Abstract
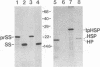
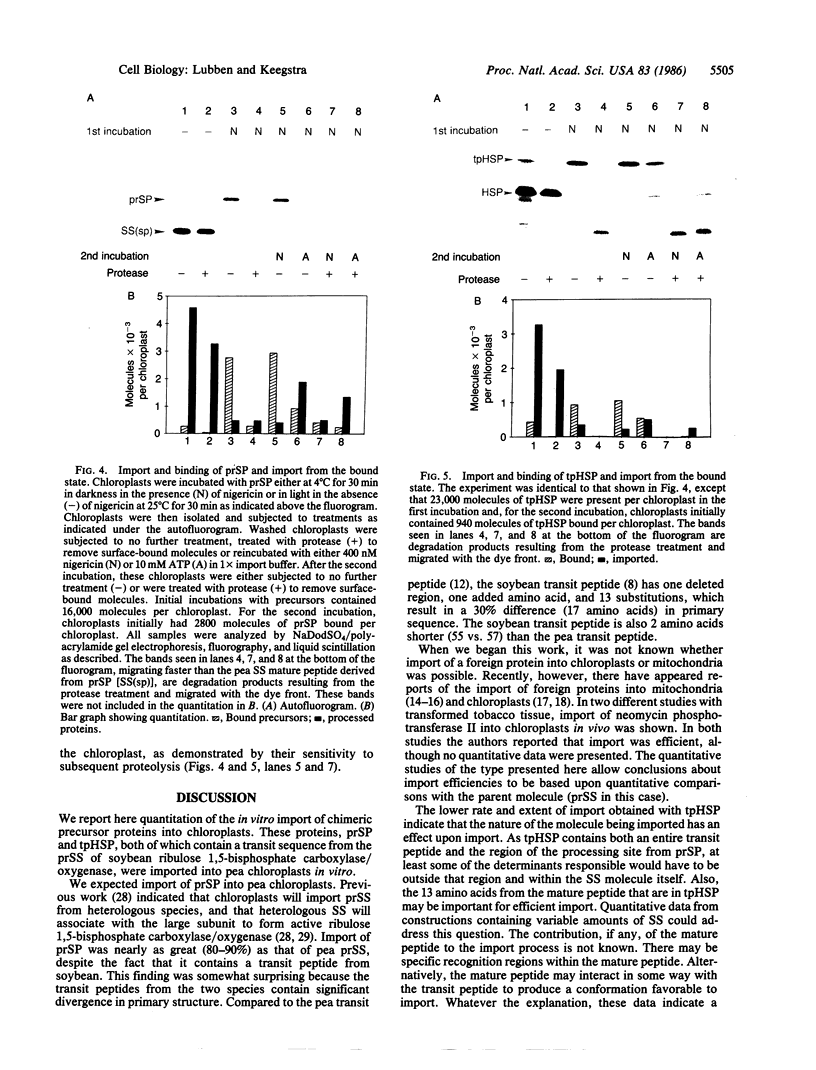
In order to further our understanding of the targeting of nuclear-encoded proteins into intracellular organelles, we have investigated the import of chimeric precursor proteins into pea chloroplasts. Two different chimeric precursor proteins were produced by in vitro expression of chimeric genes. One chimeric precursor contained the transit peptide of the small subunit of soybean ribulose 1,5-bisphosphate carboxylase and the mature peptide of the same protein from pea. The second contained the same transit peptide plus 13 amino acids of the pea mature peptide fused to a cytosolic heat shock protein. The extent of import and binding of the two chimeric proteins was examined by using quantitative assays and was compared to the import of pea small subunit precursor. Both precursor proteins imported well into pea chloroplasts, although the extent of import observed with the chimeric small-subunit-heat shock precursor was less than that observed with the soybean-pea small subunit precursor. The heat shock protein alone did not import into nor bind to chloroplasts. The binding of both the chimeric small-subunit-heat shock protein and the soybean-pea small subunit precursor to chloroplasts was physiologically significant, as shown by the fact that when chloroplasts with bound precursors were isolated, these bound precursors could subsequently be imported.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater M., Bywater R., Hellman L. A novel chromatographic procedure for purification of bacterial plasmids. Anal Biochem. 1983 Jul 1;132(1):219–224. doi: 10.1016/0003-2697(83)90451-7. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Czarnecka E., Gurley W. B., Nagao R. T., Mosquera L. A., Key J. L. DNA sequence and transcript mapping of a soybean gene encoding a small heat shock protein. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3726–3730. doi: 10.1073/pnas.82.11.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Geller B. L., Emr S. D. Intracellular targeting and import of an F1-ATPase beta-subunit-beta-galactosidase hybrid protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3983–3987. doi: 10.1073/pnas.81.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Gurley W. B., Czarnecka E., Nagao R. T., Key J. L. Upstream sequences required for efficient expression of a soybean heat shock gene. Mol Cell Biol. 1986 Feb;6(2):559–565. doi: 10.1128/mcb.6.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind M. L., Wessler S. R., Schmidt G. W. Functional determinants in transit sequences: import and partial maturation by vascular plant chloroplasts of the ribulose-1,5-bisphosphate carboxylase small subunit of Chlamydomonas. J Cell Biol. 1985 Jan;100(1):226–234. doi: 10.1083/jcb.100.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Seftor E. A., Schell J., Bohnert H. J. The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J. 1985 Jan;4(1):25–32. doi: 10.1002/j.1460-2075.1985.tb02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Tobin E. M. Nucleotide sequence encoding the precursor of the small subunit of ribulose 1,5-bisphosphate carboxylase from Lemna gibba L.G-3. Nucleic Acids Res. 1983 Nov 25;11(22):8051–8061. doi: 10.1093/nar/11.22.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]