Abstract
Histone H2A participates in host defense responses by producing antimicrobial peptides (AMPs). The present study deals with identification of a putative antimicrobial sequence, Himanturin from the histone H2A of Round Whip Ray, Himantura pastinacoides. A 204 bp fragment encoding 68 amino acid residues was amplified from cDNA of Round Whip Ray, H. pastinacoides. Himanturin exhibited high similarity to previously reported histone H2A derived AMPs indicating the presence of an antimicrobial sequence motif. Physicochemical properties of Himanturin suggest it to be a potential antimicrobial candidate.
Keywords: Antimicrobial peptide, Himantura pastinacoides, Elasmobranch, Histone H2A, Himanturin
1. Introduction
Antimicrobial peptides (AMPs) are an important component in the innate immune system of almost all multicellular organisms [11,4,28,35,13,18,9]. They are generally defined as low molecular weight, amphipathic peptides which are mostly cationic. AMPs show wide divergence in their amino acid composition, size and conformational structures but exhibit striking similarity in their mode of action [22,5,29,17,33,23]. They have retained their antimicrobial activity against a broad spectrum of pathogenic organisms, despite of their ancient and wide spread presence in nature [41,22]. Remarkable specificity to prokaryotes with low toxicity to eukaryotic cells has favored their investigation and exploitation as new antibiotics [40]. Aquatic organisms rely mainly on their innate immune defenses for protection against pathogenic organisms and hence should be considered as potential sources of antimicrobial peptides.
AMPs derived from histone proteins form an important category of peptide antibiotics [14]. Traditionally, histones are known as major components of the nucleosome structures in eukaryotic cells. Histone proteins play a key role in the innate immune defense of organisms by forming AMPs. These AMPs are derived from their histone precursors by the action of proteolytic enzymes. Histone derived AMPs with potent activity has been isolated and reported from various organisms [25,26,30,2,8,27,20,32,7]. Buforin I isolated from Asian toad Bufo bufo was the first report of a histone H2A derived AMP [25,7]. In the case of marine invertebrates, histone derived AMPs have been reported from Pacific White Shrimp Litopenaeus vannamei [27], Scallop Chlamys farreri [20] and Disk Abalone Haliotis discus discus [7]. From fishes histone derived antimicrobial peptides have been reported from Catfish Parasilurus asotus [26], Atlantic salmon Salmon salar [30], Rainbow Trout Oncorhynchus mykiss [8], and Atlantic Halibut Hippoglossus hippoglossus [2]. Present study was carried out to identify novel antimicrobial peptides from Rays as part of their innate immunity. Here we report the identification of an antimicrobial peptide sequence from the histone H2A of Round Whip Ray, Himantura pastinacoides. This is the first report of a histone H2A derived AMP from Elasmobranchs.
2. Materials and methods
2.1. Sample collection
Live Round Whip Ray, H. pastinacoides was caught off Vypeen, Kochi, Kerala, India. Samples were transported to laboratory in live condition. Blood was collected from the lamellar artery near gill region using specially designed capillary tubes (RNase free) rinsed in precooled anticoagulant solution (RNase free 10% sodium citrate, pH 7).
2.2. cDNA synthesis
Total RNA was isolated from blood cells using TRI® reagent and following manufacturer's instructions. Purity and quality of RNA was checked on 0.8% agarose gel. RNA was quantified by spectrophotometry at 260 and 280 nm. Only RNAs with absorbance ratios (A260:A280) equal to or greater than 1.8 were used for the present work. First strand cDNA was generated in a 20 μl reaction volume containing 5 μg total RNA, 1× RT buffer, 2 mM dNTP, 2 mM oligo (dT)20 20 U of RNase inhibitor and 100 U of MMLV Reverse transcriptase (New England Biolabs, USA). The reaction was conducted at 42 °C for 1 h followed by an inactivation step at 85 °C for 15 min.
2.3. PCR amplification
Amplification of a Hipposin- like antimicrobial peptide from cDNA of H. pastinacoides was done using sense primer (5′-ATGTCCGGRMGMGGSAARAC-3′) and antisense primer (5′-GGGATGATGCGMGTCTTCTTGTT-3′) [2]. PCR amplification of 1 μl of cDNA was performed in a 25 μl reaction volume containing 1× standard Taq buffer (10 mM Tris–HCl, 50 mM KCl, pH 8.3), 1.5 mM MgCl2, 200 mM dNTPs, 0.4 mM each primer and 1U Taq DNA polymerase (New England Biolabs, USA). The thermal profile used was an initial denaturation at 94 °C for 2 min followed by 35 cycles of 94 °C for 15 s, 60 °C for 30 s and 68 °C for 30 s and a final extension at 68 °C for 10 min. PCR product was analyzed by electrophoresis in 1.5% agarose gel in TBE buffer, stained with SYBR® safe and visualized under UV light. Sequencing of purified PCR product was performed with an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit on an ABI Prism 377 DNA sequencer (Applied Biosystem) at SciGenom Sequencing Facility, India.
2.4. Taxonomic identification
For taxonomic identification of the species, genomic DNA was isolated from gills using ‘salting out’ technique as described by Miller et al. [24]. The concentration of isolated DNA was estimated using a UV–vis Spectrophotometer (Hitachi U-2900). The DNA was diluted to a final concentration of 100 ng/μl. The Cytochrome Oxidase-I (COI) gene was amplified in a 25 μl reaction volume containing the above said PCR reagents in same concentration. 1 μl of genomic DNA was used as template. The primers used for the amplification of COI gene were Forward (5′-TCGACTAATCATAAAGATATGGGCCAC-3′) and Reverse (5′-ACTTCAGGGTGACCGAAGAATCAGAA-3′) [38]. The thermal regime consisted of an initial denaturation at 95 °C for 5 min followed by 35 cycles of 95 °C for 45 s, 50 °C for 30 s and 72 °C for 45 s and a final extension at 72 °C for 10 min. Amplicons obtained were sequenced using ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit on an ABI Prism 377 DNA sequencer (Applied Biosystem) at SciGenom Sequencing Facility, India.
The COI primers amplified a readable 600 bp region of the gene mitochondrial cytochrome oxidase subunit I (GenBank ID JN982361). BLAST analysis (http://www.ncbi.nlm.nih.gov/blast) of nucleotide sequences confirmed the identity of the Ray as H. pastinacoides showing 97% similarity to GenBank ID: EU398852.1 H. pastinacoides.
2.5. Analysis of peptide properties
The nucleotide sequence and deduced amino acid sequence of peptide was subjected to BLAST at the NCBI (http://www.ncbi.nlm.nih.gov/blast). Translation of the cDNA was performed using the Expert Protein Analysis System (http://au.expasy.org/). Physicochemical parameter of the deduced peptide was calculated by the ProtParam tool (http://cn.expasy.org/tools/protparam.html). Multiple sequence alignment of the peptide with previously reported histone derived AMPs from other animals, was performed with ClustalW. Phylogenetic tree was constructed by the Neighbor-joining (NJ) and Maximum Likelihood (ML) method based on nucleic acid sequences. Phylogenetic tree was drawn using MEGA version 5.05. Homology searches were performed using BLASTn and BLASTp at National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Three dimensional structure of the peptide was predicted based on template PDB No. 1TzyA using SWISS-MODEL [1,10,31].
3. Results
A 204 bp fragment cDNA encoding 68 amino acids from the mRNA of blood cells of H. pastinacoides was obtained by RT-PCR (Fig. 1). BLAST analysis of the nucleotide and deduced amino acid sequences revealed that the peptide belonged to histone H2A family. The obtained nucleotide and deduced amino acid sequences were deposited in GenBank database (GenBank ID: HQ720150). Multiple-sequence alignment of the amino acid sequence of the peptide with previously reported histone H2A derived AMPs revealed that the first 39 amino acid sequence from N-terminal of the deduced peptide showed similarity to histone H2A derived AMPs i.e. Hipposin, Buforin I, Buforin II, Parasin I, Abhisin and those reported from Oncorhynchus mykiss, L. vannamei and Chlamys farreri (Fig. 2). This H2A derived peptide sequence from H. pastinacoides was termed as ‘Himanturin’ and here onwards will be denoted by the term. The 39 amino acid Himanturin was found to have a predicted molecular weight of 4.27 kDa and a theoretical isoelectric point (pI) of 11.73 as predicted by PROTPARAM software. Himanturin was found to be rich in arginine (15.4%), glycine (12.8%), alanine (12.8%), leucine (10.3%), valine (10.3%) and lysine (7.7%) as reported in all other histone H2A derived AMPs. Himanturin was found to have one negative residue (Glu) as against nine positive residues (Arg+Lys), thereby having a net charge of +8. Hydrophobicity of peptide was found to be +34.68 Kcal/mol (35%) as predicted by PepDraw. The hydrophilic index plot of Himanturin was analyzed using Kyte and Doolittle method [16]. The result showed the presence of both hydrophilic and hydrophobic domains in Himanturin, indicating the amphipathic nature of the peptide. N-terminus was found to be hydrophilic and C-terminus hydrophobic. Bootstrap distance tree calculated confirmed the similarity of the obtained nucleotide sequence to the previously reported histone H2A nucleotide sequences (Fig.3). SWISS-MODEL predicted an alpha helical structure for Himanturin (Fig.4). Analysis of Himanturin for its antimicrobial activity was carried out with Antimicrobial Peptide Database (http://aps.unmc.edu/AP/main.php).
Fig. 1.

Nucleotide and amino acid sequences of histone H2A. The underlined amino acid sequences indicate Himanturin from Round Whip Ray. H. pastinacoides.
Fig. 2.

ClustalW multiple alignment of Himanturin (H. pastinacoides) with hipposin (Hippoglossus hippoglossus), Buforin I and II (Bufo bufo gargarizans), Parasin I (Parasilurus asotus), Rainbow Trout H2A (Oncorhynchus mykiss), Litopenaeus AMP (Litopenaeus vannamei), Scallop AMP (Chlamys farreri) and abhisin (Haliotis discus).
Fig. 3.

A bootstrapped neighbor-joining tree obtained using MEGA version 5.05 illustrating relationships between the nucleotide sequence of H. pastinacoides to the neucleotide sequences of previously reported histone H2A from different organisms: Gallus gallus, Meleagris gallopavo, Taeniopygia guttata, Homo sapiens, Oryctolagus cuniculus, Mus musculus, Ornithorhychus anatinus, Tetradon nigroviridis, Rainbow trout, Oreochromus niloticus, Cynaglossus sp., Perca flavescens, Gasterosteus aculeatus, Oreochromis niloticus, Bufo bufo gagarizans, Xenopus tropicalis, Xenopus laevis, Aplysia californica, Sunetta scripta, Litopenaeus vannamei, Penaeus monodon, Lepeophtheirus salmonis, Haliotis discus, Chlamys farreri, Solen marginatus, Mytilus californianus, Mytilus chilensis, Mytilus galloprovincialis, Mytilus edulis and Venerupis pullastra.
Fig. 4.
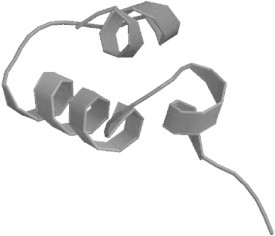
Predicted 3-dimentional structure of Himanturin from Round Whip Ray H. pastinacoides.
4. Discussion
Histone H2A derived antimicrobial peptides reported from various animals have activity against both Gram-positive and Gram-negative bacteria and fungi [25,26,30,2,27,20,14,7]. In Asian Toad, Bufo bufo gargarizans, the intact histone H2A protein is secreted into the stomach and Buforin I is produced by the action of pepsin isozymes cleaving the Try 39 – Ala 40 bond of intact protein [15]. Similarly in Catfish (Parasilurus asotus), Parasin I is produced by cleavage of Ser19-Arg20 bond of histone H2A by Cathepsin D found in skin mucus of the fish [6]. Action of enzyme pepsin on 68 amino acid sequence obtained from Round Whip Ray was analyzed using PeptideCutter tool (http://web.expasy.org/peptide_cutter/), which predicts pepsin to have a potential cleavage site at amino acid position 39 thereby resulting in formation of Himanturin. These findings suggest the possibility that N-terminus of histone H2A of H. pastinacoides could be an active antimicrobial peptide assisting in innate immunity of the Ray. In Drosophila secretion of antimicrobial peptides is mediated by two distinct pathways, the Toll pathway and Immune Deficiency (IMD) pathway. The Toll pathway is activated primarily in response to fungal and Gram positive bacterial infections, whereas the IMD pathway is activated predominantly in response to Gram negative and other Gram positive bacterial infections [19,12]. Toll activates expression of antifungal peptide genes, Dorsomycin and Metchnikowin, whereas, IMD induces transcription of genes, which encode the antibacterial peptides i.e., Diptericins, Cecropins, Drosocins and Attacins. A pathway similar to that of Drosophila IMD, termed as LvIMD was reported from L. vannamei. Expression of LvIMD mRNA is influenced by LPS and Gram negative Vibrio alginolyticus and expression of LvIMD could induce a 3 fold increase in the expression of PEN 4 [36]. Presence of Toll-like receptor in L. vannamei (Lv Toll1) was first reported by Yang et al. [39]. Two more Toll-like receptors, Lv Toll2 and Lv Toll3 were reported by Wang et al. [37]. In comparison to Lv Toll1 and Lv Toll3, Lv Toll2 was found to be more significant in the activation of AMP promoters in L. vannamei [37]. Mechanisms similar to these might be involved in the cleavage of precursor derived antimicrobial peptides.
In case of 51-mer Hipposin, fragment containing 1 to 19 amino acid residues from the N terminal did not exhibit marked antimicrobial activity, whereas fragment consisting of 16–39 amino acid (similar to buforin II) had such activity indicating that this part of Hipposin possesses antimicrobial sequence motif and the activity was found enhanced by the presence of the fragment having 40–51 amino acid residues [3]. Fragment consisting of 4–39 amino acid residues from N-terminal of Himanturin exhibit striking similarity to 16–39 amino acid fraction of Hipposin which has been shown to contain the antimicrobial sequence motif. Almost all previously reported histone H2A derived AMPs have fragments similar to this fraction of Hipposin and it could be deduced that their activity is mainly due to this portion. Fraction 4–39 of Himanturin is similar to 16 to 51 amino acid fraction of hipposin except for Thr at position 16 of Hipposin which has been replaced by Ser (position 4) in Himanturin and His at position 41 of Hipposin being replaced by Glu (position 29) in Himanturin. Presence of Ser instead of Thr at position 16 of Hipposin can also be seen in Abhisin and histone H2A derived AMPs reported from L. vannamei and Chlamys farreri. Histone H2A AMPs reported from marine organisms exhibit broad spectrum antimicrobial activity. Hipposin and Parasin I are the most studied Histone H2A derived antimicrobial peptides. Hipposin showed strong antibacterial activity against several Gram positive and Gram negative bacteria and the activity could be detected down to concentrations of 1.6 μg/ml [2]. Parasin I was found to exhibit a minimum inhibitory concentration of 1 to 4 μg/ml against an array of Gram positive and Gram negative bacteria without any haemolytic activity [26]. Parasin I was also found to exhibit antifungal activity against Cryptococcus neoformans, Saccharomyces cerevisiae and Candida albicans with minimum inhibitory concentration of 2 μg/ml [26]. Abhisin, an antimicrobial peptide derived from histone H2A of Disk Abalone Haliotis discus was found to be active against Listeria monocytogenes (G+), Vibrio ichthyoenteri (G−), and yeast, Pityrosporum ovale. Abhisin treatment (50 μg/ml) decreased the viability of THP-1 leukemia cancer cells approximately by 25% without any effect on the normal Vero cells, suggesting that Abhisin has cytotoxicity against cancer cells but not normal cells [7]. Histone H2A derived AMPs exhibit strong activity against aquatic and human pathogens. Himanturin exhibit strong similarity to these highly potent antimicrobial peptides. Antimicrobial Peptide Database (http://aps.unmc.edu/AP/main.php) predicts Himanturin to be an Antimicrobial peptide since it form alpha helices and have at least 7 residues on the same hydrophobic surface which allows the peptide to interact with membranes.
The phylogenetic relationship of H. pastinacoides to other organisms is shown in Fig. 3. The molecular phylogenetic tree based on nucleotide sequences of previously reported histone H2A sequences demonstrate that the members of the family are derived from a common ancestor by a series of evolutionary changes. The boot strap distance tree calculated reveals that H. pastinacoides cluster under the group Fishes and Amphibians. Histone genes evolve very slowly and therefore, evolutionary analyses of histones should be informative with regard to the phylogenetic relationships of distantly related organisms [34]. At the nucleotide level, the variability in histone genes appears to be the result of a larger amount of non-synonymous variation, which affects to a lesser extent, the structural domain of the protein comprising the histone fold [21]. Because the topology of major histone H2A phylogeny is similar to the eukaryotic phylogeny, histone H2A can be used as a molecular marker for classification. More data on Ray histone H2A sequences will decipher the relationship of Ray H2A to other vertebrate and invertebrate H2A.
5. Conclusion
A peptide containing antimicrobial sequence motif from the histone H2A of Round Whip Ray, H. pastinacoides was identified and named as Himanturin. High similarity of Himanturin to other histone H2A derived AMPs with proven antimicrobial activity and its physicochemical properties in agreement with those of traditional antimicrobial peptides strongly endorse it to be an antimicrobial peptide. Since the peptide is reported from a “food grade” source, the Round Whip Ray, it has the potential to be developed into an effective antimicrobial agent with broad application potential.
Acknowledgments
The authors are grateful to the Director, Centre for Marine Living Resources and Ecology (CMLRE) and Ministry of Earth Sciences (MoES), Govt. of India for the research grant (MoES/10-MLR/2/2007) and scientific support for the work.
References
- 1.Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modelling. Bioinformatics (Oxford, England) 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 2.Birkemo A.G., Luders T., Andersen O., Nes I.F., Meyer J.N. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.) Biochimica et Biophysica Acta. 2003;646:207–215. doi: 10.1016/s1570-9639(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 3.Birkemo A.G., Mantzilas D., Luders T., Nes I.F., Meyer J.N. Identification and structural analysis of the antimicrobial domain in hipposin, a 51-mer antimicrobial peptide isolated from Atlantic halibut. Biochimica et Biophysica Acta. 2004;169:9221–9227. doi: 10.1016/j.bbapap.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Boman H.G. Antibacterial peptides: basic facts and emerging concepts. Journal of Internal Medicine. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 5.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nature Reviews Microbiology. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 6.Cho J.H., Park I.Y., Kim H.S., Lee W.T., Kim M.S., Kim S.C. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB Journal. 2002;16:429–431. doi: 10.1096/fj.01-0736fje. [DOI] [PubMed] [Google Scholar]
- 7.De Zoysa M., Nikapitiya C., Whang I., Lee J.S., Lee J. Abhisin: a potential antimicrobial peptide derived from histone H2A of disk abalone (Haliotis discus discus) Fish and Shellfish Immunology. 2009;27:639–646. doi: 10.1016/j.fsi.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes J.M.O., Molle G., Kemp G.D., Smith V.J. Isolation and characterisation of oncorhyncin II, a histone H1-derived antimicrobial peptide from skin secretions of rainbow trout, Oncorhynchus mykiss. Developmental and Comparative Immunology. 2004;28:127–138. doi: 10.1016/s0145-305x(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 9.Guani-guerra E., Santos-mendoza T., Lugo-reyes S.O., Teran L.M. Antimicrobial peptides: General overview and clinical implications in human health and disease. Clinical Immunology. 2010;135(1):1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Guex N., Peitsch M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R.E.W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann J.A., Reichhart J.M. Drosophila innate immunity: an evolutionary perspective. Nature Immunology. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 13.Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clinical Microbiology Reviews. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki H., Iwamuro S. Potential roles of histones in host defense as antimicrobial agents Infect. Disorders Drug Targets. 2008;8(3):195–205. doi: 10.2174/1871526510808030195. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.S., Yoon H., Minn I., Park C.B., Lee W.T., Zasloff M. Pepsin- mediated processing of the cytoplasmic histone H2A to strong antimicrobial peptide buforin I. Journal of Immunology. 2000;165:3268–3274. doi: 10.4049/jimmunol.165.6.3268. [DOI] [PubMed] [Google Scholar]
- 16.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 17.Labadie O.W., Picman J., Hincke M.T. Avian antimicrobial proteins: structure, distribution and activity. Poultry Science. 2007;63:421–438. [Google Scholar]
- 18.Lai Y., Gallo R.L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends in Immunology. 2009;30(2):131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaitre B., Reichhart J.M., Hoffmann J.A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proceedings of National Academy of Sciences USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Song L., Zhao J., Zhu L., Zou H., Zhang H. The preliminary study on a potential antibacterial peptide derived from histone H2A in hemocytes of scallop Chlamys farreri. Fish and Shellfish Immunology. 2007;22:663–672. doi: 10.1016/j.fsi.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Lopez J.M.E., Ishibashi T., Ausio J. H2A Bbd: a quickly evolving hypervariable mammalian histone that destabilizes nucleosomes in an acetylation-independent way. The FASEB Journal. 2008:316–326. doi: 10.1096/fj.07-9255com. [DOI] [PubMed] [Google Scholar]
- 22.Marshall S.H., Arenas G. Antimicrobial peptides: A natural alternative to chemical antibiotics and a potential for applied biotechnology. Electronic Journal of Biotechnology. 2003;6(2):271–284. [Google Scholar]
- 23.Mihajlovic M., Lazaridis T. Antimicrobial peptides bind more strongly to membrane pores. Biochimica et Biophysica Acta. 2010;1798:1494–1502. doi: 10.1016/j.bbamem.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller S., Dykes D., Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Research. 1998;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C., Kim M., Kim S. A novel antimicrobial peptide from Bufo bufo gargarizans. Biochemical and Biophysical Research Communications. 1996;218(1):408–413. doi: 10.1006/bbrc.1996.0071. [DOI] [PubMed] [Google Scholar]
- 26.Park I.Y., Park C.B., Kim M.S., Kim S.C. Parasin I, an antimicrobial peptide derived from histone H2A in the cat fish, Parasilurus asotus. FEBS Letters. 1998;43:7258–7262. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- 27.Patat S., Carnegie R.B., Kingsbury C., Gross P.S., Chapman R. Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. European Journal of Biochemistry/FEBS. 2004;271:4825–4833. doi: 10.1111/j.1432-1033.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 28.Patrzykat A., Douglas S.E. Gone gene fishing: how to catch novel marine antimicrobials. Trends in Biotechnology. 2003;21(8):362–369. doi: 10.1016/S0167-7799(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 29.Pazgier M., Hoover D.M., Yang D., Lu W., Lubkowski J. Review human β -defensins. Cellular and Molecular Life Sciences: CMLS. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards R.C., O'Neil D.B., Thibault P., Ewart K.V. Histone H1: an antimicrobial protein of Atlantic salmon (Salmo salar) Biochemical and Biophysical Research Communications. 2001;284:549–555. doi: 10.1006/bbrc.2001.5020. [DOI] [PubMed] [Google Scholar]
- 31.Schwede T., Kopp J., Guex N., Peitsch M.C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic acids research. 2003;3:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sook Y., Min J., Yup I., Jo B., Jang S.A., Kim K-sun. Structure – activity relations of parasin I, a histone H2A-derived antimicrobial peptide. Peptides. 2008;29:1102–1108. doi: 10.1016/j.peptides.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Stromstedt A, Ringstad L, Schmidtchen A, Malmsten M. Interaction between amphiphilic peptides and phospholipid membranes. Current Opinion in Colloid and Interface Science. 2010;15(6):467–478. [Google Scholar]
- 34.Thatcher T.H., Gorovsky M.A. Phylogenetic analysis of the core histones H2A, H2B, H3, and H4. Nucleic Acids Research. 1994;22:174–179. doi: 10.1093/nar/22.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tincu J.A., Taylor S.W. Antimicrobial peptides from marine invertebrates. Antimicrobial Agents and Chemotherapy. 2004;48:3645–3654. doi: 10.1128/AAC.48.10.3645-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P.H., Gu Z.H., Huang X.D., Liu B.D., Deng X.X., Ai H.S., Wang J. An immune deficiency homolog from the white shrimp, Litopenaeus vannamei, activates antimicrobial peptide genes. Molecular Immunology. 2009;46:1897–1904. doi: 10.1016/j.molimm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Wang P.H., Liang J.P., Gu Z.H., Wan D.H., Pang L.R., Weng S.P., Yu X.Q. Molecular cloning, characterization and expression analysis of two novel Tolls (LvToll2 and LvToll3) and three putative Spatzle-like Toll ligands (LvSpz1-3) from Litopenaeus vannamei. Developmental and Comparative Immunology. 2012;36:359–371. doi: 10.1016/j.dci.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Ward R.D., Zemlak T.S., Innes B.H., Last P.R., Hebert P.D.N. DNA barcoding Australia's fish species Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L.S., Yin Z.X., Liao J.X., Huang X.D., Guo C.J., Weng S.P., Chan S.M. A Toll receptor in shrimp. Molecular Immunology. 2007;44:1999–2008. doi: 10.1016/j.molimm.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Zasloff M. Antibiotic peptides as mediators of innate immunity. Current Opinion in Immunology. 1992;4:3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]
- 41.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]


