Abstract
Objective:
To evaluate the likelihood of response to IV immunoglobulin (IVIg) by studying consecutive patients presenting with progressive, asymmetric, pure lower motor neuron (LMN) limb weakness, and to determine the clinical phenotype of those who respond.
Methods:
Thirty-one consecutive patients with progressive, focal-onset LMN limb weakness, without evidence of clinical upper motor neuron signs; sensory, respiratory, or bulbar involvement; or evidence of motor nerve conduction block on electrodiagnostic studies, were prospectively included in this study. Each patient underwent treatment with IVIg (2 g/kg) for a minimum of 3 months. Electrodiagnostic studies, a neuromuscular symptom score, and expanded Medical Research Council sum score were documented before and after IVIg treatment. The final diagnosis was determined after prolonged clinical follow-up.
Results:
Only 3 of 31 patients (10%) responded to IVIg. All responders demonstrated distal upper limb–onset weakness, EMG abnormalities confined to the clinically weak muscles, and a normal creatine kinase. This set of features was also identified in 31% of nonresponders presenting with distal upper limb weakness. Sex, age at onset, number of involved limb regions, and the duration of symptoms before treatment were not significantly different between groups.
Conclusion:
The findings of the present study do not support uniform use of IVIg in patients presenting with progressive asymmetric LMN limb weakness. It is suggested that IVIg treatment be limited to patients who demonstrate clinical and laboratory features suggestive of multifocal motor neuropathy.
Classification of evidence:
This study provides Class IV evidence that IVIg will not improve muscle function in 90% of patients with progressive, asymmetric, pure LMN weakness.
Treatable progressive lower motor neuron syndromes (PLMNS), such as multifocal motor neuropathy (MMN), remain difficult to distinguish from untreatable causes of focal limb weakness early in the course of the disease. In particular, standard electrophysiologic studies may not identify characteristic conduction block (CB) in patients with MMN, or CB may disappear over time.1,2 Small case series demonstrating successful treatment of selected patients with asymmetric limb weakness who do not meet the diagnostic criteria of MMN with CB3–6 have generated the suggestion that patients with progressive and asymmetric distal lower motor neuron (LMN) limb weakness may warrant a trial of IV immunoglobulin (IVIg).1 However, uniform treatment with IVIg of all patients with an asymmetric distal PLMNS would produce a significant burden on health care resources, and thus appropriate patient selection is necessary. The present study was undertaken to determine the rate of response to IVIg therapy in a cohort of consecutive patients presenting with PLMNS without motor nerve CB on electrodiagnostic studies, and to determine the phenotypic features of those patients who do respond to IVIg treatment, in order to help guide rational treatment selection.
METHODS
Between 2006 and 2012, 31 consecutive patients evaluated in the neuromuscular clinic at the University of California, San Francisco, were included in this study. These patients demonstrated evidence of progressive, asymmetric, focal-onset limb weakness with isolated LMN signs (muscle wasting and weakness) on clinical examination. Patients were excluded if there was clinical evidence on history or examination of bulbar or respiratory weakness, sensory abnormalities, or upper motor neuron (UMN) signs (spasticity, hyperreflexia or reflex spread, and Babinski or Hoffmann signs) on any evaluation before treatment with IVIg.
Standard protocol approvals, registrations, and patient consents.
The study was exempt from Committee on Human Research approval because IVIg is a routine part of clinical care for patients with pure LMN syndromes and all data analyses were performed without patient identifiers.
Study hypothesis/classification of evidence.
The study hypothesis was that patients with PLMNS demonstrate improvements in muscle strength and neuromuscular symptoms after treatment with IVIg. This study provides Class IV evidence of the benefit of IVIg treatment of patients with progressive, asymmetric, pure LMN weakness.
Methodology and treatment.
Motor and sensory nerve conduction studies (NCS) in a minimum of 2 limbs were performed in each patient using standard techniques.7 Motor nerve studies included the median, ulnar, peroneal, and tibial nerves. Median and ulnar motor NCS included stimulation at the axilla. F-wave latencies were recorded from each nerve. Sensory NCS included superficial radial, median, ulnar, superficial peroneal, and sural nerves. EMG was performed in each patient with sampling of muscles in the upper and lower limbs, thoracic paraspinals, and genioglossus.
Patients were included if electrodiagnostic studies identified a purely motor process with normal sensory NCS, and neurogenic abnormalities on EMG studies, with or without reduced compound muscle action potential (CMAP) amplitudes, and without evidence of CB, temporal dispersion, nerve conduction slowing, or prolongation of F-wave latencies on motor NCS. The definitions of CB and temporal dispersion used by Katz et al.4 were applied in this study, with a reduction of CMAP amplitude or area of >30%, or increase of the CMAP duration of >30% in any segment considered significant.
Additional clinical evaluations performed at baseline included a neuromuscular symptom score (NSS)8 that evaluated 20 frequently performed activities using specific muscle groups, including of the upper and lower limbs and trunk, for a total score of 60. In addition, muscle strength of bilateral upper and lower limb muscle groups (deltoid, biceps, wrist extensors, finger flexors, finger extensors, finger abductors, hip flexors, quadriceps, tibialis anterior, and extensor hallucis longus) was assessed using the Medical Research Council scoring system and incorporated into an expanded Medical Research Council sum score (EMRCSS) developed for the detection of muscle weakness in patients with amyotrophic lateral sclerosis (ALS), with a total score of 100.9
Laboratory investigations were performed to exclude other treatable causes of weakness including primary muscle disease, infection, vasculitis, systemic autoimmune disease, and malignancy. Anti-ganglioside M1 (GM1) antibodies and CSF were not routinely tested because they have not been shown to predict response to IVIg treatment.6 Each patient underwent MRI studies of the spine to exclude structural causes of their weakness. Subclinical respiratory muscle weakness at baseline was excluded with forced vital capacity and maximum inspiratory pressure measurements.
Each patient received treatment with IVIg at a dose of 2 g/kg, which was repeated for a minimum of 3 monthly treatments before reevaluation. The majority of patients (84%) received IVIg in the home by Crescent HealthCare, a Walgreens Company, and the remainder received IVIg in a local treatment center. After this initial treatment period, each patient was reassessed with clinical evaluations including a complete neurologic examination, NSS and EMRCSS, and repeat electrodiagnostic studies. Evidence of improvement was defined as a 2-point increase in the EMRCSS or NSS. Each patient was also asked to give their subjective impression of the benefit of the IVIg treatment, and side effects during IVIg treatment were recorded.
IVIg treatment was ceased in those patients who reported no benefit or significant progression of weakness after 3 months of therapy (20 patients). IVIg treatment was continued in the remaining patients and only subsequently ceased when there was definite evidence of disease progression (8 patients, mean IVIg courses 9.9 ± 1.8, range 4–17). Irrespective of subsequent treatment, each patient was then followed in a specialized neuromuscular clinic for an average of 32.6 ± 5.0 months from symptom onset (range 8–108 months), and the duration of follow-up for responders after initiation of treatment was a minimum of 12 months.
Statistical analysis.
Student t test was used to compare continuous variables and the Fisher exact test to compare categorical variables. Probability levels <0.05 were taken to be significant.
RESULTS
Only 3 of 31 patients (9.7%, 95% confidence interval 3.3%–24.9%, Wilson method) treated with IVIg in the present cohort demonstrated objective improvement after the 3-month treatment period (table 1). Each of these responders reported subjective improvement. Two patients with isolated hand weakness noted partial improvement in strength and function with mild to moderate residual deficits. One patient with a 35-year history of weakness, commencing with distal upper limb (UL) weakness and progressing to weakness in all 4 limbs before treatment, experienced normalization of speech and swallowing function, and definite improvement in limb strength. Despite treatment, this patient remained confined to a wheelchair and dependent for activities of daily living. Objective improvements in the responding subjects were generally modest (NSS 0%–13% improvement, EMRCSS 1.7%–3.3% improvement). Subjective improvement after treatment was also reported by 34% of nonresponders (10 patients), and 7 of these 10 patients had unchanged NSS and/or EMRCSS between baseline and posttreatment visits.
Table 1.
Change in the NSS and EMRCSS determined at baseline and after 3 months of IVIg treatment
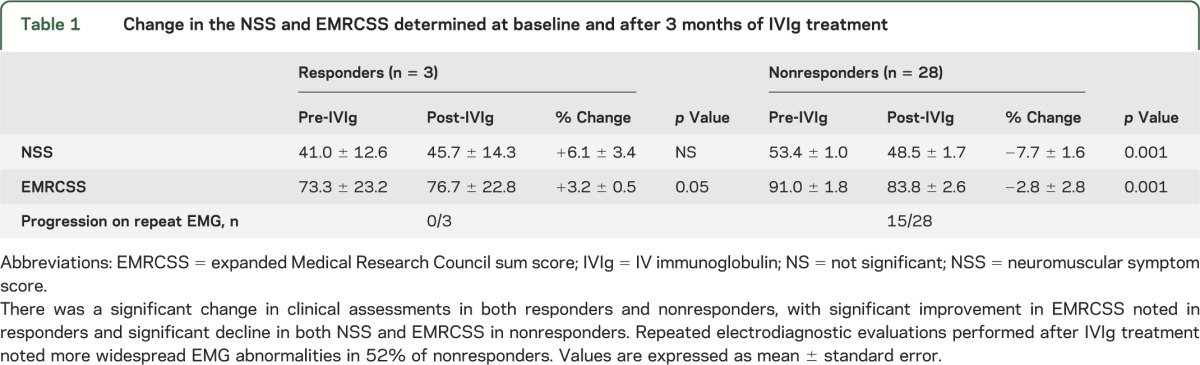
None of the patients who improved after the initial treatment period subsequently developed progressive weakness, UMN signs, or respiratory or bulbar weakness over the follow-up period. Each responder stabilized with ongoing IVIg treatment but it was noted that the magnitude of functional gains was generally modest (table 1). No patient developed CB on repeat EMG studies.
In the nonresponder group, 12 patients (43%) developed clinical UMN signs after an average of 10.4 ± 1.5 months (range 3–20 months) following initiation of IVIg treatment and were classified as having ALS. A further 9 patients (32%) subsequently developed bulbar symptoms (3 patients, mean 5.3 months from IVIg treatment, range 3–10 months) and/or respiratory symptoms (7 patients, mean 24 months from IVIg treatment, range 3–73 months) without UMN signs and were classified as having progressive muscular atrophy (PMA). The remaining 7 patients (25%) had ongoing progression of LMN limb weakness, with spread to other limbs, but without bulbar, respiratory, or UMN features, and were classified as having probable PMA. Of the 10 nonresponders who reported subjective benefit after IVIg treatment, the final diagnosis was ALS in 4 patients, PMA in 3 patients, and probable PMA in 3 patients.
Regarding the characteristics of the responder group, each patient presented with distal UL-onset weakness, as compared with the nonresponder group, in which 48% presented with distal UL weakness, 38% with distal lower limb weakness, and 14% with proximal UL weakness (table 2). Other defining characteristics were normal serum creatine kinase (CK) levels in all responders and EMG studies demonstrating neurogenic abnormalities confined to muscles demonstrating clinical weakness without abnormalities of the thoracic paraspinal muscles. Anti-GM1 antibodies were detected in 2 responders (titers 1:3,200) and 0 of 9 nonresponders in whom the antibody was tested. Sex, age at onset, number of involved limb regions at the time of treatment, and the duration of symptoms before treatment were not significantly different between groups.
Table 2.
Clinical and laboratory characteristics of responders and nonresponders
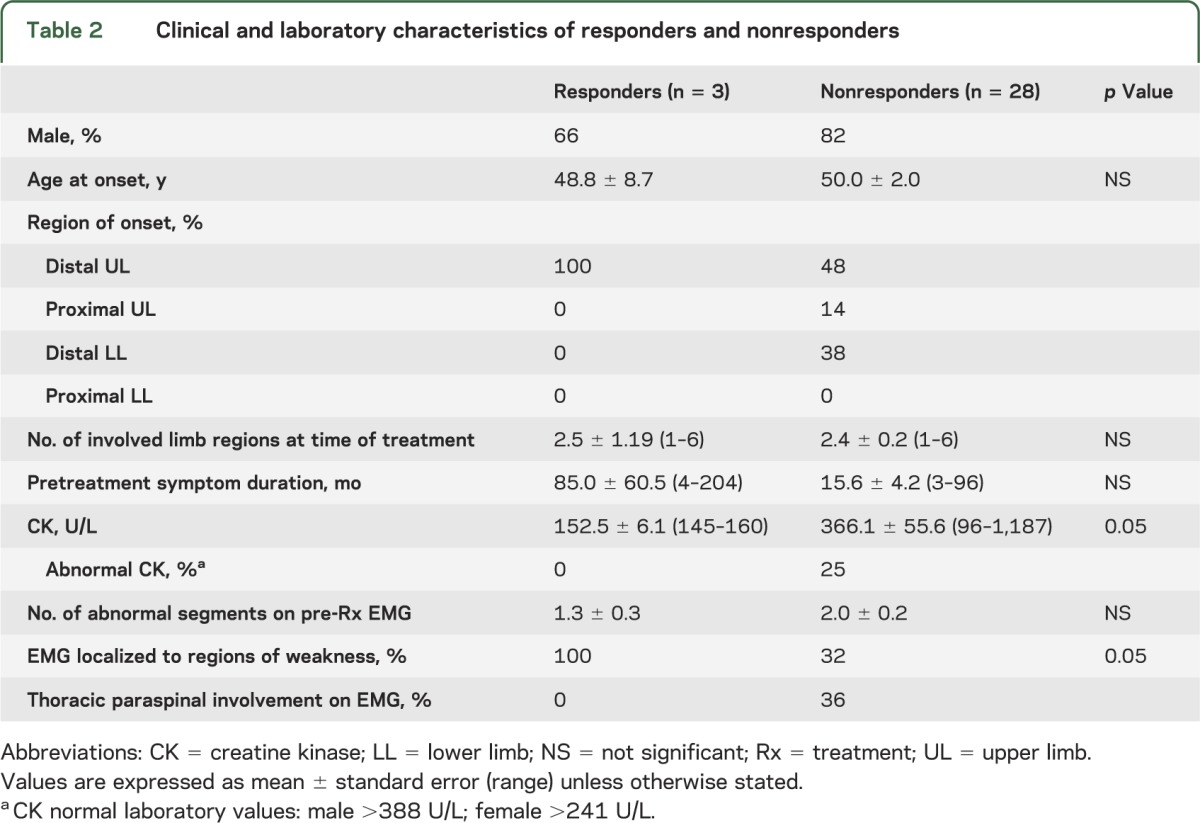
The characteristics of the 16 patients (3 responders and 13 nonresponders) with distal UL-onset weakness were analyzed to determine features that may predict a positive clinical response to IVIg treatment. Regarding clinical weakness at the time of treatment, 7 of 13 nonresponders (54%) had weakness confined to the distal UL (6 unilateral and 1 bilateral involvement). Neurogenic EMG abnormalities were confined to the limb demonstrating clinical weakness in 46% of this subgroup of 13 patients (all patients with unilateral distal UL weakness), and none of these patients had abnormalities on EMG of thoracic paraspinal muscles.
The distribution of clinical weakness and EMG abnormalities was further divided into peripheral nerve territories (median, ulnar, radial, musculocutaneous, and axillary nerves), and considered diffuse if more than 3 territories were involved. Mean number of involved nerve territories was 3.3 in nonresponders and 2.3 in responders (p < 0.05). In 31% of nonresponders with distal UL-onset weakness, EMG abnormalities matched the distribution of clinical weakness, including in 50% of patients with isolated distal UL weakness. None of the responders had EMG abnormalities outside of the distribution of clinical weakness.
IVIg therapy was associated with reported side effects in 36% of patients, including rash in 13% (chronic in 6%, transient in 6%), transient headaches during and after the infusion in 13%, laboratory-confirmed aseptic meningitis in 6%, recurrent local limb infusion reactions in 3%, and palpitations in 3%.
DISCUSSION
In the present study, 31 consecutive patients presenting with progressive, focal-onset, isolated LMN limb weakness, without CB on motor NCS, were treated with IVIg. The reported cohort represents the largest consecutively recruited series of such patients to date. Only a small minority of patients responded to IVIg treatment. The diagnosis of responding patients was most likely MMN without CB as the patients demonstrated a phenotype consistent with MMN, specifically distal UL-onset weakness restricted to the distribution of individual peripheral nerve territories, with EMG abnormalities limited to weak muscles.1 The findings of the present study suggest that there is a low rate of response of patients with PLMNS to IVIg treatment, and do not support uniform administration of IVIg in all patients presenting with asymmetric LMN limb weakness without CB on motor NCS. Rather, selection of appropriate patients based on the combination of findings suggestive of MMN on clinical, laboratory, and electrodiagnostic examinations is suggested.
The rate of response to IVIg in the present study was much lower than in previously published studies evaluating the role of IVIg in PLMNS, where response rates varied between 40% and 74%.3–6 This discrepancy may be attributable to previous studies reporting selected groups of patients rather than prospective, consecutive series of patients as described here. In addition, most previous studies reporting response rates for patients with PLMNS without CB included patients with electrophysiologic features of demyelination but without meeting the diagnostic criteria for MMN with CB,3,5,6 hence increasing the likelihood of an underlying inflammatory etiology.
Previous series have observed that patients with a characteristic clinical phenotype of MMN, specifically slowly progressive distal UL-onset weakness, are more likely to respond to IVIg.2–6 Only 33% of the patients in this series with distal UL-onset weakness responded, and this increased to 50% when only patients with EMG abnormalities restricted to the muscles demonstrating clinical weakness and normal serum CK were considered. It is noted that distal UL weakness may be a prominent and early feature of ALS,10 and hence it may be difficult to distinguish degenerative anterior horn cell disorders from MMN early in the disease course. While predictive clinical and laboratory features serve as a guide to select patients for treatment with IVIg, there appears to be no clear combination of features that can determine the likelihood of a positive treatment response with very high sensitivity. Careful patient selection based on clinical, laboratory, and EMG findings as recommended here is expected to reduce the rate of inappropriate IVIg treatments, while avoiding missed treatment opportunities.
Additional investigations may be useful in the workup of patients before treatment. High-titer serum anti-GM1 antibodies were detected in 2 of 3 responders, and anti-GM1 antibodies are identified in 20% to 80% of patients with MMN.11 While the presence of high-titer anti-GM1 antibodies may contribute to treatment decisions in patients presenting with progressive asymmetric LMN limb weakness, it is important to note that low titers of anti-GM1 antibodies are identified in patients with ALS and other noninflammatory LMN disorders.12,13 Hence, the presence of low-titer anti-GM1 antibodies in patients with PLMNS without other predictors of a positive treatment response described above may not provide adequate support for a trial of IVIg.
High-resolution ultrasonography (HRUS) may also provide additional useful information. HRUS demonstrates enlargement of UL peripheral nerves in patients with MMN, even in the absence of clinical or electrodiagnostic abnormalities.14 This is in contrast with ALS, where mild reductions of nerve cross-sectional area are noted.15 HRUS was not included in the present study, and further investigation of the sensitivity and specificity of this technology in the discrimination of MMN from mimic disorders is warranted.
A group of patients reported symptomatic improvement after IVIg treatment, and 70% of this group were noted to plateau on clinical assessments during IVIg treatment. Transient improvement after IVIg treatment in patients with ALS has been reported16 and this may represent a transient improvement in secondary inflammatory processes.17 The lack of progression in this patient group over the 3-month treatment interval may also reflect more slowly progressive disease, which may be observed in LMN-predominant ALS and PMA.18–20
Finally, although IVIg therapy is largely considered to be safe,21 the high rate of side effects noted in the present study, higher than reported in other studies, suggests that the impact of IVIg treatment on the well-being of the patient should be considered before its use as a “nothing to lose” treatment trial. IVIg has both direct adverse effects as reported here, as well as indirect adverse effects such as the often considerable time burden associated with the infusions, prolongation of the period of diagnostic uncertainty for the patient, delay of institution of other appropriate treatments, and the lingering fatigue that often accompanies IVIg treatment22 that may compound this common symptom in ALS.23,24 Hence, based on the findings of the present study, IVIg treatment is recommended for those patients in whom there is a possibility of a positive response, specifically those patients with a normal CK, EMG abnormalities confined to the areas of weakness, and distal, asymmetric limb weakness.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- CB
conduction block
- CK
creatine kinase
- CMAP
compound muscle action potential
- EMRCSS
expanded Medical Research Council sum score
- GM1
ganglioside M1
- HRUS
high-resolution ultrasonography
- IVIg
IV immunoglobulin
- LMN
lower motor neuron
- MMN
multifocal motor neuropathy
- NCS
nerve conduction studies
- NSS
neuromuscular symptom score
- PLMNS
progressive lower motor neuron syndrome
- PMA
progressive muscular atrophy
- UL
upper limb
- UMN
upper motor neuron
AUTHOR CONTRIBUTIONS
Dr. Simon: data acquisition, analysis and interpretation, drafting and revision of the manuscript. Ms. Ayer: data acquisition and critical revision of the manuscript for important intellectual content. Dr. Lomen-Hoerth: study design, data acquisition, analysis and interpretation, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
N.G.S. gratefully acknowledges funding from the National Health and Medical Research Council of Australia and the Motor Neurone Disease Research Institute of Australia (grant 1039520).
DISCLOSURE
Dr. Simon reports no disclosures. Ms. Ayer is employed with the Walgreens IG program, which provides in-home infusion services. Dr. Lomen-Hoerth reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Chaudhry V, Swash M. Multifocal motor neuropathy: is conduction block essential? Neurology 2006;67:558–559 [DOI] [PubMed] [Google Scholar]
- 2.Taylor BV, Wright RA, Harper CM, Dyck PJ. Natural history of 46 patients with multifocal motor neuropathy with conduction block. Muscle Nerve 2000;23:900–908 [DOI] [PubMed] [Google Scholar]
- 3.Ellis CM, Leary S, Payan J, et al. Use of human intravenous immunoglobulin in lower motor neuron syndromes. J Neurol Neurosurg Psychiatry 1999;67:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz JS, Barohn RJ, Kojan S, et al. Axonal multifocal motor neuropathy without conduction block or other features of demyelination. Neurology 2002;58:615–620 [DOI] [PubMed] [Google Scholar]
- 5.Burrell JR, Yiannikas C, Rowe D, Kiernan MC. Predicting a positive response to intravenous immunoglobulin in isolated lower motor neuron syndromes. PLoS One 2011;6:e27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strigl-Pill N, Konig A, Schroder M, et al. Prediction of response to IVIg treatment in patients with lower motor neurone disorders. Eur J Neurol 2006;13:135–140 [DOI] [PubMed] [Google Scholar]
- 7.Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 4th ed New York: Oxford University Press; 2013 [Google Scholar]
- 8.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 1993;329:1993–2000 [DOI] [PubMed] [Google Scholar]
- 9.Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain 2011;134:2582–2594 [DOI] [PubMed] [Google Scholar]
- 10.Menon P, Kiernan MC, Yiannikas C, Stroud J, Vucic S. Split-hand index for the diagnosis of amyotrophic lateral sclerosis. Clin Neurophysiol 2013;124:410–416 [DOI] [PubMed] [Google Scholar]
- 11.Meuth SG, Kleinschnitz C. Multifocal motor neuropathy: update on clinical characteristics, pathophysiological concepts and therapeutic options. Eur Neurol 2010;63:193–204 [DOI] [PubMed] [Google Scholar]
- 12.Niebroj-Dobosz I, Janik P, Kwiecinski H. Serum IgM anti-GM1 ganglioside antibodies in lower motor neuron syndromes. Eur J Neurol 2004;11:13–16 [DOI] [PubMed] [Google Scholar]
- 13.Taylor BV, Gross L, Windebank AJ. The sensitivity and specificity of anti-GM1 antibody testing. Neurology 1996;47:951–955 [DOI] [PubMed] [Google Scholar]
- 14.Beekman R, van den Berg LH, Franssen H, Visser LH, van Asseldonk JT, Wokke JH. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology 2005;65:305–307 [DOI] [PubMed] [Google Scholar]
- 15.Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve 2011;44:346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalakas MC, Stein DP, Otero C, Sekul E, Cupler EJ, McCrosky S. Effect of high-dose intravenous immunoglobulin on amyotrophic lateral sclerosis and multifocal motor neuropathy. Arch Neurol 1994;51:861–864 [DOI] [PubMed] [Google Scholar]
- 17.Smith RG, Appel SH. Immunosuppression and anti-inflammatory agents in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1(suppl 4):33–42 [PubMed] [Google Scholar]
- 18.Wijesekera LC, Mathers S, Talman P, et al. Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology 2009;72:1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caroscio JT, Mulvihill MN, Sterling R, Abrams B. Amyotrophic lateral sclerosis: its natural history. Neurol Clin 1987;5:1–8 [PubMed] [Google Scholar]
- 20.Christensen PB, Hojer-Pedersen E, Jensen NB. Survival of patients with amyotrophic lateral sclerosis in 2 Danish counties. Neurology 1990;40:600–604 [DOI] [PubMed] [Google Scholar]
- 21.Dalakas MC, Clark WM. Strokes, thromboembolic events, and IVIg: rare incidents blemish an excellent safety record. Neurology 2003;60:1736–1737 [DOI] [PubMed] [Google Scholar]
- 22.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev 2007;6:257–259 [DOI] [PubMed] [Google Scholar]
- 23.Vucic S, Cheah BC, Kiernan MC. Maladaptation of cortical circuits underlies fatigue and weakness in ALS. Amyotroph Lateral Scler 2011;12:414–420 [DOI] [PubMed] [Google Scholar]
- 24.Vucic S, Krishnan AV, Kiernan MC. Fatigue and activity dependent changes in axonal excitability in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2007;78:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]


