Abstract
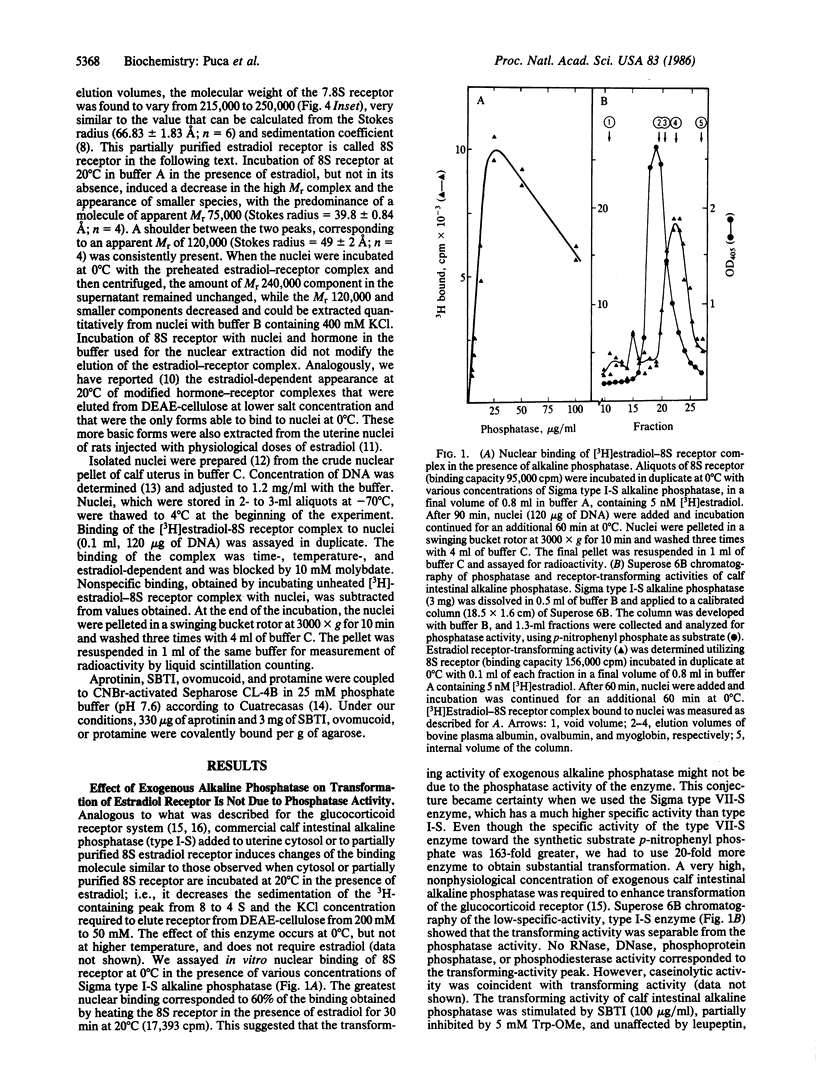
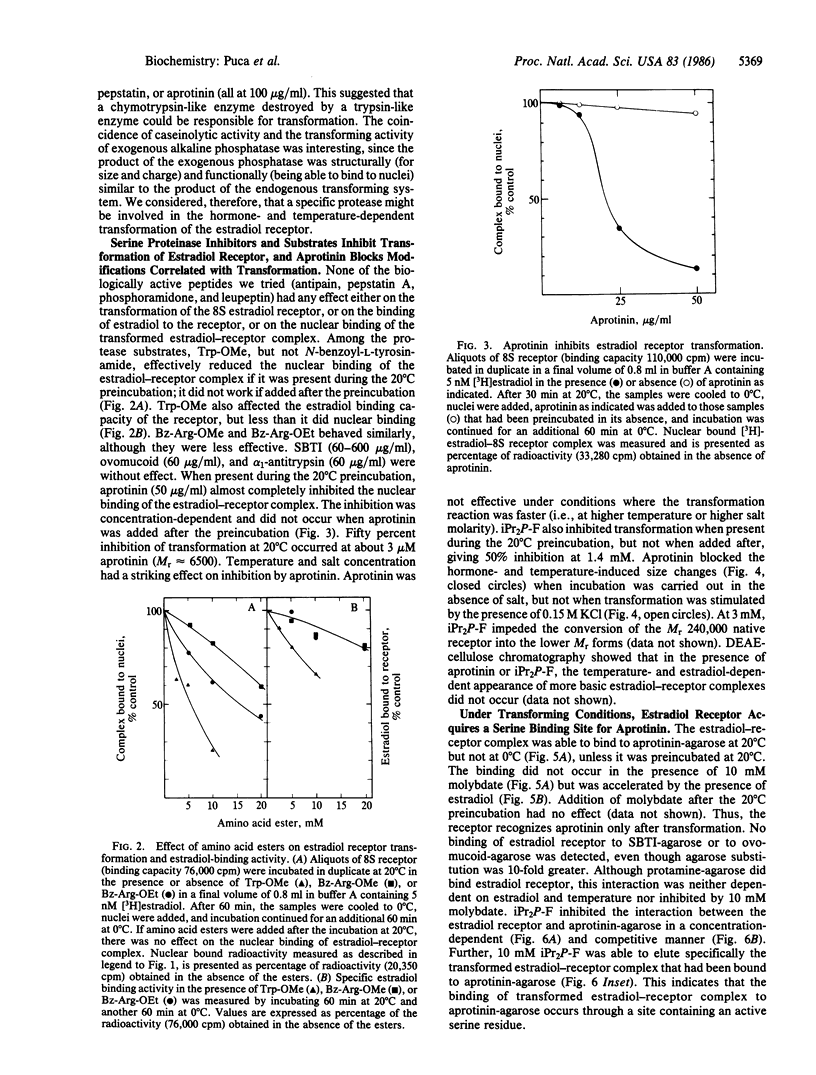
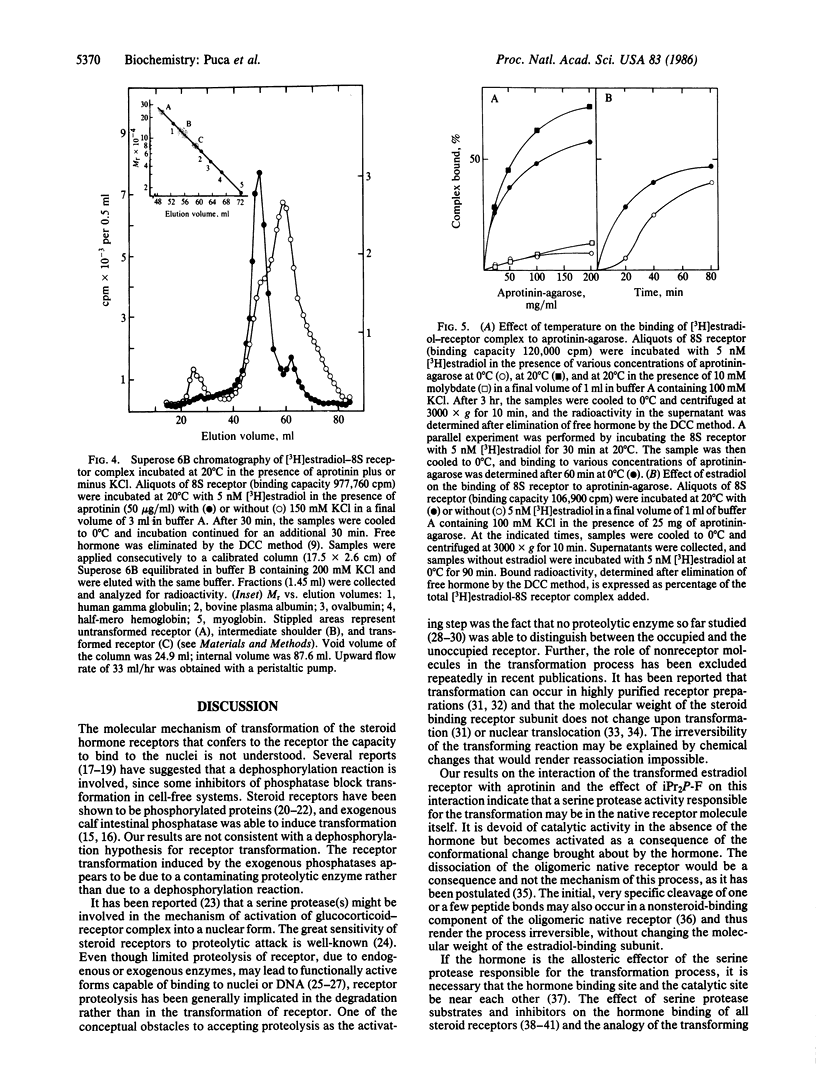
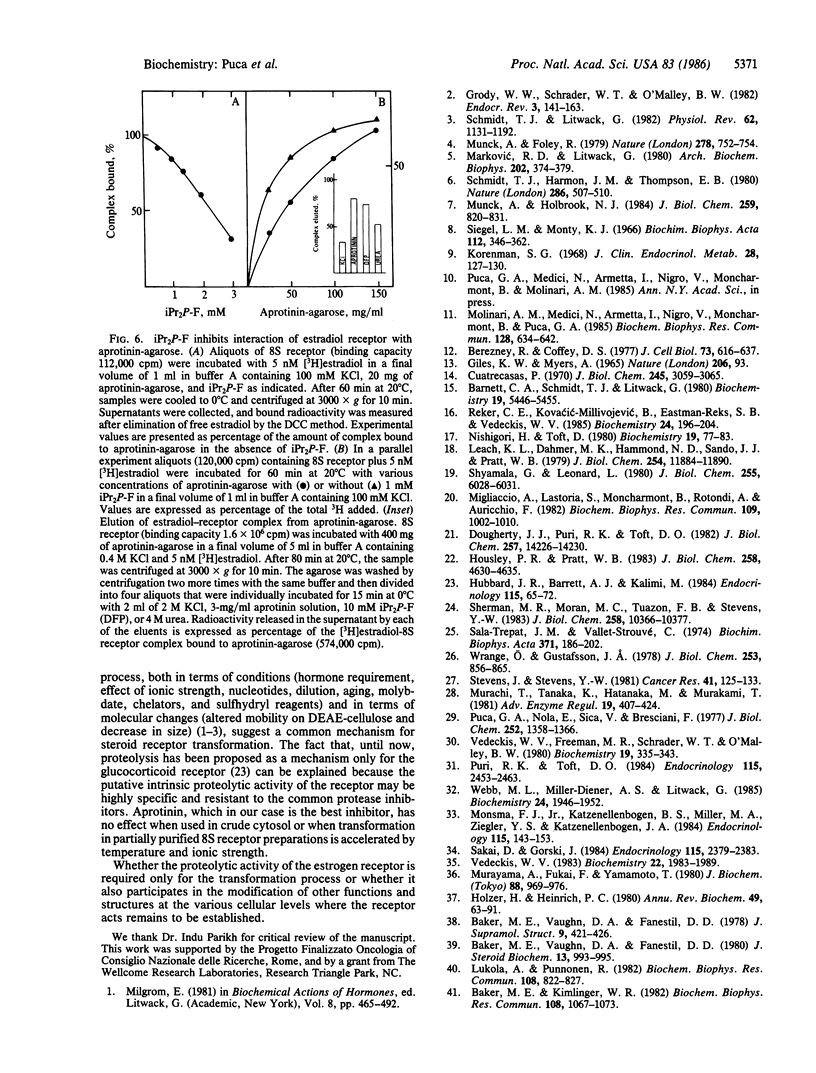
We have investigated the effect of various protease inhibitors and substrates on the hormone- and temperature-dependent binding of partially purified estradiol-receptor complex to isolated nuclei. Only serine protease substrates and inhibitors significantly depressed estradiol receptor transformation. At 20 degrees C, we observed 50% inhibition with about 3 microM aprotinin or with 1.4 mM diisopropyl fluorophosphate. Aprotinin also blocked those size and charge modifications of receptor that are characteristic of the transformation process. The estradiol receptor was able to bind to aprotinin-agarose only under transforming conditions; i.e., the interaction was hormone- and temperature-dependent and inhibited by molybdate. Diisopropyl fluorophosphate, a covalent reagent for serine esterases, competitively inhibited the binding and specifically eluted the estradiol-receptor complex that had been bound to aprotinin-agarose. These results indicate that estradiol receptor transformation is due to the effect of a serine protease and that the receptor itself is endowed with this catalytic activity, which is triggered by the steroid.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. E., Kimlinger W. R. Protease substrates inhibit binding of 3H-R5020 to the G-fragment in chick oviduct cytosol. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1067–1073. doi: 10.1016/0006-291x(82)92108-8. [DOI] [PubMed] [Google Scholar]
- Baker M. E., Vaughn D. A., Fanestil D. D. Competitive inhibition of dexamethasone binding to the glucocorticoid receptor in HTC cells by tryptophan methyl ester. J Steroid Biochem. 1980 Aug;13(8):993–995. doi: 10.1016/0022-4731(80)90175-2. [DOI] [PubMed] [Google Scholar]
- Baker M. E., Vaughn D. A., Fanestil D. D. Inhibition by protease inhibitors of binding of adrenal and sex steroid hormones. J Supramol Struct. 1978;9(3):421–426. doi: 10.1002/jss.400090312. [DOI] [PubMed] [Google Scholar]
- Barnett C. A., Schmidt T. J., Litwack G. Effects of calf intestinal alkaline phosphatase, phosphatase inhibitors, and phosphorylated compounds on the rate of activation of glucocorticoid-receptor complexes. Biochemistry. 1980 Nov 11;19(23):5446–5455. doi: 10.1021/bi00564a046. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem. 1982 Dec 10;257(23):14226–14230. [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Holzer H., Heinrich P. C. Control of proteolysis. Annu Rev Biochem. 1980;49:63–91. doi: 10.1146/annurev.bi.49.070180.000431. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Pratt W. B. Direct demonstration of glucocorticoid receptor phosphorylation by intact L-cells. J Biol Chem. 1983 Apr 10;258(7):4630–4635. [PubMed] [Google Scholar]
- Hubbard J. R., Barrett A. J., Kalimi M. Tosyl-lysyl chloromethane alters glucocorticoid-receptor complex nuclear binding and physical properties. Endocrinology. 1984 Jul;115(1):65–72. doi: 10.1210/endo-115-1-65. [DOI] [PubMed] [Google Scholar]
- Korenman S. G. Radio-ligand binding assay of specific estrogens using a soluble uterine macromolecule. J Clin Endocrinol Metab. 1968 Jan;28(1):127–130. doi: 10.1210/jcem-28-1-127. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Dahmer M. K., Hammond N. D., Sando J. J., Pratt W. B. Molybdate inhibition of glucocorticoid receptor inactivation and transformation. J Biol Chem. 1979 Dec 10;254(23):11884–11890. [PubMed] [Google Scholar]
- Lukola A., Punnonen R. Interaction of serine protease inhibitors and substrates with human uterine estrogen receptor. Biochem Biophys Res Commun. 1982 Sep 30;108(2):822–827. doi: 10.1016/0006-291x(82)90903-2. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Marković R. D., Litwack G. Activation of liver and kidney glucocorticoid-receptor complexes occurs in vivo. Arch Biochem Biophys. 1980 Jul;202(2):374–379. doi: 10.1016/0003-9861(80)90440-3. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Lastoria S., Moncharmont B., Rotondi A., Auricchio F. Phosphorylation of calf uterus 17 beta-estradiol receptor by endogenous Ca2+-stimulated kinase activating the hormone binding of the receptor. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1002–1010. doi: 10.1016/0006-291x(82)92039-3. [DOI] [PubMed] [Google Scholar]
- Molinari A. M., Medici N., Armetta I., Nigro V., Moncharmont B., Puca G. A. Particulate nature of the unoccupied uterine estrogen receptor. Biochem Biophys Res Commun. 1985 Apr 30;128(2):634–642. doi: 10.1016/0006-291x(85)90093-2. [DOI] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Katzenellenbogen B. S., Miller M. A., Ziegler Y. S., Katzenellenbogen J. A. Characterization of the estrogen receptor and its dynamics in MCF-7 human breast cancer cells using a covalently attaching antiestrogen. Endocrinology. 1984 Jul;115(1):143–153. doi: 10.1210/endo-115-1-143. [DOI] [PubMed] [Google Scholar]
- Munck A., Foley R. Activation of steroid hormone-receptor complexes in intact target cells in physiological conditions. Nature. 1979 Apr 19;278(5706):752–754. doi: 10.1038/278752a0. [DOI] [PubMed] [Google Scholar]
- Munck A., Holbrook N. J. Glucocorticoid-receptor complexes in rat thymus cells. Rapid kinetic behavior and a cyclic model. J Biol Chem. 1984 Jan 25;259(2):820–831. [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Murayama A., Fukai F., Yamamoto T. Estrogen receptor of cow uterus. III. Molecular constitution of estrogen receptor-binding factors. J Biochem. 1980 Oct;88(4):969–976. doi: 10.1093/oxfordjournals.jbchem.a133085. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Toft D. Inhibition of progesterone receptor activation by sodium molybdate. Biochemistry. 1980 Jan 8;19(1):77–83. doi: 10.1021/bi00542a012. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen binding proteins of calf uterus. Molecular and functional characterization of the receptor transforming factor: A Ca2+-activated protease. J Biol Chem. 1977 Feb 25;252(4):1358–1366. [PubMed] [Google Scholar]
- Puri R. K., Toft D. O. Transformation of highly purified avian progesterone receptor. Endocrinology. 1984 Dec;115(6):2453–2463. doi: 10.1210/endo-115-6-2453. [DOI] [PubMed] [Google Scholar]
- Reker C. E., Kovacic-Milivojević B., Eastman-Reks S. B., Vedeckis W. V. Transformed mouse glucocorticoid receptor: generation and interconversion of the 3.8S, monomeric and 5.2S, oligomeric species. Biochemistry. 1985 Jan 1;24(1):196–204. doi: 10.1021/bi00322a028. [DOI] [PubMed] [Google Scholar]
- Sakai D., Gorski J. Reversible denaturation of the estrogen receptor and estimation of polypeptide chain molecular weight. Endocrinology. 1984 Dec;115(6):2379–2383. doi: 10.1210/endo-115-6-2379. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Vallet-Strouvé C. Binding of the estradiol receptor from calf uterus to the chromatin: active forms. Biochim Biophys Acta. 1974 Nov 5;371(1):186–202. doi: 10.1016/0005-2795(74)90168-8. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Harmon J. M., Thompson E. B. 'Activation-labile' glucocorticoid-receptor complexes of a steroid-resistant variant of CEM-C7 human lymphoid cells. Nature. 1980 Jul 31;286(5772):507–510. doi: 10.1038/286507a0. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Litwack G. Activation of the glucocorticoid-receptor complex. Physiol Rev. 1982 Oct;62(4 Pt 1):1131–1192. doi: 10.1152/physrev.1982.62.4.1131. [DOI] [PubMed] [Google Scholar]
- Sherman M. R., Moran M. C., Tuazon F. B., Stevens Y. W. Structure, dissociation, and proteolysis of mammalian steroid receptors. Multiplicity of glucocorticoid receptor forms and proteolytic enzymes in rat liver and kidney cytosols. J Biol Chem. 1983 Sep 10;258(17):10366–10377. [PubMed] [Google Scholar]
- Shyamala G., Leonard L. Inhibition of uterine estrogen receptor transformation by sodium molybdate. J Biol Chem. 1980 Jul 10;255(13):6028–6031. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W. Influence of limited proteolysis on the physicochemical and DNA-binding properties of glucocorticoid receptors from corticoid-sensitive and -resistant mouse lymphoma P1798. Cancer Res. 1981 Jan;41(1):125–133. [PubMed] [Google Scholar]
- Vedeckis W. V., Freeman M. R., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct: partial purification and characterization of a calcium-activated protease which hydrolyzes the progesterone receptor. Biochemistry. 1980 Jan 22;19(2):335–343. doi: 10.1021/bi00543a014. [DOI] [PubMed] [Google Scholar]
- Vedeckis W. V. Subunit dissociation as a possible mechanism of glucocorticoid receptor activation. Biochemistry. 1983 Apr 12;22(8):1983–1989. doi: 10.1021/bi00277a038. [DOI] [PubMed] [Google Scholar]
- Webb M. L., Miller-Diener A. S., Litwack G. Purification, characterization, and activation of the glucocorticoid-receptor complex from rat kidney cortex. Biochemistry. 1985 Apr 9;24(8):1946–1952. doi: 10.1021/bi00329a022. [DOI] [PubMed] [Google Scholar]
- Wrange O., Gustafsson J. A. Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis. J Biol Chem. 1978 Feb 10;253(3):856–865. [PubMed] [Google Scholar]


