Abstract
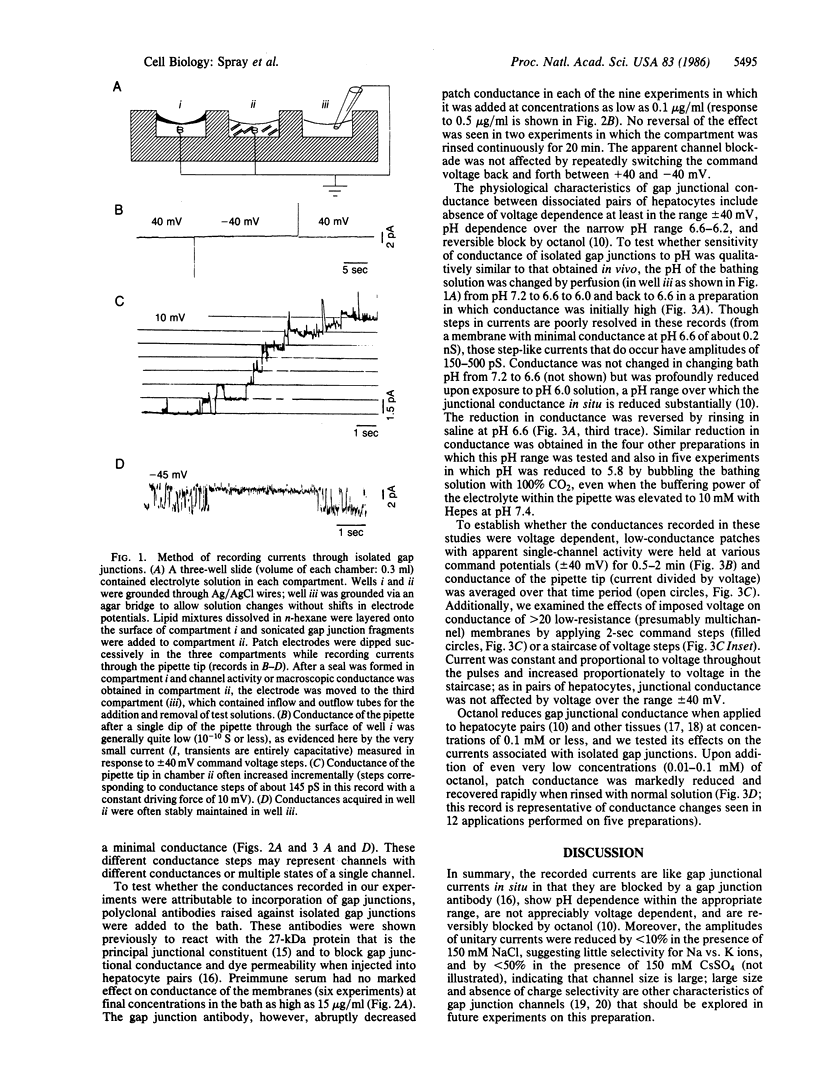
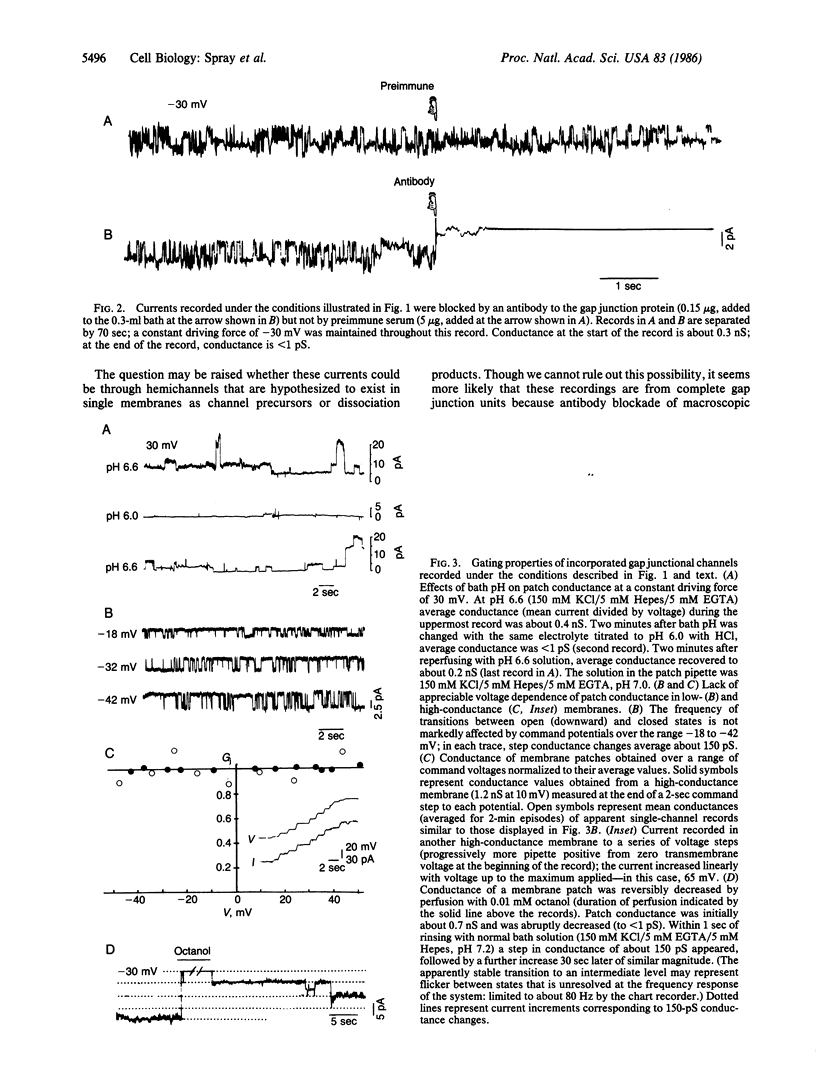
We have shown previously that conductance of rat liver gap junctions is blocked by an affinity-purified polyclonal antibody generated against rat liver junctional membranes, is not affected by moderate transjunctional or transmembrane potentials, and is reversibly decreased by cytoplasmic acidification and perfusion with octanol. We have now recorded currents from isolated liver gap junctions using patch electrodes dipped through a layer of mixed lipids whose concentrations match those of isolated liver appositional membranes. These currents are blocked by the same polyclonal antibody, are insensitive to moderate voltages imposed across the pipette tip, and are reversibly blocked by similar concentrations of H ions and octanol as are junctions in situ. The currents are likely to be gap junctional in origin; their block by low pH and other agents indicates that the gating mechanisms are intrinsic to the gap junctions themselves and presumably result from conformational change in the channel-forming protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Spira M. E., Spray D. C. Permeability of gap junctions between embryonic cells of Fundulus: a reevaluation. Dev Biol. 1978 Jul;65(1):114–125. doi: 10.1016/0012-1606(78)90184-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Hertzberg E. L., Skibbens R. V. A protein homologous to the 27,000 dalton liver gap junction protein is present in a wide variety of species and tissues. Cell. 1984 Nov;39(1):61–69. doi: 10.1016/0092-8674(84)90191-0. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Spray D. C., Bennett M. V. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2412–2416. doi: 10.1073/pnas.82.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Ramón F. Electrotonic coupling in internally perfused crayfish segmented axons. J Physiol. 1981 Aug;317:509–518. doi: 10.1113/jphysiol.1981.sp013840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Kremmer T., Wisher M. H., Evans W. H. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim Biophys Acta. 1976 Dec 14;455(3):655–664. doi: 10.1016/0005-2736(76)90039-0. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Bernardini G., Peracchia L. L. Is calmodulin involved in the regulation of gap junction permeability? Pflugers Arch. 1983 Oct;399(2):152–154. doi: 10.1007/BF00663912. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Nicholson B. J., Yancey S. B. Chemistry of gap junctions. Annu Rev Physiol. 1985;47:263–279. doi: 10.1146/annurev.ph.47.030185.001403. [DOI] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann G., Wiegandt H., Rose B., Zimmerman A., Ben-Haim D., Loewenstein W. R. Diameter of the cell-to-cell junctional membrane channels as probed with neutral molecules. Science. 1981 Jul 31;213(4507):551–553. doi: 10.1126/science.7244653. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Bennett M. V. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Spray D. C., White R. L., Mazet F., Bennett M. V. Regulation of gap junctional conductance. Am J Physiol. 1985 Jun;248(6 Pt 2):H753–H764. doi: 10.1152/ajpheart.1985.248.6.H753. [DOI] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G. A., Hall J. E., Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]


