Abstract
Ewing’s sarcoma (EWS) is a pediatric cancer that is conventionally treated by surgery, chemotherapy, and radiation therapy. Innovative immunotherapies to treat EWS are currently under development. Unfortunately for EWS patients, when the disease is found to be resistant to current therapeutic approaches, the prognosis is predictably grim. Radiation therapy and immunotherapy could potentially synergize in the eradication of EWS, as some studies have previously shown that irradiation increases the presence of immune receptors, including MHC class I molecules, on the surface of tumor cells. However, EWS cells have been reported to express low levels of MHC class I molecules, a phenotype that would inhibit T-cell mediated lysis. We have previously demonstrated that the transgene-driven overexpression of amyloid β (A4) precursor-like protein 2 (APLP2) reduces the expression of MHC class I molecules on the surface of human cervical carcinoma HeLa cells. We thus examined whether endogenously expressed APLP2 downregulates MHC class I expression on EWS cells, particularly upon irradiation. We found that irradiation induces the relocalization of APLP2 and MHC class I molecules on the surface of EWS cells, redistributing cells from subpopulations with relatively low APLP2 and high MHC class I into subpopulations with relatively high APLP2 and low MHC class I surface expression. Consistent with these findings, the transfection of an APLP2-targeting siRNA into EWS cells increased MHC class I expression on the cell surface. Furthermore, APLP2 was found by co-immunoprecipitation to bind to MHC class I molecules. Taken together, these findings suggest that APLP2 inhibits MHC class I expression on the surface of irradiated EWS cells by a mechanism that involves APLP2/MHC class I interactions. Thus, therapeutic strategies that limit APLP2 expression may boost the ability of T cells to recognize and eradicate EWS in patients.
Keywords: Ewing’s sarcoma, HLA, MHC class I molecule, amyloid β (A4) precursor-like protein 2, immune evasion, immunotherapy, pediatric cancer, radiation therapy
Introduction
Ewing sarcoma (EWS) is a pediatric cancer that most often develops in the second decade of life.1 Typically, EWS is driven by chromosomal translocations generating EWS-FLI1 fusions, which encode aberrant transcription factors.2 The current approach to EWS involves surgery, radiation, and high-dose chemotherapy, all treatments with harsh side effects. While the 5-y survival rate for non-metastasized EWS is now around 60–70%, metastatic EWS only has a 25% 5-y survival rate.3,4 Thus, improved therapeutic approaches with high efficacy and limited side effects are urgently needed for EWS patients.
Many immunotherapeutic strategies are under development as alternative approaches to EWS, including cell-based strategies.5,6 Even in the absence of immunization, cytotoxic T lymphocytes (CTLs) that recognize EWS-associated antigens in the context of MHC class I molecules have been found in late-stage patients.7 However, EWS cells have been observed to exhibit variable, and often low, expression levels of MHC class I molecules on their surface.8,9 Because MHC class I molecules bind intracellular peptides and migrate to the cell surface for presenting such peptides to CTLs, reduced MHC class I expression levels constitute a means for cancer cells to avoid immunodetection and eradication.10,11 Notably, patients with cancers of the EWS family that express few or no MHC class I molecules appear to exhibit significantly reduced survival rates.12 Patients bearing osteosarcomas that express relatively low levels of MHC class I molecules were likewise found to exhibit poor survival rates.13 Thus, determining the molecular mechanisms by which sarcoma cells are able to limit MHC class I expression on the cell surface is necessary to develop therapeutic strategies that allow for their eradication by CTLs.
We have identified amyloid β (A4) precursor-like protein 2 (APLP2) as a protein that is amply expressed by several types of cancer cell lines, including EWS cell lines.14 APLP2 is a member of the amyloid precursor protein (APP) family, which has 3 members in mammals (APP, APLP1, and APLP2).15-17 These proteins have biochemical functions related to transcription, homeostasis, cell survival, growth, and migration.17-25 In addition, recent clues indicate that this family of proteins may regulate endocytosis. For example, APP has been demonstrated to facilitate the internalization of the high-affinity choline transporter.26 Furthermore, studies from our laboratory using HeLa (a human cervical adenocarcinoma cell line) have demonstrated that APLP2 increases the endocytosis of MHC class I molecules.14,27-31 Additional data from our previous studies indicate that APLP2 displays MHC allotype specificity. For example, we have reported that APLP2 binds more strongly to HLA-A2 than to HLA-A24 molecules in humans. Similarly, in mice APLP2 interacts more robustly with Kd, Db, and Dq than with Ld molecules.14,28
Based on the aforementioned findings, we hypothesized that APLP2 is responsible, at least in part, for restricting MHC class I expression on the surface of EWS cells, thus potentially contributing to EWS immune evasion. We report here that 2 EWS cell lines (TC71 and A673 cells) comprise cellular subpopulations displaying reciprocal surface expression levels of MHC class I molecules and APLP2. Since radiation therapy has previously been shown to upregulate surface-exposed MHC class I molecules and is clinically used to treat EWS patients,1,32,33 we also examined the impact of ionizing radiation on the expression of APLP2 and MHC class I molecules on the cell surface.
Altogether, our data indicate that APLP2 limits the expression of MHC class I molecules on the surface of EWS TC71 and A673 cells. Of particular clinical relevance, such a reduction was noted to persist even upon irradiation, a stimulus that effectively upregulated MHC class I expression on EWS cell subsets characterized by lower APLP2 levels but not on those with relatively high APLP2 surface expression. Thus, APLP2 might allow EWS cells to evade recognition by T cells, hence interfering with the ability of radiation therapy to facilitate T cell-mediated elimination of EWS. Our findings suggest that treatments reducing APLP2 expression may offer a means to improve the efficacy of endogenous immune responses and T cell-based immunotherapies against EWS. The identification of an endogenous subpopulation of APLP2high EWS cells that expresses low levels of MHC class I molecules even upon irradiation adds to the growing body of knowledge on tumor immunoevasion, which is necessary to understand in order to design effective immunotherapeutic regimens for EWS patients.
Results
APLP2 associates with MHC class I molecules in EWS cells
Previously, we demonstrated by immunoblotting that APLP2 is abundantly expressed by a variety of cancer cell lines, including EWS-derived cells.14 Furthermore, in an earlier study using HeLa cells, we found that APLP2 binds to MHC class I molecules at the cell surface and facilitates their endocytosis and routing to lysosomes.30 We surmised that if APLP2 also modulates the expression of MHC class I molecules on EWS cells, then their association would be demonstrable also in this cellular setting. To assess whether there is an interaction between APLP2 and MHC class I molecules in EWS cell lines, TC71 and A673 cells were lysed and MHC class I molecules were immunoprecipitated with the pan-human MHC class I antibody W6/32. As a control, the W6/32 immunoprecipitates were probed with an antibody recognizing MHC class I heavy chains (HC10) (Fig. 1, top panel). To detect MHC class I-associated APLP2, the immunoblots were probed with an APLP2-specific antiserum (Fig. 1, bottom panel). The presence of APLP2 in immunoprecipitates obtained with anti-MHC class I antibodies indicates that APLP2 is complexed with MHC class I molecules in EWS cells (Fig. 1).
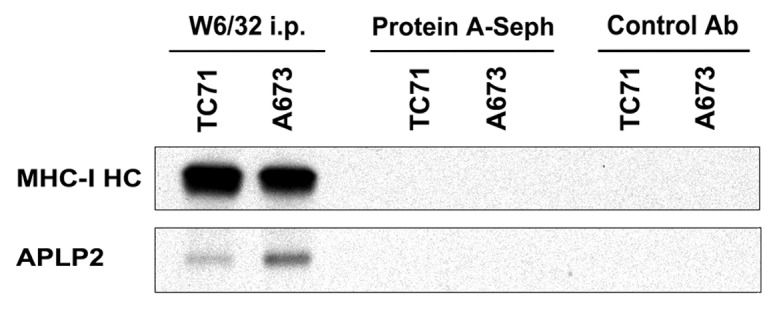
Figure 1. APLP2 is associated with MHC class I molecules in EWS cells. The W6/32 antibody was used to immunoprecipitate folded, MHC class I molecules from TC71 and A673 cell lysates. As a control, the procedure was also performed with no immunoprecipitating antibody (lanes labeled as “Protein A-Sepharose”) and with an irrelevant IgG (isotype control) antibody (anti-CD3 antibody, lanes labeled as “Control Ab”). Immunoprecipitates were then probed by immunoblotting with the HC10 antibody against the MHC class I heavy chain (MHC-I HC) and APLP2. The results shown are representative of 6 separate experiments.
APLP2 is expressed on the surface of EWS cells and ionizing radiation causes increased distribution of APLP2 to the surface of EWS cells.
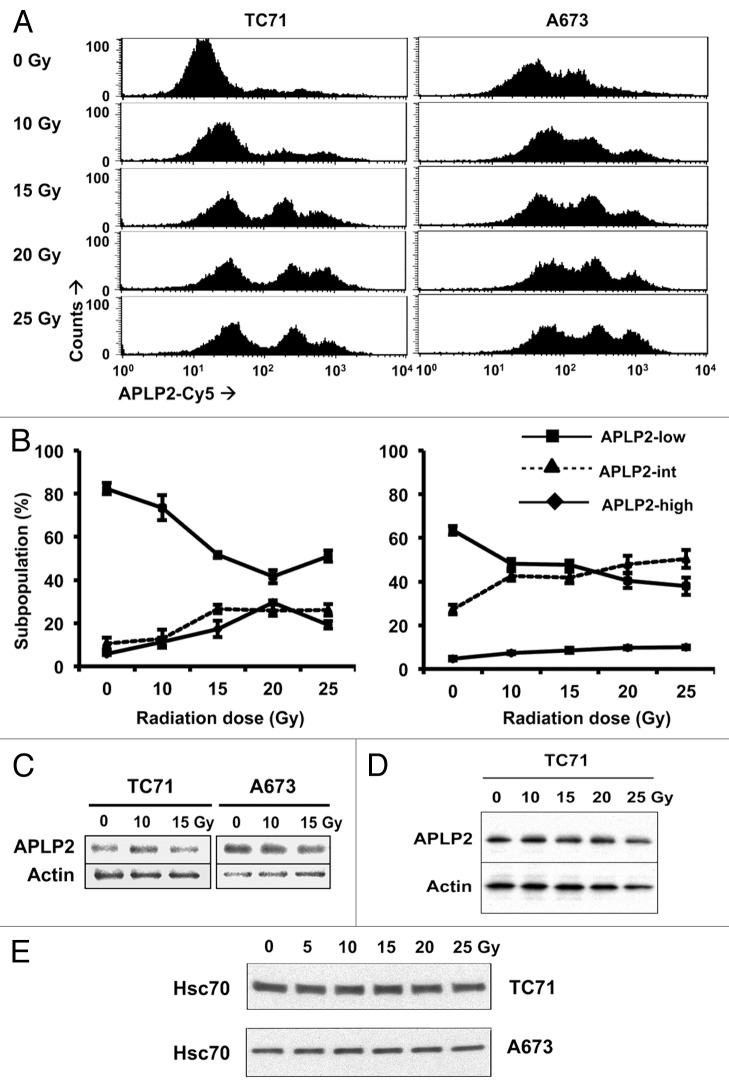
We also postulated that if APLP2 influences MHC class I expression by EWS cells through an endocytic mechanism, then APLP2 should be detectable on the surface of EWS cells. To determine whether APLP2 can be found at the surface of EWS cells, we examined the TC71 and A673 cell lines for APLP2 expression by flow cytometry. For both cell lines, three distinct profiles of surface APLP2 expression could be identified, observed as 3 regions on flow cytometry histograms, and representing three sub-populations of cells: low (APLP2low), intermediate (APLP2int) and high expressers (APLP2high) (Fig. 2A and B). Control data for these experiments, as obtained with isotype-matched antibodies, can be found in Figure S1.
Figure 2. APLP2 is expressed on the surface of EWS cells and can be redistributed by ionizing radiation. (A-E) Radiation shifts EWS cells toward subpopulations with high levels of surface-exposed APLP2, but does not increase total APLP2 expression. EWS cells were irradiated with the indicated dose of γ rays and cultured for 24 h prior to analysis. (A) Representative histograms of surface APLP2 expression on TC71 (left) and A673 (right) cells 24 h post-irradiation as determined by flow cytometry. (B) The mean frequencies of EWS cells with APLP2low (box), APLP2int (triangle) and APLP2high (diamond) phenotypes are shown with respect to radiation intensity. Error bars denote SD (n = 6 experimental replicates within 1 experiment that yielded results representative of several experiments (2 experiments including both TC71 and A673, 2 with A673 but not TC71, and 3 with TC71 but not A673). (C–E) Irradiated TC71 and A673 cells were lysed, and the lysate supernatants were subjected to immunoblotting to determine total expression levels of APLP2 (C and D). Actin levels were monitored to ensure equal lane loading in C and D. Separate experiments were performed to demonstrate that there is no decrease in the expression of another loading control, HSC70, in TC71 and A673 cells exposed to 25 Gy (E). Immunoblotting data are representative of n = 2-3 independent experiments yielding similar results.
Since radiation therapy is a standard approach to EWS, we also examined the effect of γ radiation on APLP2 surface expression. The majority of non-irradiated TC71 and A673 cells resided within the APLP2low subpopulation (Fig. 2A and B). By 24 h post-irradiation, fractions of both TC71 and A673 cells had shifted into subpopulations with considerably higher APLP2 surface levels, and even the APLP2low subpopulations showed slightly higher APLP2 surface expression (Fig. 2A and B). The observed redistributions from the APLP2low to the APLP2high subpopulations were dose-dependent in the range of 0 to 15 Gy, with relatively little alterations when high doses were employed (15–25 Gy) (Fig. 2A and B). The changes in the expression of APLP2 on the surface of irradiated EWS cells could not be ascribed to increased total APLP2 levels, as the immunoblotting of whole cell lysates showed no substantial alteration in total APLP2 amounts in either EWS cell line irradiated with up to 25 Gy (Fig. 2C and D). Although in cells exposed to 25 Gy there was a slight decrease in the levels of both APLP2 and actin, there was no decrease in an additional control protein, heat shock 70kDa protein 8 (HSPA8, also known as HSC70) (Fig. 2E). (The effect of irradiation on the survival of TC71 and A673 cells is illustrated in Figure S2. Additional data on this matter can be found in a recent publication from our laboratory,34 demonstrating that TC71 cells, but not A673 cells, exhibit a dose-dependent apoptotic response to irradiation.) Our results suggest that ionizing radiation increases the frequency of EWS cells that display high levels of APLP2 on their surface due to APLP2 relocalization from an intracellular compartment.
The level of cell-surface MHC class I molecules is reduced on APLP2high EWS cells
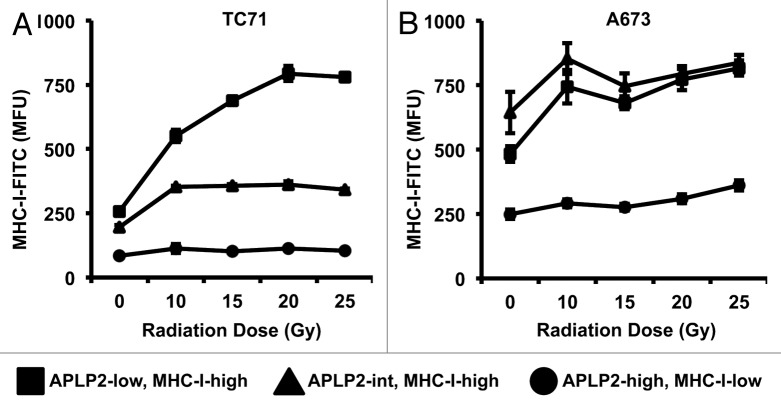
Several laboratories have reported that EWS cells express low amounts of MHC class I molecules on their surface.8,9,12 Since we had noted 3 subpopulations of EWS cells varying from each other with respect to surface APLP2 levels (Fig. 2A and B), and we had previously found that APLP2 can inhibit MHC class I expression on the surface of HeLa cells,30,31 we next investigated whether such 3 EWS subpopulations would exhibit distinct MHC class I expression profiles. In line with our previous findings, lower levels of MHC class I molecules were observed on the surface of APLP2high cells, as compared with APLP2low cells, in both the TC71 and A673 cell lines (Fig. 3, gating shown in Figure S3). Since irradiation resulted in a shift of EWS cells toward APLP2high cell subpopulations (Fig. 2A and B), we co-assessed MHC class I and APLP2 expression on irradiated TC71 and A673 cells. We identified 3 distinct subpopulations of cells within both EWS cell lines: APLP2lowMHCIhigh, APLP2intMHCIhigh, and APLP2highMHCIlow cells (Fig. 3; Figure S3). Ionizing radiation increased the amount of surface-exposed MHC class I molecules, especially on APLP2low cells (Fig. 3; Figure S3). MHC class I levels rose steeply on the surface of irradiated APLP2lowMHCIhigh TC71 (Fig. 3A, squares) and, to a lesser extent, A673 cells (Fig. 3B, squares). In contrast, the expression levels of MHC class I molecules on the surface of APLP2high MHCIlow cells did not markedly increase upon irradiation (Fig. 3A and B, circles), not even when the cells were exposed to 20–25 Gy. This indicates that EWS cell subpopulations that express high amounts of APLP2 fail to increase surface MHC class I molecules upon irradiation. Thus, even after exposure to ionizing radiation, APLP2highMHCIlow EWS cells maintain reduced expression of MHC class I molecules.
Figure 3. The APLP2high phenotype identifies EWS cells with consistently relatively low MHC class I surface expression upon irradiation. (A-B) EWS cells exposed to the indicated doses of γ rays were examined by flow cytometry for the co-expression of APLP2 and folded MHC class I molecules (using the W6/32 antibody) on their surface. Three cell subpopulations were identified: APLP2highMHCIlow, APLP2intMHCIhigh, and APLP2lowMHCIhigh cells. MHC class I-dependent fluorescence (in terms of mean fluorescence units, MFU) is depicted for each subpopulation of TC71 (A) and A673 (B) cells. Error bars denote SD (n = 6 experimental replicates within 1 representative experiment out of 3 independent ones performed that yielded similar results, with 1 experiment including both TC71 and A673, and 2 experiments including 1 of the 2 cell lines). Representative dot plots can be found in Figure S3.
Reducing APLP2 expression in EWS cells by siRNA increases the expression of MHC class I molecules on the cell surface
To further investigate whether APLP2 actively inhibits MHC class I expression on EWS cells, we downregulated APLP2 expression in TC71 and A673 cells by transiently transfecting them with an APLP2-specific siRNA. At 48 h post-transfection, the transfected cells were harvested, and reduced APLP2 expression was confirmed by immunoblotting (Fig. 4A). The downregulation of APLP2 increased MHC class I expression on the surface of both TC71 and A673 cells, as demonstrated by flow cytometry analysis of cells immunostained with the anti-human MHC class I antibody W6/32 or an antibody specific for the HLA-A2 allotype (BB7.2) (Fig. 4B). Taken together with our findings on naturally occurring EWS cell subsets characterized by the reciprocal expression of APLP2 and MHC class I molecules, these functional data implicate endogenous APLP2 in the downregulation of MHC class I expression on the surface of EWS cells.
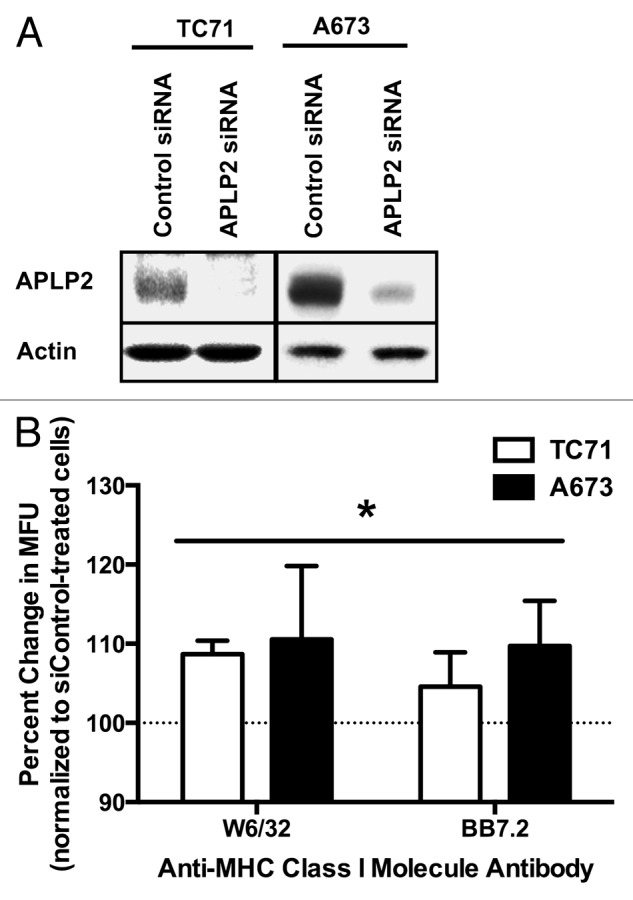
Figure 4. APLP2 downregulation increases MHC class I surface expression on EWS cells. (A-B) TC71 and A673 EWS cells were transfected with a siRNA pool targeting APLP2 or a non-targeting siRNA pool (as a negative control) for 48 h. (A) APLP2 downregulation upon transfection with APLP2-specific siRNA was confirmed by immunoblotting. Actin levels were monitored to ensure proper lane loading. (B) Surface-exposed MHC class I molecules were detected by flow cytometry on TC71 (black bars) and A673 (white bars) cells transfected with APLP2-specific or control siRNAs. The pan-human MHC-reactive W6/32 antibody and the allotype-specific BB7.2 antibody were used to detect MHC class I molecules and HLA-A2 molecules, respectively. The bar graphs depict the percent change (means ± SD) in MHC class I-dependent fluorescence (in terms of mean fluorescence units, MFU) elicited with APLP2-specific siRNAs (with cells transfected with control siRNA represented by 100%, as indicated by the dashed line). Individual MFUsiAPLP2/MFUsiControl results were obtained from paired samples, and data from 3 independent experiments were pooled. For each cell line and each antibody, siAPLP2 transfection (compared to siControl transfection) resulted in a significantly higher surface MHC class I level. Statistical significance was determined by means of unpaired Student t-test (*p < 0.05). The values used for derivation of this graph are presented in Table S1.
Discussion
Here, we have reported the identification of potentially clinically relevant endogenous subpopulations of EWS cells that express distinct levels of surface APLP2 and MHC class I molecules. We found that the TC71 and A673 subsets with the lowest (APLP2low) and second lowest (APLP2int) levels of surface APLP2 express slightly higher amounts of surface APLP2 in response to irradiation (as evinced by the rightward shift in the histograms illustrated in Figure 2A). Furthermore, upon irradiation, the proportion of APLP2low TC71 and A673 cells drops, while the fraction of cells in the APLP2int and APLP2high subsets rises. Although this phenotype is consistent for both of these cell lines, the extent of the redistribution from the APLP2low to the APLP2int and APLP2high subpopulations is more dramatic for TC71 than for A673 cells.
Among these 3 cell subsets with disparate levels of surface-exposed APLP2, 2 subpopulations (APLP2low and APLP2int) exhibit relatively high expression of MHC class I molecules on the surface. Conversely, APLP2high cells display low amounts of MHC class I molecules on their surface (Fig. 3; and Figure S3). APLP2 binds to MHC class I molecules in EWS cell lines even in the absence of irradiation (Fig. 1). Upon irradiation, the expression of APLP2 and MHC class I molecules on the surface of EWS cells is altered, resulting in a shift of cells from the APLP2lowMHCIhigh to the APLP2highMHCIlow subset (Fig. 3; and Figure S3). Although in our experiments the expression of these proteins on the cell surface was not tracked chronologically, the existence of an APLP2intMHCIhigh subpopulation suggests that high surface APLP2 levels may precede the reduction of surface MHC class I expression. This scenario is consistent with our model, theorizing that the presence of APLP2 at the cell surface is required for the APLP2-mediated endocytosis of MHC class I molecules.29-31
The fact that irradiation redistributes EWS cells among subpopulations with distinct levels of surface-exposed APLP2 and MHC class I molecules (Fig. 3; Figure S3) suggests that EWS cells exhibit a high degree of plasticity, rather than a static phenotypic profile, allowing them to exchange dynamically among these subsets. Further investigation into the plasticity of these cell subpopulations could generate insights of potential therapeutic relevance. In this context, it is of particular importance that the expression of MHC class I molecules was upregulated by irradiation only on APLP2lowMHCIhigh cells, and not on APLP2highMHCIlow cells.
The radiation-induced upregulation of MHC class I molecules on the surface of APLP2low and APLP2int EWS cells may reflect multiple alternative (but not mutually exclusive) mechanisms. For APLP2low and APLP2int cells, the relative lack of APLP2 molecules available to bind to MHC class I molecules at the surface and increase their endocytosis may allow the rise in cell-surface MHC class I expression after irradiation; this would be consistent with our previous findings with HeLa cells.30 Cytokine secretion following irradiation could be an alternative explanation, since an increase in the levels of MHC class I molecules on the surface of irradiated breast cancer cells has been attributed to the release of interferon (IFN)β upon DNA damage.35 In another study based a mouse melanoma model, local radiation was shown to result in the release of IFNγ into the tumor microenvironment, a signal that led to the upregulation of MHC class I molecules on the surface of the malignant cells.36 Reits et al. demonstrated that the amounts of MHC class I molecules are elevated on the surface of irradiated melanoma and colon adenocarcinoma cells due to increased intracellular protein degradation (resulting in increased production of MHC class I-binding peptides), and also as a result of enhanced translation upon the activation of the mammalian target of rapamycin (mTOR) pathway.32 Other studies have found that irradiation increases the expression of MHC class I molecules, as well as other immune receptors, on the surface of cancer cells in situ or in culture.33,37
Studies based on melanoma models have demonstrated that even a partial reduction of surface-exposed MHC class I molecules can decrease the functional recognition of malignant cells by CTLs.10,11 Many tumors exhibit a partial downregulation of MHC class I molecules in vivo, since the complete absence of these proteins may leave malignant cells unprotected from attacks by natural killer (NK) cells.38 Indeed, minor changes in the surface expression of MHC class I molecules within a particular range have recently been shown to have a dramatic impact on the ability of NK cells to lyse EWS cells.39 Therefore, owing to reduced levels of surface MHC class I molecules, APLP2highMHCIlow cells may be relatively resistant to CTLs, but susceptible to the cytotoxic activity of NK cells. In agreement with this reasoning, mice inoculated with the ES8 EWS cell line exhibited improved survival rates when radiation therapy was combined with immunotherapy using expanded NK cells.40
In our studies, we have examined total MHC class I surface expression, but not yet whether particular MHC class I allotypes, such as HLA-A2, are evenly distributed throughout the APLP2-MHC class I-defined EWS cell subpopulations. Allotypic differences would be consistent with the model proposing that APLP2-mediated endocytosis reduces the levels of surface-exposed MHC class I molecules, since we have previously shown APLP2 to have an avidity for MHC class I molecules that varies with allotype.26 Earlier studies of MHC class I expression on EWS have shown that MHC molecules tend to be present on cells from primary sarcomas, but that their expression level is highly variable and can be particularly low on advanced sarcomas.41,42 These findings may relate to the facts that the downregulation of MHC class I molecules on cancer cells is often specific for particular HLA alleles,8 and that various EWS cell lines manifest differential, allotype-specific increases in MHC class I molecule expression in response to IFNγ.43
Changes in the expression levels of immune-related molecules that occur upon radiation therapy are clinically relevant. Here, we examined the impact of ionizing radiation on the expression of APLP2 and MHC class I molecules at the surface of EWS cells, finding that the presence of elevated levels of surface APLP2 correlates with the inability of EWS cells to upregulate MHC class I at the surface in response to irradiation. We further demonstrated that APLP2 expression levels inversely correlate with the amount of MHC class I molecules exposed on the surface of EWS cells. EWS cells (and other cancer cells) must maintain the levels of surface-exposed MHC class I molecules within a tight threshold, to avoid antitumor immune defenses. Our analyses suggest that APLP2 has a regulatory role in restricting MHC class I expression on the surface of EWS cells. In support of this notion, we have demonstrated that APLP2 binds to MHC class I molecules in EWS cells, and that the siRNA-mediated knockdown of APLP2 increases the amount of surface-exposed MHC class I molecules.
MHC class I molecules are so critical for the recognition of EWS and other neoplasms by CTLs that cancer cells often downmodulate the expression of these proteins to evade immunosurveillance. The downregulation of MHC class I expression on EWS cells by epigenetic mechanisms has been previously described,43,44 and our findings indicate that the APLP2-mediated turnover of MHC class I molecules at the cell surface may represent an additional mechanism of post-transcriptional reduction of surface MHC class I molecules. Our data add to the compendium of knowledge elucidating how the expression of MHC class I molecules is regulated in malignant cells, and are expected to facilitate the development of efficient CTL- and NK cell-based immunotherapeutic strategies to combat EWS.
Materials and Methods
Cell culture
The EWS cell line A673 was purchased from ATCC (Cat. No. CRL-1598), and the EWS TC71 cell line was a generous gift from Dr. Eugenie Kleinerman at MD Anderson Cancer Center.45 The A673 cell line was cultured in Dulbecco’s modified Eagle medium (DMEM, from Life Technologies, Cat. No. 11965) supplemented with 10% v/v fetal bovine serum (Atlanta Biologicals, Cat. No. S11150) and the following additives (from Invitrogen): 1 mM sodium pyruvate (Cat. No. 11360), 2 mM l-glutamine (Cat. No. 25030), and 100 units/mL penicillin plus 100 μg/mL streptomycin (Cat. No. 15140). The TC71 cell line was cultured in RPMI 1640 medium (Thermo Fisher Scientific, Cat. No. 10-04-CV) supplemented with 15% v/v fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine, and 100 units/mL penicillin plus 100 μg/mL streptomycin.
Antibodies
For the detection of MHC class I molecules by flow cytometry, the W6/32 and BB7.2 antibodies were used, while the HC10 antibody was used for immunoblotting. HC10 is a monoclonal antibody that recognizes the unfolded heavy chain of the human MHC class I molecule.46-48 W6/32 is a conformation-specific monoclonal antibody specific for folded, β2-microglobulin-associated, MHC class I molecules.48-50 BB7.2 is an antibody recognizing HLA-A2.51 HC10, W6/32, and BB7.2 antibodies were produced from hybridomas donated by Dr. Ted Hansen (Washington University, St. Louis, MO). The anti-APLP2 antibody was purchased from Calbiochem/EMB Chemicals (Cat. No. 171617). The anti-actin monoclonal antibody (clone AC-40) was purchased from Novus Biologicals (Cat. No. NBP1–41294), and was used as a loading control. The anti-HSC70 antibody was obtained from Enzo (Cat. No. ALX-804-067-R050), and the anti-CD3 antibody was acquired from Caltag (Cat. No. RM3400). Secondary antibodies used for immunoblotting analysis were peroxidase-conjugated AffiniPure goat anti-mouse IgG light chain or peroxidase-conjugated IgG fraction mouse anti-rabbit IgG light chain purchased from Jackson ImmunoResearch Laboratories, Inc. (Cat. No. 115–035–174 and 211–032–171, respectively). AlexaFluor 488-conjugated goat anti-rabbit IgG (H+L) chain (Invitrogen, Cat. No. A-11008), and phycoerythrin (PE)-conjugated goat anti-mouse IgG F(ab')2 and Cy5-conjugated goat anti-rabbit IgG (both purchased from Jackson ImmunoResearch Laboratories, Inc., Cat. No. 115–035–071 and Cat. No. 111–175–144, respectively) were used as secondary antibodies for flow cytometry experiments.
Transfections
For siRNA transfections, 1 × 105 cells were plated in 35-mm dishes 24 h prior to transfection. An siRNA pool against APLP2 was purchased from Thermo Fisher Scientific Inc. (ON-TARGETplus SMARTpool Human APLP2, Cat. No. L-004179–00). ON-TARGETplus non-targeting pool oligonucleotides (Thermo Fisher Scientific, Cat. No. D0018101050) were used as negative controls. All siRNA transfections were performed using DharmaFECT 1 (Thermo Fisher Scientific, Cat. No. T-2001). Following transfection with either control or APLP2-specific siRNAs, the expression of APLP2 was analyzed by immunoblotting.
Flow cytometry
Flow cytometry experiments were performed at 24 h post-irradiation and 48 h post-transfection. Cells from 6-well plates were trypsinized, resuspended and evenly distributed between 2 96-well plates. Following centrifugation at 450 × g for 5 min at 4°C in an Eppendorf 5810R centrifuge, cells were incubated with the desired primary antibody for 30 min on ice, washed, incubated with fluorescently labeled secondary antibody, washed again, and analyzed on a FACS Calibur (BD Biosciences). The percent change in MHC class I expression in response to siRNA-mediated APLP2 knockdown was derived by the following formula: MFUsiAPLP2/MFUsiControl × 100. Data collection and analysis were performed at the University of Nebraska Medical Center Cell Analysis Core Facility using ModFit or Cell Quest software.
Immunoprecipitation
Ten million cells were harvested, pelleted by centrifugation (at 450 × g, 5 min), and incubated with precipitating antibody in a lysis buffer containing 1% CHAPS in 0.01 M Tris-HCl (pH 7.4), 0.15 M NaCl supplemented with 20 mM iodoacetamide and 0.2 mM phenylmethanesulfonyl fluoride for 1 h on ice. W6/32 antibodies were used to capture MHC class I molecules, and then the lysates were spun in a microcentrifuge at 14,000 rpm at 4°C for 30 min. The supernatants were added to Amersham SPA Scintillation Beads - Protein A (GE Healthcare/Amersham Biosciences, Cat. No. 17–0780–01) and incubated with occasional gentle mixing (without inversion) for 45 min on ice. Beads were washed twice with 5 mL wash buffer (0.1% CHAPS in 0.01 M Tris-HCl, 0.15 M NaCl, pH 7.4, 20 mM iodoacetamide) followed by immediate centrifugation at 450 × g, 4°C, 5 min, and then washed twice more with centrifugation delayed to 10 min after the wash. Proteins were eluted with a Protein Elution Buffer containing 0.125 M Tris (pH 6.8), 2% w/v SDS, 12% w/v glycerol and 0.02% w/v bromophenol blue by boiling the tubes for 5 min. Samples were centrifuged as described above, and protein-containing supernatants were recovered.
Immunoblotting
To produce cell lysates for immunoblotting, 1 × 107 cells were pelleted at 450 × g, 4°C, 5 min, and resuspended in a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA (EDTA) and 0.5% Triton X-100. Resuspended cells were incubated on ice for 1 h with occasional vortexing, and then stored at −80 °C overnight. The next day, lysates were thawed on ice, and spun in a microcentrifuge at maximum speed for 30 min at 4°C. Supernatants were collected in fresh tubes and stored at −80 °C.
Aliquots of cell lysate supernatants (mixed with 5 × SDS loading dye containing 250 mM Tris-HCl pH 6.8, 10% SDS, 30% glycerol, 0.02% bromophenol blue, and 5% freshly added β-mercaptoethanol) or immunoprecipitation samples were boiled for 5 min and then loaded onto 4–20% Tris-glycine pre-cast gels (Invitrogen; Cat. No. EC60285). Electrophoresis was performed under reducing conditions in an Invitrogen minigel apparatus at 125 V for 2 h and 15 min at room temperature. Proteins were then transferred onto Millipore polyvinylidene fluoride Immobilon-P membranes (Cat. No. IPVH000010) with 30 V for 2 h. Following overnight blocking in 10% w/v non-fat dry milk, blots were incubated with primary antibodies (in 10% non-fat dry milk). Thereafter, 3 washes of 15 min with 0.05% Tween 20 in PBS were performed and blots were incubated with secondary antibodies (diluted 1:5,000 in 0.05% Tween 20) for 1 h. Three washes, of 15 min each, were performed with 0.3% Tween 20. Finally, membranes were soaked in Pierce ECL immunoblotting substrate from Thermo Scientific (Cat. No. 32106), and developed on Kodak BioMax MR film.
Irradiation and cell cycle analysis
Flow cytometry data were obtained from TC71 and A673 cells growing in log phase and exposed to γ radiation. The radiation doses that result in maximum subG1 content and G2/M arrest were previously determined for TC71 and A673 cells (data not shown), and a range of doses (up to and including these maximally effective doses) of γ radiation was used in this study. Following irradiation with a Mark I 68A Cesium-137 Irradiator (J. L. Shepherd and Associates), EWS cells were cultured at 37°C, 5% CO2 for 24 h, fixed, stained with propidium iodide, then analyzed for DNA content by flow cytometry on a BD FACS Calibur with ModFit software (Verity Software House, Inc.) as described previously.52 Cell cycle analysis was performed on ≥ 20,000 cells per experimental condition.
Statistical analysis
Statistical significance was determined by means of the unpaired, 2-tailed Student’s t-test. Unless otherwise specified, data are reported as means ± SD.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Brittney Smith, Poomy Pandey, Erin Triplet, and Dr Xiaojian Wang for excellent technical assistance, Dr Janina Baranowska-Kortylewicz for assistance in the irradiation experiments, and Dr Eugenie Kleinerman and Dr Ted Hansen for providing cell lines and antibodies. We also gratefully acknowledge the assistance of the personnel of the University of Nebraska Medical Center Cell Analysis Core Facility (funded in part from National Cancer Institute Cancer Center Support Grant P30 CA036727). This work was supported by NIH R03CA176557 (to J.C.S.), by an Eppley Cancer Center Pediatric Cancer Research Pilot Grant (to J.C.S.), by Nebraska Department of Health and Human Services Cancer and Smoking Disease Research Grants (to Y.Y. and J.C.S.), and by NIH COBRE NCCS (P20 RR018759/P20GM103489) and SPORE (P50CA127297) Developmental Grants (to J.C.S.). Support for this work was also provided by University of Nebraska Medical Center Graduate Studies Office Emley and Regents Tuition Fellowships (to H.P.), a National Cancer Institute Training Grant T32 CA009476 Fellowship (to H.P.), and a Department of Education Graduate Assistance in Areas of National Need Fellowship (to H.P.).
Glossary
Abbreviations:
- APLP2
amyloid β (A4) precursor-like protein 2
- APP
amyloid precursor protein
- CTL
cytotoxic T lymphocyte
- EWS
Ewing’s sarcoma
- IFN
interferon
Citation: Peters H, Yan Y, Solheim J. APLP2 regulates the expression of MHC class I molecules on irradiated Ewing’s sarcoma cells. OncoImmunology 2013; 2:e26293; 10.4161/onci.26293
Supplemental Materials
Supplemental materials may be found here: https://www.landesbioscience.com/journals/oncoimmunology/article/26293/
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26293
References
- 1.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–19. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 2.Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. doi: 10.1016/j.canlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Karosas AO. Ewing’s sarcoma. Am J Health Syst Pharm. 2010;67:1599–605. doi: 10.2146/ajhp090526. [DOI] [PubMed] [Google Scholar]
- 4.Paulussen M, Bielack S, Jürgens H, Casali PG, ESMO Guidelines Working Group Ewing’s sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):140–2. doi: 10.1093/annonc/mdp155. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N, Heslop HE, Mackall CL. T-cell-based therapies for malignancy and infection in childhood. Pediatr Clin North Am. 2010;57:83–96. doi: 10.1016/j.pcl.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wachtel M, Schäfer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat Rev. 2010;36:318–27. doi: 10.1016/j.ctrv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Merchant MS, Chua KS, Khanna C, Helman LJ, Telford B, Ward Y, Summers J, Toretsky J, Thomas EK, et al. Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther. 2003;2:579–86. doi: 10.4161/cbt.2.5.545. [DOI] [PubMed] [Google Scholar]
- 8.Borowski A, van Valen F, Ulbrecht M, Weiss EH, Blasczyk R, Jürgens H, Göbel U, Schneider EM. Monomorphic HLA class I-(non-A, non-B) expression on Ewing’s tumor cell lines, modulation by TNF-alpha and IFN-gamma. Immunobiology. 1999;200:1–20. doi: 10.1016/S0171-2985(99)80029-1. [DOI] [PubMed] [Google Scholar]
- 9.Berghuis D, de Hooge AS, Santos SJ, Horst D, Wiertz EJ, van Eggermond MC, van den Elsen PJ, Taminiau AH, Ottaviano L, Schaefer KL, et al. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol. 2009;218:222–31. doi: 10.1002/path.2537. [DOI] [PubMed] [Google Scholar]
- 10.Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149–57. [PMC free article] [PubMed] [Google Scholar]
- 11.Cormier JN, Panelli MC, Hackett JA, Bettinotti MP, Mixon A, Wunderlich J, Parker LL, Restifo NP, Ferrone S, Marincola FM. Natural variation of the expression of HLA and endogenous antigen modulates CTL recognition in an in vitro melanoma model. Int J Cancer. 1999;80:781–90. doi: 10.1002/(SICI)1097-0215(19990301)80:5<781::AID-IJC24>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabe H, Tsukahara T, Kawaguchi S, Wada T, Torigoe T, Sato N, Terai C, Aoki M, Hirose S, Morioka H, et al. Prognostic significance of HLA class I expression in Ewing’s sarcoma family of tumors. J Surg Oncol. 2011;103:380–5. doi: 10.1002/jso.21829. [DOI] [PubMed] [Google Scholar]
- 13.Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006;97:1374–80. doi: 10.1111/j.1349-7006.2006.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuli A, Sharma M, Wang X, Simone LC, Capek HL, Cate S, Hildebrand WH, Naslavsky N, Caplan S, Solheim JC. Amyloid precursor-like protein 2 association with HLA class I molecules. Cancer Immunol Immunother. 2009;58:1419–31. doi: 10.1007/s00262-009-0657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slunt HH, Thinakaran G, Von Koch C, Lo ACY, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse β-amyloid precursor protein (APP) J Biol Chem. 1994;269:2637–44. [PubMed] [Google Scholar]
- 17.Walsh DM, Minogue AM, Sala Frigerio C, Fadeeva JV, Wasco W, Selkoe DJ. The APP family of proteins: similarities and differences. Biochem Soc Trans. 2007;35:416–20. doi: 10.1042/BST0350416. [DOI] [PubMed] [Google Scholar]
- 18.Needham BE, Wlodek ME, Ciccotosto GD, Fam BC, Masters CL, Proietto J, Andrikopoulos S, Cappai R. Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol. 2008;215:155–63. doi: 10.1002/path.2343. [DOI] [PubMed] [Google Scholar]
- 19.Bellingham SA, Ciccotosto GD, Needham BE, Fodero LR, White AR, Masters CL, Cappai R, Camakaris J. Gene knockout of amyloid precursor protein and amyloid precursor-like protein-2 increases cellular copper levels in primary mouse cortical neurons and embryonic fibroblasts. J Neurochem. 2004;91:423–8. doi: 10.1111/j.1471-4159.2004.02731.x. [DOI] [PubMed] [Google Scholar]
- 20.Cappai R, Mok SS, Galatis D, Tucker DF, Henry A, Beyreuther K, Small DH, Masters CL. Recombinant human amyloid precursor-like protein 2 (APLP2) expressed in the yeast Pichia pastoris can stimulate neurite outgrowth. FEBS Lett. 1999;442:95–8. doi: 10.1016/S0014-5793(98)01635-4. [DOI] [PubMed] [Google Scholar]
- 21.Guo J, Thinakaran G, Guo Y, Sisodia SS, Yu FX. A role for amyloid precursor-like protein 2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1998;39:292–300. [PubMed] [Google Scholar]
- 22.Li XF, Thinakaran G, Sisodia SS, Yu FS. Amyloid precursor-like protein 2 promotes cell migration toward fibronectin and collagen IV. J Biol Chem. 1999;274:27249–56. doi: 10.1074/jbc.274.38.27249. [DOI] [PubMed] [Google Scholar]
- 23.Thinakaran G, Kitt CA, Roskams AJ, Slunt HH, Masliah E, von Koch C, Ginsberg SD, Ronnett GV, Reed RR, Price DL, et al. Distribution of an APP homolog, APLP2, in the mouse olfactory system: a potential role for APLP2 in axogenesis. J Neurosci. 1995;15:6314–26. doi: 10.1523/JNEUROSCI.15-10-06314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin X, Ouyang S, Xu W, Zhang X, Fok KL, Wong HY, Zhang J, Qiu X, Miao S, Chan HC, et al. YWK-II protein as a novel G(o)-coupled receptor for Müllerian inhibiting substance in cell survival. J Cell Sci. 2007;120:1521–8. doi: 10.1242/jcs.001230. [DOI] [PubMed] [Google Scholar]
- 25.McLoughlin DM, Miller CCJ. The FE65 proteins and Alzheimer’s disease. J Neurosci Res. 2008;86:744–54. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Yang L, Wang Z, Zheng H. Amyolid precursor protein mediates presynaptic localization and activity of the high-affinity choline transporter. Proc Natl Acad Sci U S A. 2007;104:14140–5. doi: 10.1073/pnas.0704070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris CR, Petersen JL, Vargas SE, Turnquist HR, McIlhaney MM, Sanderson SD, Bruder JT, Yu YY, Burgert HG, Solheim JC. The amyloid precursor-like protein 2 and the adenoviral E3/19K protein both bind to a conformational site on H-2Kd and regulate H-2Kd expression. J Biol Chem. 2003;278:12618–23. doi: 10.1074/jbc.M208203200. [DOI] [PubMed] [Google Scholar]
- 28.Tuli A, Sharma M, Naslavsky N, Caplan S, Solheim JC, Solheim JC. Specificity of amyloid precursor-like protein 2 interactions with MHC class I molecules. Immunogenetics. 2008;60:303–13. doi: 10.1007/s00251-008-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuli A, Sharma M, McIlhaney MM, Talmadge JE, Naslavsky N, Caplan S, Solheim JC. Amyloid precursor-like protein 2 increases the endocytosis, instability, and turnover of the H2-K(d) MHC class I molecule. J Immunol. 2008;181:1978–87. doi: 10.4049/jimmunol.181.3.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuli A, Sharma M, Capek HL, Naslavsky N, Caplan S, Solheim JC. Mechanism for amyloid precursor-like protein 2 enhancement of major histocompatibility complex class I molecule degradation. J Biol Chem. 2009;284:34296–307. doi: 10.1074/jbc.M109.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters HL, Tuli A, Sharma M, Naslavsky N, Caplan S, MacDonald RG, Solheim JC. Regulation of major histocompatibility complex class I molecule expression on cancer cells by amyloid precursor-like protein 2. Immunol Res. 2011;51:39–44. doi: 10.1007/s12026-011-8238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiriva-Internati M, Grizzi F, Pinkston J, Morrow KJ, D’Cunha N, Frezza EE, Muzzio PC, Kast WM, Cobos E. Gamma-radiation upregulates MHC class I/II and ICAM-I molecules in multiple myeloma cell lines and primary tumors. In Vitro Cell Dev Biol Anim. 2006;42:89–95. doi: 10.1290/0508054.1. [DOI] [PubMed] [Google Scholar]
- 34.Peters HL, Yan Y, Nordgren TM, Cutucache CE, Joshi SS, Solheim JC. Amyloid precursor-like protein 2 suppresses irradiation-induced apoptosis in Ewing sarcoma cells and is elevated in immune-evasive Ewing sarcoma cells. Cancer Biol Ther. 2013;14:752–60. doi: 10.4161/cbt.25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 37.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–36. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes TD, El-Sherbiny YM, Davison A, Clough SL, Blair GE, Cook GP. A human NK cell activation/inhibition threshold allows small changes in the target cell surface phenotype to dramatically alter susceptibility to NK cells. J Immunol. 2011;186:1538–45. doi: 10.4049/jimmunol.1000951. [DOI] [PubMed] [Google Scholar]
- 40.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–9. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mechtersheimer G, Barth T, Ludwig R, Staudter M, Möller P. Differential expression of leukocyte differentiation antigens in small round blue cell sarcomas. Cancer. 1993;71:237–48. doi: 10.1002/1097-0142(19930101)71:1<237::AID-CNCR2820710137>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Lipinski M, Braham K, Philip I, Wiels J, Philip T, Goridis C, Lenoir GM, Tursz T. Neuroectoderm-associated antigens on Ewing’s sarcoma cell lines. Cancer Res. 1987;47:183–7. [PubMed] [Google Scholar]
- 43.Shamamian P, Mancini M, Kawakami Y, Restifo NP, Rosenberg SA, Topalian SL. Recognition of neuroectodermal tumors by melanoma-specific cytotoxic T lymphocytes: evidence for antigen sharing by tumors derived from the neural crest. Cancer Immunol Immunother. 1994;39:73–83. doi: 10.1007/BF01525312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berghuis D, de Hooge AS, Santos SJ, Horst D, Wiertz EJ, van Eggermond MC, van den Elsen PJ, Taminiau AH, Ottaviano L, Schaefer KL, et al. Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol. 2009;218:222–31. doi: 10.1002/path.2537. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z, Jia S-F, Hung M-C, Kleinerman ES. E1A sensitizes HER2/neu-overexpressing Ewing’s sarcoma cells to topoisomerase II-targeting anticancer drugs. Cancer Res. 2001;61:3394–8. [PubMed] [Google Scholar]
- 46.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–306. [PubMed] [Google Scholar]
- 47.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–88. doi: 10.1016/S0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 48.Carreno BM, Hansen TH. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur J Immunol. 1994;24:1285–92. doi: 10.1002/eji.1830240607. [DOI] [PubMed] [Google Scholar]
- 49.Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979;123:342–9. [PubMed] [Google Scholar]
- 50.Ladasky JJ, Shum BP, Canavez F, Seuánez HN, Parham P. Residue 3 of beta2-microglobulin affects binding of class I MHC molecules by the W6/32 antibody. Immunogenetics. 1999;49:312–20. doi: 10.1007/s002510050498. [DOI] [PubMed] [Google Scholar]
- 51.Parham P, Brodsky FM. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–99. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 52.Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH. BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene. 2005;24:3285–96. doi: 10.1038/sj.onc.1208492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.