Abstract
Sorghum [Sorghum bicolor (L.) Moench] grain yield is severely affected by abiotic and biotic stresses during post-flowering stages, which has been aggravated by climate change. New parental lines having genes for various biotic and abiotic stress tolerances have the potential to mitigate this negative effect. Field studies were conducted under irrigated and dryland conditions with 128 exotic germplasm and 12 adapted lines to evaluate and identify potential sources for post-flowering drought tolerance and stalk and charcoal rot tolerances. The various physiological and disease related traits were recorded under irrigated and dryland conditions. Under dryland conditions, chlorophyll content (SPAD), grain yield and HI were decreased by 9, 44 and 16%, respectively, compared to irrigated conditions. Genotype RTx7000 and PI475432 had higher leaf temperature and grain yield, however, genotype PI570895 had lower leaf temperature and higher grain yield under dryland conditions. Increased grain yield and optimum leaf temperature was observed in PI510898, IS1212 and PI533946 compared to BTx642 (B35). However, IS14290, IS12945 and IS1219 had decreased grain yield and optimum leaf temperature under dryland conditions. Under irrigated conditions, stalk and charcoal rot disease severity was higher than under dryland conditions. Genotypes IS30562 and 1790E R had tolerance to both stalk rot and charcoal rot respectively and IS12706 was the most susceptible to both diseases. PI510898 showed combined tolerance to drought and Fusarium stalk rot under dryland conditions. The genotypes identified in this study are potential sources of drought and disease tolerance and will be used to develop better adaptable parental lines followed by high yielding hybrids.
Electronic supplementary material
The online version of this article (doi:10.1186/2193-1801-2-650) contains supplementary material, which is available to authorized users.
Keywords: Sorghum bicolor, Climate change, Drought, Chlorophyll fluorescence, Charcoal rot, Fusarium stalk rot, Principle component analysis
Introduction
By the end of the 21st century, global surface temperatures are predicted to increase in the range of +1.4 to +5.8°C (IPCC 2007). Increased drought frequency is an important characteristic of predicted climate change and is the most important yield limiting abiotic stress worldwide (Araus et al. 2002; Meehl et al. 2007). Sorghum (Sorghum bicolor L. Moench) originated in Sub-Saharan Africa, and is a major food crop in arid and semi-arid regions of the world (Balota et al. 2008). Although sorghum has a wide range of adaptability and can be grown in a wide series of environments, including heat, drought, salinity and flooding (Ejeta and Knoll, 2007), this crop is usually affected by water stress at both pre- and post-flowering stages of development and has the most adverse effect on yield during and after anthesis (Tuinstra et al. 1997; Kebede et al. 2001; Blum 2004). Post-flowering drought stress reduces the number and size of the seeds per plant (Rosenow and Clark 1995) by 36 and 55%, respectively, which are the main causes for lower grain yield in sorghum (Assefa et al. 2010).
Due to its polygenic nature, drought tolerance is a complex trait. Drought is highly influenced by environmental conditions which makes breeding selection cycles difficult at pre- and post-flowering stages in sorghum (Tuinstra et al. 1996). However, progress in developing drought tolerant genotypes through breeding efforts is tremendous. Secondary component traits with moderate to high heritability values under drought stress include stay green, chlorophyll content, chlorophyll fluorescence, canopy temperature and transpiration efficiency (Harris et al. 2007; Kumar et al. 2008; Roháček et al. 2008; Liu et al. 2010; Kapanigowda 2011; Mutava et al. 2011; Talebi 2011). Improving these traits can increase drought tolerance potential in sorghum (Prasad et al. 2006; Borell et al. 2010). Kapanigowda (2011) observed high broad-sense heritability (0.77 to 0.90) for transpiration efficiency in sorghum and showed this trait could be improved through breeding. Mutava et al. (2011) studied diversity for chlorophyll content, leaf temperature, chlorophyll a fluorescence (PS II quantum yield), harvest index (HI) and yield among 300 genotypes from different races of sorghum and reported wide variability in sorghum.
Post-flowering drought stress is associated with charcoal rot and Fusarium stalk rot disease which leads to significant lodging and yield loss (Rosenow and Clark 1995; Tesso et al. 2004). However, the disease severity is high under hot and humid conditions (Tesso et al. 2012). Stalk rot is characterized by degradation of pith tissue near the base of the stalk as a result of senescence of the stalk pith cells (Tesso et al. 2012) resulting in reduced transportation of nutrients and water, and breakage of the stalk at the zone of infection causing lodging (Hundekar and Anahahosur 1994). Charcoal rot of sorghum is caused by Macrophomina phaseolina (Tassi) Goid. and is a serious problem under high soil temperature and low moisture by a prolonged dry period, particularly during the grain filling stage (Hassan et al. 1996). Fusarium stalk rot (caused by Fusarium spp.) is more severe when drought and high temperature stress occurs during grain development followed by wet, cool conditions near physiological maturity (Tesso et al. 2012). Several studies have been conducted to understand the influence of drought stress (Diourte et al. 1995; Seetharama et al. 1991; Tenkouano et al. 1993), nitrogen and plant growth (Cloud and Rupe 1994) and inheritance of resistance to Fusarium spp. and M. phaseolina. Seetharama et al. (1991) reported high incidence of charcoal rot and low grain yield in drought stressed sorghum plants. However, there was little attempt to identify genotypes in exotic germplasm collections that might serve as potential resistance sources to Fusarium stalk and charcoal rot for breeding programs. Even though drought and disease stresses commonly occur in sorghum production environments (Rosenow et al. 1996), there is little information on the interaction of drought on disease severity and drought tolerance in exotic sorghum germplasms. Of the total (44,773) accessions in the world sorghum germplasm collections, only 9,889 (22%) are photoperiod insensitive which are extensively used in breeding programs. The available genetic resources in sorghum are still under unexploited for diseases and drought stress tolerance (Rosenow and Dahlberg 2000). Thus, it is necessary to screen genotypes under both irrigated and dryland conditions to document environments that favor disease development. Evaluation of new germplasm based upon secondary traits that are associated with drought and disease tolerace is of paramount importance.. In the present study, 44 plant introductory (PI) and 84 minicore photoperiod insensitive germplasm accessions were evaluated along with 12 adapted lines. We hypothesized that there is genetic variation among the PI lines and sorghum minicore germplasm for post-flowering drought stress, Fusarium stalk rot (F. thapsinum) and charcoal rot (M. phaseolina) resistance. Identifying potential sources with post-flowering drought tolerance traits will combat disease development and result in increased grain yield. This study was conducted with the following objectives: (i) evaluate and identify sorghum exotic germplasm for drought, Fusarium stalk rot, and charcoal rot tolerance, and (ii) to identify traits confering tolerance to drought and stalk rots diseases.
Materials and methods
Genotypes and experimental design
A total of 245 minicore germplasm (10% from the 2247 core and 1% of the entire 37,000 world collections) were evaluated and identified in 2001 at ICRISAT (International Crops Research Institute for the Semi-Arid Tropics, Hyderabad, India) using hierarchical cluster analysis based on 11 qualitative and 10 quantitative traits (Upadhyaya et al. 2009). Of the 245 minicore germplasm, 84 photoperiod insensitive accessions with diverse origins and wide variability in flowering and plant height were used in this study. An additional 44 plant introductory (PI) exotic germplasm (fertility restoring R lines) of diverse origins were selected based on a preliminary drought stress field screening at the Agricultural Research Center, Hays, Kansas in 2008 and 2009 (data not presented) and 12 adapted B (male sterile maintainer) and R (fertility restorer) check lines: SC399B and BTx399 (parental lines used in hybrid seed production), RTx7078 (pre-flowering drought tolerant and post-flowering drought susceptible), SC599R (stalk rot resistant and post-flowering drought tolerant), AjabsidoR and KS19R (post-flowering drought tolerant), SC35R (charcoal rot resistant), 1790E R and BTx642 (stay-green), RTx7000 (susceptible to stalk rot and charcoal rot), BTx3042 (large seeded) and Laing Tang Ai R (high transpiration efficiency; Xin et al. 2009) were included in this study. The details of the genotypes used in this study are presented in Table 1.
Table 1.
List of exotic germplasm and adapted sorghum lines used in the study
| Entry* | Genotype | Origin | Entry | Genotype | Origin | Entry | Genotype | Origin |
|---|---|---|---|---|---|---|---|---|
| Photoperiod insensitive minicore germplasm (84) | Other exotic germplasm (44) | |||||||
| 1 | IS608 | USA | 47 | IS26737 | South Africa | 85 | PI257309 | Argentina |
| 2 | IS995 | USA | 48 | IS26749 | South Africa | 86 | PI295121 | Australia |
| 3 | IS1212 | China | 49 | IS28449 | Yemen | 87 | PI236278 | Australia |
| 4 | IS1219 | China | 50 | IS28451 | Yemen | 88 | PI510898 | Botswana |
| 5 | IS1233 | China | 51 | IS28614 | Yemen | 89 | PI510920 | Botswana |
| 6 | IS2205 | India | 52 | IS29187 | Swaziland | 90 | PI291382 | China |
| 7 | IS2389 | South Africa | 53 | IS29233 | Swaziland | 91 | PI408822 | China |
| 8 | IS2397 | South Africa | 54 | IS29304 | Swaziland | 92 | PI548007 | China |
| 9 | IS2426 | Afghanistan | 55 | IS29314 | Swaziland | 93 | PI548034 | China |
| 10 | IS2864 | South Africa | 56 | IS29326 | Swaziland | 94 | PI391652 | China |
| 11 | IS2872 | Egypt | 57 | IS29335 | Swaziland | 95 | PI610730 | China |
| 12 | IS3946 | India | 58 | IS29358 | Lesotho | 96 | PI276797 | Ehiopia |
| 13 | IS3971 | India | 59 | IS29468 | Lesotho | 97 | PI576380 | Ehiopia |
| 14 | IS4515 | India | 60 | IS59519 | Lesotho | 98 | PI262568 | Fmr. Soviet Union |
| 15 | IS4631 | India | 61 | IS29582 | Lesotho | 99 | PI267109 | Fmr. Soviet Union |
| 16 | IS4698 | India | 62 | IS29627 | South Africa | 100 | PI550685 | Fmr. Soviet Union |
| 17 | IS5094 | India | 63 | IS29654 | China | 101 | PI585330 | Hungary |
| 18 | IS8348 | Pakistan | 64 | IS29689 | Zimbabwe | 102 | PI586440 | Hungary |
| 19 | IS8777 | Uganda | 65 | IS29733 | Zimbabwe | 103 | PI267392 | India |
| 20 | IS12302 | Zimbabwe | 66 | IS30231 | Zimbabwe | 104 | PI267379 | India |
| 21 | IS12706 | USA | 67 | IS30383 | China | 105 | PI533946 | india |
| 22 | IS12735 | Saudi Arabia | 68 | IS30507 | Korea | 106 | PI562891 | India |
| 23 | IS12804 | Turkey | 69 | IS30508 | Korea | 107 | PI536516 | Maldives |
| 24 | IS12883 | India | 70 | IS30533 | Korea | 108 | PI264451 | Spain |
| 25 | IS12945 | Nicaragua | 71 | IS30536 | Korea | 109 | PI534138 | Sudan |
| 26 | IS13782 | South Africa | 72 | IS30562 | Korea | 110 | PI562166 | Sudan |
| 27 | IS14010 | South Africa | 73 | IS32295 | India | 111 | PI550590 | Ukraine |
| 28 | IS14090 | Argentina | 74 | IS33844 | India | 112 | PI534052 | Uganda |
| 29 | IS14290 | Botswana | 75 | IS473 | India | 113 | PI591002 | USA |
| 30 | IS17941 | India | 76 | IS602 | India | 114 | PI562723 | USA |
| 31 | IS19389 | Bangladesh | 77 | IS27912 | South Africa | 115 | PI475432 | Yemen |
| 32 | IS19445 | Botswana | 78 | IS20743 | India | 116 | PI533916 | Zaire |
| 33 | IS19450 | Botswana | 79 | IS20727 | India | 117 | PI565174 | Zimbabwe |
| 34 | IS20697 | USA | 80 | IS19676 | India | 121 | PI570959 | Sudan |
| 35 | IS20816 | USA | 81 | IS16151 | India | 122 | PI570895 | Sudan |
| 36 | IS21863 | Syria | 82 | IS19262 | Sudan | 123 | PI568992 | Sudan |
| 37 | IS22294 | Botswana | 83 | ICSR89058 | India | 124 | PI 571032 | Sudan |
| 38 | IS22616 | Myanmar | 84 | IS4581 | Yemen | 125 | PI 563146 | Sudan |
| 39 | IS23992 | Yemen | 126 | PI571165 | Sudan | |||
| 40 | IS24348 | India | 127 | PI569810 | Sudan | |||
| 41 | IS24365 | India | 128 | PI568323 | Sudan | |||
| 42 | IS24453 | South Africa | 129 | PI569809 | Sudan | |||
| 43 | IS24463 | South Africa | 130 | PI534052 | Uganda | |||
| 44 | IS24492 | South Africa | 131 | PI563253 | Uganda | |||
| 45 | IS26694 | South Africa | ||||||
| 46 | IS26701 | South Africa | Adapted B and R check lines (12) | |||||
| 118 | BTx399 | Texas, USA | ||||||
| 119 | Laing TangAi R | China | ||||||
| 120 | Ajabsido R | Sudan | ||||||
| 132 | KS19R | Kansas, USA | ||||||
| 133 | SC599R | Converted line | ||||||
| 134 | 1790E R | Texas, USA | ||||||
| 135 | BTx642 (B35) | Texas, USA | ||||||
| 136 | SC35R | Converted line | ||||||
| 137 | SC399B | Converted line | ||||||
| 138 | BTx3042 | Texas, USA | ||||||
| 139 | RTx7078 | Texas, USA | ||||||
| 140 | RTx7000 | Texas, USA | ||||||
Experiments were conducted at the Agricultural Research Center, Hays, Kansas (38.979°N latitude, 99.326°W longitude; 611 m elevation above sea level), under irrigated and dryland conditions in summer 2011. A total of 140 genotypes were sown in a randomized complete block design with two replications. The soil type at the experimental site was a Harney silt loam (fine smectitic mesic Typic Argiustoll) and was fertilized with 89.6 kg N ha-1 during planting. Seeds of each genotype were treated with fungicide (ethanethiol or ethyl mercaptan (Captan) @ 2 mL kg-1 seed) and were planted in single-row plots with 9.14 m length and 0.76 m width with 100 seeds per row.
Irrigation and environmental conditions
Experiments under irrigated conditions were managed by providing additional irrigation of 35.0, 32.7 and 40.8 mm at 40 (14 July 2011), 52 (26 July 2011) and 86 (29 Aug 2011) days after planting (DAP), respectively through a skip furrow-row irrigation system. Irrigation was provided before the plants showed wilting symptoms (leaf rolling). The dryland experiment was rainfall dependant. The rainfall received was 276 mm during the crop growth period and it came within 34 days of cropping season. The number of rainy days (> 5 mm) received during the cropping season were 16 with 3, 3, 4, 1 and 5 days in June, July, Aug, Sep and Oct, respectively (Additional file 1: Figure S1). Therefore, the crop experienced drought stress during 80 to 110 DAP. In addition, there was a 19% decrease in total rainfall during the cropping season compared to the normal average over 30 years. The average air temperature during seedling and vegetative stages of the cropping season ranged from 25 to 29°C (Additional file 1: Figure S1), while the temperature during flowering and grain filling ranged from 18 to 27°C.
Drought stress
Three plants per genotype in each replication, both in the irrigated and dryland experiments, were selected based on phenotypic uniformity namely flowering date, plant height and panicle exsertion. The plants were tagged to record PS (photosystem) II quantum yield (Fv/Fm), canopy temperature and chlorophyll content (SPAD). Chlorophyll content was measured at 59, 76 and 103 DAP; PS II quantum yield at 67 and 83 DAP; and leaf temperature at 61 and 81 DAP. Chlorophyll content was recorded on the flag leaf using the SPAD (Soil and Plant Analytical Development) meter (Minolta SPAD 502, Spectrum Technologies, Inc., Plainfield, Illinois, USA). Leaf temperature was measured from the flag leaf on each tagged plant using a forward-looking infrared (FLIR) camera (EXTECH i5, Extech Instruments Corp., Nashua, New Hampshire, USA) on clear, hot sunny days between 11.00 am and 2.00 pm. PS II quantum yield was measured from 30-min dark-adapted flag leaves (Prasad et al. 2008) using a pulse modulated, hand held, OS-30 p chlorophyll fluorometer (Opti-Sciences, Hudson, New Hampshire, USA). Plant height (cm) was measured from ground to the tip of the panicle on the main stem. Days to mid-anthesis was recorded as number of days from planting until 50% of the panicles were at mid-anthesis on a whole plot basis. At harvest, grain yield and biomass were obtained from each genotype by arbitrarily sampling three uniform plants and calculating harvest index (HI) using the procedure of Prasad et al. (2008).
Disease stress
Inoculum preparation
Inoculum suspensions of Fusarium thapsinum (stalk rot) and Macrophomina phaseolina (charcoal rot) pure cultures were initiated in potato dextrose broth. The suspensions were incubated on a shaker at room temperature until conidia (F. thapsinum) or mycelium (M. phaseolina) was produced. F. thapsinum suspensions were strained through four layers of cheesecloth to separate conidia from mycelial masses. M. phaseolina did not produce conidia, therefore the mycelium was blended, and the suspension was strained through cheesecloth, as described for F. thapsinum, to separate small hyphal fragments. Concentrations of conidia or hyphal fragments were determined using a hemacytometer or counting chamber. The final concentration of both pathogens was adjusted to 2 × 105 conidia or hyphal fragments mL-1 by diluting with 10 mM phosphate-buffered saline (pH 7.2).
Field inoculation and measurements
During anthesis, a total of nine plants per genotype in each replication in both dryland and irrigated experiments were tagged for artificial inoculation with F. thapsinum, M. phaseolina, and sterile water (control), respectively (i.e. three plants per treatment). Plants were inoculated at 10 days after flowering at the rate of 1 mL of inoculum per plant between the bottom-most node and brace node. Artificial inoculation was performed using a syringe and pre-wounding the plant with a fine-bit drill. Plants were evaluated for resistance to stalk and charcoal rot by measuring the lesion length by splitting the stem at 28 days post-inoculation (DPI) and measuring the spread of red discoloration along the length of the pith from the point of inoculation. Panicles were harvested from the same plants to compare the yield reduction (%) due to stalk and charcoal rot.
Data analysis
Analysis of variance was carried out using the PROC GLM procedure of SAS (version 9.1.3) by evaluating genotype effects for each environment. A randomized complete block design was used with two replications. Means separation was carried out by environment for both drought and disease related traits using Fischer’s protected LSD (SAS, v 9.1.3). The data on chlorophyll content, leaf temperature and PS II quantum yield at various stages were averaged to get the main effects of genotype. Genotypes were grouped into tolerant and susceptible for each trait based on their mean performance across the environments. Combined analyses (both irrigated and dryland) were done to identify tolerant and susceptible genotypes for each trait. Relative performance of a genotype was calculated by comparing to the check genotype BTx642 for drought related traits. Variance components for genotype were estimated and used to calculate repeatability estimates to increase precision in selection based on repeatability (Knapp et al. 1987 and represents the proportion of total variance in multiple measurements of a trait that is due to differences among genotypes (Dohm 2002). Repeatability values were obtained by subtracting the fraction of the total phenotypic variance attributable to variance between repetitions (Falconer and Mackay 1996) using type III sum of squares in PROC GLM in SAS 9.1 (SAS Institute, Cary, NC). Principle component analysis (PCA) was performed to evaluate associations among the genotypes and among the variable using the XLStat (Addinsoft, Paris, France) software package.
Results
Genotype reaction to drought stress
Mean performance and analysis of variance of the genotypes for chlorophyll content, PS II quantum yield and leaf temperature for both environments averaged over different stages of observation and other agronomic traits are presented in Additional file 2: Tables S1 and S3 and were used to understand the reaction of the genotypes to drought stress as it showed significant differences among all genotypes for all the traits. The mean plant height among genotypes over two environments was 119 cm. Genotypes exhibited an average plant height of 175 and 223 cm under dryland and irrigated conditions, respectively (Table 2). Of the 140 genotypes, 105 exhibited mean plant heights ranging from 150 to 331 cm and the remaining were < 150 cm (Additional file 2: Table S1). Across environments, genotypes IS23992, IS28449, and PI408822 were the tallest (288 to 331 cm), and BTx399, SC299, and BTx3042 were the shortest (75 to 82 cm) (Additional file 2: Table S1). Days to flowering ranged from 61 to 105 days over two environments. Most of the exotic germplasm accessions were in the range of 82 to 106 days to 50% flowering. All adapted lines flowered between 61 and 75 DAP. Genotypes IS8777, IS26694 and IS14290 were late (100 to 106 days) and IS12804, IS12706 and PI291382 reached early 50% flowering (Additional file 2: Table S3).
Table 2.
Mean performance of agronomic and drought stress related physiological traits
| Environment | Plant height (cm) | Chlorophyll content (SPAD value) | PS II quantum yield | LT (°C) | Days to flowering | Grain yield (g plant-1) | HI |
|---|---|---|---|---|---|---|---|
| Dry land | 175.3b* | 44.59b | 0.751b | 37.06a | 81.89a | 20.79b | 0.35b |
| (60.10-297.51) † | (22.66-61.92) | (0.70-0.78) | (33.83 - 40.4) | (59.10-101.2) | (4.81-66.40) | (0.02-0.37) | |
| Irrigated | 222.2a | 48.84a | 0.759a | 36.09b | 81.53a | 37.62a | 0.42a |
| (72.53-365.21) | (25.04-63.65) | (0.61-0.79) | (34.23-42.46) | (60.12-104.41) | (7.12-77.97) | (0.03-0.45) | |
| LSD (0.05) | 5.72 | 0.71 | 0.0045 | 0.58 | 0.91 | 0.88 | 0.1 |
SPAD = chlorophyll content; LT = leaf temperature; HI = harvest index; *Means followed by same letters in a column are not significantly different at LSD (P < 0.05); †Values in the parentheses indicate the mean range.
Drought stress in dryland conditions decreased chlorophyll content (SPAD value), grain yield and HI by 9, 44 and 17%, respectively, over irrigated conditions (Table 2). Based on the average of three measurements, genotypes PI264451, PI267109 and BTx3042 had increased chlorophyll content between 15 and 21% compared to the check BTx642. In contrast, genotypes SC 399, IS29654, IS1219 and IS995 had decreased (28 to 55%) chlorophyll content compared to BTx642 under dryland conditions. Similarly, the genotypes, IS28451, RTx7000 and BTx642 had increased PS II quantum yield (0.78 to 0.79) and IS2426, SC399 and IS1219 had decreased PS II quantum yield (0.67 to 0.71; Additional file 2: Table S3) under dryland conditions.
Under dryland conditions, genotypes RTx7000, PI562723, IS24453, PI475432, and IS2397 had increased leaf temperature (41 to 42°C) and Ajabsido, KS19, PI570895, IS29654 and IS26694 had decreased leaf temperature (34 to 36°C) (Table 3). The check (BTx642) had a leaf temperature of 40°C. However, there was not much variation in leaf temperature (35 to 37°C) among these genotypes under irrigated condition (Table 3). Under dryland conditions, genotypes with increased leaf temperature had a grain yield of 15 to 35 g plant-1. However, the genotypes with lower leaf temperatures had a grain yield of 7.7 to 43 g plant-1 (Table 3). The top five genotypes showed a 2.6 to 3.8% increase in leaf temperature, while the bottom five showed a 6 to 16% decrease in leaf temperature when compared to BTx642 (Additional file 2: Table S3). Genotypes PI510898, IS1212 and PI533946 had higher yields under dryland conditions which is 57, 38 and 38% increase over the check BTx642. Similarly, genotypes PI408822, Ajabsido and IS26737 had high yields under irrigated conditions (Additional file 2: Table S3). Mean HI was high (0.42) in irrigated when compared to dryland (0.35) conditions (Table 2). Genotypes PI475432, IS3971 and IS30562 exhibited high (0.48 to 0.57) and IS26694, IS14290 and IS12945 exhibited low (<0.1) HI under dryland conditions (Additional file 2: Table S3) when compared to other genotypes. Genotypes PI570959, PI565174, IS17941 and IS12883 showed a 6 to 52% decrease in HI when compared to BTx642 under the dryland environment.
Table 3.
Mean performance of genotypes based on leaf temperature (LT), grain yield and harvest index (HI) under dryland and irrigated environments
| Dryland environment | Irrigated environment | |||||
|---|---|---|---|---|---|---|
| Genotype | LT (°C) | Grain yield (g plant-1) | HI | LT (°C) | Grain yield (g plant-1) | HI |
| Top five genotypes | ||||||
| RTx7000 | 42.3 | 30.4 | 0.33 | 35.4 | 40.1 | 0.36 |
| PI562723 | 42.3 | 22.3 | 0.34 | 35.2 | 14.6 | 0.40 |
| IS24453 | 41.5 | 15.0 | 0.19 | 36.8 | 49.2 | 0.13 |
| PI475432 | 41.5 | 34.4 | 0.37 | 35.1 | 39.0 | 0.16 |
| IS2397 | 41.4 | 15.5 | 0.18 | 36.6 | 42.2 | 0.30 |
| Bottom five genotypes | ||||||
| Ajabsido | 34.8 | 27.2 | 0.37 | 36.1 | 71.1 | 0.29 |
| KS19R | 35.8 | 24.3 | 0.44 | 36.6 | 55.7 | 0.31 |
| PI570895 | 36.3 | 43.3 | 0.40 | 36.0 | 55.3 | 0.26 |
| IS29654 | 36.4 | 7.7 | 0.16 | 37.2 | 1.16 | 0.04 |
| IS26694 | 36.4 | 12.0 | 0.10 | 36.5 | 45.0 | 0.13 |
PCA for drought related traits
PC1 separated the genotypes based on plant height and days to flowering. PC2 separated the genotypes based on chlorophyll content, PS II quantum yield, leaf temperature and HI (data not shown). The tallest (IS23992, IS5094, IS28451 and IS28614) and the shortest (BTx399 and 1790E) genotypes were differentiated by the PC1 loading value. A clear differentiation was observed between genotypes (PI267109, PI264451 and BTx3042) with increased and decreased (IS29326) chlorophyll content and HI. The PC2 loading value differentiated the genotypes with increased PSII quantum yield (IS28451, IS23992 and IS28614) and PC1 loading value with decreased PSII quantum value and delayed flowering (IS29326 and IS2670) (Figure 1).
Figure 1.
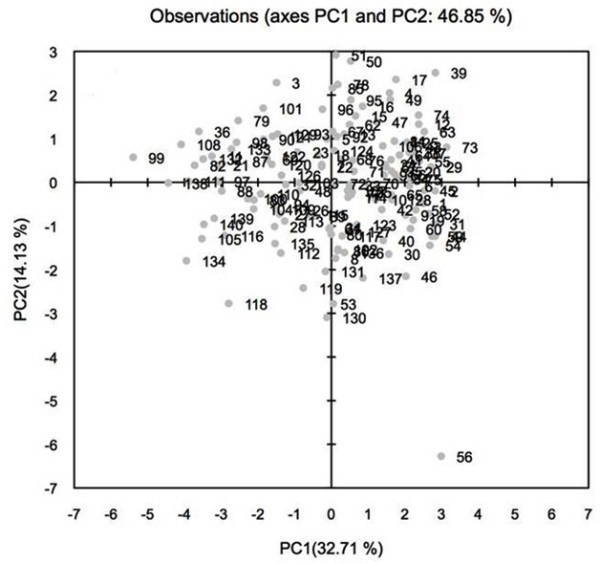
Two dimensional plot of PC1 (principle component) versus PC2 for 140 sorghum genotypes segregating for drought related traits under both environments. Note: List of 140 genotypes described in Table 1.
Genotype reaction to Fusarium stalk rot and Charcoal rot inoculation
Significant effects of genotypes were observed for Fusarium stalk rot and charcoal rot lesion length under both environments (Additional file 2: Table S2). Mean lesion length among the genotypes for both pathogens inoculations were 8.4 and 9.3 cm in dryland and 10.4 and 13.0 cm in irrigated environments, respectively (Table 4).
Table 4.
Mean performance of Fusarium stalk rot and charcoal rot on lesion length and grain yield in sorghum exotic germplasm and adapted lines
| Environment | Lesion length (cm) | Grain yield (g plant-1) | ||||
|---|---|---|---|---|---|---|
| F. thapsinum | M. phaseolina | Control | F. thapsinum | M. phaseolina | Control | |
| Dryland | 8.44b*(0.11-38.10) | 9.39b (0.10-35.58) | 2.90b (0.11-4.55) | 31.52b (0.85-72.55) | 35.73b (6.91-78.86) | 33. 55b (0.23-79.15) |
| Irrigated | 10.47a (0.12-40.50) | 13.01a (0.11-64.10) | 4.67a (0.02-5.56) | 42.06a (2.43-79.20) | 43.94a (5.25-84.89) | 40.47a(1.25-87.47) |
| LSD (0.05) | 0.92 | 0.86 | 0.93 | 2.97 | 3.51 | 2.87 |
*Means followed by same letters are not significantly different according to LSD (P < 0.05) within trait and between environments; Values in parentheses indicate the mean range.
Under dryland conditions, genotypes IS30562, IS14010 and PI510920R had lower lesion length of (2.7 to 4.5 cm) for Fusarium stalk rot with grain yield ranging from 31.4 to 37.3 g plant-1. However, the genotypes IS30536, IS29654, and IS12706 had lesion lengths of 14.0 to 29.2 cm with grain yield ranging from 7.6 to 14.0 g plant-1 (Table 5). Similarly, the top three genotypes were with lower lesion length (2.8 to 3.0 cm) for M. phaseolina with a grain yield ranging from 16.4 to 47.7 g plant-1. The genotypes with increased lesion length (17.4 to 38.0 cm) had a grain yield ranging from 18.3 to 28.9 g plant-1. It is obvious from the present study that there are genotypes with lower lesion lengths and higher grain yield (Table 5).
Table 5.
Mean performance of genotypes (top and bottom) for lesion length and grain yield in response to Fusarium stalk and charcoal rot under the dryland environment
| Genotype | F. thapsinum | Genotype | M. phaseolina | ||||
|---|---|---|---|---|---|---|---|
| Lesion length (cm) | F. thapsinum grain yield (g plant-1) | Control grain yield (g plant-1) | Lesion length (cm) | M. phaseolina grain yield (g plant-1) | Control grain yield (g plant-1) | ||
| Top five genotypes | |||||||
| IS30562 | 4.50 | 37.30 | 47.45 | 1790E R | 2.77 | 16.43 | 20.36 |
| IS14010 | 4.42 | 34.98 | 46.45 | IS 26749 | 3.00 | 37.75 | 49.25 |
| PI510920R | 2.75 | 31.43 | 35.73 | BTx399 | 3.03 | 47.73 | 55.20 |
| BTx399 | 6.92 | 30.28 | 55.20 | IS19445 | 3.58 | 29.1 | 38.25 |
| PI510898R | 3.50 | 29.45 | 58.65 | IS24463 | 4.33 | 39.98 | 42.95 |
| Bottom five genotypes | |||||||
| IS30536 | 29.17 | 7.60 | 17.33 | PI568323R | 38.00 | 26.00 | 28.85 |
| IS29654 | 14.00 | 10.45 | 27.75 | PI548034R | 28.25 | 18.30 | 46.85 |
| IS12706 | 19.20 | 14.04 | 34.14 | IS12706 | 17.42 | 25.71 | 34.14 |
| PI548007R | 18.67 | 15.25 | 28.89 | IS16151 | 14.92 | 28.88 | 30.75 |
| RTx7078 | 12.30 | 25.01 | 30.29 | PI391652R | 10.97 | 25.19 | 48.88 |
Under irrigated conditions, it was observed that different sets of genotypes were found to be tolerant and susceptible for both pathogens when compared to dryland conditions (Table 6). The control plants (water inoculated) had higher grain yield than plants inoculated with either pathogen. Genotypes IS59519, PI562166R and IS14090 had the lowest lesion length (F. thapsinum inoculated) of 5.9 to 7.3 cm with a grain yield ranging from 38.6 to 54.3 g plant-1 when compared to genotypes IS30533, IS608 and IS22616 having the highest lesion length of 18.1 to 28.3 cm with the lowest grain yield ranging from 12.8 to 24.1 g plant-1 (Table 6). Similarly, genotypes KS19R, IS22294 and IS12706 had the lowest lesion length (M. phaseolina inoculated) of 2.5 to 6.5 cm with the highest grain yield ranging from 35.1 to 42.1 g plant-1 when compared to genotypes IS33844, PI570895R and PI548007R, which had the highest lesion length of 15.5 to 33.0 cm, with the lowest grain yield ranging from 15.3 to 28.9 g plant-1 (Table 6).
Table 6.
Mean performance of genotypes (top and bottom) for lesion length and grain yield in response to Fusarium stalk rot and charcoal rot under the irrigated environment
| Genotype | F. thapsinum | Genotype | M. phaseolina | ||||
|---|---|---|---|---|---|---|---|
| Lesion length (cm) | F. thapsinum Grain yield (g plant-1) | Control Grain yield (g plant-1) | Lesion length (cm) | M. phaseolina Grain yield (g plant-1) | Control Grain yield (g plant-1) | ||
| Top five genotypes | |||||||
| IS59519 | 7.25 | 54.33 | 64.20 | KS19R | 2.50 | 42.13 | 42.43 |
| PI562166R | 7.00 | 44.13 | 69.05 | IS22294 | 6.50 | 35.90 | 66.05 |
| IS14090 | 5.88 | 38.60 | 49.08 | IS12706 | 6.25 | 35.15 | 40.62 |
| PI576380R | 6.50 | 37.33 | 44.78 | IS23992 | 6.00 | 33.85 | 68.25 |
| IS29582 | 8.13 | 35.97 | 27.64 | PI267379R | 5.75 | 30.98 | 41.08 |
| Bottom five genotypes | |||||||
| IS30533 | 28.25 | 12.78 | 61.98 | IS33844 | 33.00 | 15.35 | 62.85 |
| IS608 | 18.13 | 22.18 | 34.10 | PI570895R | 32.88 | 26.05 | 49.38 |
| IS22616 | 18.50 | 24.13 | 36.10 | PI548007R | 17.00 | 28.90 | 47.00 |
| Laing TangAi R | 21.50 | 26.05 | 32.10 | IS20743 | 19.00 | 16.30 | 18.04 |
| PI548034R | 25.50 | 31.30 | 83.05 | IS608 | 15.50 | 23.25 | 34.10 |
PCA for disease related traits
Disease related traits revealed 76.3% of the variability explained by the first two PCA components. PC1 and PC2 respectively accounted 50.5 and 25.8% of the total variability for lesion length (Figure 2). Component loading revealed the highest positive values of PC1 with increased lesion length due to F. thapsinum and M. phaseolina inoculation indicating susceptibility to these pathogens (data not shown). Lower negative PC1 and positive PC2 values represent shorter lesion length and indicate genotypes with tolerance to Fusarium stalk rot and charcoal rot.
Figure 2.
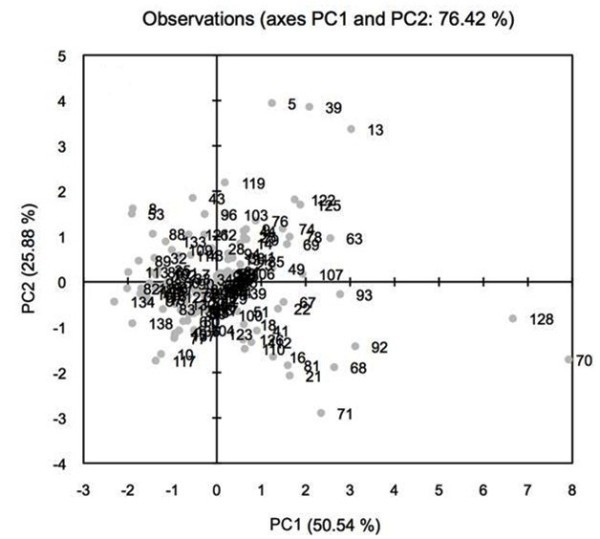
Two dimensional plot of PC1 (principle component) versus PC2 for 140 sorghum genotypes segregating for stalk rot and charcoal rot lesion length. Note: List of 140 genotypes described in Table 1.
Discussion
The present study showed wide genetic variability among the sorghum genotypes for drought and disease tolerance, which provides more opportunities for effective selection. Post-flowering drought stress significantly increased chlorophyll loss and decreased PS II quantum yield in all the genotypes (Table 2). The reduction in chlorophyll content and PS II quantum yield activities under drought stress could be due to thylakoid membrane damage caused by increased production of reactive oxygen species (Tambussia et al. 2000). Enzymes involved in chlorophyll biosynthesis are bound to membranes (Tewai and Tripathy 1998); and dilation of thylakoids by drought stress can also cause chlorophyll loss (Bukhov et al. 1999). Drought stress decreased PS II quantum yield indicating increased permeability of thylakoid membranes resulting in proton leakage across the thylakoid membrane which decreases ATP and NADPH production and decreased photochemical efficiency (Schrader et al. 2004). Miyashita et al. (2005) found that PS II quantum yield level was decreased in kidney beans (Phaseolus vulgaris L.) due to drought stress, suggesting reduction in electron transfer to photosystem II. Mutava et al. (2011) observed similar results in grain sorghum genotypes. In the present study, genotypes PI264451 and PI267109 had increased chlorophyll content, and IS28451 and RTx7000 had increased PS II quantum yield under dryland condition (Additional file 2: Table S1). The above-mentioned genotypes can be used in drought tolerance breeding for introgression of the stay green trait.
Seversike et al. (2012) showed that air temperature altered transpiration responses to vapor pressure deficit (VPD). Controlled transpiration rate under moisture limited condition shows drought tolerance in crop plants. Under environments where VPD is high, genotypes having limited transpiration rate may yield more due to significant water savings. Leaf temperature is positively correlated with transpiration rate and can be of a surrogate measure for drought tolerance. Our data showed that genotypes RTx7000 and PI475432 had increased leaf temperature (42 to 41°C) in the dryland environment with slightly higher grain yield (30 to 34 g plant-1) relative to the other genotypes. Since, RTx7000 is a non-stay green-genotype, it contrasts with the finding of Jordan et al. (2012) that the stay-green trait may result from decreased water use early in the season, allowing water to be conserved to sustain a longer period of grain filling. However, it confirms to the finding of Choudharya et al. (2013) that genotypes expressing the breakpoint in transpiration rate with increasing VPD were found among both stay-green and non-stay-green genotypes. However, the genotype PI570895 had a leaf temperature of 36°C and grain yield of 43 g plant-1 (Additional file 2: Table S3) indicating that this genotype may have availed more soil moisture and cooled the canopy favors higher grain yield (Table 3). This type of genetic variability was also observed by Mutava et al. (2011) on sorghum. Similar extreme variation was observed by Gholipoor et al. (2010) among sorghum genotypes in transpiration response to vapor pressure deficit (VPD). They identified seventeen sorghum genotypes exhibiting a breakpoint in their VPD response in the range from 1.6 to 2.7 kPa, above which there was little or no increase in transpiration and concluded the possibility of soil water conservation upon utilizing these genotypes. Overall, considering leaf temperature, chlorophyll content, and PS II quantum yield, genotypes PI510898, IS1212, PI533946, PI550590, IS2872 and PI562166 may have drought tolerance ability as evidenced by higher grain yield under dryland conditions (Additional file 2: Table S3). Repeatability estimates for SPAD, PS II quantum yield, plant height, days to flowering, lesion length for Fusarium stalk rot, charcoal rot and grain yield were moderate to higher for both environments (Additional file 2: Tables S1 and S2). These repeatability estimates indicated that these traits can be improved through selection as this tool quantifying the extent to which one trait’s performance remains consistent over environments.
Growing environment significantly affected Fusarium stalk rot. The weather during the cropping season was characterized by intermittent rainfall and high temperature stress early in the season. However, later in the season wet and cool weather was followed by dry conditions (Additional file 1: Figure S1), which favors disease development (Tesso et al. 2012). Under dryland condition, moisture stress later in the season was associated with significantly lower lesion length compared to the irrigated environment (Table 4). These results were consistent with the results reported earlier by Tesso et al. (2004), which showed that lower mean disease score was due to lack of adequate late season moisture in sorghum. Significant differences in mean lesion length were noted between genotypes (Table 4). These differences are assumed to reflect differences in disease susceptibility of the genotypes through mobilization of stalk carbohydrate reserves to developing seed during grain development (Tesso et al. 2012) rather than differences in host defense responses. Because of lost reserves, the stalk tissue might become senescent earlier to favor stalk and charcoal rot infection. Lower lesion lengths for both stalk rot and charcoal rot inoculation were observed in the genotype IS30562 and 1790E R, respectively under the dryland environment. The common susceptible sources for both diseases were IS12706 with high mean lesion length under the dryland condition (Table 5). Similarly, IS59519 and PI562166R were tolerant to Fusarium stalk rot and KS19R and IS22294 were tolerant to M. phaseolina rot under irrigated conditions (Table 6). The genotypes IS30533 and IS33844 were found to be susceptible to Fusarium stalk rot and charcoal rot under irrigated conditions (Table 6).
BTx399, a stay-green line showed a high degree of tolerance to M. phaseolina, with low lesion length relative to other adapted lines, (Tuinstra et al. 1996; Xu et al. 2000; Kebede et al. 2001). This line was also drought tolerant and Fusarium stalk rot resistant (Tao et al. 1993). This may be due to the retention of a higher proportion of assimilates in the stem by stay green lines later in the growing season (Seetharama et al. 1991). Tesso et al. (2004) reported that SC599 shows high level of resistance to F. proliferatum, and it can be used as a potential source of resistance to both Fusarium stalk rot disease and charcoal rot. Tenkouano et al. (1993) also reported that B35, which is a close relative of SC35 and SC599 and their hybrids showed high levels of resistance to M. phaseolina. These results indicate that stalk resistance in SC599 and B35 are different and cannot be equated (Tenkouano et al. 1993). The simplest explanation for these differences is that different genes are involved in regulating stalk rot resistance in these two genetic backgrounds (Tesso et al. 2005). Similarly, BTx399 may also have a different genetic background, which governs the resistance to Fusarium stalk rot and charcoal rot.
PCA on drought related traits revealed that PC1and PC2 accounted 34.5% and 14.3% of the total variability respectively. Further, PCA revealed a significant negative correlation between days to flowering and HI. This makes sense since late flowering results in a shorter grain filling period in sorghum, where the duration of the reproductive stage is fixed (Barnabas et al. 2008). In this study, plant height exhibited significant positive correlation with days to flowering (r = 0.37, P < 0.0001) and negative correlation with harvest index (r = −0.56, P < 0.0001). A similar relationship was observed in earlier studies by Murray et al. (2008), Ritter et al. (2008) and Zhao et al. (2009) in sorghum. These authors concluded that taller sorghums have the advantages of accumulating more biomass due to greater translocation of photosynthates from the vegetative tissues resulting in late maturity and low grain yield. Also, some tall sorghums may be prone to lodging, which results in low harvest index (Rooney 2004; Murray et al. 2009). Genotypes of this group may utilize the available soil water for vegetative development, leaving no moisture for the grain filling stage concomitant with lower current photosynthesis during post-flowering stages and decreased grain yield. A significant positive association was observed between chlorophyll content under dryland and HI under both environments. Richards (2000) suggested that increased photosynthetic rate through improved leaf chlorophyll content can improve grain productivity and HI of the genotype. Leaf temperature had lower negative values of PC2 in both environments and chlorophyll content and PS II quantum yield had positive values. This clearly indicates that genotypes with extreme leaf temperatures (>35°C) might experience damage to leaf photosynthetic apparatus and chlorophyll loss (Djanaguiraman et al. 2011).
PCA on combined drought and disease related traits showed positive correlation between PS II quantum yield, chlorophyll content and HI indicating these traits are associated with drought and disease tolerance (Nazir Mir et al. 1998). However, these have to be verified using proven disease tolerant and susceptible genotypes under controlled and field experiments. Plant height was positively correlated with lesion length indicating that disease development is directly proportional to plant height which was also confirmed by component loading value (Bazzalo et al. 1991). Genotypes PI267109, IS12706, PI533916, BTx3042 and PI291382 were separated from others and showed increased PS II quantum yield (0.74 to 0.77), SPAD (50 to 60) and HI (0.30 to 0.47) with early maturity (61 to 66 days to flowering) under dryland condition (Additional file 2: Table S3). Likewise, genotype IS24463 showed increased tolerance to both pathogens. Combined PCA analysis of drought and disease related traits clearly indicated that chlorophyll content, PS II quantum yield and HI can be used as a screening index for post-flowering drought and disease tolerance (Figure 3). The aforementioned sources can be used to develop potential lines for post-flowering drought tolerance, Fusarium stalk and charcoal rot resistance.
Figure 3.
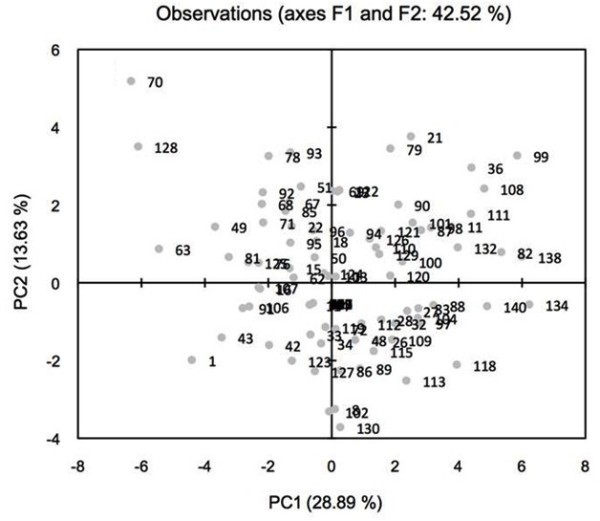
Two dimensional plot of PC1 (principle component) versus PC2 for 140 sorghum genotypes segregating for combined drought and disease related traits. Note: List of 140 genotypes described in Table 1.
Conclusions
Wide variability was observed between the genotypes for chlorophyll content, PS II quantum yield and leaf temperature as well as HI and Fusarium stalk rot and charcoal rot lesion length and grain yield in the sorghum minicore germplasm. Genotypes PI510898, IS1212 and PI533946 showed increased grain yield; modest values of chlorophyll content, PS II quantum yield, and leaf temperature; and early maturity under dryland conditions. Genotypes IS14290, IS12945 and IS1219 showed greater susceptibility to drought with lower grain yield and HI along with late maturity. Genotype IS24463 expressed a high degree of tolerance to both Fusarium stalk rot and charcoal rot and IS12706 and IS16151 were highly susceptible based on mean lesion length and grain yield under dryland conditions. Lesion length and grain yield were negatively correlated with chlorophyll content, PS II quantum yield and HI under both diseases indicating that higher pathogen infection resulted in lower plant health and reduced grain yield. Genotype PI510898 showed combined tolerance to drought and Fusarium stalk rot under dryland conditions. The aforementioned genotypes can be a potential sources for improvement of drought and disease tolerance in future sorghum breeding.
Electronic supplementary material
Additional file 1: Figure S1: Precipitation and average temperature for Hays, KS, during the crop growth period from June to October 2011. Note: *National Weather Service 30-year average (1981 to 2010); “1st (40 DAP)”, “2nd (52 DAP)”, and “3rd (86 DAP)”. (DOCX 64 KB)
Additional file 2: Table S1: Mean square and significance levels for agronomic and drought related traits in sorghum exotic germplasm and adapted lines. Table S2. Mean squares and significance levels (p) from ANOVA for lesion length and grain yield related to Fusarium stalk rot and charcoal rot in sorghum exotic germplasm and adapted lines. Table S3. Mean performance of genotypes for physiological traits and grain yield under dryland and irrigated environments. (JPEG 37 KB)
Acknowledgements
We thank the Kansas Grain Sorghum Commission, United Sorghum Check-off Program and Great Plains Sorghum Improvement and Utilization Center for continuous funding support. The authors gratefully acknowledge Wayne Aschwege and Cody Prosser, Kansas State University, Agriculture Research Center, Hays, KS, for their assistance in planting and measurements. This paper is Contribution No. 13-216-J from the Kansas Agricultural Experiment Station, Manhattan.
Abbreviations
- PCA
Principal component analysis
- DAP
Days after planting
- VPD
Vapor pressure deficit.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MHK, RP, MD, RMA, TT, PVVP and CRL designed the experiments. MHK collected the data and the data was analyzed by MHK and MD. MHK and RP drafted the manuscript and it was corrected by MD, RMA, TT, PVVP and CRL. All authors read and approved the final manuscript.
Contributor Information
Mohankumar H Kapanigowda, Email: mohanhk@k-state.edu.
Ramasamy Perumal, Email: perumal@ksu.edu.
Maduraimuthu Djanaguiraman, Email: jani@k-state.edu.
Robert M Aiken, Email: raiken@ksu.edu.
Tesfaye Tesso, Email: ttesso@k-state.edu.
PV Vara Prasad, Email: vara@k-state.edu.
Christopher R Little, Email: clittle@ksu.edu.
References
- Araus JL, Slafer GA, Reynolds MP, Royo C. Plant breeding and water relations in C3 cereals: what should we breed for? Ann Bot. 2002;89:925–940. doi: 10.1093/aob/mcf049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa Y, Staggenborg SA, Prasad PVV. Crop Manage Online. 2010. Grain sorghum water requirement and responses to drought stress: A review. [Google Scholar]
- Balota M, Payne WA, Rooney WL, Rosenow DT. Gas exchange and transpiration ratio in sorghum. Crop Sci. 2008;48:2361–2371. doi: 10.2135/cropsci2008.01.0051. [DOI] [Google Scholar]
- Barnabas B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Bazzalo ME, Dimarco P, Martinez F, Daleo GR. Indicators of resistance of sunflower plant to basal rot (Sclerotinia sclerotiorum): symptomatological, biochemical, anatomical, and morphological characters of the host. Euphytica. 1991;57:195–205. doi: 10.1007/BF00039666. [DOI] [Google Scholar]
- Blum A. Sorghum physiology. In: Nguyen HT, Blum A, editors. Physiology and Biotechnology Integration for Plant Breeding. New York: Marcel Dekker; 2004. pp. 141–223. [Google Scholar]
- Borell AK, Hammer GL, Douglas ACL. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Sci. 2010;40:1026–1037. doi: 10.2135/cropsci2000.4041026x. [DOI] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U. Heat sensitivity of chloroplasts and leaves: Leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosyn Res. 1999;59:81–93. doi: 10.1023/A:1006149317411. [DOI] [Google Scholar]
- Choudharya S, Mutava RN, Shekoofa A, Sinclair TR, Prasad PVV. Is the stay-green trait in sorghum a result of transpiration sensitivity to either soil drying or vapor pressure deficit. Crop Sci. 2013;53:2129–2134. doi: 10.2135/cropsci2013.01.0043. [DOI] [Google Scholar]
- Cloud GL, Rupe JC. Influence of nitrogen, plant growth stage, and environment on charcoal rot of grain sorghum caused by Macrophomina phaseolina (Tassi) Goid. Plant Soil. 1994;158:203–210. doi: 10.1007/BF00009495. [DOI] [Google Scholar]
- Diourte M, Starr JL, Jeger MJ. Charcoal rot (M. phaseolina) resistance and the effects of water stress on disease development in sorghum. Plant Pathol. 1995;44:196–202. doi: 10.1111/j.1365-3059.1995.tb02729.x. [DOI] [Google Scholar]
- Djanaguiraman M, Prasad PVV, Boyle DL, Schapaugh WT. High-temperature stress and soybean leaves: leaf anatomy and photosynthesis. Crop Sci. 2011;51:2125–2131. doi: 10.2135/cropsci2010.10.0571. [DOI] [Google Scholar]
- Dohm MR. Repeatability estimates do not always set an upper limit to heritability. Funct Ecol. 2002;16:273–280. doi: 10.1046/j.1365-2435.2002.00621.x. [DOI] [Google Scholar]
- Ejeta G, Knoll JE. Marker-assisted selection in sorghum. In: Varshney RK, Tuberosa R, editors. Genomic-assisted Crop Improvement Genomics Applications in Crops, vol 2. 2007. pp. 187–205. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Longman, New York: Paperback, Subsequent 4th Edition - ISBN 0582243025; 1996. [Google Scholar]
- Gholipoor M, Prasad PVV, Mutava RN, Sinclair TR. Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes. Field Crop Res. 2010;119:85–90. doi: 10.1016/j.fcr.2010.06.018. [DOI] [Google Scholar]
- Harris K, Subudhi PK, Borrell A, Jordan D, Rosenow DT, Nguyen HT, Klein P, Klein R, Mullet JM. Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J Exp Bot. 2007;58:327–338. doi: 10.1093/jxb/erl225. [DOI] [PubMed] [Google Scholar]
- Hassan MH, Salam MA, Arsan MR. Influence of certain factors on severity of stalk rot disease of grain sorghum in Upper Egypt. Aust J Agric Sci. 1996;27:179–189. [Google Scholar]
- Hundekar AR, Anahahosur KH. Pathogenicity of fungi associated with sorghum stalk rot. Karnataka J Agric Sci. 1994;7:291–295. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) fourth assessment report. Cambridge, UK: Climate change, Cambridge University Press; 2007. [Google Scholar]
- Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG. The relationship between stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Sci. 2012;52:1153–1161. doi: 10.2135/cropsci2011.06.0326. [DOI] [Google Scholar]
- Kapanigowda MH. Quantitative trait locus (QTL) mapping of transpiration efficiency related to pre-flower drought tolerance in sorghum [Sorghum bicolor (L.) Moench] College Station, Texas, USA: Dissertation, Texas A&M University; 2011. [Google Scholar]
- Kebede H, Subudhi PK, Rosenow DT, Nguyen HT. Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench) Theor Appl Genet. 2001;103:266–276. doi: 10.1007/s001220100541. [DOI] [Google Scholar]
- Knapp SJ, Ross WM, Stroup WW. Precision of genetic variance and heritability estimates from sorghum population. Crop Sci. 1987;27:265–268. doi: 10.2135/cropsci1987.0011183X002700020029x. [DOI] [Google Scholar]
- Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN. Breeding for drought tolerance: direct selection for yield, response to selection and use of drought tolerant donors in upland and lowland-adapted populations. Field Crop Res. 2008;107:221–231. doi: 10.1016/j.fcr.2008.02.007. [DOI] [Google Scholar]
- Liu X, Zheng Z, Tan Z, Li Z, He C, Liu D, Zhang G, Luo Y. QTL mapping for controlling anthesis-silking interval based on RIL population in maize. Afric J Biotechnol. 2010;9:950–955. [Google Scholar]
- Meehl GA, Arblaster JM, Tebaldi C. Geophys Res Lett. 2007. Contribution of natural and anthropogenic forcing to changes in temperature extremes over the United States. [Google Scholar]
- Miyashita K, Tanakamaru S, Maitani T, Kimura K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ Exp Bot. 2005;53:205–214. doi: 10.1016/j.envexpbot.2004.03.015. [DOI] [Google Scholar]
- Murray SC, Rooney WL, Mitchell SE, Sharma A, Klein PE, Mullet JE, Kresovich S. Genetic improvement of sorghum as a biofuel feedstock: II. QTL for stem and leaf structural carbohydrates. Crop Sci. 2008;48:2180–2193. doi: 10.2135/cropsci2008.01.0068. [DOI] [Google Scholar]
- Murray SC, Rooney WL, Mitchell SE, Kresovich S. Sweet sorghum diversity and association mapping for Brix and height. Plant Genome. 2009;2:48–62. doi: 10.3835/plantgenome2008.10.0011. [DOI] [Google Scholar]
- Mutava RN, Prasad PVV, Tuinstra MR, Kofoid KD, Yu J. Characterization of sorghum genotypes for traits related to drought tolerance. Field Crops Res. 2011;123:10–18. doi: 10.1016/j.fcr.2011.04.006. [DOI] [Google Scholar]
- Nazir Mir M, Wendorf R, Perez BRM. Chlorophyll fluorescence in relation to superficial scald development in apple. J Amer Soc Hort Sci. 1998;123:887–892. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH., Jr Adverse high temperature effects on pollen viability, seed-set, seed yield, and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric For Meteorol. 2006;139:237–251. doi: 10.1016/j.agrformet.2006.07.003. [DOI] [Google Scholar]
- Prasad PVV, Pisipati SR, Mutava RN, Tuinstra MR. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008;48:1911–1917. doi: 10.2135/cropsci2008.01.0036. [DOI] [Google Scholar]
- Richards RA. Selectable traits to increase the crop photosynthesis and yield of grain crops. J Exp Bot. 2000;51:447–458. doi: 10.1093/jexbot/51.suppl_1.447. [DOI] [PubMed] [Google Scholar]
- Ritter KB, Jordan DR, Chapman SC, Godwin ID, Mace ES, McIntyre CL. Identification of QTL for sugar-related traits in a sweet × grain sorghum (Sorghum bicolor L. Moench) recombinant inbred population. Mol Breed. 2008;22:367–384. doi: 10.1007/s11032-008-9182-6. [DOI] [Google Scholar]
- Roháček K, Soukupová J, Barták M. Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. In: Schoefs B, editor. Plant Cell Compartments - Selected Topics. Kerala, India: Research Signpost; 2008. pp. 41–104. [Google Scholar]
- Rooney W. Sorghum improvement–integrating traditional and new technology to produce improved genotypes. Adv Agron. 2004;83:37–109. doi: 10.1016/S0065-2113(04)83002-5. [DOI] [Google Scholar]
- Rosenow DT, Clark LE: Drought and lodging resistance for a quality sorghum crop. Chicago, IL: American Seed Trade Association; 1995:82–97. [Proc 50th Ann Corn and Sorghum Industry Res Conf, Dec 6 – 7, 1995]
- Rosenow DT, Dahlberg JA. Collection, conversion and utilization of sorghum. In: Smith CW, Frederiksen RA, editors. Sorghum: Origin, History. NY, USA: Technology and Production. John Wiley and Sons Inc.; 2000. pp. 309–328. [Google Scholar]
- Rosenow DT, Ejeta G, Clark LE, Gilbert ML, Henzell RG, Borell AK, Muchow RC. Proc Int Conf on Genetic Improvement of Sorghum and Pearl Millet, Sept 23–27, 1996. TX: Lubbock; 1996. Breeding for pre- and post-flowering drought stress resistance in sorghum; pp. 400–411. [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ. 2004;27:725–735. doi: 10.1111/j.1365-3040.2004.01172.x. [DOI] [Google Scholar]
- Seetharama N, Sachan RC, Huda AKS, Gill KS, Rao RN, Bidinger FR, Reddy DM. Effect of pattern and severity of moisture-deficit strew on stalk-rot incidence in sorghum. 2. Effect of source/sink relationships. Field Crop Res. 1991;26:355–374. doi: 10.1016/0378-4290(91)90011-J. [DOI] [Google Scholar]
- Seversike TM, Sermons SM, Sinclair TR, Carter TE, Jr, Rufty TW. Temperature interactions with transpiration response to vapor pressure deficit among cultivated and wild soybean genotypes. Physiol Plant. 2012;148:62–73. doi: 10.1111/j.1399-3054.2012.01693.x. [DOI] [PubMed] [Google Scholar]
- Talebi R. Evaluation of chlorophyll content and canopy temperature as indicators for drought tolerance in Durum wheat (Triticum durum) Aust J Basic Appl Sci. 2011;5:1457–1462. [Google Scholar]
- Tambussia EA, Bartolia CG, Beltranoa J, Guiameta JJ, Araus JL. Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum) Physiol Plant. 2000;108:398–404. doi: 10.1034/j.1399-3054.2000.108004398.x. [DOI] [Google Scholar]
- Tao Y, Manners JM, Ludlow MM, Henzell RG. DNA polymorphisms in grain sorghum (Sorghum bicolor (L.) Moench) Theor Appl Gent. 1993;86:679–688. doi: 10.1007/BF00222656. [DOI] [PubMed] [Google Scholar]
- Tenkouano F, Miller R, Frederiksen RA, Rosenow DT. Genetics of non-senescence and charcoal rot resistance in sorghum. Theor Appl Gen. 1993;85:644–648. doi: 10.1007/BF00220925. [DOI] [PubMed] [Google Scholar]
- Tesso T, Claflin LE, Tuinstra MR. Estimation of combining ability for resistance to Fusarium Stalk Rot in grain sorghum. Crop Sci. 2004;44:1195–1199. doi: 10.2135/cropsci2004.1195. [DOI] [Google Scholar]
- Tesso T, Claflin LE, Tuinstra MR. Analysis of stalk rot resistance and gentic diversity among drought tolerant sorghum gentoypes. Crop Sci. 2005;45:645–652. doi: 10.2135/cropsci2005.0645. [DOI] [Google Scholar]
- Tesso T, Perumal R, Little CR, Adeyanju A, Radwan GL, Prom LK, Magill CW. Sorghum pathology and biotechnology – A fungal disease perspective: Part II. Anthracnose, stalk rot, and downy mildew. Euro J Plant Sci Biotech. 2012;6:31–44. [Google Scholar]
- Tewai AK, Tripathy BC. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998;117:851–858. doi: 10.1104/pp.117.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G. Identification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum. Crop Sci. 1996;36:1337–1344. doi: 10.2135/cropsci1996.0011183X003600050043x. [DOI] [Google Scholar]
- Tuinstra MR, Grote EM, Goldsbrough PB, Ejeta G. Genetic analysis of post-flowering drought tolerance and components of grain development in Sorghum bicolor (L.) Moench. Mol Breed. 1997;3:439–348. doi: 10.1023/A:1009673126345. [DOI] [Google Scholar]
- Upadhyaya HD, Pundir RPS, Dwivedi SL, Gowda CLL, Reddy VG, Singh S. Developing a minicore collection of sorghum for diversified utilization of germplasm. Crop Sci. 2009;49:1769–1780. doi: 10.2135/cropsci2009.01.0014. [DOI] [Google Scholar]
- Xin Z, Aiken R, Burke J. Genetic diversity of transpiration efficiency in sorghum. Field Crop Res. 2009;111:74–80. doi: 10.1016/j.fcr.2008.10.010. [DOI] [Google Scholar]
- Xu W, Subudhi PK, Crasta OR, Rosenow DT, Mullet JE, Nguyen HT. Molecular mapping of QTLs conferring stay-green in sorghum. Genome. 2000;43:461–469. [PubMed] [Google Scholar]
- Zhao YL, Dolat A, Steinberger Y, Wang X, Osman A, Xie GH. Biomass yield and changes in chemical composition of sweet sorghum cultivars grown for biofuel. Field Crops Res. 2009;111:55–64. doi: 10.1016/j.fcr.2008.10.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1: Precipitation and average temperature for Hays, KS, during the crop growth period from June to October 2011. Note: *National Weather Service 30-year average (1981 to 2010); “1st (40 DAP)”, “2nd (52 DAP)”, and “3rd (86 DAP)”. (DOCX 64 KB)
Additional file 2: Table S1: Mean square and significance levels for agronomic and drought related traits in sorghum exotic germplasm and adapted lines. Table S2. Mean squares and significance levels (p) from ANOVA for lesion length and grain yield related to Fusarium stalk rot and charcoal rot in sorghum exotic germplasm and adapted lines. Table S3. Mean performance of genotypes for physiological traits and grain yield under dryland and irrigated environments. (JPEG 37 KB)


