly interesting because, in addition to having a life expectancy of more than 20 years [9], tarantula spiders are also phylogenetically very old with fossil records dating from the Devonian period (400 million years ago) [32,31].
In this work, we purified six molecules with antimicrobial activity that have never been before described from the plasma of the tarantula spider A. rondoniae. Only one molecule, rondonin, which was named in honour of the studied species, was isolated and characterised. Rondonin, a peptide characterised with antifungal activity that inhibits the growth of C. albicans MDM8 in ten minutes and exhibited fungicidal activity, like a study previous with some antifungal agents like amphotericin B, fluconazole and LY303366 showed that fluconazole and LY303366 are fungistatic and amphotericin B exhibited fungicidal activity with a reduction in ≥3 log10 compared to the starting inoculum [21]. Furthermore, rondonin showed no haemolytic activity, had identity with the C-terminal fragment of subunit “d” of haemocyanin from the tarantula Eurypelma californicum and A. gomesiana and showed 90% similarity with a fragment of subunit “f” of A. gomesiana, differing in only the second amino acid: ILIQYEGHKH. Lee et al. [24] also found a fragment of haemocyanin, named astacidin 1, in the plasma of the crayfish Pacifastacus leniusculus, which consists of 16 amino acids with the sequence FKVQNQHGQVVKIFHH-COOH, has a molecular mass of 1945.2 Da, and possesses antimicrobial activity.
Destoumieux-Garzon et al. [6] showed that the plasma of shrimp contains an original class of strictly antifungal (poly)peptides with molecular masses ranging from 2753.2 Da for a peptide from Penaeus vannamei, which consists of 23 amino acids with the sequence FEDLPNFGHIQVKVFNHGEHIHH, 7982.4 Da for a peptide of 31 amino acids from P. stylirostris (VTDGDADSAVPNLHHENTEYNHYGSHGVYPDK) and 8362.8 Da for a 32 amino acid peptide, also from P. stylirostris (LVVAVTDGDADSAVPNLHENTEYNHYGSHGVY). The two peptides from P. stylirostris revealed perfect homology with the C-terminus of the haemocyanin of Penaeus.
In this case, the authors speculated that the penaeid shrimp can use haemocyanin, which is abundant and readily available in the plasma, to produce C-terminal fragments that possess broad antifungal activities within the first hours of an infection. Therefore, haemocyanin has a potential function in crustacean immunity by serving as a substrate for the generation of antifungal (poly)peptides that could contribute to microorganism elimination in plasma. It remains to be established whether the mechanism leading to the partial cleavage of haemocyanin is part of the shrimp immune reaction and how involved this process can be in an immediate and systemic antimicrobial response in shrimp.
This result suggests that, as observed in crustaceans, the cleavage of haemocyanin and the production of peptide fragments with antimicrobial activity also occur in spiders as a first line of defence against infection. Several studies suggest that haemocyanins are involved in the arthropod immune system. The activity of the haemocyanin fragment discovered in this study reinforces that idea.
The identification and characterisation of new substances can lead to the development of new drugs that kill resistant pathogenic microorganisms. This peptide has activity against clinical isolates that cause candidiasis, one of the opportunistic pathogens responsible for nosocomial infections that colonise human mucosal surfaces [30]. Yeasts of the genus Candida are significant due to the high frequency at which they colonise and infect a human host. Candida species are found in the gastrointestinal tract in 20–80% of the healthy adult population. In addition, approximately 20–30% of women have Candida colonies in their vaginas [7]. The increase in the prevalence of yeast infections is likely due to the AIDS epidemic, cancer chemotherapy, organ and bone-narrow transplants and invasive hospital procedures [43,45] because of the overuse of antifungal agents, such as fluconazole [45]. Due to its small size, rondonin can be synthesised quickly and can kill yeast in ten minutes. Furthermore, no toxicity towards human erythrocytes was observed in this study. Therefore, rondonin may represent a new strategy for developing drugs that neutralise or inhibit pathogens.
Acknowledgements
We are grateful to Dr. Mirian A.F. Hayashi (Dept. Farmacology/UNIFESP) for providing the clinical strains. We also appreciate the financial support of Fapesp (CAT/Cepid Project 98-14307-9), Capes and Cnpq.
References
- 1.Atherton, Sheppard . IRL Press; Oxford: 1989. Solid phase peptide synthesis—a practical approach. [Google Scholar]
- 2.Boman H.G. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- 3.Boman H.G. Peptide antibiotics and their role in innate immunity. Annual Review of Immunology. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 4.Budnik B.A., Olsen J.V., Egorov T.A., Anisimova V.E., Galkina T.G., Musolyamov A.K., Grishin E.V., Zubarev R.A. De novo sequencing of antimicrobial peptides isolated from the venom glands of the wolf spider Lycosa singoriensis. Journal of Mass Spectrometry. 2004;39:193–201. doi: 10.1002/jms.577. [DOI] [PubMed] [Google Scholar]
- 5.Cociancich S., Goyffon M., Bontems F., Bulet P., Bouet F., Menez A., Hoffmann J.A. Purification and characterization of a scorpion defensin, a 4 kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochemical and Biophysical Research Communications. 1993;194:17–22. doi: 10.1006/bbrc.1993.1778. [DOI] [PubMed] [Google Scholar]
- 6.Destoumieux-Garzon D., Saulnier D., Garnier J., Jouffrey C., Bulet P., Bache're E. Crustacean immunity: antifungal peptides are generated from the C-terminus of shrimp hemocyanin in response to microbial challenge. The Journal of Biological Chemistry. 2001;276:47070–47077. doi: 10.1074/jbc.M103817200. [DOI] [PubMed] [Google Scholar]
- 7.Dignani M.C., Solomkin J.S., Anaissie E. Candida. In: Anaissie E., McGinnis M.R., Pfaller M.A., editors. Medical mycology. 1a Edição. Churchill Livingstone; Filadélfia: 2003. pp. 195–239. [Google Scholar]
- 8.Ehret-Sabatier L., Loew D., Goyffon M., Fehlbaum P., Hoffmann J.A., Van Dorsselaer A., Bulet P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. The Journal of Biological Chemistry. 1996;271:29537–29544. doi: 10.1074/jbc.271.47.29537. [DOI] [PubMed] [Google Scholar]
- 9.Foelix R.F. Harvard University Press; Cambridge, Massachussetts/London: 1996. Biology of spiders. [Google Scholar]
- 10.Fogaça A.C., Silva P.I., Jr, Miranda M.T., Bianchi A.G., Miranda A., Ribolla P.E., Daffre S. Antimicrobial activity of a bovine hemoglobin fragment in the tick Boophilus miroplus. The Journal of Biological Chemistry. 1999;274:25330–25334. doi: 10.1074/jbc.274.36.25330. [DOI] [PubMed] [Google Scholar]
- 11.Haeberli S., Kuhn-Nentwig L., Schaller J., Nentwig W. Characterization of antibacterial activity of peptides isolated from the venom of the spider Cupiennius salei (Aranae: Ctenidae) Toxicon. 2000;38:373–380. doi: 10.1016/s0041-0101(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 12.Hancock R.E.W., Diamond G. The role of cationic antimicrobial peptides in innate host defense. Trends in Microbiology. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 13.Harris F., Dennison S.R., Phenix D.A. Anionic antimicrobial peptide from eukaryotic organisms. Current Protein and Peptide Science. 2009;10:585–606. doi: 10.2174/138920309789630589. [DOI] [PubMed] [Google Scholar]
- 14.Hetru C., Bulet P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods in Molecular Biology. 1997;78(1):35–48. doi: 10.1385/0-89603-408-9:35. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman I.A., Kafatos F.C., Janeway C.A., Ezekowitz R.A.B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman J.A., Hertu C. Insect defensins: inducible antibacterial peptides. Immunology Today. 1992;13:411–415. doi: 10.1016/0167-5699(92)90092-L. [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga S., Kawabata S., Muta T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. The Journal of Biochemistry (Tokyo) 1998;123:1–15. doi: 10.1093/oxfordjournals.jbchem.a021894. [DOI] [PubMed] [Google Scholar]
- 19.Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clinical Microbiology Reviews. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamysz W., Okrój M., Lukasiak J. Novel properties of antimicrobial peptides. Acta Biochimica Polonica. 2003;50:461–469. [PubMed] [Google Scholar]
- 21.Klepser M.E., Ernest E.J., Lewis R.S., Ernest M.E., Pfaller M.A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrobial Agents and Chemotherapy. 1998;42(5):1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn-Nentwig L. Antimicrobial and cytolitic peptides of venomous arthropods. Cellular and Molecular Life Sciences. 2003;60:2651–2668. doi: 10.1007/s00018-003-3106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai R., Lomas L.O., Jonczy J., Turner P.C., Rees H.H. Two novel noncationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. The Journal of Biochemistry. 2004;379:681–685. doi: 10.1042/BJ20031429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.Y., Lee B.L., Söderhäll K. Processing of an antibacterial peptide from hemocyanin of the freshwater crayfish Pacifastacus leniusculus. The Journal of Biological Chemistry. 2002;278(10):7927–7933. doi: 10.1074/jbc.M209239200. [DOI] [PubMed] [Google Scholar]
- 25.Mak P., Wo´jcik K., Wicherek Ł., Suder P., Dubin A. Antibacterial hemoglobin peptides in human menstrual blood. Peptides. 2004;25:1839–1847. doi: 10.1016/j.peptides.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Markl J., Markl A., Schartau W., Linzen B. Subunit heterogeneity in arthropod hemocyanins: I (Chelicerata) Journal of Comparative Physiology B. 1979;130:283–292. [Google Scholar]
- 27.Markl J., Savel A., Decker H., Linzen B. Hemocyanins in spiders, IX. Homogeneity, subunit composition and the basic oligomeric structure of Eurypelma californicum hemocyanin. Hoppe-Seyler´s Zeitschrift für Physiologische Chemie. 1980;36:649–660. doi: 10.1515/bchm2.1980.361.1.649. [DOI] [PubMed] [Google Scholar]
- 28.Markl J., Decker H., Stöcker W., Savel A., Linzen B., Schutter W.G., van Bruggen E.F.J. On the role of dimeric subunits in the quaternary structure of arthropod hemocyanins. Hoppe-Seyler´s Zeitschrift für Physiologische Chemie. 1981;362:185–188. [PubMed] [Google Scholar]
- 29.Markl J. Evolution and function of structurally diverse subunits in the respiratory protein hemocyanin from arthropods. The Biological Bulletin. 1986;171:90–115. [Google Scholar]
- 30.Odds F.C., Gow N.A., Brown A.J. Fungal virulence studies come of age. Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-3-reviews1009. [p. 1009.1–.4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selden P., Shea W.A., Bonamo P.M. A spider and other arachnids from Devonian of New York, and reinterpretations of Devonian Aeaneae. Paleontology. 1991;34:241. [Google Scholar]
- 32.Shear W.A., Palmer J.M., Coddington J.A., Bonamo P.M. A Devonian spinneret: early evidences of spiders and silk use. Science. 1989;246:479. doi: 10.1126/science.246.4929.479. [DOI] [PubMed] [Google Scholar]
- 33.Schneider H.-J., Markl J., Schartau W., Linzen B. Subunit heterogeneity of Eurypelma (Dugesiella) hemocyanin, and separation of polypeptide chains. Hoppe-Seyler´s Zeitschrift für Physiologische Chemie. 1977;358:1133–1141. doi: 10.1515/bchm2.1977.358.2.1133. [DOI] [PubMed] [Google Scholar]
- 34.Silva Jr PI. Sistema Imune de Aracnídeos: Estrutura química e atividade de peptídeos antimicrobianos da Hemolinfa de Acanthoscurria gomesiana. Tese apresentada ao Instituto de Ciências Biomédicas da USP para obtenção do título de doutor em Ciências; 2000.
- 35.Silva P.I., Jr, Daffre S., Bulet P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana. Hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the Tachyplesin family. The Journal of Biological Chemistry. 2000;275(45):33464–33470. doi: 10.1074/jbc.M001491200. [DOI] [PubMed] [Google Scholar]
- 36.Silva F.D., Rezende C.A., Rossi D.C.P., Esteves E., Dyszy F.H., Schreier S., Gueiros-Filho F., Campos C.B., Pires J.R., Daffre S. Structure and mode of action of microplusin, a copper II-chelating antimicrobial peptide from the cattle tick Rhipicephalus (Boophilus) microplus. The Journal of Biological Chemistry. 2009;284:34735–34746. doi: 10.1074/jbc.M109.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Söderhäll K., Cerenius L. Role to the prophenoloxidase-activating system in invertebrate immunity. Current Opinion in Immunology. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 38.Söderhall K., Smith V.J. Separation of the haemocyte of Carcinus maenas and other decapod crustaceans and phenoloxidase distribution. Developmental and Comparative Immunology. 1983;7:229–239. doi: 10.1016/0145-305x(83)90004-6. [DOI] [PubMed] [Google Scholar]
- 39.Steffen H., Rieg S., Wiedemann I., Kalbacher H., Deeg M., Sahl H.G., Peschel A., Götz F., Garbe C., Schittek B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrobial Agents and Chemotherapy. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strominger Animal antimicrobial peptides: ancient players in innate immunity. The Journal of Immunology. 2009;182:6633–6634. doi: 10.4049/jimmunol.0990038. [DOI] [PubMed] [Google Scholar]
- 41.Ullal A.J., Litaker R.W., Noga E.J. Antimicrobial peptides derived from haemoglobin are expressed in epithelium of channel catfish (Ictalurus punctatus, Rafinesque) Developmental and Comparative Immunology. 2008;32:1301–1312. doi: 10.1016/j.dci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Voit R., Feldmmaier-Fuchs G., Schweikardt T., Decker H., Burmester T. Complete sequence of the 24-mer hemocyanin of the tarantula Eurypelma californicum. The Journal of Biological Chemistry. 2000;275(50):39339–39344. doi: 10.1074/jbc.M005442200. [DOI] [PubMed] [Google Scholar]
- 43.White T.C., Man K.A., Bowden R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistence. Clinical Microbiology Reviews. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamane ES. Estudo da atividade antimicrobiana da crotamina native e sintética, do seu análogo recombinante e fragmentos peptídicos. Dissertação apresentada a Universidade Federal de São Paulo—Escola Paulista de Medicina para obtenção do título de mestre em Ciências; 2010.
- 45.Yang Y.L., Lo H.-J. Mechanisms of antifungal agent resistance. Journal of Microbiology, Immunology and Infection. 2001;34:79–86. [PubMed] [Google Scholar]
- 46.Yigit N., Benli M. The antibacterial activity of hemolymph of spider, Agelena labyrinthica (Araneae: Agelenidae) Journal of Forestry Faculty. 2008;8:120–124. [Google Scholar]
- 47.Zhu W.L., Song Y.M., Park Y., Park K.H., Yang S.T., Kim J.I. Substitution of the leucine zipper sequence in melittin with peptoid residues affects self-association, cell selectivity, and mode of action. Biochimica et Biophysica Acta. 2007;1768(6):1506–1517. doi: 10.1016/j.bbamem.2007.03.010. [DOI] [PubMed] [Google Scholar]


