Abstract
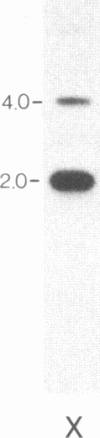
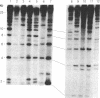
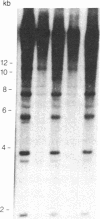
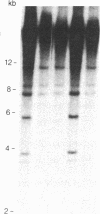
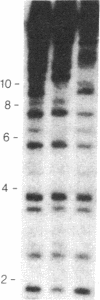
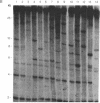
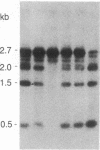
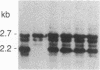
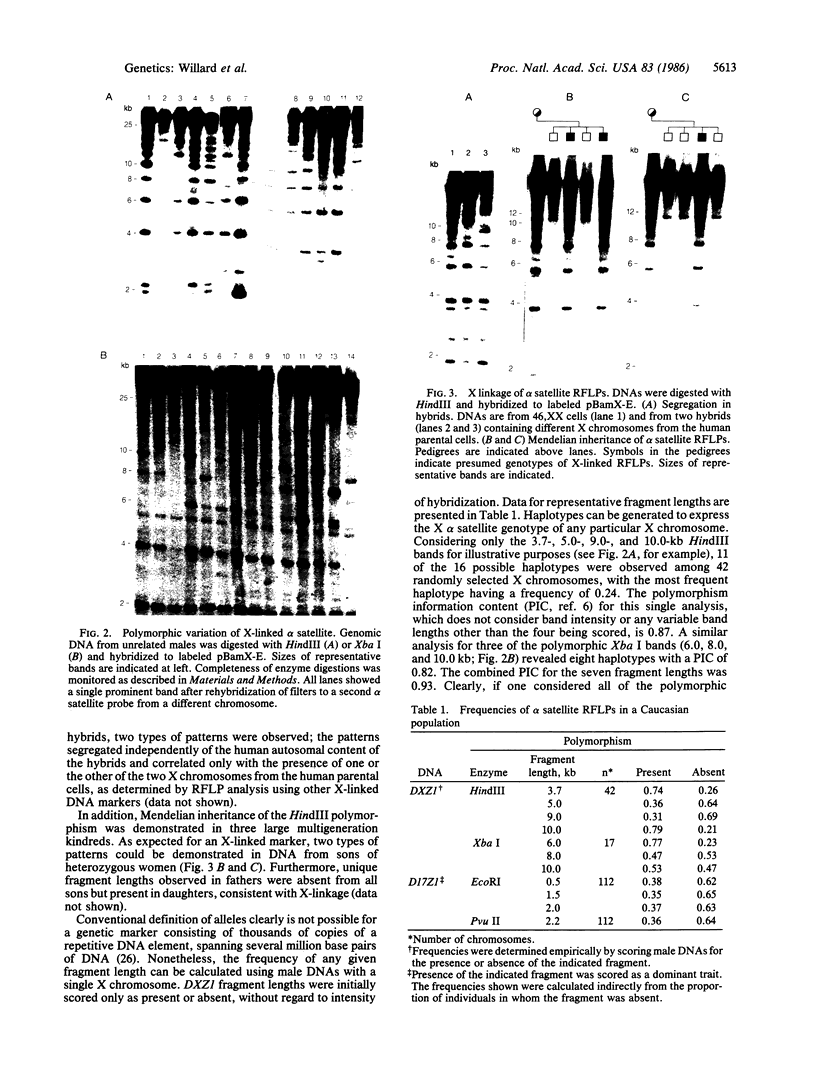
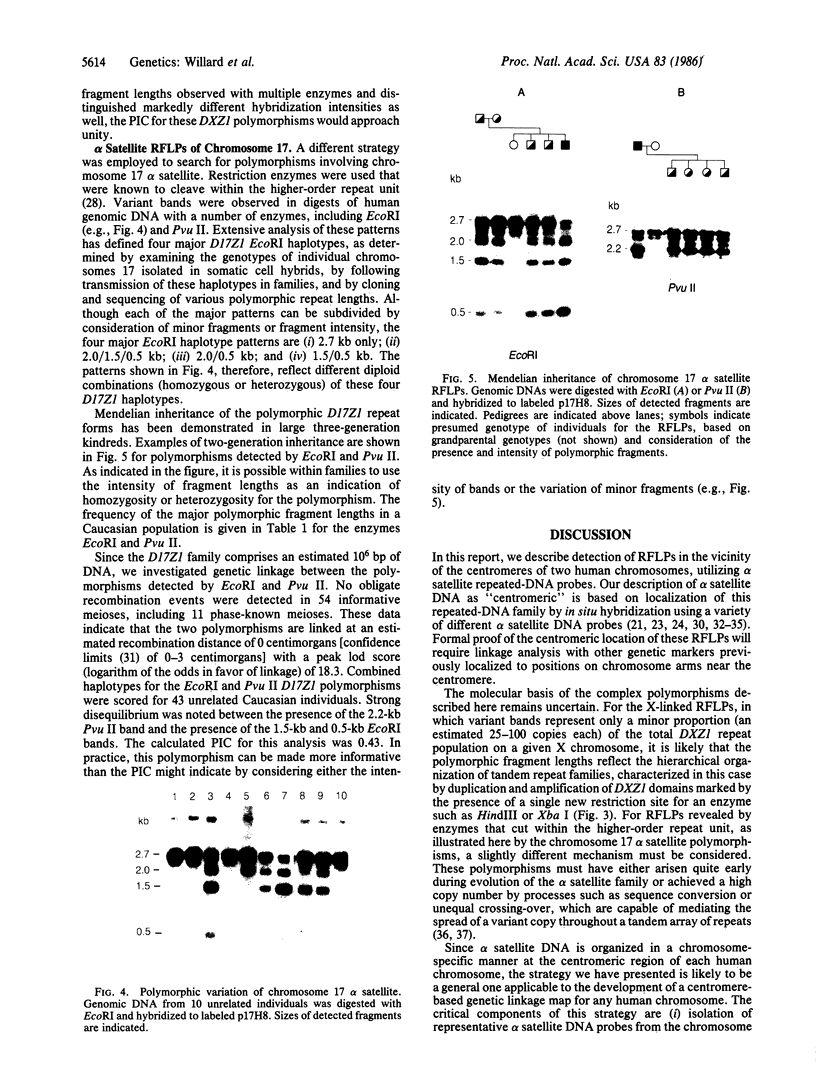
We describe a general strategy for the detection of high-frequency restriction fragment length polymorphisms in the centromeric regions of human chromosomes by molecular analysis of alpha satellite DNA, a diverse family of tandemly repeated DNA located near the centromeres of all human chromosomes. To illustrate this strategy, cloned alpha satellite repeats isolated from two human chromosomes, 17 and X, have been used under high-stringency conditions that take advantage of the chromosome-specific organization of this divergent repeated DNA family. Multiple high-frequency restriction fragment length polymorphisms are described for the centromeric region of both chromosome 17 and X chromosome. Mendelian inheritance of the variants is demonstrated. The X-linked alpha satellite polymorphisms in particular are highly informative and constitute a virtually unique centromeric DNA marker for each X chromosome examined. Since the strategy we describe is a general one, the alpha satellite family of DNA should provide a rich source of molecular variation in the human genome and should contribute to the development of centromere-based genetic linkage maps of human chromosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Conneally P. M., Edwards J. H., Kidd K. K., Lalouel J. M., Morton N. E., Ott J., White R. Report of the Committee on Methods of Linkage Analysis and Reporting. Cytogenet Cell Genet. 1985;40(1-4):356–359. doi: 10.1159/000132186. [DOI] [PubMed] [Google Scholar]
- Devilee P., Slagboom P., Cornelisse C. J., Pearson P. L. Sequence heterogeneity within the human alphoid repetitive DNA family. Nucleic Acids Res. 1986 Mar 11;14(5):2059–2073. doi: 10.1093/nar/14.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., White R. The genetic linkage map of the human X chromosome. Science. 1985 Nov 15;230(4727):753–758. doi: 10.1126/science.4059909. [DOI] [PubMed] [Google Scholar]
- Gray K. M., White J. W., Costanzi C., Gillespie D., Schroeder W. T., Calabretta B., Saunders G. F. Recent amplification of an alpha satellite DNA in humans. Nucleic Acids Res. 1985 Jan 25;13(2):521–535. doi: 10.1093/nar/13.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F., Wexler N. S., Conneally P. M., Naylor S. L., Anderson M. A., Tanzi R. E., Watkins P. C., Ottina K., Wallace M. R., Sakaguchi A. Y. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983 Nov 17;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Jabs E. W., Wolf S. F., Migeon B. R. Characterization of a cloned DNA sequence that is present at centromeres of all human autosomes and the X chromosome and shows polymorphic variation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4884–4888. doi: 10.1073/pnas.81.15.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P. A. Human chromosome heteromorphisms (variants). Prog Med Genet. 1977;2:251–274. doi: 10.1016/0378-1119(77)90004-x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Kittur S. D., Hoppener J. W., Antonarakis S. E., Daniels J. D., Meyers D. A., Maestri N. E., Jansen M., Korneluk R. G., Nelkin B. D., Kazazian H. H., Jr Linkage map of the short arm of human chromosome 11: location of the genes for catalase, calcitonin, and insulin-like growth factor II. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5064–5067. doi: 10.1073/pnas.82.15.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. Chromosomal localization of complex and simple repeated human DNAs. Chromosoma. 1978 Mar 22;66(1):23–32. doi: 10.1007/BF00285813. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Wu J. C. Homology between human and simian repeated DNA. Nature. 1978 Nov 2;276(5683):92–94. doi: 10.1038/276092a0. [DOI] [PubMed] [Google Scholar]
- McKusick V. A. The Wilhelmine E. Key 1979 Invitational Lecture: The anatomy of the human genome. J Hered. 1980 Nov-Dec;71(6):370–391. doi: 10.1093/oxfordjournals.jhered.a109392. [DOI] [PubMed] [Google Scholar]
- Mitchell A. R., Gosden J. R., Miller D. A. A cloned sequence, p82H, of the alphoid repeated DNA family found at the centromeres of all human chromosomes. Chromosoma. 1985;92(5):369–377. doi: 10.1007/BF00327469. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Middlesworth W., Colletti C. A., Aldridge J., Fischbeck K. H., Bartlett R., Pericak-Vance M. A., Roses A. D., Kunkel L. M. Detection of deletions spanning the Duchenne muscular dystrophy locus using a tightly linked DNA segment. 1985 Aug 29-Sep 4Nature. 316(6031):842–845. doi: 10.1038/316842a0. [DOI] [PubMed] [Google Scholar]
- Murray J. M., Davies K. E., Harper P. S., Meredith L., Mueller C. R., Williamson R. Linkage relationship of a cloned DNA sequence on the short arm of the X chromosome to Duchenne muscular dystrophy. Nature. 1982 Nov 4;300(5887):69–71. doi: 10.1038/300069a0. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985 Oct 10;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Ruddle F. H. A new era in mammalian gene mapping: somatic cell genetics and recombinant DNA methodologies. Nature. 1981 Nov 12;294(5837):115–120. doi: 10.1038/294115a0. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Unequal crossover and the evolution of multigene families. Cold Spring Harb Symp Quant Biol. 1974;38:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Tsui L. C., Buchwald M., Barker D., Braman J. C., Knowlton R., Schumm J. W., Eiberg H., Mohr J., Kennedy D., Plavsic N. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science. 1985 Nov 29;230(4729):1054–1057. doi: 10.1126/science.2997931. [DOI] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Schmidtke J., Watson E. A., Law H. Y., Farrall M., Cooke H. J., Eiberg H., Williamson R. Localization of cystic fibrosis locus to human chromosome 7cen-q22. 1985 Nov 28-Dec 4Nature. 318(6044):384–385. doi: 10.1038/318384a0. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome-specific alpha satellite DNA: nucleotide sequence analysis of the 2.0 kilobasepair repeat from the human X chromosome. Nucleic Acids Res. 1985 Apr 25;13(8):2731–2743. doi: 10.1093/nar/13.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Leppert M., Bishop D. T., Barker D., Berkowitz J., Brown C., Callahan P., Holm T., Jerominski L. Construction of linkage maps with DNA markers for human chromosomes. Nature. 1985 Jan 10;313(5998):101–105. doi: 10.1038/313101a0. [DOI] [PubMed] [Google Scholar]
- White R., Woodward S., Leppert M., O'Connell P., Hoff M., Herbst J., Lalouel J. M., Dean M., Vande Woude G. A closely linked genetic marker for cystic fibrosis. 1985 Nov 28-Dec 4Nature. 318(6044):382–384. doi: 10.1038/318382a0. [DOI] [PubMed] [Google Scholar]
- Willard H. F. Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet. 1985 May;37(3):524–532. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Riordan J. R. Assignment of the gene for myelin proteolipid protein to the X chromosome: implications for X-linked myelin disorders. Science. 1985 Nov 22;230(4728):940–942. doi: 10.1126/science.3840606. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Darling S. M., Erickson R. P., Craig I. W., Buckle V. J., Rigby P. W., Willard H. F., Goodfellow P. N. Isolation and characterization of an alphoid centromeric repeat family from the human Y chromosome. J Mol Biol. 1985 Apr 20;182(4):477–485. doi: 10.1016/0022-2836(85)90234-7. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Hansen S. K., Oishi K. K., Ryder O. A., Hamkalo B. A. Characterization of a cloned repetitive DNA sequence concentrated on the human X chromosome. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6593–6597. doi: 10.1073/pnas.79.21.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]