Abstract
Brassinosteroids (BRs) are growth-promoting steroid hormones that regulate diverse physiological processes in plants. Most BR biosynthetic enzymes belong to the cytochrome P450 (CYP) family. The gene encoding the ultimate step of BR biosynthesis in Arabidopsis likely evolved by gene duplication followed by functional specialization in a dicotyledonous plant-specific manner. To gain insight into the evolution of BRs, we performed a genomic reconstitution of Arabidopsis BR biosynthetic genes in an ancestral vascular plant, the lycophyte Selaginella moellendorffii. Selaginella contains four members of the CYP90 family that cluster together in the CYP85 clan. Similar to known BR biosynthetic genes, the Selaginella CYP90s exhibit eight or ten exons and Selaginella produces a putative BR biosynthetic intermediate. Therefore, we hypothesized that Selaginella CYP90 genes encode BR biosynthetic enzymes. In contrast to typical CYPs in Arabidopsis, Selaginella CYP90E2 and CYP90F1 do not possess amino-terminal signal peptides, suggesting that they do not localize to the endoplasmic reticulum. In addition, one of the three putative CYP reductases (CPRs) that is required for CYP enzyme function co-localized with CYP90E2 and CYP90F1. Treatments with a BR biosynthetic inhibitor, propiconazole, and epi-brassinolide resulted in greatly retarded and increased growth, respectively. This suggests that BRs promote growth in Selaginella, as they do in Arabidopsis. However, BR signaling occurs through different pathways than in Arabidopsis. A sequence homologous to the Arabidopsis BR receptor BRI1 was absent in Selaginella, but downstream components, including BIN2, BSU1, and BZR1, were present. Thus, the mechanism that initiates BR signaling in Selaginella seems to differ from that in Arabidopsis. Our findings suggest that the basic physiological roles of BRs as growth-promoting hormones are conserved in both lycophytes and Arabidopsis; however, different BR molecules and BRI1-based membrane receptor complexes evolved in these plants.
Introduction
The characterization of Arabidopsis dwarf (dwf) mutants revealed that brassinosteroids (BRs) are essential for normal growth and development throughout the life cycle of the plant [1], [2]. Arabidopsis dwf5 [3], dwf7 [4], and dwf1 [5], in an order of action in the biosynthetic pathways, are defective in the enzymatic steps that lead to the synthesis of campesterol, which serves as a precursor of BRs [2]. Campesterol is further modified by the BR biosynthetic enzymes defined by de-etiolated2 [6], dwf4 [7]–[9], constitutive photomorphogenesis and dwarfism [10], [11], and BR6ox [1], [12], [13]. Leaving a few steps including steroid-2-hydroxylase to be cloned, BR biosynthetic enzymes in the pathways have been mapped to a gene in Arabidopsis [2], [9].
Most of the BR biosynthetic enzymes belong to the cytochrome P450 (CYP) family [2]. CYP enzymes primarily act as monooxygenases to hydroxylate their substrates [14]. Plant CYPs form two primary clades, named A-type and non-A-type CYPs [15]. A-type CYPs mediate plant-specific metabolism, e.g., the synthesis of secondary metabolites such as lignin, whereas non-A-type CYPs participate in metabolic processes common to eukaryotes. CYP51, a sterol 14α-demethylase, is a representative ancestor of plant non-A-type CYPs [14]–[16]. CYP proteins are classified further into subgroups according to their sequence similarity: identity greater than 40% are categorized into the same numerical group and those that more than 55% into alphabetical subgroups [14], [17]. Arabidopsis BR biosynthetic CYPs belong to the CYP85 clan, which includes CYP90A1 (cpd), CYP90B1 (dwf4), CYP90C1 (rotundifolia3), CYP90D1, CYP85A1 (BR6ox1), and CYP85A2 (BR6ox2). In Selaginella moellendorffii, at least four genes cluster in the CYP85 clan, including CYP90E1, CYP90E2, CYP90E3, and CYP90F1 [14], [15], suggesting that BRs are biosynthesized using these putative BR biosynthetic enzymes.
BR signals are perceived by a plasma membrane-localized receptor kinase, BRASSINOSTEROID-INSENSITIVE1 (BRI1) [18]–[20]. BR binding activates the BRI1 kinase domain, switching its binding partner from BRI1 KINASE INHIBITOR 1 (BKI1) [21] to BRI1-ASSOCIATED-KINASE1 (BAK1) [22], [23]. The BRI1-BAK1 co-receptor then relays the phosphorylation signal to BR-SIGNALING KINASES (BSKs) [24] and CONSTITUTIVE DIFFERENTIAL GROWTH1 (CDG1) [25]. Upon receiving the BR signal, a type I protein phosphatase named BRI1 SUPPRESSOR 1 (BSU1) [2], [26] represses the function of BRASSINOSTEROID INSENSITIVE2 (BIN2), a GSK3β-like protein kinase that acts as an inhibitor in BR signaling pathways [27], [28]. BES1 and BZR1 are the substrates of BIN2; in the non-phosphorylated state, these transcription factors regulate the transcription of their target genes [29]. BR signaling results in the dephosphorylation of phosphorylated BZR1 by a phosphatase named PROTEIN PHOSPHATASE 2A (PP2A) [29], [30].
Whereas the BR biosynthetic and signaling pathways have been thoroughly characterized in Arabidopsis, our knowledge of these pathways is limited in other species. To gain insight into the evolution of BR pathways, were constituted Arabidopsis BR pathways in an ancestral vascular plant, the lycophyte Selaginella moellendorffii. Lycophytes constitute a monophyletic sister clade to the euphyllophytes, which includes ferns and seed plants. Paleobotanical evidence suggests that the two vascular plant lineages separated approximately ∼400 million years ago [31]. Most prominently, vascular patterning in the leaf-like structure (microphyll) of Selaginella is different from that in Arabidopsis, with Selaginella lacking leaf gaps. Furthermore, gibberellins are not the major growth-promoting hormone in Selaginella [32]. Because BRs are known to be important regulators of vascular patterning and growth stimulation in Arabidopsis, identifying the counterpart underlying genes in Selaginella by comparative genomics analysis would provide evolutionary insight into the roles of BR hormones.
Results and Discussion
Phylogenetic Analysis of Putative BR Biosynthetic Genes
In Arabidopsis, BRs are biosynthesized through the action of CYP proteins belonging to the CYP85 clan [33]. To establish how many of the BR biosynthetic enzymes are similar in Arabidopsis and Selaginella, we searched the Phytozome database for sequences in the Selaginella genome with similarity to BR biosynthetic CYPs in Arabidopsis. We identified four sequences showing significant similarity with Arabidopsis CYP85A1. Consistent with previous findings [34], all four of these sequences, which were previously named CYP90E1, CYP90E2, CYP90E3, and CYP90F1, clustered with CYP85A1.
According to the numbering convention of CYP sequences, the group number often reflects the catalytic function; for instance, CYP90s catalyze brassinosteroid biosynthetic reactions [16]. Arabidopsis CYP amino acid sequences ranged in identity from 32% to 83% with each other. A comparison of Arabidopsis and Selaginella CYP sequences revealed levels of identity as low as 31% between AtCYP85A1 and SmCYP90E1, and as high as 43% between AtCYP90A1 and SmCYP90F1 (Table 1).
Table 1. Percent similarity between amino acid sequences of CYPs.
| Arabidopsis thaliana | Selaginella moellendorffii | ||||||||||
| CYP85A2(BR6OX2) | CYP85A1(BR6OX1) | CYP90B1(DWF4) | CYP90A1(CPD) | CYP90C1(ROT3) | CYP90D1 | CYP90E1 | CYP90E2 | CYP90E3 | CYP90F1 | ||
| Arabidopsis thaliana | CYP85A2 (BR6OX2) | 100 | |||||||||
| CYP85A1 (BR6OX1) | 83 | 100 | |||||||||
| CYP90B1 (DWF4) | 33 | 32 | 100 | ||||||||
| CYP90A1 (CPD) | 37 | 35 | 42 | 100 | |||||||
| CYP90C1 (ROT3) | 33 | 33 | 37 | 42 | 100 | ||||||
| CYP90D1 | 35 | 35 | 36 | 41 | 52 | 100 | |||||
| Selaginella moellendorffii | CYP90E1 | 32 | 31 | 34 | 40 | 38 | 37 | 100 | |||
| CYP90E2 | 34 | 32 | 33 | 38 | 39 | 37 | 86 | 100 | |||
| CYP90E3 | 33 | 32 | 34 | 38 | 37 | 38 | 77 | 81 | 100 | ||
| CYP90F1 | 37 | 36 | 38 | 43 | 39 | 35 | 35 | 37 | 37 | 100 | |
www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi). Percent identity between Arabidopsis CYP90s ranged from 32% (between AtCYP90B1 and AtCYP85A1) to 83% (between AtCYP85A1 and AtCYP85A2). A comparison of CYP sequences in Arabidopsis and Selaginella revealed identity values ranging from 31% (between SmCYP90E1 and AtCYP85A1) to 43% (between SmCYP90F1 and AtCYP90A1). SmCYP90E consists of three members that share 77∼86% identity, whereas SmCYP90F has one member, which has 35∼37% identity with the SmCYP90Es. Sequences of known BR biosynthetic enzymes from Arabidopsis were pair-wise compared with select sequences from Selaginella. Percent identity values were calculated using the 2 BLAST program (
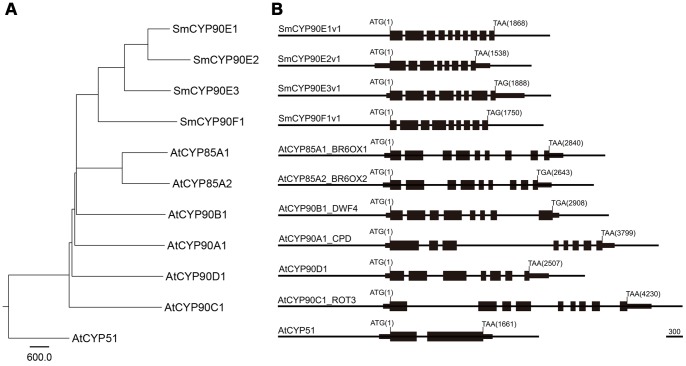
To better understand the phylogenetic relationship between the CYP sequences, we generated a phylogenetic tree based on the genomic DNA sequences of the CYP genes (Figure 1A). We found that SmCYP90F1 is ancestral to the SmCYP90Es, and that SmCYP90E1 and SmCYP90E2 were duplicated relatively recently (Figure 1A). Selaginella CYPs clustered primarily with CYP90C1 and CYP90D1 of Arabidopsis, which catalyze the C-23 hydroxylation steps in the BR biosynthetic pathways [35], [36].
Figure 1. Phylogenetic tree and schematic representation of select CYP sequences.
(A) Genomic DNA sequences were aligned using ClustalW, and the phylogenetic tree was generated using the Neighbor-joining method. The unit bar represents relative evolutionary distance. The Arabidopsis CYP51 sequence was used as an outgroup. (B) Schematic illustration of CYP genes. Filled boxes represent exons, and thinner boxes at the 5′ of 3′ ends indicate untranslated sequences. The base ‘A’ at the translation start codon was set to 1. All of the compared CYPs, except AtCYP51, consist of 7∼10 exons, characterizing BR biosynthetic genes belonging to the CYP85 clan.
Previously, it was found that genes belonging to the CYP85 clan exhibit a scattered pattern of exons and introns [7]. For instance, AtCYP51A1 has only two exons separated by one intron [37], whereas all of the CYPs of the CYP85 clan possess seven to ten exons (Figure 1B). In contrast to the dispersed positioning of a relatively large number of exons in Arabidopsis CYPs, those of Selaginella had shorter introns, such that the length of genomic DNA spanning all of the exons and introns of Selaginella was more compact than that of Arabidopsis. In addition, we observed that the CYP genes often exhibited subtle differences in exon patterning; the seventh and eighth exons in the 5′ end of SmCYP90F1 were combined into a single exon in SmCYP90E3 (Figure 1B). The degree of sequence identity and similarity in the organization of the exons and introns suggests that the four CYP90s in Selaginella might be involved in BR metabolism. Future molecular complementation tests showing that Selaginella CYP90 sequences rescue a specific Arabidopsis BR dwarf mutant may pinpoint their function in BR biosynthesis.
Subcellular Localization of CYP90E2 and CYP90F1
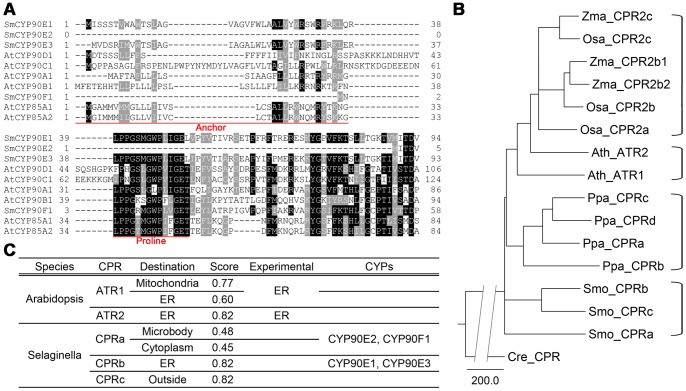
A multiple sequence alignment of the CYP sequences revealed that CYP90E2 and CYP90F1 lack signal peptides that are required for targeting to the endoplasmic reticulum (ER) (Figure 2A). Thus, we postulated that these two proteins might function elsewhere in the cell. To test this idea, we predicted the subcellular localizations of these proteins using the PSORT program. CYP90E1 and CYP90E3 were found to localize to the ER with a score of above 0.8, where 1 indicates full certainty; however, CYP90E2 and CYP90F1 had a greater probability of localizing to the microbody (peroxisome) or cytoplasm than to the ER (Figure 2B). Some ER-targeted proteins are known to lack typical signal sequences [38]. Since cytochrome P450reductases (CPRs) catalyze the transfer of electrons from NAD(P)H to CYPs and therefore co-localize with the CYPs [39], we would expect CPRs to co-localize with CYP90E2 and CYP90F1 at positions other than the ER.
Figure 2. Predicted subcellular localization of CYPs and their putative reductases.
(A) Multiple sequence alignment of CYP amino acid sequences from Selaginella and Arabidopsis. Sequences corresponding to functional domains identified in AtCYP90B1 are underlined. Box shading was carried out using BOXSHADE 3.21. Dashes represent gaps. Letters in black and gray backgrounds indicate 100% and 50% conservation among the sequences, respectively. The N-terminal regions up to the first 124 amino acids are shown. (B) Prediction of the subcellular localization of the NADPH-cytochrome P450 reductases (CPRs). The PSORT program was used to predict and score the calculation. (C) Phylogenetic tree of CPRs from Arabidopsis, maize, rice, Physcomitrella, and Selaginella. CPRs from the same species or from Zea mays (Zm) and Oryza sativa (Os) combined are bracketed on the right. The Chlamydomonas reinhardtii CPR sequence was used as an outgroup. The broken line for the outgroup indicates a genetic distance of 400. Scale bar = genetic distance of 200.
Based on amino acid sequence similarity, we found that the Selaginella genome contains three putative CPR genes (Figure 2C), whereas Arabidopsis contains two and Physcomitrella patens four (Figure 2C). Interestingly, the PSORT program predicted that each of the three Selaginella CPRs (Smo_CPRs) have different cellular localizations; type A CPR (Smo_CPRa) is predicted to localize to the cytoplasm or microbody with similar certainty, Smo_CPRb to the ER, and Smo_CPRc to the extracellular matrix (outside) (Figure 2B). The finding that both the CYPs that lack signal sequences (i.e., CYP90E2 and CYP90F1) and their putative reductases are predicted to localize to positions other than the ER suggests that these CYPs function in subcellular positions other than the ER membrane.
Brassinolide Stimulates Growth, whereas Propiconazole Suppresses Growth
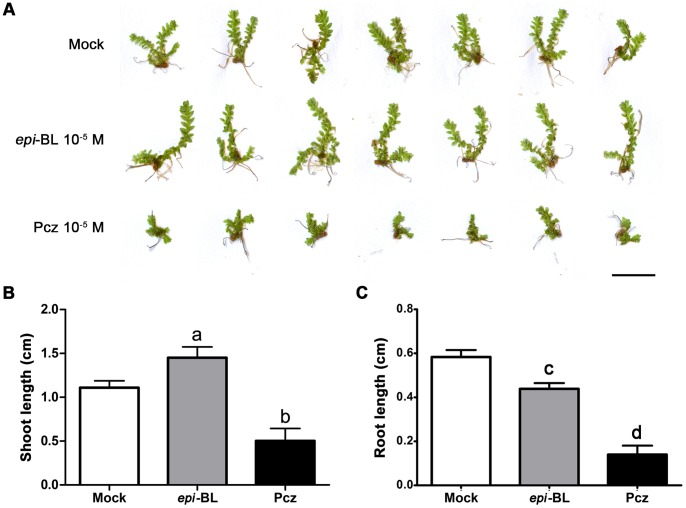
To understand how BRs affect Selaginella growth, we measured the growth responses of Selaginella after treatments with epi-brassinolide (epi-BL) and the BR biosynthetic inhibitor, propiconazole (Pcz) [40]. Compared with the mock control, the lengths of the shoots treated with 10−5 M epi-BL were longer after two weeks, whereas 10−5 M Pcz-treated seedlings were shorter (Figure 3A). Interestingly, the lengths of the two branches of mock-treated seedlings were similar, whereas epi-BL treatment tended to elongate one branch (Figure 3A). The elongation of the epi-BL-treated shoots was statistically significant (Figure 3B). Relative to the mock control, the shoots of plants treated with 10−5 M epi-BL were approximately 50% longer than those of the mock-treated control. However, the shoots of seedlings treated with10−5 M Pcz were approximately half as long as those of the control (Figure 3B). We measured the lengths of the roots, too. Different from the shoots, epi-BL-treated roots were significantly shorter than those of mock-treated plants. The Pcz-treated roots were also shorter than those of mock treatment. Inhibition of root growth by higher concentration of epi-BL is similar as what we observe in higher plants like Arabidopsis, suggesting that the physiological responses are conserved both in Selaginella and Arabidopsis. Considering that gibberellins do not induce significant elongation of Selaginella shoots [32], we hypothesize that BRs play a major role in Selaginella shoot growth.
Figure 3. Response of Selaginella to epi-brassinolide (epi-BL) and propiconazole (Pcz).
(A) Morphologies after treatment with mock, 10−5M epi-BL, and 10−5M Pcz for two weeks. Scale bar = 0.5 cm. (B) Average shoot lengths after treatment. Letters above each bar indicate significant differences compared with the mock treatment. Statistical significance was determined using one-way ANOVA (P<0.01, n = 7). Error bars are standard deviations. (C) Average root lengths after treatment. Letters above each bar indicate significant differences compared with the mock treatment. Statistical significance was determined using student t-test (P<0.01, n≥3). Error bars are standard deviations.
Analysis of Endogenous BRs
Based on our identification of putative Selaginella BR biosynthetic genes, it is likely that BRs are biosynthesized in Selaginella. We therefore searched for BRs in the aerial tissues of Selaginella; however, the levels of known BR biosynthetic intermediates were below detectable limits. Most BR intermediates found in Arabidopsis and rice were not detectable or only present at very low levels. However, there is still a possibility that uncommon BRs might be produced in Selaginella at greater levels than ordinary BRs. If this is the case, the chemical structures of the putative BRs will need to be determined before they can be quantified precisely.
Similarity-based Identification of BR Signaling Genes
Once BRs are synthesized endogenously and act as signaling molecules, specific receptors perceive and transmit the signals. To determine if the signaling components of Arabidopsis also exist in Selaginella, we searched the Selaginella database using Arabidopsis BR signaling genes as probes. To identify homologs, we considered the degree of sequence identity, the phylogenetic tree, the probability determined in the BLAST analysis, and the domain structures of the protein sequences. Table 2 summarizes the findings of our BLAST search.
Table 2. Identification of BR signaling components by comparative sequence analysis.
| Signaling component | Arabidopsis locus | Selaginella locus | Identity (%) | Probability (e value) |
| BRI1 (4) | At4G39400 | 99902 | 37.00 | 5.00E-166 |
| BAK1 (5) | At4G33430 | 85471 | 73.70 | 0 |
| 167872 | 58.70 | 3.50E-172 | ||
| 268032 | 82.40 | 1.50E-171 | ||
| 85818 | 55.80 | 5.50E-164 | ||
| BKI1 | At5G42750 | – | – | – |
| BSK1 (3) | At4G35230 | 103506 | 72.80 | 0 |
| CDG1 | At3G26940 | 13751 | 61.60 | 7.00E-126 |
| 99936 | 61.50 | 1.60E-124 | ||
| 79963 | 53.10 | 2.50E-98 | ||
| 151233 | 51.60 | 2.40E-93 | ||
| 109804 | 50.50 | 7.10E-93 | ||
| BIN2 (3) | At4G18710 | 159063 | 79.50 | 0 |
| 132906 | 78.30 | 0 | ||
| BSU1 | At1G03445 | 438528 | 45.00 | 7.10E-180 |
| BZR1 | At1G75080 | 437216 | 77.80 | 2.6E-22 |
| BES1 | At1G19350 | 437216 | 79.20 | 8.50E-23 |
| PP2A B’β | At3G09880 | 267276 | 74.10 | 1.00E-178 |
| 63731 | 71.30 | 2.30E-168 | ||
| 99313 | 70.40 | 6.20E-164 | ||
| 149261 | 64.90 | 3.20E-150 |
’β (SERINE/THREONINE PROTEIN PHOSPHATASE 2A B’β). Percent identity values were obtained after performing a BLASTX search of the Phytozome database. The number of Arabidopsis gene copies is given in parentheses after each locus name. Abbreviations: BRI1 (BRASSINOSTEROID INSENSITIVE1), BAK1 (BRI1-ASSOCIATED RECEPTOR KINASE1), BKI1 (BRI1 KINASE INHIBITOR1), BSK1 (BR-SIGNALING KINASE1), CDG1 (CONSTITUTIVE DIFFERENTIAL GROWTH1), BIN2 (BRASSINOSTEROID-INSENSITIVE 2), BSU1 (BRI1 SUPPRESSOR1), BZR1 (BRASSINAZOLE-RESISTANT1), BES1 (BRI1-EMS-SUPPRESSOR1), BSK1 (BR-SIGNALING KINASE1), and PP2A B
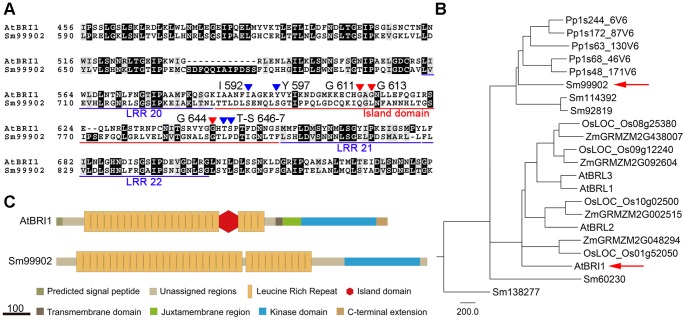
Our results suggest that it is unlikely that Selaginella has a functional homolog of Arabidopsis BRI1. The most similar protein in Selaginella, 99902, shared only 37% sequence identity with Arabidopsis BRI1 (Table 2). The amino acid residues identified as being important by mutation analysis of the BR-binding island domain were not conserved in Selaginella (Figure 4A) [41], [42], suggesting that the Selaginella protein does not contain the island domain (Figure 4B). Phylogenetic analysis also showed that the most similar protein forms a separate cluster from Arabidopsis BRI1 and BRI1-like proteins (Figure 4C). Therefore, it is not likely that 99902 has similar functions to Arabidopsis BRI1 and BRI1-like proteins.
Figure 4. Comparison of Arabidopsis BRI1 (AtBRI1) with its closest homolog from Selaginella.
(A) Multiple sequence alignment between AtBRI1 and the Selaginella sequence Sm99902 was performed using ClustalW. Underlining delimits the domains. Red triangles indicate residues identified as being functionally important by mutation studies [41] and blue triangles are key residues that participate in BL binding [42]. (B) Schematic representation of the domains identified in AtBRI1. A key for the colors and shapes used is provided below the diagram. Scale bar = 100 bp. (C) Phylogenetic tree of AtBRI1 and closely related sequences from maize, rice, Physcomitrella, and Selaginella. Arrows indicate AtBRI1 and Sm99902. Scale bar = genetic distance of 200.
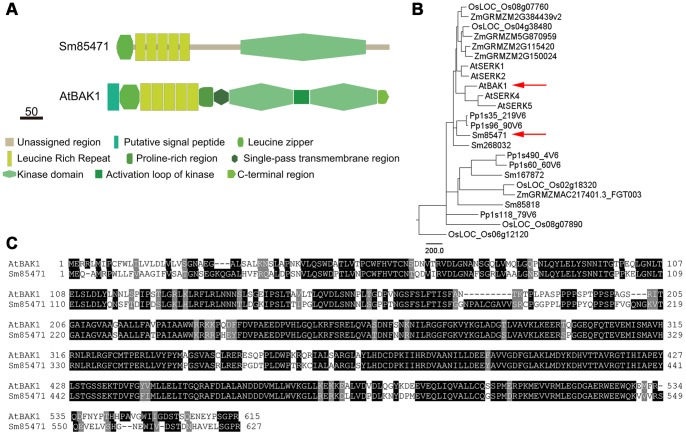
The Selaginella sequence most similar to Arabidopsis BAK1 was 85471, which shared 73% identity and a probability value of 0 (Table 2). A comparison of protein domains revealed that both BAK1 and 85471 have five leucine-rich repeats and a cytoplasmic kinase domain (Figure 5). These results suggest that 85471 is a homolog of Arabidopsis BAK1. However, in addition to its role as a BR co-receptor, BAK1 functions in the plant immune response [43]. Therefore, it would be interesting to conduct a functional analysis of 85471 to determine whether it also has multiple roles and to confirm its involvement in BR signaling.
Figure 5. Comparison of Arabidopsis BAK1 (AtBAK1) with its homologs in Selaginella.
(A) Functionally important domains, including the leucine-rich repeat and protein kinase domain, are conserved. (B) Phylogenetic analysis of BAK1-like protein sequences. Arrows indicate AtBAK1 and its close homolog Selaginella 85472 (Sm85471). (C) Multiple sequence alignment of AtBAK1 and Sm85471.
It is likely that the Selaginella sequence 438528 is a homolog of Arabidopsis BSU1, because of its relatively high level of sequence identity (45%), low probability value (7.1E-180) (Table 2), conserved domain structure, and clustering pattern in the phylogenetic tree (Figure S1). Arabidopsis BSU1 functions as a protein phosphatase that uses BIN2 as a substrate to repress its negative role in signaling pathways. Furthermore, a homolog of BIN2 seems to exist in Selaginella. A Phytozome database search using Arabidopsis BIN2 as probe identified Selaginella sequence 159063, which had an e-value of 0. In addition, Selaginella counterparts of Arabidopsis BZR1, BSK1, and PP2A are present (Table 2).
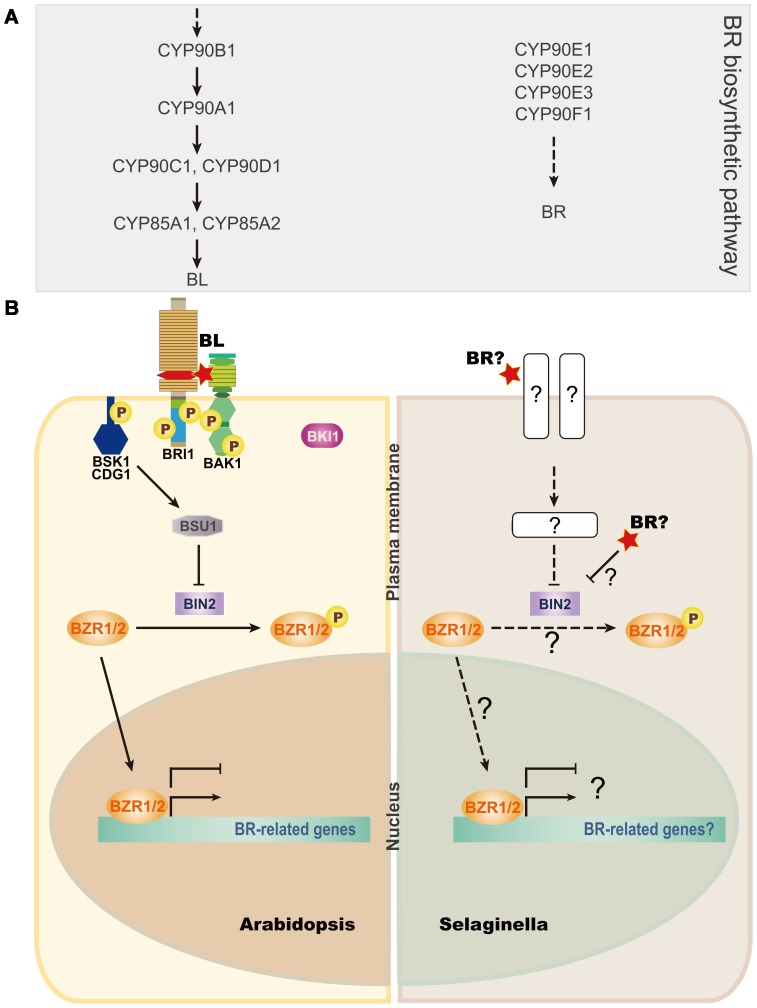
In conclusion, BR biosynthetic pathways exist in Selaginella, based on the presence of putative BR biosynthetic genes (i.e., three CYP90Es and CYP90F). Interestingly, it appears that BR biosynthesis occurs in the cytoplasm in Selaginella, as neither CPRs nor two CYP proteins (i.e., CYP90E2 and CYP90F1) are targeted to the ER, which is the site of BR biosynthesis in Arabidopsis [38]. If BRs are indeed synthesized at positions other than the ER, the transport and secretion pathways of synthesized BRs is also expected to be different. One possibility is that the synthesized BRs are immediately used in the steroidogenic cells, instead of being transported out of the cells.
Interestingly, we found that the typical membrane receptor complex for BRs in Arabidopsis, consisting of BRI1, BAK1, BKI, and BSK, is not present in Selaginella (Figure 6). However, the downstream signaling components, including BIN2, BSU1, and BZR1, seem to be present in Selaginella. Therefore, at least two possible scenarios could explain BR signaling in Selaginella. First, BRs may be perceived by a receptor complex that is possibly located in the plasma membrane, similar to that in Arabidopsis, and remains to be discovered. Second, BRs may be perceived directly by BIN2, by some unknown mechanism, and the signals may be transmitted to regulate gene expression through BR-specific transcription factors, including BZR1. In either case, it seems likely that BR biosynthetic and signaling pathways have evolved in Arabidopsis to include additional BR biosynthetic enzymes and a plasma membrane-localized receptor complex. In fact, it was shown that the ultimate step in the BR biosynthetic pathways in Arabidopsis is absent in monocot plants, including rice [44], suggesting that BR biosynthetic pathways evolved to meet the needs of individual plant species. Functional analyses of the putative BR genes in Selaginella identified here will provide insight into the evolution and roles of BRs in Selaginella and Arabidopsis.
Figure 6. Parallel comparison of BR biosynthetic and signaling pathways.
(A) Pathway showing the order in which Arabidopsis BR biosynthetic CYPs function. Selaginella CYP90s are shown to the right. (B) Model comparing Arabidopsis (left) with Selaginella (right) signaling pathways. Abbreviations are given beneath Table 2. P indicates phosphorylation. Solid lines indicate established signaling routes, whereas broken lines show possible routes. Flat-tipped and pointed arrows indicate inhibitory and positive actions, respectively. Question marks indicate steps that remain to be characterized.
Methods
Plant Growth and Morphometric Analysis
Aerial tissues of Selaginella moellendorffii grown in soil for two years in a greenhouse were harvested for analysis of endogenous BR levels. The tissues, including strobili, microphylls, ligules, spores, and bulbils, were extracted twice with 300 mL of methyl alcohol, and spiked with deuterium-labeled internal standards for quantification of BR biosynthetic intermediates. Purification and quantification of BRs were performed according to a previously described method [45].
For sterile growth, bulbils were sterilized for 12 min by shaking in a sterilization solution consisting of 50% Clorox and 0.1%Triton X-20, and then washed five times with sterile water. The bulbils were sprinkled on 0.65% agar-solidified media containing 0.5X Murashige and Skoog salts (pH 6.5). Plates containing bulbils were grown on the bench top at room temperature and illuminated with light at 7 µmol m−2 s−1. After two weeks of growth, germinated bulbils were transferred to media supplemented with 10−5 M propiconazole (Pcz) or epi-brassinolide (epi-BL). Bulbils were grown on the supplemented media for two more weeks and then subjected to morphometric analysis.
Phylogenetic and Schematic Analysis
The amino acid sequences of the genes involved in the BR biosynthetic and response pathways were extracted from the Phytozome database (http://www.phytozome.net). Using the ClustalW function of the Jalview program in Phytozome, a multiple sequence alignment was performed and analyzed, and phylogenetic trees were generated using the Neighbor-joining method based on a BLOSOM62 matrix. To visualize the phylogenetic trees, the Figtree program was used [46].
To draw the exon map shown in Figure 1, the cDNA sequences were aligned with the genomic DNA sequences to delimit the borders of exons and introns. To estimate the subcellular localizations of the proteins, the protein sequences were submitted to the PSORT program (http://psort.hgc.jp/). The results are summarized in Figure 2B. To analyze functional similarities of the compared proteins, schematics of the proteins showing defined protein domains were generated [7], [47], [48].
Supporting Information
Comparison of Arabidopsis BSU1 (AtBSU1) and BES1 (AtBES1) with their close homologs in Selaginella. (A) Schematic comparison of AtBSU1 and Selaginella 438528 (Sm438528). (B) Phylogenetic analysis of BSU1-like protein sequences. Arrows indicate AtBSU1 and its close homolog Sm438528. (C) Schematic comparison of AtBES1 and Selaginella 437216 (Sm437216). (D) Phylogenetic analysis of BES1-like protein sequences. Arrows indicate AtBES1 and its close homolog Sm437216. Scale bars represent 100 and 50 bp, respectively, in (A) and (C). Scale bar = genetic distance of 200.
(TIF)
Acknowledgments
We would like to thank J. Banks and G. Na for providing Selaginella tissues, and S. Takatsuto for supplying deuterium-labeled internal standards for BR analysis.
Funding Statement
This research was supported, in part, by grants from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ008051), Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ906910), funded by the Rural Development Administration (to S.C.); Technology Development Program (110033-5) for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea (to S.C.); the BK21 Research Fellowships (to J.C.), funded by the Ministry of Education, Republic of Korea; and a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19380069 and 23380066 to S.F.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kwon M, Choe S (2005) Brassinosteroid biosynthesis and dwarf mutants. J Plant Biol 48: 1–15. [Google Scholar]
- 2. Chung Y, Choe S (2013) The Regulation of Brassinosteroid Biosynthesis in Arabidopsis. Crit Rev Plant Sci 32: 396–410. [Google Scholar]
- 3. Choe S, Tanaka A, Noguchi T, Fujioka S, Takatsuto S, et al. (2000) Lesions in the sterol delta reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J 21: 431–443. [DOI] [PubMed] [Google Scholar]
- 4. Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, et al. (1999) The Arabidopsis dwf7/ste1 mutant is defective in the delta7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell 11: 207–221. [PMC free article] [PubMed] [Google Scholar]
- 5. Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, et al. (1999) The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol 119: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401. [DOI] [PubMed] [Google Scholar]
- 7. Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, et al. (1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, et al. (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26: 573–582. [DOI] [PubMed] [Google Scholar]
- 9. Kim B, Kim G, Fujioka S, Takatsuto S, Choe S (2012) Overexpression of 3beta-hydroxysteroid dehydrogenases/C-4 decarboxylases causes growth defects possibly due to abnormal auxin transport in Arabidopsis. Mol Cells 34: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, et al. (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182. [DOI] [PubMed] [Google Scholar]
- 11. Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, et al. (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602. [DOI] [PubMed] [Google Scholar]
- 12. Kim TW, Hwang JY, Kim YS, Joo SH, Chang SC, et al. (2005) Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 17: 2397–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, et al. (1999) The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci U S A 96: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizutani M, Ohta D (2010) Diversification of P450 genes during land plant evolution. Annu Rev Plant Biol 61: 291–315. [DOI] [PubMed] [Google Scholar]
- 15. Hamberger B, Bak S (2013) Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Philos Trans R Soc Lond B Biol Sci 368: 20120426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson D, Werck-Reichhart D (2011) A P450-centric view of plant evolution. Plant J 66: 194–211. [DOI] [PubMed] [Google Scholar]
- 17. Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, et al. (1996) P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6: 1–42. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938. [DOI] [PubMed] [Google Scholar]
- 19. He Z, Wang ZY, Li J, Zhu Q, Lamb C, et al. (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363. [DOI] [PubMed] [Google Scholar]
- 20. Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, et al. (1999) Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Chory J (2006) Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 22. Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212. [DOI] [PubMed] [Google Scholar]
- 23. Chung Y, Choe V, Fujioka S, Takatsuto S, Han M, et al. (2012) Constitutive activation of brassinosteroid signaling in the Arabidopsis elongated-D/bak1 mutant. Plant Mol Biol 80: 489–501. [DOI] [PubMed] [Google Scholar]
- 24. Tang W, Kim TW, Oses-Prieto JA, Sun Y, Deng Z, et al. (2008) BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim TW, Guan S, Burlingame AL, Wang ZY (2011) The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell 43: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46: 701–724. [DOI] [PubMed] [Google Scholar]
- 27. Choe S, Schmitz RJ, Fujioka S, Takatsuto S, Lee MO, et al. (2002) Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3beta-like kinase. Plant Physiol 130: 1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maharjan PM, Schulz B, Choe S (2011) BIN2/DWF12 Antagonistically Transduces Brassinosteroid and Auxin Signals in the Roots of Arabidopsis. J Plant Biol 54: 126–134. [Google Scholar]
- 29. He JX, Gendron JM, Yang Y, Li J, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A 99: 10185–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang W, Yuan M, Wang R, Yang Y, Wang C, et al. (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banks JA (2009) Selaginella and 400 million years of separation. Annu Rev Plant Biol 60: 223–238. [DOI] [PubMed] [Google Scholar]
- 32. Hirano K, Nakajima M, Asano K, Nishiyama T, Sakakibara H, et al. (2007) The GID1-mediated gibberellin perception mechanism is conserved in the Lycophyte Selaginella moellendorffii but not in the Bryophyte Physcomitrella patens. Plant Cell 19: 3058–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohnishi T, Yokota T, Mizutani M (2009) Insights into the function and evolution of P450s in plant steroid metabolism. Phytochemistry 70: 1918–1929. [DOI] [PubMed] [Google Scholar]
- 34. Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, et al. (2011) The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332: 960–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim GT, Fujioka S, Kozuka T, Tax FE, Takatsuto S, et al. (2005) CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J 41: 710–721. [DOI] [PubMed] [Google Scholar]
- 36. Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, et al. (2006) C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18: 3275–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim HB, Schaller H, Goh CH, Kwon M, Choe S, et al. (2005) Arabidopsis cyp51 mutant shows postembryonic seedling lethality associated with lack of membrane integrity. Plant Physiol 138: 2033–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szczesna-Skorupa E, Chen CD, Rogers S, Kemper B (1998) Mobility of cytochrome P450 in the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A 95: 14793–14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen K, Moller BL (2010) Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 71: 132–141. [DOI] [PubMed] [Google Scholar]
- 40. Hartwig T, Chuck GS, Fujioka S, Klempien A, Weizbauer R, et al. (2011) Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci U S A 108: 19814–19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vert G, Nemhauser JL, Geldner N, Hong F, Chory J (2005) Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol 21: 177–201. [DOI] [PubMed] [Google Scholar]
- 42. She J, Han Z, Kim TW, Wang J, Cheng W, et al. (2011) Structural insight into brassinosteroid perception by BRI1. Nature 474: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, et al. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A 104: 12217–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim BK, Fujioka S, Takatsuto S, Tsujimoto M, Choe S (2008) Castasterone is a likely end product of brassinosteroid biosynthetic pathway in rice. Biochem Biophys Res Commun 374: 614–619. [DOI] [PubMed] [Google Scholar]
- 45. Fujioka S, Takatsuto S, Yoshida S (2002) An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiol 130: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rambaut A (2007) FigTree [Online]. Available at http://tree.bio.ed.ac.uk/software/figtree/.
- 47. Weston SJ, Leistikow RL, Reddy KR, Torres M, Wertheimer AM, et al. (2005) Reconstitution of hepatitis C virus-specific T-cellmediated immunity after liver transplantation. Hepatology 41: 72–81. [DOI] [PubMed] [Google Scholar]
- 48. Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61: 681–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of Arabidopsis BSU1 (AtBSU1) and BES1 (AtBES1) with their close homologs in Selaginella. (A) Schematic comparison of AtBSU1 and Selaginella 438528 (Sm438528). (B) Phylogenetic analysis of BSU1-like protein sequences. Arrows indicate AtBSU1 and its close homolog Sm438528. (C) Schematic comparison of AtBES1 and Selaginella 437216 (Sm437216). (D) Phylogenetic analysis of BES1-like protein sequences. Arrows indicate AtBES1 and its close homolog Sm437216. Scale bars represent 100 and 50 bp, respectively, in (A) and (C). Scale bar = genetic distance of 200.
(TIF)