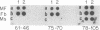Abstract
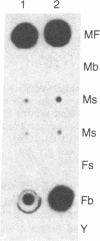
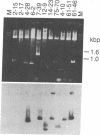
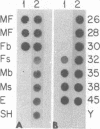
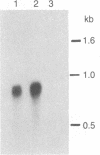
We have isolated and characterized a cDNA clone that is derived from a developmentally regulated mRNA found only in mature female schistosomes. The mRNA is approximately 950 nucleotides in length and is not detectable in immature female schistosomes isolated from single-sex infections, in male worms, or in eggs. During normal bisexual infections, the mRNA species is first detected 28 days after infection (the time of worm pairing) and increases to a high level 35 days after infection, coinciding with the start of egg production. The nucleotide sequence of the cDNA shows two large open reading frames in the coding strand. Several features of the clone, including the deduced sequence of the polypeptide encoded by one of the reading frames, suggest a relationship to the silk moth chorion (egg shell) gene family. The isolation of this clone provides us with a probe for further studies of female schistosome development and is a first step toward a detailed understanding of this process at the molecular level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. C. MATING BEHAVIOR AND DEVELOPMENT OF SCHISTOSOMES IN THE MOUSE. J Parasitol. 1965 Aug;51:605–616. [PubMed] [Google Scholar]
- Aronstein W. S., Strand M. Gender-specific and pair-dependent glycoprotein antigens of Schistosoma mansoni. J Parasitol. 1984 Aug;70(4):545–557. [PubMed] [Google Scholar]
- Aronstein W. S., Strand M. Identification of species-specific and gender-specific proteins and glycoproteins of three human schistosomes. J Parasitol. 1983 Dec;69(6):1006–1017. [PubMed] [Google Scholar]
- Atkinson B. G., Atkinson K. H. Schistosoma mansoni: one- and two-dimensional electrophoresis of proteins synthesized in vitro by males, females, and juveniles. Exp Parasitol. 1982 Feb;53(1):26–38. doi: 10.1016/0014-4894(82)90089-3. [DOI] [PubMed] [Google Scholar]
- Atkinson K. H., Atkinson B. G. Biochemical basis for the continuous copulation of female Schistosoma mansoni. Nature. 1980 Jan 31;283(5746):478–479. doi: 10.1038/283478a0. [DOI] [PubMed] [Google Scholar]
- Basch P. F., Basch N. Intergeneric reproductive stimulation and parthenogenesis in Schistosoma mansoni. Parasitology. 1984 Oct;89(Pt 2):369–376. doi: 10.1017/s0031182000001372. [DOI] [PubMed] [Google Scholar]
- Byram J. E., Senft A. W. Structure of the schistosome eggshell: amino acid analysis and incorporation of labelled amino acids. Am J Trop Med Hyg. 1979 May;28(3):539–547. doi: 10.4269/ajtmh.1979.28.539. [DOI] [PubMed] [Google Scholar]
- CLEGG J. A. IN VITRO CULTIVATION OF SCHISTOSOMA MANSONI. Exp Parasitol. 1965 Apr;16:133–147. doi: 10.1016/0014-4894(65)90037-8. [DOI] [PubMed] [Google Scholar]
- Capron A., Biguet J., Rose F., Vernes A. Les antigènes de Schistosoma mansoni. II. Etude immunoélectrophorétique comparée de divers stades larvaires et des adults des deux sexes. Aspects immunologiques des relations hote-parasite de la cervaire et de l'adulte de S. mansoni. Ann Inst Pasteur (Paris) 1965 Nov;109(5):798–810. [PubMed] [Google Scholar]
- Clough E. R. Morphology and reproductive organs and oogenesis in bisexual and unisexual transplants of mature Schistosoma mansoni females. J Parasitol. 1981 Aug;67(4):535–539. [PubMed] [Google Scholar]
- Cordeiro M. N., Gazzinelli G. Schistosoma mansoni: resolution and molecular weight estimates of tegument glycoproteins by polyacrylamide gel electrophoresis. Exp Parasitol. 1979 Dec;48(3):337–344. doi: 10.1016/0014-4894(79)90117-6. [DOI] [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- Erasmus D. A. A comparative study of the reproductive system of mature, immature and "unisexual" female Schistosoma mansoni. Parasitology. 1973 Oct;67(2):165–183. doi: 10.1017/s0031182000046394. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R., Kafatos F. C. Developmentally regulated genes in silkmoths. Annu Rev Genet. 1984;18:443–487. doi: 10.1146/annurev.ge.18.120184.002303. [DOI] [PubMed] [Google Scholar]
- Knight M., Simpson A. J., Payares G., Chaudri M., Smithers S. R. Cell-free synthesis of Schistosoma mansoni surface antigens: stage specificity of their expression. EMBO J. 1984 Jan;3(1):213–219. doi: 10.1002/j.1460-2075.1984.tb01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Michaels R. M. Mating of Schistosoma mansoni in vitro. Exp Parasitol. 1969 Aug;25(1):58–71. doi: 10.1016/0014-4894(69)90052-6. [DOI] [PubMed] [Google Scholar]
- Popiel I., Basch P. F. Putative polypeptide transfer from male to female Schistosoma mansoni. Mol Biochem Parasitol. 1984 Apr;11:179–188. doi: 10.1016/0166-6851(84)90064-1. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ruppel A., Cioli D. A comparative analysis of various developmental stages of Schistosoma mansoni with respect to their protein composition. Parasitology. 1977 Dec;75(3):339–343. doi: 10.1017/s003118200005188x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Shaw J. R., Marshall I., Erasmus D. A. Schistosoma mansoni: in vitro stimulation of vitelline cell development by extracts of male worms. Exp Parasitol. 1977 Jun;42(1):14–20. doi: 10.1016/0014-4894(77)90056-x. [DOI] [PubMed] [Google Scholar]
- Shaw J. R. Schistosoma mansoni: pairing in vitro and development of females from single sex infections. Exp Parasitol. 1977 Feb;41(1):54–65. doi: 10.1016/0014-4894(77)90129-1. [DOI] [PubMed] [Google Scholar]
- Short R. B. Presidential address: Sex and the single schistosome. J Parasitol. 1983 Feb;69(1):3–22. [PubMed] [Google Scholar]
- Simpson A. J., Knight M. Cloning of a major developmentally regulated gene expressed in mature females of Schistosoma mansoni. Mol Biochem Parasitol. 1986 Jan;18(1):25–35. doi: 10.1016/0166-6851(86)90047-2. [DOI] [PubMed] [Google Scholar]
- de Cicco D. V., Spradling A. C. Localization of a cis-acting element responsible for the developmentally regulated amplification of Drosophila chorion genes. Cell. 1984 Aug;38(1):45–54. doi: 10.1016/0092-8674(84)90525-7. [DOI] [PubMed] [Google Scholar]
- van Keulen H., Loverde P. T., Bobek L. A., Rekosh D. M. Organization of the ribosomal RNA genes in Schistosoma mansoni. Mol Biochem Parasitol. 1985 May;15(2):215–230. doi: 10.1016/0166-6851(85)90121-5. [DOI] [PubMed] [Google Scholar]